Subjects with a family history of hypertension (FHH) demonstrate a significantly greater muscle sympathetic nerve activity (MSNA) reactivity to mental stress than those without FHH. This greater sympathoexcitation was observed despite comparable blood pressure (BP) reactivity between groups, and both groups demonstrate a marked dissociation between MSNA and BP responsiveness.
Keywords: family history of hypertension, muscle sympathetic nerve activity, blood pressure, cardiovascular reactivity hypothesis
Abstract
A number of recent studies have highlighted large interindividual variability of muscle sympathetic nerve activity (MSNA) responsiveness to mental stress in humans. The purpose of this study was to examine blood pressure (BP) and MSNA responsiveness to mental stress in a large and generalizable cohort of young adults with and without family history of hypertension (FHH). We hypothesized that subjects with FHH would demonstrate greater sympathoexcitation to mental stress than subjects without FHH. A total of 87 subjects (55 men and 32 women, 18–40 yr of age) from recently published (n = 45) and ongoing (n = 42) studies were examined; 57 subjects (19 with FHH and 38 without FHH) had complete MSNA recordings at baseline. Heart rate (HR), BP, and MSNA were recorded during 5 min of supine rest and 5 min of mental stress (mental arithmetic). Resting MSNA and HR were not statistically different between subjects with and without FHH (P > 0.05), whereas resting mean arterial pressure was higher in subjects with FHH (86 ± 2 vs. 80 ± 1 mmHg, P < 0.05). Mental stress increased MSNA in subjects with FHH (Δ5 ± 1 bursts/min), but not in subjects without FHH [Δ1 ± 1 burst/min, P < 0.01 (time × group)]. Mental stress increased mean arterial pressure (Δ12 ± 1 and Δ10 ± 1 mmHg, P < 0.001) and HR (Δ19 ± 2 and Δ16 ± 2 beats/min, P < 0.001) in subjects with and without FHH, but these increases were not different between groups [P ≥ 0.05 (time × group)]. MSNA and BP reactivity to mental stress were not correlated in either group. In conclusion, FHH was associated with heightened MSNA reactivity to mental stress, despite a dissociation between MSNA and BP responsiveness.
Listen to this article's corresponding podcast at http://ajpheart.podbean.com/e/sympathetic-reactivity-and-family-history-of-hypertension/
NEW AND NOTEWORTHY
Subjects with a family history of hypertension (FHH) demonstrate a significantly greater muscle sympathetic nerve activity (MSNA) reactivity to mental stress than those without FHH. This greater sympathoexcitation was observed despite comparable blood pressure (BP) reactivity between groups, and both groups demonstrate a marked dissociation between MSNA and BP responsiveness.
twenty years ago, Noll et al. (35) examined muscle sympathetic nerve activity (MSNA) responsiveness to mental stress in young adults with and without a family history of hypertension (FHH). It was reported that MSNA reactivity was significantly augmented in 10 medical students with a parental FHH compared with 8 medical students who were offspring of two normotensive parents. The authors correctly framed these novel findings within the context of limited generalizability (i.e., 18 young, healthy medical students).
Over the past 20 years, a number of key advancements regarding MSNA reactivity and mental stress in humans shed new light and interest on the findings of Noll et al. (35). First, MSNA responsiveness to mental stress can be widely variable in humans (7). Second, although early evidence suggested that self-perceptions of stress might explain the large interindividual variability associated with MSNA reactivity to mental stress (4), more recent studies have challenged this paradigm (6, 9). Third, and perhaps most importantly, our laboratory has documented a dissociation between MSNA and blood pressure (BP) reactivity to mental stress in young, healthy adults (9). This dissociation between MSNA and BP reactivity is relevant, given a recent meta-analysis that suggests an association of BP reactivity to mental stress with future risk of hypertension (11); however, mechanisms underlying the exaggerated BP reactivity to mental stress remain unresolved.
A recent review highlights the lack of test-retest studies establishing within-subject reproducibility, despite a total of 55 publications on MSNA reactivity to mental stress over the past >40 years (7). Given the large interindividual variability of MSNA responsiveness to mental stress (7) and the recent calls for increased scientific rigor and reproducibility (12), we conducted a traditional test-retest study and reported significant reproducibility of MSNA responses to mental stress, as well as cold pressor test, within a single laboratory visit and across laboratory visits separated by ∼1 mo (15). Interestingly, Greaney et al. (18) recently reported that MSNA reactivity to cold pressor test was augmented in subjects with FHH.
Accordingly, we have a renewed scientific interest in MSNA reactivity to mental stress, particularly what might be underlying the high interindividual variability and what it could potentially represent from a clinical perspective. We recently proposed a “MSNA reactivity hypothesis,” and the first step to advancing that hypothesis was the reported within-subject reproducibility (15). In the present study we have taken the next logical step to determine if the findings of Noll et al. (35) can be reproduced in a larger and more generalizable cohort of young, healthy adults. We hypothesize that subjects with a parental FHH will have a greater MSNA responsiveness to mental stress than subjects without a FHH.
METHODS
Subjects
Eighty-seven healthy adults (55 men and 32 women) between 18 and 40 yr of age participated in the study. Subjects were recruited retrospectively from research studies completed within the past 6 years (6, 10, 25, 45) and prospectively from ongoing research studies. All subjects completed a questionnaire regarding the hypertension history of their parents (self-report, no medical record confirmation) that included the age at which their parents were clinically diagnosed. Subjects were required to abstain from participating in regular exercise and from taking caffeine or alcohol for 12 h prior to the experiment. Exclusion criteria included pregnancy, breastfeeding, and oral contraceptive use. All participants underwent the same mental stress protocol (see Experimental Design). The testing procedures were explained to all subjects before we obtained their written informed consent to participate in the study, which was approved by the Michigan Technological University Institutional Review Board.
Experimental Design
On the testing day, subjects arrived at the laboratory, where three seated BP measurements were obtained after ≥5 min of quiet rest. After the BP recording, the subjects were instrumented for microneurography and other measurements in the supine position. After 5 min of supine baseline measurements, subjects were subjected to 5 min of mental stress via mental arithmetic. Briefly, the mental arithmetic involved the continuous subtraction of the number 6 or 7 (randomly chosen) from a two- or three-digit number. Subjects answered verbally and were pressured by investigators to answer as quickly and accurately as possible. A new number to subtract from was provided by a single investigator every 5–10 s. MSNA, heart rate (HR), and beat-to-beat BP were recorded at rest and during a 5-min mental stress task.
Measurements
BP and HR.
Two separate techniques were used to obtain measurements of arterial BP: 1) seated resting arterial BP was measured three consecutive times (separated by ∼1-min intervals) using an automated sphygmomanometer (model HEM-907XL, Omron Health Care), and 2) beat-to-beat arterial BP was recorded continuously using a Finometer (Finapres Medical Systems, Amsterdam, The Netherlands). Arterial BPs were expressed as systolic, diastolic (DAP), and mean (MAP) arterial BPs; HR was recorded continuously via a three-lead electrocardiogram.
Microneurography.
Multifiber recordings of MSNA were obtained by insertion of a tungsten microelectrode (FHC, Bowdoin, ME) into the peroneal nerve of the right leg, while a reference electrode was inserted 2–3 cm subcutaneously from the recording electrode. Both electrodes were connected to a differential preamplifier and then to an amplifier (total gain of 80,000), where the nerve signal was band-pass-filtered (700-2,000 Hz) and integrated (time constant 0.1 s). Recordings of MSNA were required to be spontaneous, pulse-synchronous bursts that increased during end-expiratory apnea and remained unchanged during auditory stimulus or stroking of the skin.
Data Analysis
Data were imported and analyzed in the WinCPRS software program (Absolute Aliens, Turku, Finland). R waves were detected and marked in the time series. Bursts of MSNA were automatically detected on the basis of amplitude with the use of a signal-to-noise ratio of 3:1, within a 0.5-s search window centered on a 1.3-s expected burst peak latency from the previous R wave. Potential bursts were displayed and edited by one trained investigator. MSNA was expressed as burst frequency (bursts/min), burst incidence (bursts/100 heartbeats), and total MSNA (i.e., the sum of the normalized burst areas/min).
Statistical Analysis
All data were analyzed statistically using commercial software (SPSS 22.0, IBM SPSS, Armonk, NY). The Shapiro-Wilk test revealed that the normality assumption for the data was not violated. One-way analysis of variance with FHH as the between-factor was used to compare the baseline variables. The stress reactivity was calculated as the stress response (5-min mean value for mental stress minus the corresponding 5-min mean baseline). The reactivity to mental stress was analyzed using an analysis of covariance with parental FHH as the between-factor and body mass index (BMI) as covariate. Baseline MAP (which was also different between groups) was not included as a covariate in the analysis, because it was significantly correlated to BMI. Pearson correlations were used to examine the correlations between baseline BP and BMI, as well as BP and MSNA reactivity to mental stress. Baseline data are presented as means ± SE, while cardiovascular and MSNA reactivity data are presented as adjusted means ± SE (adjusted for BMI).
RESULTS
Baseline Characteristics
When all subjects were analyzed (n = 87), participants with FHH had higher BMI (26 ± 1 vs. 24 ± 1 kg/m2, P = 0.036) and resting MAP (85 ± 2 vs. 78 ± 1 mmHg, P = 0.001) than participants without FHH. Age (23 ± 1 vs. 22 ± 1 yr, P = 0.329) and resting HR (66 ± 2 vs. 65 ± 2 beats/min, P = 0.887) were not significantly different between groups. BMI and baseline MAP were significantly correlated (r = 0.417, P < 0.001). The average age of the parents at the time of clinical diagnosis of hypertension was 43 ± 2 yr.
Table 1 depicts subject characteristics, baseline hemodynamics, and MSNA during supine rest in the subset of 57 subjects with baseline MSNA. Consistent with our larger sample, subjects with FHH had higher BMI and resting MAP than subjects without FHH, while age and resting HR were not different between groups.
Table 1.
Neural and hemodynamic measurements at rest
| Variable | FHH+ (n = 19) | FHH− (n = 38) | P Value |
|---|---|---|---|
| Sex | 16 M, 3 F | 24 M, 14 F | |
| Age, yr | 23 ± 1 | 21 ± 1 | 0.121 |
| BMI, kg/m2 | 27 ± 1 | 25 ± 1 | 0.017* |
| SAP, mmHg | 119 ± 2 | 111 ± 2 | 0.009* |
| DAP, mmHg | 70 ± 2 | 65 ± 1 | 0.036* |
| MAP, mmHg | 86 ± 2 | 80 ± 1 | 0.007* |
| HR, beats/min | 62 ± 2 | 59 ± 1 | 0.284 |
| MSNA | |||
| bursts | 11 ± 2 | 12 ± 1 | 0.518 |
| bursts/100 heartbeats | 18 ± 2 | 21 ± 2 | 0.367 |
Values are means ± SE for all trials. M, male; F, female; FHH, family history of hypertension; BMI, body mass index; SAP, DAP, and MAP, systolic, diastolic, and mean arterial blood pressure; HR, heart rate; MSNA, muscle sympathetic nerve activity.
P < 0.05.
Cardiovascular Reactivity to Mental Stress
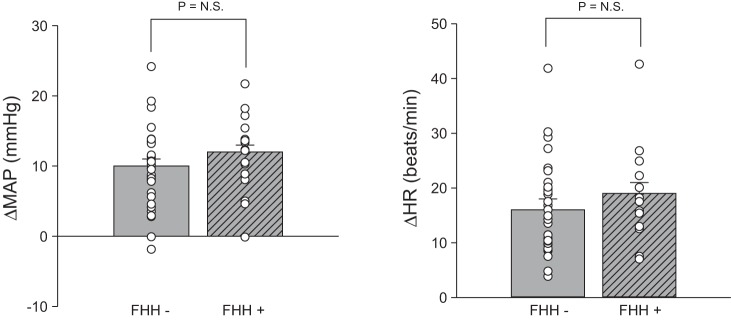
Figure 1 shows BP and HR responsiveness to mental stress in the subset of subjects in which microneurography was sustained throughout both baseline and mental stress (n = 49: 16 with FHH and 33 without FHH). Characteristics for this subset of 49 subjects were consistent with larger data sets of subjects with (15 male and 1 female, 23 ± 1 yr, 27 ± 1 kg/m2) and without (20 male and 13 female, 21 ± 1 yr, 25 ± 1 kg/m2) FHH, including a significant group difference in BMI (P = 0.011). Mental stress significantly increased MAP and HR in both groups (P < 0.001). MAP (Δ10 ± 1 vs. Δ12 ± 1 mmHg, P = 0.205) and HR (Δ16 ± 2 vs. Δ19 ± 2 beats/min, P = 0.355) reactivity were comparable in subjects with and without FHH. These findings remained consistent when BP and HR responses to mental stress were determined using the entire dataset (n = 87) and the subgroup with baseline MSNA, but not MSNA during mental stress (n = 57).
Fig. 1.
Changes in mean arterial pressure (MAP) and heart rate (HR) during 5 min of mental stress. MAP and HR responses to mental stress were not different between subjects with (+) and without (−) family history of hypertension (FHH). NS, not significant.
Sympathetic Neural Reactivity to Mental Stress
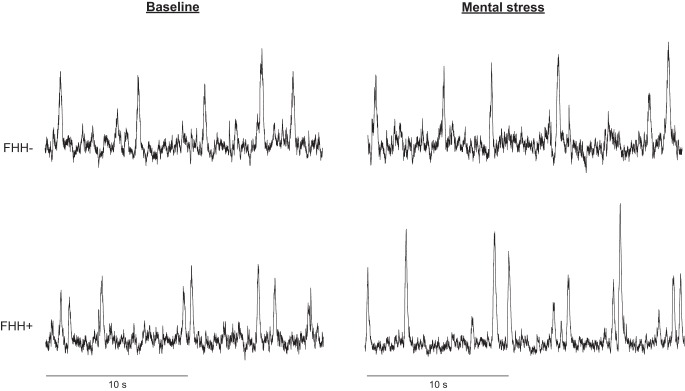
Figure 2 depicts the MSNA responsiveness to mental stress. MSNA reactivity was significantly higher in subjects with FHH than subjects without FHH (Δ5 ± 1 vs. Δ1 ± 1 burst/min, P = 0.005). Post hoc analysis revealed a significant increase in MSNA in subjects with FHH (P = 0.004), but not in subjects without FHH (P = 0.132). Likewise, MSNA burst incidence (Δ4 ± 2 vs. Δ−3 ± 1 bursts/100 heartbeats, P = 0.009) and total MSNA (Δ107 ± 24 vs. Δ48 ± 16%, P = 0.042) reactivity to mental stress were significantly higher in subjects with FHH than subjects without FHH. Figure 3 illustrates representative neurogram traces from subjects with and without FHH during baseline and mental stress.
Fig. 2.
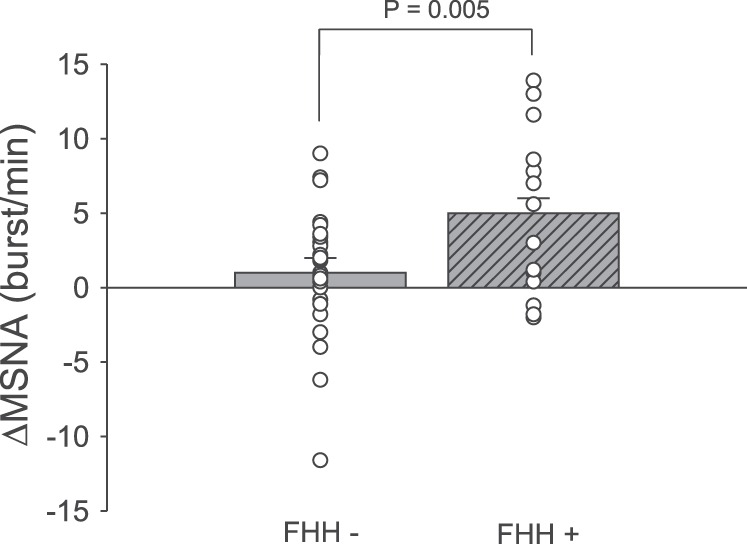
Changes in mean muscle sympathetic nerve activity (MSNA) burst frequency during 5 min of mental stress. MSNA response to mental stress was significantly augmented in subjects with (FHH+) compared with subjects without (FHH−) family history of hypertension.
Fig. 3.
Representative neurogram traces of 2 subjects: 1 with (FHH+) and 1 without (FHH−) family history of hypertension.
Relationships Between BP and MSNA Reactivity
Pearson's correlations between DAP and MSNA reactivity to mental stress according to FHH are shown in Fig. 4. Regardless of FHH status, there was no correlation between ΔMSNA and ΔDAP (P > 0.05). The dissociation between MSNA and BP reactivity remained with ΔMAP (r = 0.207, P > 0.05).
Fig. 4.
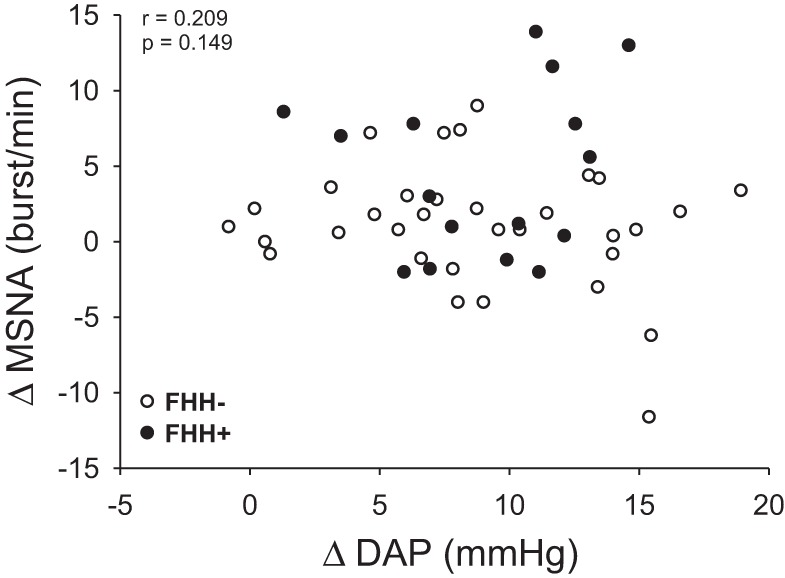
Correlation between changes in diastolic arterial pressure (DAP) and changes in muscle sympathetic nerve activity (MSNA) during mental stress. MSNA and DAP reactivity to mental stress were not significantly correlated in subjects with (FHH+) and without (FHH−) family history of hypertension.
Ratings of Perceived Stress
Ratings of perceived stress during the mental stress trial were not different between subjects with and without FHH (P = 0.810). Moreover, ratings of perceived stress were not significantly correlated to changes in MAP, HR, or MSNA during mental stress (P > 0.05). The lack of MAP or HR correlation with ratings of perceived stress was observed in the entire dataset (n = 87) and the subgroup analyses (n = 57 and n = 49).
DISCUSSION
The present study examined sympathetic neural and cardiovascular reactivity to mental stress in subjects with and without parental FHH, and we report several novel findings that advance the original work of Noll et al. (35). First, more generalizable cohort of young adults yielded a group of FHH adults with significantly elevated BMI and baseline BP. Second, “cardiovascular reactivity” (i.e., BP and HR reactivity) to mental stress was not significantly different between subjects with and without FHH. Third, MSNA responsiveness to mental stress was significantly greater in young healthy subjects with FHH than individuals without FHH, even when adjusted for BMI. Fourth, BP reactivity to mental stress was not correlated to MSNA reactivity in either group, illustrating an important dissociation between MSNA and BP reactivity to mental stress in humans that is highly relevant to the “cardiovascular reactivity hypothesis.” In summary, these findings significantly advance our understanding of both sympathetic neural and cardiovascular control during acute mental stress in humans with FHH and shed new light on the potential role of the sympathetic nervous system in the pathogenesis of high BP in offspring of hypertensive parents.
Since the early 1970s, the familial incidence of hypertension has been intensely investigated, yet basal characteristics of individuals with and without FHH remain controversial. Consistent with the findings of Lopes et al. (30) and Grewen et al. (20), FHH participants in our study had higher BMI and a greater resting BP. Other studies report higher resting BP (17, 23, 27, 32, 40, 42), but similar BMI, in subjects with FHH than those without FHH. These differences across studies are likely due to a number of methodological details and higher degree of interindividual variability. In contrast to the reported variability of BMI and resting BP in subjects with and without FHH, there appears to be consistency across laboratories regarding resting MSNA and FHH status. Specifically, six of seven prior studies investigating MSNA and FHH status have reported that resting MSNA is not statistically different in subjects with and without FHH (3, 18, 22, 26, 35, 44). In one study (31), subjects with extreme and complicated FHH (i.e., concomitant grade III or IV retinopathy) demonstrated higher resting MSNA than subjects without FHH; however, the impact of the underlying retinopathy in that study remains unclear (31). Consistent with these prior studies, we observed similar baseline levels of MSNA in participants with and without FHH. We believe that this reproducibility of resting MSNA across FHH studies and laboratories is highly relevant, given the much greater baseline hemodynamic variability across studies examining the cardiovascular reactivity to mental stress.
Numerous cross-sectional and longitudinal studies have been conducted to explore the associations between cardiovascular reactivity to acute mental stress and hypertension (7, 24, 41, 46). A recent meta-analysis by Chida and Steptoe (11) showed that greater BP reactivity to acute laboratory mental stress was longitudinally associated with several adverse cardiovascular outcomes, including hypertension. This meta-analysis has reinvigorated the cardiovascular reactivity hypothesis, which advances the concept that exaggerated BP and/or HR reactivity to mental stress provides potential predictive insights into the risk of future hypertension.
Some studies (14, 16, 19, 23, 34), including the work of Noll et al. (35), have reported a greater BP responsiveness to mental stress in subjects with FHH than those without FHH. However, other studies (1, 27, 33, 38, 39, 43), including the data presented in the current study, report similar BP reactivity to mental stress in subjects with and without FHH. There are several potential explanations for the divergent cardiovascular reactivity literature in subjects with FHH. First, it has been suggested that the degree of cardiovascular reactivity can be influenced by the intensity of the stressor (36). There can be tremendous heterogeneity in the administration of laboratory mental stress. For example, is the task being administered by a graduate student, postdoctoral student, technician, or the authoritative principal investigator? How aggressively are subjects encouraged to get the right answer and/or answer quickly? These details are often difficult to ascertain from methods sections but could impact the “intensity” of the stress task. Second, sex differences might contribute to cardiovascular reactivity inconsistencies among FHH studies. Lawler et al. (28) reported a higher BP and HR responsiveness to a reaction time task in men with FHH than women with FHH. We have also observed higher BP reactivity to mental stress in men than women in a population where FHH status was unknown (9). In the present study we were not powered to explore potential sex difference, yet despite a higher percentage of men in the FHH group, we observed no group differences in BP reactivity. Therefore, our sex distribution is unlikely to be a major confounding factor in the present study interpretation.
Noll et al. (35) demonstrated a significantly greater pressor and MSNA responsiveness to mental stress in subjects with FHH than those without FHH. The simultaneous increases in both BP and MSNA reactivity have led some to suggest MSNA reactivity as a potential mechanism contributing to the cardiovascular reactivity. However, the findings of Noll et al. (35) need to be framed within the context of some key limitations. First, subject recruitment is not extensively described by Noll et al. (35), but the nearly 50:50 ratio of individuals with and without FHH suggests that there may have been some targeted recruitment. In the present study, recruitment was random and untargeted; consequently, we obtained an overall distribution that was consistent with national norms (i.e., approximately one-third of parents were hypertensive). Second, the participants in the study of Noll et al. (35) were limited to young, healthy medical students of a narrow age range, thus limiting generalizability. Third, Noll et al. (35) did not report the correlations between MSNA and BP reactivity in their subjects. This is relevant, because since this initial work of Noll et al. (35), our laboratory has demonstrated that BP reactivity to mental stress is not significantly associated with MSNA reactivity (9).
In the present study we successfully reproduced the MSNA reactivity findings of Noll et al. (35), but this augmented sympathoexcitation during mental stress in FHH adults was not accompanied by a greater pressor response. Moreover, changes in MSNA were not correlated to changes in BP, and this dissociation between MSNA and BP reactivity was observed regardless of FHH status. This dissociation significantly advances the work of Noll et al. (35) and clearly indicates that other mechanisms are involved in the pressor response associated with mental stress. Nevertheless, the consistently higher MSNA reactivity to mental stress in adults with FHH than those without FHH supports our emerging MSNA reactivity hypothesis that advances the concept that we might be able to gain distinctive insight into future hypertension risk from MSNA reactivity studies.
Mental stress elicits a well-documented, and somewhat paradoxical, forearm vasodilation (37). While there is some evidence supporting the role of forearm MSNA withdrawal in this forearm vasodilation (21), this does not appear to be required (8). Other mechanisms, such as circulating epinephrine (29) and nitric oxide (5, 13), are also suspected to contribute to forearm vasodilation during mental stress. It is possible that in some subjects the transduction of MSNA into vasoconstriction is inhibited/offset by epinephrine- or nitric oxide-induced vasodilation and that this might help explain similar pressor responses to mental stress, despite significantly different MSNA reactivity in our two groups. For example, Fredrickson et al. (17) reported that FHH was associated with exaggerated epinephrine excretion in response to mental arithmetic. Moreover, Anderson et al. (2) reported that, despite similar increases in BP, forearm blood flow responses to mental stress were significantly increased in children with at least one hypertensive parent compared with those with no FHH. The current study did not assess epinephrine or nitric oxide responses to mental stress. Moreover, the sympathetic nervous system is highly regionalized, and the present study only examined MSNA to the lower leg. Other areas in which we cannot assess sympathetic neural activity (i.e., renal and splanchnic) may contribute to the similar BP reactivity, despite significantly different MSNA reactivity.
The impact of FHH on MSNA reactivity to other acute laboratory stressors remains equivocal. Greaney et al. (18) recently reported a greater MSNA reactivity to cold pressor test in adults with FHH than those without FHH. However, others have reported that MSNA reactivity to cold pressor test was not significantly influenced by FHH (3) or was actually heightened in subjects without FHH compared with those with FHH (26). In contrast, two independent studies report greater MSNA reactivity to handgrip exercise in subjects with FHH than those without FHH (18, 30). So while evidence is accumulating to support a MSNA reactivity hypothesis, particularly during certain laboratory stressors, more rigorous studies are necessary. The type of stressor (i.e., mental stress, cold pressor test, etc.) may be an important factor, and the field would benefit from longitudinal study designs.
The strength of this study resides in the random selection of participants, the normal distribution of our sample, and the number of participants with complete MSNA data. The FHH questionnaire was given to all subjects screened and tested in our laboratory for a variety of studies. We did not attempt to match groups in size, age, sex, or BMI, which allowed us to have a sample more representative of the population from which it was derived. A study limitation we want to acknowledge was that self-report of parental FHH was not confirmed by medical records. However, this practice of self-report is utilized by the vast majority of previously published work. Given advances in electronic medical records, we believe it will be increasingly important that future FHH studies take a more objective approach and not simply rely on self-report. Finally, of the 16 FHH subjects with MSNA reactivity data, 5 reported that both parents were hypertensive, 2 reported maternal hypertension, and 9 reported paternal hypertension. These sample sizes were not sufficient to run subanalyses that might explore the potential role of number of hypertensive parents or parental sex (i.e., maternal vs. paternal influence) on MSNA reactivity, but we hope future work will consider such factors. To date, the majority of FHH studies with a neural cardiovascular focus have not accounted for such parental details.
In conclusion, subjects with FHH demonstrate a significantly greater MSNA reactivity to mental stress than those without FHH. This greater sympathoexcitation is observed despite comparable BP reactivity between groups. Moreover, both groups demonstrate a dissociation between MSNA and BP responsiveness. These findings significantly advance our understanding of the complex relationships between MSNA and hemodynamic responsiveness to acute mental stress and the potential role of FHH in this relationship.
GRANTS
This study was supported in part by National Heart, Lung, and Blood Institute Grant HL-122919-01.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
I.T.F. and J.R.C. performed the experiments; I.T.F., M.W., and J.R.C. analyzed the data; I.T.F., M.W., and J.R.C. interpreted the results of the experiments; I.T.F. prepared the figures; I.T.F. and J.R.C. drafted the manuscript; I.T.F., M.W., and J.R.C. edited and revised the manuscript; I.T.F., M.W., and J.R.C. approved the final version of the manuscript; J.R.C. developed the concept and designed the research.
ACKNOWLEDGMENTS
The authors thank Christopher Schwartz, John Durocher, Huan Yang, Robert Larson, Jenna Klein, and Kristen Reed for assistance with various aspects of data collection.
REFERENCES
- 1.al'Absi M, Everson SA, Lovallo WR. Hypertension risk factors and cardiovascular reactivity to mental stress in young men. Int J Psychophysiol 20: 155–160, 1995. [DOI] [PubMed] [Google Scholar]
- 2.Anderson EA, Mahoney LT, Lauer RM, Clarke WR. Enhanced forearm blood flow during mental stress in children of hypertensive parents. Hypertension 10: 544–549, 1987. [DOI] [PubMed] [Google Scholar]
- 3.Calhoun DA, Mutinga ML. Race, family history of hypertension, and sympathetic response to cold pressor testing. Blood Press 6: 209–213, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Callister R, Suwarno NO, Seals DR. Sympathetic activity is influenced by task difficulty and stress perception during mental challenge in humans. J Physiol 454: 373–387, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cardillo C, Kilcoyne CM, Quyyumi AA, Cannon RO 3rd, Panza JA. Role of nitric oxide in the vasodilator response to mental stress in normal subjects. Am J Cardiol 80: 1070–1074, 1997. [DOI] [PubMed] [Google Scholar]
- 6.Carter JR, Durocher JJ, Kern RP. Neural and cardiovascular responses to emotional stress in humans. Am J Physiol Regul Integr Comp Physiol 295: R1898–R1903, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carter JR, Goldstein DS. Sympathoneural and adrenomedullary responses to mental stress. Compr Physiol 5: 119–146, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carter JR, Kupiers NT, Ray CA. Neurovascular responses to mental stress. J Physiol 564: 321–327, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carter JR, Ray CA. Sympathetic neural responses to mental stress: responders, nonresponders and sex differences. Am J Physiol Heart Circ Physiol 296: H847–H853, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carter JR, Schwartz CE, Yang H, Joyner MJ. Fish oil and neurovascular reactivity to mental stress in humans. Am J Physiol Regul Integr Comp Physiol 304: R523–R530, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chida Y, Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: a meta-analysis of prospective evidence. Hypertension 55: 1026–1032, 2010. [DOI] [PubMed] [Google Scholar]
- 12.Collins FS, Tabak LA. NIH plans to enhance reproducibility. Nature 505: 612–613, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dietz NM, Rivera JM, Eggener SE, Fix RT, Warner DO, Joyner MJ. Nitric oxide contributes to the rise in forearm blood flow during mental stress in humans. J Physiol 480: 361–368, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferrara LA, Moscato TS, Pisanti N, Marotta T, Krogh V, Capone D, Mancini M. Is the sympathetic nervous system altered in children with familial history of arterial hypertension? Cardiology 75: 200–205, 1988. [DOI] [PubMed] [Google Scholar]
- 15.Fonkoue IT, Carter JR. Sympathetic neural reactivity to mental stress in humans: test-retest reproducibility. Am J Physiol Regul Integr Comp Physiol 309: R1380–R1386, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frazer NL, Larkin KT, Goodie JL. Do behavioral responses mediate or moderate the relation between cardiovascular reactivity to stress and parental history of hypertension? Health Psychol 21: 244–253, 2002. [PubMed] [Google Scholar]
- 17.Fredrickson M, Tuomisto M, Bergman-Losman B. Neuroendocrine and cardiovascular stress reactivity in middle-aged normotensive adults with parental history of cardiovascular disease. Psychophysiology 28: 656–664, 1991. [DOI] [PubMed] [Google Scholar]
- 18.Greaney JL, Matthews EL, Wenner MM. Sympathetic reactivity in young women with a family history of hypertension. Am J Physiol Heart Circ Physiol 308: H816–H822, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenberg W, Shapiro D. The effects of caffeine and stress on blood pressure in individuals with and without a family history of hypertension. Psychophysiology 24: 151–156, 1987. [DOI] [PubMed] [Google Scholar]
- 20.Grewen KM, Girdler SS, Hinderliter A, Light KC. Depressive symptoms are related to higher ambulatory blood pressure in people with a family history of hypertension. Psychosom Med 66: 9–16, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Halliwill JR, Lawler LA, Eickhoff TJ, Dietz NM, Nauss LA, Joyner MJ. Forearm sympathetic withdrawal and vasodilatation during mental stress in humans. J Physiol 504: 211–220, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hausberg M, Sinkey CA, Mark AL, Hoffman RP, Anderson EA. Sympathetic nerve activity and insulin sensitivity in normotensive offspring of hypertensive parents. Am J Hypertens 11: 1312–1320, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Johnson EH. Cardiovascular reactivity, emotional factors, and home blood pressures in black males with and without a parental history of hypertension. Psychosom Med 51: 390–403, 1989. [DOI] [PubMed] [Google Scholar]
- 24.Kamarck TW, Lovallo WR. Cardiovascular reactivity to psychological challenge: conceptual and measurement considerations. Psychosom Med 65: 9–21, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Klein JC, Crandall CG, Brothers RM, Carter JR. Combined heat and mental stress alters neurovascular control in humans. J Appl Physiol 109: 1880–1886, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lambert EA, Schlaich MP. Reduced sympathoneural responses to the cold pressor test in individuals with essential hypertension and in those genetically predisposed to hypertension. No support for the “pressor reactor” hypothesis of hypertension development. Am J Hypertens 17: 863–868, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Lawler KA, Kline K, Seabrook E, Krishnamoorthy J, Anderson SF, Wilcox ZC, Craig F, Adlin R, Thomas S. Family history of hypertension: a psychophysiological analysis. Int J Psychophysiol 28: 207–222, 1998. [PubMed] [Google Scholar]
- 28.Lawler KA, Lacy J, Armstead CA, Lawler JE. Family history of hypertension, gender, and cardiovascular responsivity during stress. J Behav Med 14: 169–186, 1991. [DOI] [PubMed] [Google Scholar]
- 29.Lindqvist M, Davidsson S, Hjemdahl P, Melcher A. Sustained forearm vasodilation in humans during mental stress is not neurogenically mediated. Acta Physiol Scand 158: 7–14, 1996. [DOI] [PubMed] [Google Scholar]
- 30.Lopes HF, Bortolotto LA, Szlejf C, Kamitsuji CS, Krieger EM. Hemodynamic and metabolic profile in offspring of malignant hypertensive parents. Hypertension 38: 616–620, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Lopes HF, Consolim-Colombo FM, Barreto-Filho JA, Riccio GM, Negrao CE, Krieger EM. Increased sympathetic activity in normotensive offspring of malignant hypertensive parents compared to offspring of normotensive parents. Braz J Med Biol Res 41: 849–853, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Maver J, Strucl M, Accetto R. Autonomic nervous system activity in normotensive subjects with a family history of hypertension. Clin Auton Res 14: 369–375, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Morell MA, Myers HF, Shapiro D, Goldstein I, Armstrong M. Psychophysiological reactivity to mental arithmetic stress in black and white normotensive men. Health Psychol 7: 479–496, 1988. [DOI] [PubMed] [Google Scholar]
- 34.Neus H, Godderz W, Otten H, Ruddel H, von Eiff AW. Family history of hypertension and cardiovascular reactivity to mental stress—effects of stimulus intensity and environment. J Hypertens 3: 31–37, 1985. [DOI] [PubMed] [Google Scholar]
- 35.Noll G, Wenzel RR, Schneider M, Oesch V, Binggeli C, Shaw S, Weidmann P, Luscher TF. Increased activation of sympathetic nervous system and endothelin by mental stress in normotensive offspring of hypertensive parents. Circulation 93: 866–869, 1996. [DOI] [PubMed] [Google Scholar]
- 36.Pierce TW, Grim RD, King JS. Cardiovascular reactivity and family history of hypertension: a meta-analysis. Psychophysiology 42: 125–131, 2005. [DOI] [PubMed] [Google Scholar]
- 37.Roddie IC. Human responses to emotional stress. Ir J Med Sci 146: 395–417, 1977. [DOI] [PubMed] [Google Scholar]
- 38.Salerno L, Corrao S, Curiale B, Salli L, Scalici G, D'Angelo A, Arnone S. [Cardiovascular response to sympathetic stimulation in normal subjects with or without familial hypertension]. Cardiologia 35: 919–923, 1990. [PubMed] [Google Scholar]
- 39.Schmieder RE, Ruddel H, Schachinger H, Bruns J, Schulte W. Renal hemodynamics and cardiovascular reactivity in the prehypertensive stage. Behav Med 19: 5–12, 1993. [DOI] [PubMed] [Google Scholar]
- 40.Schulte W, Diederich N, von Eiff AW, Neus H. Cardiovascular reactivity to methacholine in normotensives with genetic risk of hypertension. Clin Exp Hypertens A 6: 717–730, 1984. [DOI] [PubMed] [Google Scholar]
- 41.Swain A, Suls J. Reproducibility of blood pressure and heart rate reactivity: a meta-analysis. Psychophysiology 33: 162–174, 1996. [DOI] [PubMed] [Google Scholar]
- 42.Walia V, Ahuja L, Kaur P. Cardiovascular reactivity to cold pressor test in offspring of normotensive and hypertensive parents. Indian J Med Res 99: 38–41, 1994. [PubMed] [Google Scholar]
- 43.Wright CE, O'Donnell K, Brydon L, Wardle J, Steptoe A. Family history of cardiovascular disease is associated with cardiovascular responses to stress in healthy young men and women. Int J Psychophysiol 63: 275–282, 2007. [DOI] [PubMed] [Google Scholar]
- 44.Yamada Y, Miyajima E, Tochikubo O, Matsukawa T, Shionoiri H, Ishii M, Kaneko Y. Impaired baroreflex changes in muscle sympathetic nerve activity in adolescents who have a family history of essential hypertension. J Hypertens Suppl 6: S525–S528, 1988. [DOI] [PubMed] [Google Scholar]
- 45.Yang H, Durocher JJ, Larson RA, Dellavalla JP, Carter JR. Total sleep deprivation alters cardiovascular reactivity to acute stressors in humans. J Appl Physiol 113: 903–908, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zanstra YJ, Johnston DW. Cardiovascular reactivity in real life settings: measurement, mechanisms and meaning. Biol Psychol 86: 98–105, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]