Abstract
The identification of modifiable risk factors for glaucoma progression is needed. Our objective was to determine whether maladaptive coping styles are associated with recent glaucoma progression or worse visual field mean deviation.
A hospital-based case–control study was conducted in the Glaucoma Service of Maisonneuve-Rosemont Hospital in Montreal, Canada. Patients with primary open angle glaucoma or normal tension glaucoma with ≥4 years of follow-up and ≥5 Humphrey visual fields were included. Cases had recent visual field progression as defined according to the Early Manifest Glaucoma Trial pattern change probability maps. Controls had stable visual fields. The Brief Cope questionnaire, a 28-item questionnaire about 14 different ways of coping with the stress of a chronic disease, was asked. Questions were also asked about demographic and medical factors, and the medical chart was examined. Outcomes included glaucoma progression (yes, no) and visual field mean deviation. Logistic and linear regressions were used.
A total of 180 patients were included (82 progressors and 98 nonprogressors). Although none of the 14 coping scales were associated with glaucoma progression (P > 0.05), higher denial was correlated with worse visual field mean deviation (r = −0.173, P = 0.024). In a linear regression model including age, sex, education, depression, intraocular pressure, and family history of glaucoma, greater levels of denial (β = −1.37, 95% confidence interval [CI] −2.32, −0.41), Haitian ethnicity (β = −7.78, 95% CI −12.52, −3.04), and the number of glaucoma medications (β = −1.20, 95% CI −2.00, −0.38) were statistically significantly associated with visual field mean deviation.
The maladaptive coping mechanism of denial was a risk factor for worse visual field mean deviation. Further prospective research will be required to verify the pathways by which denial may exert an effect on glaucomatous visual field loss.
Keywords: Coping, Denial, Epidemiology, Glaucoma, Risk factor
1. Introduction
Glaucoma is a leading cause of blindness in the world.[1] Untreated, the average time to blindness due to open-angle glaucoma is estimated to be 25 years.[2] Intraocular pressure (IOP) is the primary modifiable risk factor that has been identified for glaucoma progression.[3–7] With treatment to lower the IOP such as eye drops or surgery, some people can have little progression of the disease (−0.2 dB/y), whereas others may have substantial progression (−1 dB/y) despite treatment.[4] The identification of additional modifiable risk factors is needed.
The diagnosis of a lifelong, potentially blinding eye disease like glaucoma and the ensuing treatment regimen can lead to different psychological responses in people. Coping, according to Lazarus and Folkman,[8] involves “cognitive and behavioral efforts to manage specific external and/or internal demands that are appraised as taxing or exceeding the resources of the person.” There are adaptive coping strategies (e.g., positive reframing and active coping) and maladaptive coping strategies (e.g., substance use and denial). Research has been done on coping strategies and their relationship to the progression of chronic diseases or conditions like human immunodeficiency virus (HIV), dementia, and pain[9–12] but very little in the field of ophthalmology.[13,14] We are not aware of any studies that have examined coping strategies and the risk of glaucoma progression. The identification of maladaptive coping strategies as risk factors for glaucoma progression could lead to increased surveillance of these at-risk patients or interventions to target these coping strategies and replace them with more adaptive coping strategies. Interventions to target maladaptive coping strategies such as denial have been efficacious in HIV and chronic kidney disease.[15]
We hypothesized that people who were more likely to make use of maladaptive coping strategies would be more likely to have progressive glaucoma and more severe visual field damage.
2. Methods
2.1. Study design and population
A hospital-based case–control study was conducted from 2012 to 2015 at Maisonneuve-Rosemont Hospital in Montreal, Canada. Patients seen within the last 6 years in the Glaucoma Service with ≥4 years of follow-up were identified from the Eye Clinic administrative database. Inclusion criteria were a clinical diagnosis of glaucoma as documented in the medical record, a minimum of 4 years of follow-up, ≥5 Humphrey visual fields taken at intervals of ≤12 months, and a best corrected visual acuity of 20/40 or better. Exclusion criteria included systemic diseases with known effects on the visual field, a distance refraction >6.00 diopters (D) (spherical equivalent) or >2.50 D of astigmatism, or visually significant eye disease other than glaucoma which could affect the participant's performance on visual field testing. Significant nonglaucomatous eye disease was defined as a best corrected visual acuity of <20/100 due to diabetic retinopathy (moderate or more severe nonproliferative diabetic retinopathy), optic neuropathy, or exudative macular degeneration. One eye per patient was enrolled. If both eyes were eligible, the eye with the most glaucoma progression was enrolled. Eligible patients were called to come for a follow-up examination and were approached during the clinic visit about participation in the study. Written informed consent was obtained, and ethics approval was given by the hospital ethics committee. The research followed the tenets of the Declaration of Helsinki.
2.2. Case and control definitions
Successive Humphrey visual fields were analyzed according to the Early Manifest Glaucoma Trial pattern deviation change probability maps, which are well described.[16] These maps analyze change based on pattern standard deviation (SD), which removes the effects of progressive media opacity. These maps identify each test point as nonchanging, significantly deteriorating, or improving as compared with the average of 2 baseline fields. Cases having visual field progression were defined as having a minimum of 3 significantly (P < 0.05) progressing points confirmed on 3 consecutive follow-up visual field examinations.[16] Controls had stable or improving visual fields.
2.3. Outcomes
This study had 2 outcomes: glaucoma progression (yes, no), which was an evaluation of recent changes in the visual field, and visual field mean deviation (dB), which indicated overall damage to the visual field, regardless of when it occurred.
2.4. Data collection
Coping was measured using the Brief Cope questionnaire.[17,18] This questionnaire has 14 scales each assessing a different coping strategy for dealing with the participant's glaucoma: active coping, planning, using instrumental support, using emotional support, venting, behavioral disengagement, self-distraction, self-blame, positive reframing, humor, denial, acceptance, religion, and substance use. Each scale has 2 items. For example, the items from the denial scale are: “I have been saying to myself that this isn’t real” and “I have been refusing to believe that it has happened.” An item from the behavioral disengagement scale is “I have been giving up trying to deal with it.” Participants were asked to evaluate how often they use a given coping strategy to deal with their glaucoma (4-point response scale: not at all, little bit, medium amount, a lot). Responses from each item are summed to create a range of scores of 1 to 8 for each scale. This questionnaire has been validated and is widely used.[17,18]
Depression was measured using the Center for Epidemiologic Studies Depression (CES-D) Scale.[19] Demographic factors such as age, sex, ethnicity, and education were obtained by self-report. We asked about a family history of glaucoma in parents, siblings, or children. The medical record was used to provide information on Humphrey visual fields (pattern SD and mean deviation) and IOP obtained via Goldman applanation tonometry for the enrollment visit and the 3 previous visits.
2.5. Statistical power and analysis
With 82 cases and 98 controls, we had 80% power to detect a difference in average Brief Cope scores of 0.6 (SD = 1.4) between progressors and nonprogressors assuming a Type 1 error rate of 5%.
The normality of the continuous variables was checked using normal probability plots. Progressors were compared with nonprogressors using Wilcoxon rank-sum tests, t tests, χ2 tests, or Fisher exact tests, as appropriate. The correlation of the Brief Cope scales was checked using Spearman correlation coefficients. A logistic regression model was used to determine independent risk factors for progression. A linear regression model was used to examine independent risk factors for visual field mean deviation. To avoid putting too many moderately correlated variables in the model together, Brief Cope scale variables were only added to the regression model if they were statistically significant in Wilcoxon rank-sum tests. Demographic, systemic health, and ocular variables were included as confounding variables in the analysis based on prior literature and biological plausibility. A P value of 0.05 was considered statistically significant. Stata version 11 (StataCorp, College Station, TX) was used for all analyses.
3. Results
A total of 180 people with glaucoma were recruited including 155 with primary open-angle glaucoma, 19 with normal tension glaucoma, and 6 with other subtypes (steroid-induced glaucoma, pigmentary glaucoma, chronic angle closure glaucoma, juvenile open-angle glaucoma, neovascular glaucoma, and mixed mechanism glaucoma). The mean age was 71 years (SD = 12), 87% were white, 6% were Haitian, and 7% were classified as “other” due to small numbers (e.g., Asian and Hispanic).
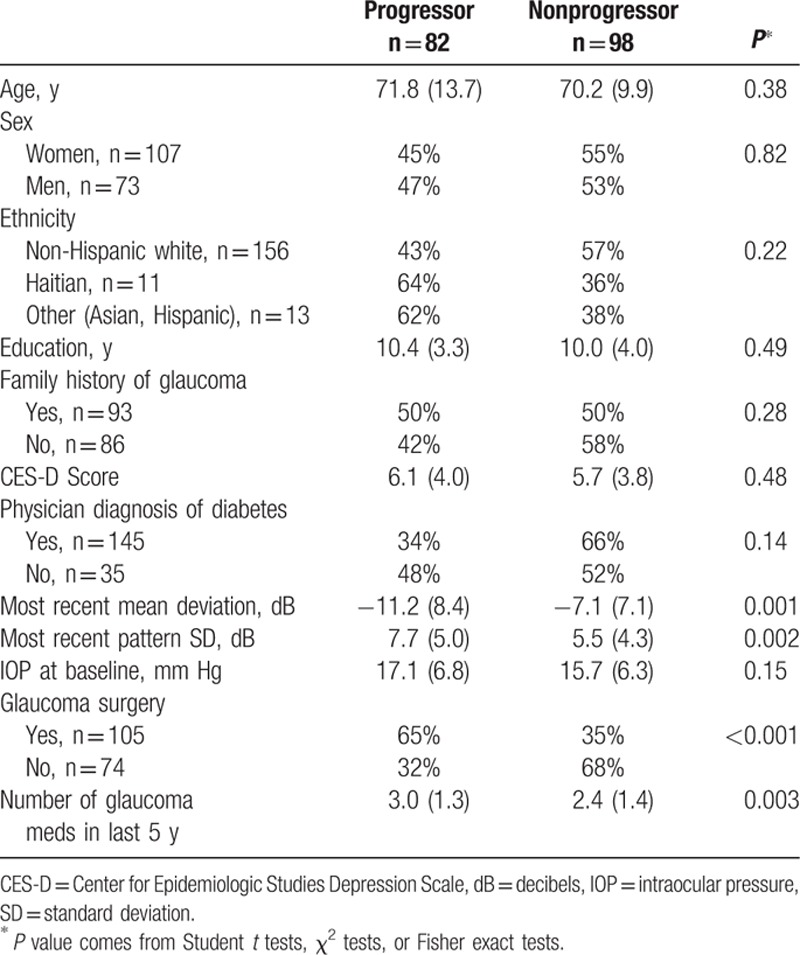
Eighty-two people, who had experienced glaucomatous visual field progression over the previous 4 years, were recruited, whereas 98 people did not experience progression. Progressors were fairly similar to nonprogressors (Table 1) except that progressors had worse mean deviation (−11.2 vs −7.1 dB) and pattern SD (7.7 vs 5.5 dB) than nonprogressors (P < 0.01). They were also more likely to have had glaucoma surgery (65% vs 35%) and to have taken more glaucoma medications in the last 5 years (3.0 vs 2.4) than nonprogressors (P < 0.01).
Table 1.
Description of study population by glaucoma progression status.
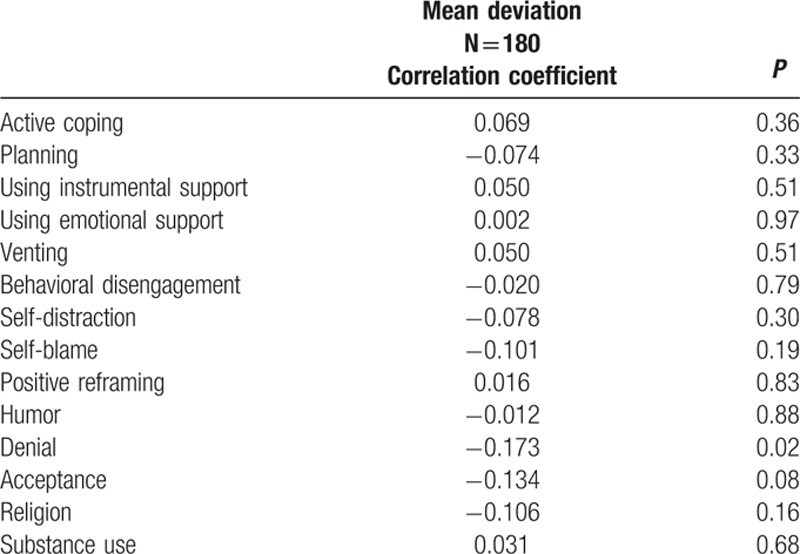
Brief Cope scale scores in our sample of patients with glaucoma were the highest for acceptance (median = 6, interquartile range [IQR] = 5), and active coping (median = 4, IQR = 3.5). The 14 scales of the Brief Cope questionnaire were not statistically significantly different between progressors and nonprogressors (Table 2). By contrast, higher denial scores were significantly negatively correlated with visual field mean deviation (r = −0.173, P = 0.024, Table 3). The other 13 Brief Cope scales were not significantly correlated with visual field mean deviation.
Table 2.
Brief Cope questionnaire scores by glaucoma progression status.
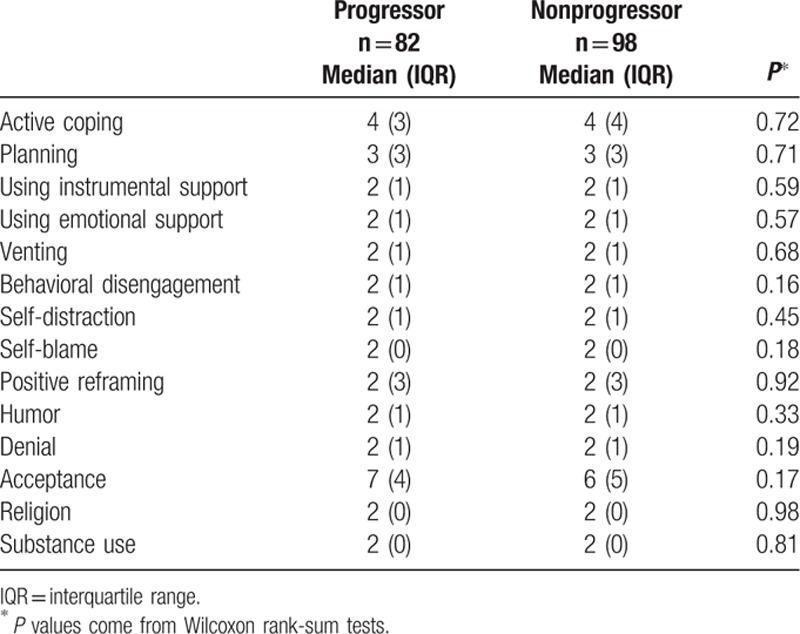
Table 3.
Correlation of Brief Cope questionnaire scale scores with visual field severity.
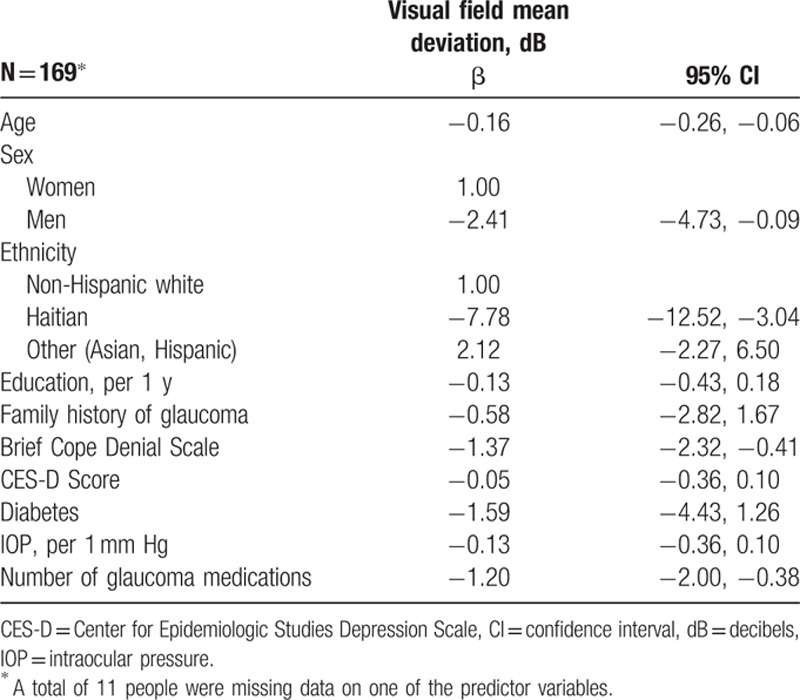
Denial scores remained significantly associated with visual field mean deviation after adjustment (Table 4). Each 1-point increase in the denial score was associated with a 1.37 dB lower visual field mean deviation on average (95% confidence interval [CI] −2.32, −0.41). Haitian older adults had an average 7.78 dB worse visual field mean deviation than non-Hispanic white older adults on average (95% CI −12.52, −3.04). Older age, male sex, and a higher number of medications were also associated with worse visual field mean deviation (P < 0.05, Table 4).
Table 4.
Variables and their relationship with visual field mean deviation in a multiple linear regression model.
4. Discussion
We have identified denial as a new modifiable risk factor for damage to visual field mean deviation but not glaucoma progression. Worse scores to the visual field mean deviation indicate damage that has been done over the lifetime, whereas glaucoma progression, as we have defined it, indicates damage over the last few years while in the care of an ophthalmologist. Our results suggest that denial may have a larger impact on delaying the initial visit of a person with suspected glaucoma to an ophthalmologist rather than impeding treatment success once in the care of an ophthalmologist. Although in the short term denial may give a person a temporary reprieve from the stress of a difficult diagnosis or the fear of blindness, in the long term, denial can lead to worse health outcomes.[20] For example, in addition to its relationship with glaucomatous visual field loss, denial has also been associated with worse outcomes for a number of other chronic diseases such as Type 1 diabetes,[21] asthma,[22] and HIV.[11,23]
In fact, educational and self-care interventions have successfully decreased maladaptive coping styles like denial in HIV patients.[15,24] For example, Carrico et al[15] found that a cognitive behavioral stress management program reduced denial and depression scores in a 10-week randomized controlled trial of HIV-positive gay men. Educational initiatives directed at glaucoma patients have improved medication adherence and persistence rates, but their effect on coping strategies or glaucomatous visual field loss are unknown.[25,26] Further research in this area is needed.
In addition to denial, Haitian ethnicity was also associated with glaucomatous visual field loss, which is similar to other findings.[27,28] Explanations for this relationship require further investigation but may have to do with ethnic differences in central corneal thickness,[29] IOP, stage at diagnosis, or medication adherence,[30,31] which themselves are risk factors for glaucoma or glaucoma progression.[5,6,32] Haitian participants did have a somewhat higher treated maximum IOP (mean = 19.5) than white participants (mean = 18.7) but not statistically significantly so. Denial scores were equal in Haitians (median = 2, IQR = 0) and Caucasians (median = 2, IQR = 1). Neither IOP nor denial explained the relationship between Haitian ethnicity and glaucomatous visual field loss. Prospective studies are needed to better explain this association.
We also found that using a greater number of glaucoma medications, which indicates uncontrolled IOP, was associated with glaucomatous visual field loss, which is consistent with other studies.[33,34] We did not find that IOP was a risk factor for glaucoma progression or glaucomatous visual field loss as many others have.[3–6] This finding is likely due to our hospital department being a referral center for glaucoma surgery. Most patients have already been treated with maximal medical therapy by a general ophthalmologist who has already identified glaucoma. When patients present to our service for a surgical consult, the documented IOP is not the lifetime maximal IOP of the patient. In addition, we relied on the IOP readings from the medical chart rather than measuring IOP ourselves in a standardized and reproducible manner. This may have led to higher variability. We would have had >80% power to detect a mean difference of 2.8 mm Hg in IOP between progressors and nonprogressors, whereas we only saw a mean difference of 1.4 mm Hg.
Strengths of this study were the use of the Early Manifest Glaucoma Trial Pattern Change Probability Maps to determine glaucoma progression, the large numbers of people with and without glaucoma progression, the use of the validated Brief Cope questionnaire to measure coping, and the identification of a novel modifiable risk factor (denial) for glaucomatous visual field loss. A limitation of this study is that the case–control design does not allow us to establish the temporality of the denial and the glaucomatous visual field loss. Furthermore, we only had 11 Haitian participants. Although this was enough to give a statistically significant result due to the large effect size, such a small sample of Haitian patients resulted in a more unstable estimate with very wide CIs. Finally, we did not collect or have access to data on why denial is related to visual field mean deviation such as compliance with medications, number of missed appointments, or severity of glaucoma damage at first diagnosis. Further prospective research should be done to confirm our findings in other populations and to verify the pathways by which denial may exert an effect on glaucomatous visual field loss.
This study may lead to the development of interventions that can target maladaptive coping strategies like denial in order to prevent glaucomatous visual field loss. Some patients with eye disease may need to be empowered through outreach, education, and self-care training in order to maximally benefit from advances in medical treatment and to avoid poor health outcomes.
Footnotes
Abbreviations: CES-D = Center for Epidemiologic Studies Depression, CI = confidence interval, D = diopters, dB = decibels, HIV = human immunodeficiency virus, IOP = intraocular pressure, IQR = interquartile range, SD = standard deviation.
This project was funded by grants from the Canadian Glaucoma Research Society and the Quebec Glaucoma Foundation. The funding organizations had no role in the design or conduct of this research.
Meeting Presentation: American Glaucoma Society, March 2016.
EF, ML, PH, DD, and GL assisted in the design of the study, the interpretation of the results, and a review of the manuscript. VF and HK assisted in the collection of the data, the interpretation of the results, and a review of the manuscript. EF wrote the manuscript. EF had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors have no conflicts of interest to disclose.
References
- 1.Resnikoff S, Pascolini D, Etya’ale D, et al. Global data on visual impairment in the year 2002. Bull World Health Organ 2004; 82:844–851. [PMC free article] [PubMed] [Google Scholar]
- 2.Heijl A, Bengtsson B, Hyman L, et al. Natural history of open-angle glaucoma. Ophthalmology 2009; 116:2271–2276. [DOI] [PubMed] [Google Scholar]
- 3.Coleman AL, Miglior S. Risk factors for glaucoma onset and progression. Surv Ophthalmol 2008; 53 suppl 1:S3–S10. [DOI] [PubMed] [Google Scholar]
- 4.De Moraes CG, Juthani VJ, Liebmann JM, et al. Risk factors for visual field progression in treated glaucoma. Arch Ophthalmol 2011; 129:562–568. [DOI] [PubMed] [Google Scholar]
- 5.Leske MC, Heijl A, Hyman L, et al. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology 2007; 114:1965–1972. [DOI] [PubMed] [Google Scholar]
- 6.Chauhan BC, Mikelberg FS, Balaszi AG, et al. Canadian Glaucoma Study: 2. Risk factors for the progression of open-angle glaucoma. Arch Ophthalmol 2008; 126:1030–1036. [DOI] [PubMed] [Google Scholar]
- 7.The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. The AGIS Investigators. Am J Ophthalmol 2000; 130:429–440. [DOI] [PubMed] [Google Scholar]
- 8.Lazarus R, Folkman S. Stress, Appraisal and Coping. New York: Springer; 1984. [Google Scholar]
- 9.Kremer H, Ironson G, Kaplan L, et al. Spiritual coping predicts CD4-cell preservation and undetectable viral load over four years. AIDS Care 2015; 27:71–79. [DOI] [PubMed] [Google Scholar]
- 10.Tschanz JT, Piercy K, Corcoran CD, et al. Caregiver coping strategies predict cognitive and functional decline in dementia: the Cache County Dementia Progression Study. Am J Geriatr Psychiatry 2013; 21:57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Temoshok LR, Wald RL, Synowski S, et al. Coping as a multisystem construct associated with pathways mediating HIV-relevant immune function and disease progression. Psychosom Med 2008; 70:555–561. [DOI] [PubMed] [Google Scholar]
- 12.Carroll LJ, Cassidy JD, Cote P. The role of pain coping strategies in prognosis after whiplash injury: passive coping predicts slowed recovery. Pain 2006; 124:18–26. [DOI] [PubMed] [Google Scholar]
- 13.Maca SM, Schiesser AW, Sobala A, et al. Distress, depression and coping in HLA-B27-associated anterior uveitis with focus on gender differences. Br J Ophthalmol 2011; 95:699–704. [DOI] [PubMed] [Google Scholar]
- 14.Pappa C, Hyphantis T, Pappa S, et al. Psychiatric manifestations and personality traits associated with compliance with glaucoma treatment. J Psychosom Res 2006; 61:609–617. [DOI] [PubMed] [Google Scholar]
- 15.Carrico AW, Antoni MH, Duran RE, et al. Reductions in depressed mood and denial coping during cognitive behavioral stress management with HIV-Positive gay men treated with HAART. Ann Behav Med 2006; 31:155–164. [DOI] [PubMed] [Google Scholar]
- 16.Leske MC, Heijl A, Hyman L, et al. Early Manifest Glaucoma Trial: design and baseline data. Ophthalmology 1999; 106:2144–2153. [DOI] [PubMed] [Google Scholar]
- 17.Carver CS. You want to measure coping but your protocol's too long: consider the brief COPE. Int J Behav Med 1997; 4:92–100. [DOI] [PubMed] [Google Scholar]
- 18.Doron J, Trouillet R, Gana K, et al. Examination of the hierarchical structure of the brief COPE in a French sample: empirical and theoretical convergences. J Pers Assess 2014; 96:567–575. [DOI] [PubMed] [Google Scholar]
- 19.Irwin M, Artin KH, Oxman MN. Screening for depression in the older adult: criterion validity of the 10-item Center for Epidemiological Studies Depression Scale (CES-D). Arch Intern Med 1999; 159:1701–1704. [DOI] [PubMed] [Google Scholar]
- 20.Goldbeck R. Denial in physical illness. J Psychosom Res 1997; 43:575–593. [DOI] [PubMed] [Google Scholar]
- 21.Jaser SS, White LE. Coping and resilience in adolescents with type 1 diabetes. Child Care Health Dev 2011; 37:335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barton C, Clarke D, Sulaiman N, et al. Coping as a mediator of psychosocial impediments to optimal management and control of asthma. Respir Med 2003; 97:747–761. [DOI] [PubMed] [Google Scholar]
- 23.Leserman J, Petitto JM, Golden RN, et al. Impact of stressful life events, depression, social support, coping, and cortisol on progression to AIDS. Am J Psychiatry 2000; 157:1221–1228. [DOI] [PubMed] [Google Scholar]
- 24.Jones DL, Ishii M, LaPerriere A, et al. Influencing medication adherence among women with AIDS. AIDS Care 2003; 15:463–474. [DOI] [PubMed] [Google Scholar]
- 25.Waterman H, Evans JR, Gray TA, et al. Interventions for improving adherence to ocular hypotensive therapy. Cochrane Database Syst Rev 2013; 4:CD006132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Djafari F, Lesk MR, Giguere CE, et al. Impact of a brief educational intervention on glaucoma persistence: a randomized controlled clinical trial. Ophthalmic Epidemiol 2015; 22:380–386. [DOI] [PubMed] [Google Scholar]
- 27.Lichter PR, Musch DC, Gillespie BW, et al. Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology 2001; 108:1943–1953. [DOI] [PubMed] [Google Scholar]
- 28.Lee JM, Caprioli J, Nouri-Mahdavi K, et al. Baseline prognostic factors predict rapid visual field deterioration in glaucoma. Invest Ophthalmol Vis Sci 2014; 55:2228–2236. [DOI] [PubMed] [Google Scholar]
- 29.Wang SY, Melles R, Lin SC. The impact of central corneal thickness on the risk for glaucoma in a large multiethnic population. J Glaucoma 2014; 23:606–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sleath B, Ballinger R, Covert D, et al. Self-reported prevalence and factors associated with nonadherence with glaucoma medications in veteran outpatients. Am J Geriatr Pharmacother 2009; 7:67–73. [DOI] [PubMed] [Google Scholar]
- 31.Rees G, Chong XL, Cheung CY, et al. Beliefs and adherence to glaucoma treatment: a comparison of patients from diverse cultures. J Glaucoma 2014; 23:293–298. [DOI] [PubMed] [Google Scholar]
- 32.Rao HL, Addepalli UK, Jonnadula GB, et al. Relationship between intraocular pressure and rate of visual field progression in treated glaucoma. J Glaucoma 2013; 22:719–724. [DOI] [PubMed] [Google Scholar]
- 33.Hollo G, Quaranta L, Cvenkel B, et al. Risk factors associated with progression in exfoliative glaucoma patients. Ophthalmic Res 2012; 47:208–213. [DOI] [PubMed] [Google Scholar]
- 34.Stewart WC, Kolker AE, Sharpe ED, et al. Factors associated with long-term progression or stability in primary open-angle glaucoma. Am J Ophthalmol 2000; 130:274–279. [DOI] [PubMed] [Google Scholar]


