Chemical stimulation of the arcuate nucleus (ARCN) elicited increases in interscapular brown adipose tissue sympathetic nerve activity and temperature of brown adipose tissue. These responses were mediated via the release of α-melanocyte-stimulating hormone (α-MSH) and glutamate in the dorsomedial nucleus (DMN) and the paraventricular nucleus. Subpopulations of ARCN neurons projecting directly to the DMN and containing proopiomelanocortin, α-MSH, and vesicular glutamate transporter-3 were identified.
Keywords: bat nerve activity, brown adipose tissue temperature, dorsomedial nucleus, glutamate receptors, interscapular brown adipose tissue, melanocortin receptors, paraventricular nucleus, proopiomelanocortin
Abstract
Hypothalamic arcuate nucleus (ARCN) stimulation elicited increases in sympathetic nerve activity (IBATSNA) and temperature (TBAT) of interscapular brown adipose tissue (IBAT). The role of hypothalamic dorsomedial (DMN) and paraventricular (PVN) nuclei in mediating these responses was studied in urethane-anesthetized, artificially ventilated, male Wistar rats. In different groups of rats, inhibition of neurons in the DMN and PVN by microinjections of muscimol attenuated the increases in IBATSNA and TBAT elicited by microinjections of N-methyl-d-aspartic acid into the ipsilateral ARCN. In other groups of rats, blockade of ionotropic glutamate receptors by combined microinjections of D(-)-2-amino-7-phosphono-heptanoic acid (D-AP7) and NBQX into the DMN and PVN attenuated increases in IBATSNA and TBAT elicited by ARCN stimulation. Blockade of melanocortin 3/4 receptors in the DMN and PVN in other groups of rats resulted in attenuation of increases in IBATSNA and TBAT elicited by ipsilateral ARCN stimulation. Microinjections of Fluoro-Gold into the DMN resulted in retrograde labeling of cells in the ipsilateral ARCN, and some of these cells contained proopiomelanocortin (POMC), α-melanocyte-stimulating hormone (α-MSH), or vesicular glutamate transporter-3. Since similar projections from ARCN to the PVN have been reported by us and others, these results indicate that neurons containing POMC, α-MSH, and glutamate project from the ARCN to the DMN and PVN. Stimulation of ARCN results in the release of α-MSH and glutamate in the DMN and PVN which, in turn, cause increases in IBATSNA and TBAT.
NEW & NOTEWORTHY
Chemical stimulation of the arcuate nucleus (ARCN) elicited increases in interscapular brown adipose tissue sympathetic nerve activity and temperature of brown adipose tissue. These responses were mediated via the release of α-melanocyte-stimulating hormone (α-MSH) and glutamate in the dorsomedial nucleus (DMN) and the paraventricular nucleus. Subpopulations of ARCN neurons projecting directly to the DMN and containing proopiomelanocortin, α-MSH, and vesicular glutamate transporter-3 were identified.
brown adipose tissue (BAT) plays a critical role in nonshivering thermogenesis in which activation of sympathetic nervous system stimulates β3-adrenergic receptors in the adipocyte membrane causing lipolysis of triacylglycerol in BAT adipocytes. Fatty acids released during this lipolysis activate an uncoupling protein (UCP1), which is highly expressed in the inner mitochondrial membrane of the BAT adipocytes (7, 57), and protons flow through UCP1 from the intermembrane space into the mitochondrial matrix, generating energy that is dissipated as heat (45). Increase in the BAT nerve activity results in thermogenesis in BAT, and energy expenditure is increased. Thus, neural regulation of BAT thermogenesis is involved in the regulation of body temperature, as well as energy balance. Increase in BAT thermogenesis has been reported in response to cold exposure, fever generation, and recovery from hibernation (9, 34).
Cutaneous cooling results in increase in BAT nerve activity and BAT thermogenesis. Signals from the cutaneous thermal sensory receptors are transmitted to primary sensory neurons located in the dorsal root ganglia, and this information is relayed to second-order thermal sensory neurons located in the dorsal horn (DH) of the spinal cord. Activation of glutamatergic cool sensory DH neurons results in stimulation of glutamatergic third-order thermal sensory neurons in the external lateral subnucleus of lateral parabrachial nucleus (LPBN). GABAergic interneurons located in the median preoptic subnucleus (MnPO) of the preoptic area are activated resulting in inhibition of BAT-regulating GABAergic warm-sensitive neurons located in the medial preoptic area (MPA). The warm-sensitive GABAergic neurons in the MPA normally inhibit tonically active glutamatergic BAT sympathoexcitatory neurons located in the hypothalamic dorsomedial nucleus (DMN). Inhibition of the GABAergic MPA neurons results in disinhibition of BAT sympathoexcitatory neurons in the DMN which, in turn, causes excitation of glutamatergic BAT sympathetic premotor neurons located in the rostral raphe pallidus nucleus (rRPa) and para-pyramidal area (PaPy). BAT sympathetic preganglionic neurons located in the spinal intermediolateral column are activated, and sympathetic outflow in the BAT nerve is increased to elicit BAT thermogenesis (18, 33, 34).
Cutaneous warming results in a decrease in BAT nerve activity and decrease in BAT thermogenesis. Activation of glutamatergic warm sensory DH neurons results in stimulation of glutamatergic third-order thermal sensory neurons in the dorsal subnucleus of LPBN (38). Glutamatergic interneurons located in the MnPO are activated resulting in excitation of BAT-regulating GABAergic warm-sensitive neurons located in MPA. Increase in the activity of warm-sensitive GABAergic neurons in the MPA results in decrease in the activity of glutamatergic BAT sympathoexcitatory neurons located in the DMN which, in turn, causes decrease in the activity of glutamatergic BAT sympathetic premotor neurons located in the rRPa and PaPy. Consequently, the activity of BAT sympathetic preganglionic neurons, BAT nerve sympathetic outflow, and BAT thermogenesis are decreased (18, 33, 34, 38).
Hypothalamic MnPO, MPA, and DMN play a crucial role in the regulation of BAT thermogenesis (18, 33, 34). However, evidence indicates that other hypothalamic areas are also involved in regulating BAT nerve activity and thermogenesis. For example, stimulation of the hypothalamic paraventricular nucleus (PVN) reverses cooling-evoked BAT sympathetic nerve activity, indicating that this nucleus contains BAT sympathoinhibitory neurons (30). The physiological stimuli that activate BAT sympathoinhibitory neurons located in the PVN are not known. The perifornical lateral hypothalamus is also involved in BAT thermogenesis; orexinergic/glutamatergic neurons located in the perifornical lateral hypothalamus project to the rRPa and PaPy and exert an excitatory effect on BAT thermogenesis (28, 56).
Although it is known that the hypothalamic arcuate nucleus (ARCN) plays an important role in the control of feeding behavior and energy expenditure (14, 15, 17, 47, 54), very little information is available regarding the role of this nucleus in regulating BAT nerve activity and BAT thermogenesis. In the rat, cold exposure has been reported to evoke c-Fos expression in the ARCN, suggesting that this nucleus plays a role in temperature regulation (9). ARCN contains two distinct neuronal populations, proopiomelanocortin/cocaine- and amphetamine-regulated transcript-containing neurons (POMC/CART neurons) and neuropeptide Y/agouti-related peptide containing neurons (NPY/AGRP neurons). Activation of POMC/CART neurons results in suppression of appetite (anorexigenic) and increase in energy expenditure (60) and activation of NPY/AGRP neurons results in hyperphagia (orexigenic) and suppression of energy expenditure (2). Retrograde trans-synaptic tracing studies using pseudorabies virus have shown an anatomical link of the hypothalamic ARCN, PVN, and DMN nuclei to interscapular BAT (IBAT) (10). IBAT receives dense innervation from the sympathetic nervous system via the BAT nerve (7), and sympathetic nerve fibers make nest-like networks around adipocytes (49). On the basis of this information, in the present study, we tested the hypothesis that chemical stimulation of the ARCN may increase IBAT sympathetic nerve activity (IBATSNA) and BAT thermogenesis (TBAT) via activation of neurons in the PVN and DMN.
METHODS
General procedures.
Adult male Wistar rats (Charles River Laboratories, Wilmington, MA), weighing 300–360 g, were used in this study. The animals were housed under controlled conditions with a 12:12 light-dark cycle. Food and water were available to the animals ad libitum. The experiments were designed according to the National Institutes of Health's “Guide for the Care and Use of Laboratory Animals” and approved by the Institutional Animal Care and Use Committee of the Rutgers New Jersey Medical School.
The details of all the procedures used in the present study are reported in our earlier publications (3, 13, 22, 23, 39). Initially, the rats were anesthetized with inhalation of isoflurane (2–3% in 100% oxygen), using a vaporizer (Fluotec-3, Cyprane, UK). The trachea was cannulated, and the rats were artificially ventilated using a rodent respirator (model 683; Harvard Apparatus, Holliston, MA). The femoral vein and artery on one side were cannulated. Rats were administered urethane (1.2–1.4 gm/kg iv), using a solution containing 800 mg/ml of urethane, which was injected intravenously in 6–9 aliquots (each 0.05–0.1 ml containing 40–80 mg of urethane) at 2-min intervals. Isoflurane inhalation was discontinued after the administration of 4–5 aliquots of urethane. Administration of the total anesthetic dose of urethane was completed within 12–18 min. The adequate depth of anesthesia was indicated by the absence of an increase in blood pressure (BP) and/or withdrawal of the limb in response to hind paw pinch. The depth of anesthesia was periodically checked until the end of the experiment. BP and heart rate (HR) recordings were stored on a computer hard drive using 1401 plus A/D converter and Spike2 software (Cambridge Electronic Design, Cambridge, UK). Colonic temperature (Tcore) was monitored using a rectal probe consisting of a copper-constantan thermocouple, which was inserted 5–6 cm into the rectum. Tcore was maintained between 36.5 and 37.5°C using a temperature controller (model TCAT-2A; Physitemp Instruments, Clifton, NJ).
A total number of 48 rats were used. Alterations in the BP, HR, IBATSNA, and TBAT were studied in response to 1) repeated microinjections of N-methyl-d-aspartic acid (NMDA) into the ARCN (n = 5), 2) chemical stimulation of the ARCN before and after inhibition of neurons in the ipsilateral DMN (n = 6) and ipsilateral PVN (n = 7), 3) chemical stimulation of the ARCN before and after blockade of ionotropic glutamate receptors (iGLURs) in the ipsilateral DMN (n = 7) and ipsilateral PVN (n = 5), 4) chemical stimulation of the ARCN before and after blockade of melanocortin 3/4 receptors (MC3/4Rs) in the ipsilateral DMN (n = 5) and ipsilateral PVN (n = 5), and 5) microinjections of α-MSH into the DMN (n = 4). Retrograde tracing of projections from the ARCN to the DMN combined with immunohistochemistry was done in four rats.
Microinjections.
Our experiments required microinjections into two nuclei (ARCN and DMN or ARCN and PVN) in the same animal. The rats were placed in a prone position with the bite bar 3.3 mm below the interaural line in a stereotaxic instrument (David Kopf Instruments, Tujunga, CA). In all experiments involving microinjections into the brain tissue, a hole (10–12 mm in diameter) was drilled in the midline at the junction of the two parietal bones caudal to the bregma. The barrels of the micropipettes used in these experiments were connected to a picospritzer (General Valve, Fairfield, NJ). All microinjections were unilateral, and the volumes of microinjections were 30 nl in ARCN and 50 nl in DMN and PVN. The volume of microinjections was visually confirmed by the displacement of fluid meniscus in the micropipette-barrel using a modified binocular horizontal microscope (model PZMH; World Precision Instruments, Sarasota, FL) with a graduated reticule in one eye-piece. The duration of microinjections was 5–10 s. ARCN, DMN, and PVN were always identified by the microinjections of NMDA (10 mM). To allow the changes in IBATSNA elicited by ARCN stimulation to return to basal level, the interval between microinjections into the ARCN and DMN or PVN was at least 20 min. Artificial cerebrospinal fluid (aCSF; pH 7.4) was used as a vehicle. After the ARCN, DMN, or PVN were identified by microinjections of NMDA, the micropipette tips remained at the microinjection sites throughout the duration of the experiment.
Microinjections into the ARCN and DMN.
To approach the ARCN on one side, a three-barrel glass micropipette (tip size: 20–40 μm) was mounted on a micromanipulator (David Kopf Instruments, Tujunga, CA) and fixed at an acute angle (10°) pointing caudally. The micropipette was introduced into the brain through the previously drilled hole using the following coordinates: 1.1–1.3 mm caudal to the bregma, 0.2–1 mm lateral to the midline and 9.6–10.1 mm ventral to the dura. Using this approach, we ensured that the tip of the micropipette reached the ARCN at the following coordinates: 1.92–3.72 caudal to the bregma, 0.2–0.6 lateral to the midline, and 9.6–10.1 ventral to the dura (43). To approach the DMN in the same rat, a three- or four-barrel glass-micropipette (tip size 20–40 μm) was mounted on a micromanipulator and lowered into the brain tissue perpendicularly using the following coordinates: 2.28–3.72 caudal to the bregma, 0.2–0.8 lateral to the midline, and 8.6–9.2 ventral to the dura (43).
Microinjections into the ARCN and PVN.
To approach the ARCN, a three-barrel glass-micropipette (tip size: 20–40 μm) was mounted on a micromanipulator and fixed at an acute angle (10°) pointing rostrally. The micropipette was lowered into the brain tissue using the following coordinates: 4–5.5 mm caudal to the bregma, 0.2–1 mm lateral to the midline, and 9.6–10.1 mm ventral to the dura. Using this approach, we ensured that the tip of the micropipette reached the ARCN at the following coordinates: 1.92–3.72 caudal to the bregma, 0.2–0.6 lateral to the midline, and 9.6–10.1 ventral to the dura (43). To approach the PVN in the same rat, a three- or four-barrel glass-micropipette (tip size: 20–40 μm) was mounted on a micromanipulator and introduced into the brain tissue perpendicularly using the following coordinates: 0.60–2.28 caudal to the bregma, 0.2–0.8 lateral to the midline, and 7.6–7.8 ventral to the dura (43).
IBATSNA recording.
Rats were placed prone in a stereotaxic instrument with a spinal clamp on the 10th thoracic vertebra. They were paralyzed with d-tubocurarine (0.6 mg iv initial dose, supplemented with 0.3 mg as needed) and artificially ventilated with room air. The end-tidal CO2 was maintained between 4 and 5%. The IBAT pad was exposed by removing overlying skin, divided along the midline, and the fat pad ipsilateral to the ARCN selected for stimulation was reflected laterally to expose its ventral surface. Under an operating microscope (model OPMI; Carl Zeiss, Dublin, CA), a small nerve bundle was dissected free from the ventral surface of this fat pad, sectioned distally, a small length (2–4 mm) of the central cut end of the nerve bundle was desheathed and placed on bipolar hook electrode. The nerve was immersed in a pool of mineral oil. The electrode was connected to a head stage (model Super-Z; CWE, Ardmore, PA), and whole nerve activity was filtered (100-5,000 Hz) and amplified (10,000–20,000 times) using model BMA-830 amplifier (CWE). The amplified signals were digitized and recorded on a computer hard drive using a data acquisition interface (1401 plus) and Spike2 software [Cambridge Electronic Design (CED), Cambridge, UK]. Digitized signals were full-wave rectified and integrated over consecutive 1-s intervals using Spike 2 software (CED). When the nerve recording was completed, the IBAT nerve was sectioned at a level located central to the recording site, and the remaining activity was considered to be the noise level.
IBAT temperature recording.
TBAT was continuously monitored by inserting a copper-constantan thermocouple (diameter: 0.635mm, model IT-18, Physitemp Instruments, Clifton, NJ) into the portion of the divided IBAT pad located contralateral to the ARCN selected for stimulation; innervation of this portion of the IBAT pad remained undisturbed. The TBAT signal was digitized and stored on a computer hard drive using a data acquisition interface (1401 plus) and Spike2 software (CED). The increases or decreases in TBAT were determined by comparison with the baseline values.
Skin cooling.
The trunk of the animal was placed in a custom-made Plexiglas tray (inner dimensions: 4.5 inches wide × 8.5 inches long × 0.5 inch deep). When skin cooling of the rat was needed, the Plexiglas tray was rapidly filled with ice-cold water (4°C), immersing the ventral half of the rat's body, while the dorsal half of the body remained unexposed to cold water. After 2–3 min, the cold water in the tray was rapidly removed by suction. Physiological variables (IBATSNA, TBAT, BP, HR, and Tcore) were continuously recorded during the cold water immersion of the rat. The heating lamp of the temperature controller was turned off during the cold water immersion. A skin cooling test was performed at the beginning of each experiment to confirm that activity was recorded from the BAT nerve (37). After the completion of the skin-cooling test, the animal was rewarmed, and the experiment was started after a minimum 30-min time interval to allow the increases in IBATSNA and TBAT and decreases in Tcore induced by skin cooling to recover to basal levels.
Histological verification of microinjection sites.
At the end of each experiment, microinjections of green retrobeads (Lumafluor, 0.2% wt/vol) were made into the ARCN (30 nl), DMN, and PVN (50 nl each) to mark the microinjection sites. The animals were then perfused with heparinized normal saline and paraformaldehyde, and the brains were removed and prepared for making sections. The microinjection sites of green retrobeads in the ARCN, DMN, and PVN were visualized under a microscope, photographed using Neurolucida software and compared with a standard atlas (43).
Retrograde tracing of ARCN projections and immunohistochemistry.
The rats were anesthetized with intraperitoneal injections of pentobarbital sodium (50 mg/kg) and a microinjection of Fluoro-Gold (FG; 2%, 5 nl) was made into the DMN (n = 4). After a 5-day recovery, the rats were again anesthetized with pentobarbital sodium, and colchicine (120 μg, 10 μl) was injected into the lateral ventricle unilaterally (the coordinates for the lateral ventricle: 0.8–0.9 mm caudal to the bregma, 1.7–1.8 mm lateral to the midline, and 3.8–4.0 mm deep from the dura) to facilitate the immunostaining of POMC, α-melanocyte stimulating hormone (α-MSH) and vesicular glutamate transporter-3 (VGLUT3) in the cell bodies. The rats were allowed to recover for 2 days after the injection of colchicine. During the recovery period, an antibiotic (cefazolin, 30 mg/kg) and analgesic (buprenorphine, 1 mg/kg) were administered. Seven days after the microinjection of FG into the DMN, the rats were deeply anesthetized with pentobarbital sodium (80 mg/kg ip), standard fixation procedures were used and serial sections of the hypothalamic area were cut (40 μm) in a vibratome (model 1000 Plus, The Vibratome, St. Louis, MO). The microinjection site of FG in the DMN and the retrogradely labeled cells in the ARCN were visualized under a microscope (model AX70; Olympus Provis, Middlebush, NJ) using an ultraviolet filter. The sections were photographed (Neurolucida software, version 7.5, MicroBrightField, Williston, VT) and compared with a standard atlas (43). In separate experiments, the sections containing the ARCN were used for immunostaining of either POMC, α-MSH, or VGLUT3. The primary antibodies for POMC, α-MSH, and VGLUT3 were rabbit anti-POMC (1:5,000; Phoenix Pharmaceuticals, Burlingame, CA), rabbit anti-alpha-MSH (1:1,000; Immunostar, Hudson, WI), and guinea pig anti-VGLUT3 (1:1,000; EMD Millipore, MA), respectively. The secondary antibody for visualizing POMC and α-MSH staining was Cy3-goat anti-rabbit IgG (1:200; Jackson Immuno-Research Laboratories, West Grove, PA) and Cy3-goat anti-guinea pig IgG (1:200; Jackson Immuno-Research Laboratories, West Grove, PA) was used to visualize VGLUT3. After the completion of incubation with the primary and secondary antibodies, the sections were rinsed, mounted on subbed slides, covered with Citifluor mountant medium (Ted Pella, Redding, CA), and the images were captured, 1 μm apart, using a laser-scanning confocal microscope (AIR confocal microscope, Nikon Instruments, Melville, NY). Controls for immunohistochemistry consisted of omission of the primary antibody from the protocol and preabsorption of the antibody with control peptide from the manufacturer. In neither one of these control procedures was staining observed. The proportion of POMC-, α-MSH-, and VGLUT3-containing cell bodies was quantified by counting the number of immunostained cells in the tissue sections from different rats using NIS-Elements Viewer (version 3.20) software.
Drugs and chemicals.
The following drugs and chemicals were used in this study: α-MSH amide, D-AP7 [D(-)-2-amino-7-phosphono-heptanoic acid; NMDA receptor antagonist], Fluoro-Gold, green retrobeads solution, isoflurane, NBQX disodium salt (2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo-[f]quinoxaline-7-sulfonamide disodium), non-NMDA receptor antagonist, muscimol hydrobromide, SHU9119 [Ac-Nle-cyclo(-Asp-His-d-2-Nal-Arg-Trp-Lys)-NH2, a potent MC3/4 receptor antagonist], and urethane. The sources of these agents were as follows: green retrobeads solution (Lumafluor, Durham, NC), Fluoro-Gold (Fluorochrome, Denver, CO), α-MSH (American Peptide, Sunnyvale, CA), isoflurane (Piramal Critical Care, Bethlehem, PA), and SHU9119 (Tocris Bioscience, Ellisville, MO). All other drugs were purchased from Sigma-Aldrich Chemicals (St. Louis, MO). All of the solutions for the microinjections were freshly prepared in aCSF (pH 7.4).
Statistical analyses.
Baseline values of IBATSNA were the averages of the amplitudes of integrated IBATSNA during the 60-s periods before all treatments. The responses elicited by microinjections of NMDA into the ARCN represented averages of maximal changes in IBATSNA amplitude during 60-s periods. The noise level, measured after sectioning the BAT nerve at a site located central to the recording site, was subtracted from the baseline values of IBATSNA, as well as IBATSNA responses elicited by ARCN stimulation. The changes in IBATSNA induced by microinjections of NMDA into the ARCN, before and after pharmacological manipulations in the DMN and PVN, were expressed as a percentage of baseline IBATSNA activity. Tcore was maintained between 36.5 and 37.5°C during IBATSNA recording. Student's paired t-test was used for comparison of IBATSNA and TBAT responses in following experiments: 1) microinjections of NMDA into the ARCN before and after microinjections of muscimol, D-AP7 and NBQX, SHU9119, and aCSF into the DMN and PVN and 2) microinjections of α-MSH into the DMN before and after microinjections of SHU9119 into the DMN. In experiments, testing for possible tachyphylaxis of responses, comparisons of the maximum increases in IBATSNA and TBAT elicited by three microinjections of NMDA into the ARCN were made by using repeated-measures ANOVA followed by Tukey-Kramer's multiple-comparison test. Group values of IBATSNA and TBAT were expressed as means ± SE. In all cases, the differences were considered significant at P < 0.05.
RESULTS
Reproducibility of NMDA responses.
Tachyphylaxis of IBATSNA and TBAT responses was not observed after repeated microinjections of NMDA into the ARCN when the interval between microinjections was at least 20 min. The increases in IBATSNA elicited by three consecutive microinjections of NMDA (10 mM) into the ARCN at 20-min intervals were 34.86 ± 4%, 35.61 ± 3%, and 34.7 ± 3%, respectively (P > 0.05). Similarly, the increases in TBAT elicited by these microinjections were 0.81 ± 0.13°C, 0.88 ± 0.12°C, and 1.14 ± 0.1°C, respectively (P > 0.05) (n = 5).
Effect of DMN inhibition.
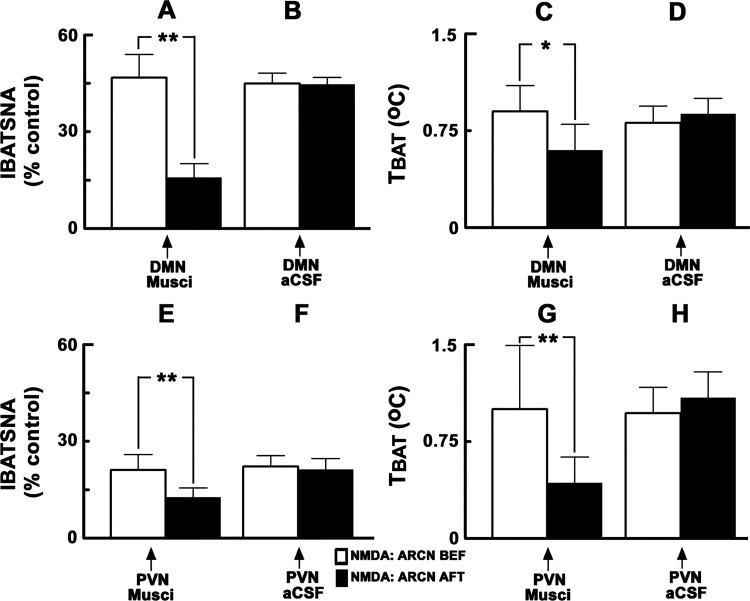
Typical tracings of the effects of inhibition of DMN neurons on the increases in IBATSNA and TBAT elicited by ARCN stimulation are shown in Fig. 1. Group data regarding the effects of ARCN stimulation on IBATSNA and TBAT are shown in Fig. 2, A and B and Fig. 2, C and D, respectively (n = 6). Unilateral microinjections of NMDA (10 mM) into the ARCN elicited increases in IBATSNA (46.7 ± 7.2%) (Fig. 2A) and TBAT (0.9 ± 0.2°C) (Fig. 2C). The times of onset, peak, and duration of the IBATSNA and TBAT responses were 16.5 ± 1.6 s, 1.7 ± 0.1 min, and 4.8 ± 0.5 min, respectively. In the same rats, ipsilateral DMN was identified by microinjections of NMDA (10 mM). Twenty minutes later, muscimol (1 mM) was microinjected into the previously identified ipsilateral DMN. Inhibition of DMN neurons by muscimol did not elicit significant changes in baseline IBATSNA and TBAT. Within 2–3 min of muscimol microinjections into the DMN, NMDA (10 mM) was microinjected again into the ARCN. Inhibition of ipsilateral DMN neurons by muscimol microinjections resulted in a significant attenuation of increases in IBATSNA and TBAT elicited by the NMDA microinjections into the ARCN; the increases in IBATSNA elicited by ARCN stimulation before and after the inhibition of the ipsilateral DMN were 46.7 ± 7.2% and 15.8 ± 4.3% (Fig. 2A), respectively (P < 0.01), and the increases in TBAT elicited by ARCN stimulation before and after the inhibition of the ipsilateral DMN were 0.9 ± 0.2 and 0.6 ± 0.2°C (Fig. 2C), respectively (P < 0.05).
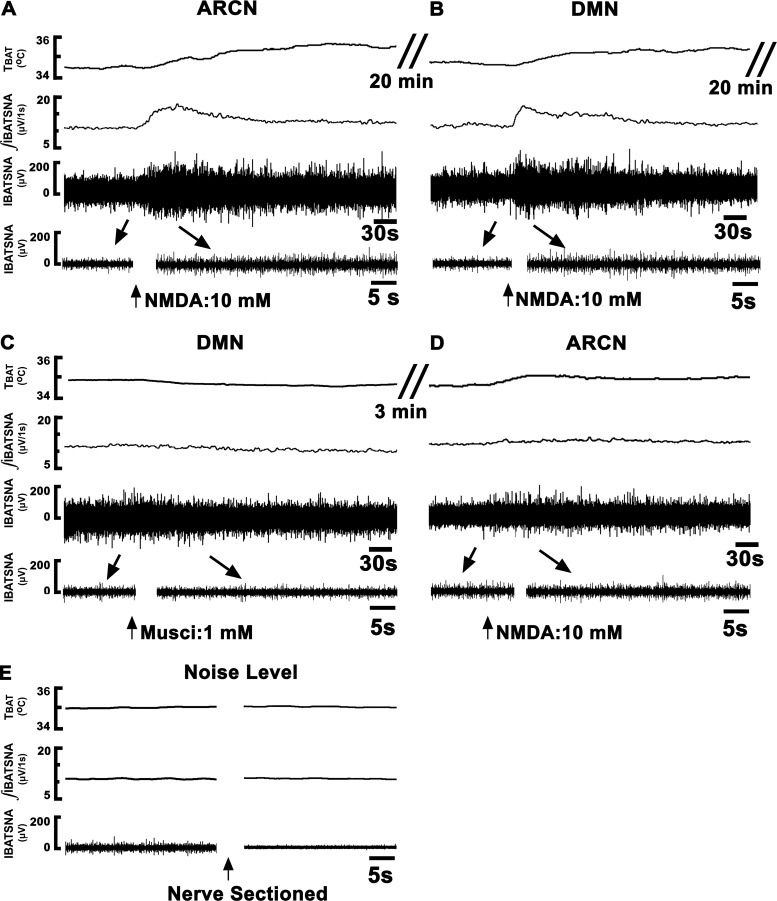
Fig. 1.
Tracings showing the effect of dorsomedial nucleus (DMN) inhibition on the responses elicited from the arcuate nucleus (ARCN). Top trace: brown adipose tissue temperature (TBAT) (°C). Second trace: integrated sympathetic nerve activity of interscapular brown adipose tissue (IBATSNA; μV/1 s). Third trace: IBATSNA whole nerve activity (μV) (time bar = 30 s). Bottom trace: expanded traces of baseline and peak activation of IBATSNA (μV) (arrows) (time bar = 5 s). Concentrations of N-methyl-d-aspartic acid (NMDA) and muscimol were 10 mM and 1 mM, respectively. The volumes of microinjection into the ARCN and DMN were 30 nl and 50 nl, respectively. A: microinjection of NMDA into the ARCN on one side (arrow) elicited increases in all recorded variables. B: twenty minutes later, ipsilateral DMN was identified by microinjection of NMDA, which elicited increases in all variables. C: twenty minutes later, muscimol was microinjected into the DMN. D: within 3 min, NMDA was again microinjected into the previously identified ARCN site; all responses were attenuated (compare with A). E: nerve activity remaining after sectioning of the BAT nerve was considered as noise level, which was subtracted from the baseline nerve activity, as well as evoked responses. ARCN, hypothalamic arcuate nucleus; DMN, hypothalamic dorsomedial nucleus; Musci, muscimol; NMDA, N-methyl-d-aspartic acid.
Fig. 2.
Effect of inhibition of neurons in the DMN and PVN on ARCN responses. A and C: unilateral microinjections of NMDA (10 mM, 30 nl) into the ARCN elicited increases in integrated IBATSNA and TBAT (open bars). Twenty minutes later, ipsilateral DMN was identified by microinjections of NMDA, and after NMDA effects subsided, muscimol (1 mM, 50 nl) was microinjected into the DMN (arrow); inhibition of DMN neurons did not elicit significant changes in IBATSNA and TBAT (not shown). Within 2–3 min, NMDA was again microinjected into the ARCN; increases in IBATSNA and TBAT were significantly attenuated after inhibition of DMN neurons (black bars). B and D: in the same animals, microinjections of artificial cerebrospinal fluid (aCSF) (50 nl) into the DMN did not alter significantly NMDA-induced responses in IBATSNA and TBAT. E and G: procedures used were identical to those mentioned in A and C, except that muscimol was microinjected into the PVN previously identified by microinjections of NMDA. F and H: procedures used were identical to those mentioned in B and D, except that aCSF was microinjected into the PVN. Microinjections of muscimol, but not aCSF, into the PVN significantly attenuated increases in IBATSNA and TBAT elicited by ARCN stimulation. *P < 0.05; **P < 0.01.
In this and other series of experiments involving the DMN, microinjections of aCSF into the DMN did not alter baseline IBATSNA or TBAT. Moreover, microinjections of aCSF into the DMN did not alter NMDA-induced responses elicited from the ARCN; the increases in IBATSNA elicited by ARCN stimulation before and after the microinjection of aCSF into the ipsilateral DMN were 44.9 ± 3.2% and 44.7 ± 2.1% (Fig. 2B), respectively (P > 0.05), and the increases in TBAT elicited by ARCN stimulation before and after the microinjection of aCSF into the DMN were 0.81 ± 0.1 and 0.9 ± 0.1°C (Fig. 2D), respectively (P > 0.05).
Effect of PVN inhibition.
The effects of inhibition of PVN neurons on increases in IBATSNA and TBAT elicited by ARCN stimulation are shown in Fig. 2, E and F and Fig. 2, G and H, respectively (n = 7). Stimulation of ARCN by microinjections of NMDA (10 mM) elicited increases in IBATSNA (Fig. 2E) and TBAT (Fig. 2G). The ipsilateral PVN was identified by microinjections of NMDA (10 mM). Twenty minutes later, muscimol (1 mM) was microinjected into the previously identified site in the PVN. Inhibition of PVN neurons by muscimol did not elicit significant changes in baseline IBATSNA and TBAT. Within 2–3 min of muscimol microinjections into the PVN, NMDA (10 mM) was microinjected again into the previously identified site in the ARCN. Inhibition of ipsilateral PVN neurons by muscimol microinjections resulted in significant attenuation of increases in IBATSNA and TBAT elicited by NMDA microinjections into the ARCN. The increases in IBATSNA elicited by ARCN stimulation before and after the inhibition of the ipsilateral PVN were 21.1 ± 4.8% and 12.7 ± 2.9% (Fig. 2E), respectively (P < 0.01) and the increases in TBAT elicited by ARCN stimulation before and after the inhibition of the ipsilateral PVN were 1 ± 0.5 and 0.4 ± 0.2°C (Fig. 2G), respectively (P < 0.01).
In this and other series of experiments involving the PVN, microinjections of aCSF into the PVN did not alter baseline IBATSNA and TBAT. Likewise, NMDA-induced responses from the ARCN were not altered by microinjections of aCSF in the PVN; the increases in IBATSNA elicited by ARCN stimulation before and after the microinjection of aCSF into the ipsilateral PVN were 22.2 ± 3.3% and 21.3 ± 3.4% (Fig. 2F), respectively (P > 0.05), and the increases in TBAT elicited by ARCN stimulation before and after the microinjection of aCSF into the PVN were 1 ± 0.2°C and 1.1 ± 0.2°C (Fig. 2H), respectively (P > 0.05).
Effect of iGLUR blockade in the DMN.
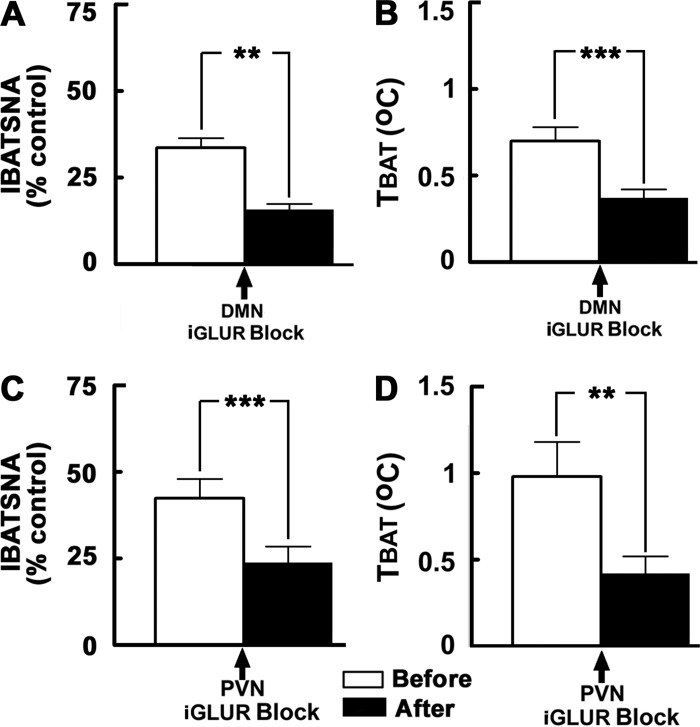
The effects of blockade of iGLURs in the DMN on increases in IBATSNA and TBAT elicited by ARCN stimulation are shown in Fig. 3, A and B, respectively (n = 7). Stimulation of ARCN by microinjections of NMDA (10 mM) elicited increases in IBATSNA (33.6 ± 2.8%) and TBAT (0.7 ± 0.1°C). Next, the ipsilateral DMN was identified by microinjections of NMDA (10 mM). Twenty minutes later, D-AP7 (5 mM) and NBQX (2 mM) were microinjected into the previously identified DMN; the interval between the two microinjections was 1–2 min. Blockade of iGLURs in the DMN did not result in significant changes in baseline IBATSNA and TBAT. Within 2–3 min, NMDA (10 mM) was microinjected again into the previously identified ARCN site. Blockade of iGLURs in the ipsilateral DMN by D-AP7 and NBQX microinjections resulted in a significant attenuation of increases in IBATSNA and TBAT elicited by NMDA microinjections into the ARCN. The increases in IBATSNA elicited by ARCN stimulation before and after the blockade of iGLURs in the ipsilateral DMN were 33.6 ± 2.8 and 15.7 ± 1.7% (Fig. 3A), respectively (P < 0.01), and the increases in TBAT elicited by ARCN stimulation before and after the blockade of iGLURs in the ipsilateral DMN were 0.7 ± 0.1 and 0.4 ± 0.1°C (Fig. 3B), respectively (P < 0.001).
Fig. 3.
Effect of blockade of iGLURs in the DMN and PVN on ARCN responses. A and B: unilateral microinjections of NMDA (10 mM, 30 nl) into the ARCN elicited increases in integrated IBATSNA and TBAT (open bars). Twenty minutes later, ipsilateral DMN was identified by microinjections of NMDA, and after NMDA effects subsided, D-AP7 (5 mM) and NBQX (2 mM) were microinjected into the DMN (arrow); blockade of iGLURs in the DMN did not elicit significant changes in IBATSNA and TBAT (not shown). NMDA was again microinjected into the ARCN within 2–3 min; increases in IBATSNA and TBAT were significantly attenuated by iGLUR blockade in the DMN (black bars). In the same animals, microinjections of aCSF (50 nl) into the DMN did not alter significantly NMDA-induced responses in IBATSNA and TBAT (not shown). C and D: procedures used were identical to those mentioned in A and B, except that iGLUR antagonists and aCSF were microinjected into the PVN, previously identified by microinjections of NMDA. Microinjections of iGLUR antagonists, but not microinjections of aCSF, into the PVN significantly attenuated increases in IBATSNA and TBAT elicited by ARCN stimulation. **P < 0.01; ***P < 0.001.
Effect of iGLUR blockade in the PVN.
The effects of blockade of iGLURs in the PVN on increases in IBATSNA and TBAT elicited by ARCN stimulation are shown in Fig. 3, C and D, respectively (n = 5). Simulation of ARCN by microinjections of NMDA (10 mM) into the ARCN elicited increases in IBATSNA (42.5 ± 5.6%) and TBAT (1 ± 0.2°C). The ipsilateral PVN was identified in the same rats by microinjections of NMDA (10 mM). Twenty minutes later, D-AP7 (5 mM) and NBQX (2 mM) were microinjected into the previously identified PVN site. Blockade of iGLURs by D-AP7 and NBQX microinjections into the ipsilateral PVN did not result in significant changes in baseline IBATSNA and TBAT. Within 2–3 min, NMDA (10 mM) was microinjected again into the previously identified ARCN site. Blockade of iGLURs in the ipsilateral PVN resulted in a significant attenuation of increase in IBATSNA and TBAT elicited by NMDA microinjections into the ARCN. The increases in IBATSNA elicited by ARCN stimulation before and after the blockade of iGLURs in the ipsilateral PVN were 42.5 ± 5.6% and 23.8 ± 4.6% (Fig. 3C), respectively (P < 0.001), and the increases in TBAT elicited by ARCN stimulation before and after the blockade of iGLURs in the ipsilateral PVN were 1 ± 0.2 and 0.4 ± 0.1°C (Fig. 3D), respectively (P < 0.01).
Effect of MCR blockade in the DMN.
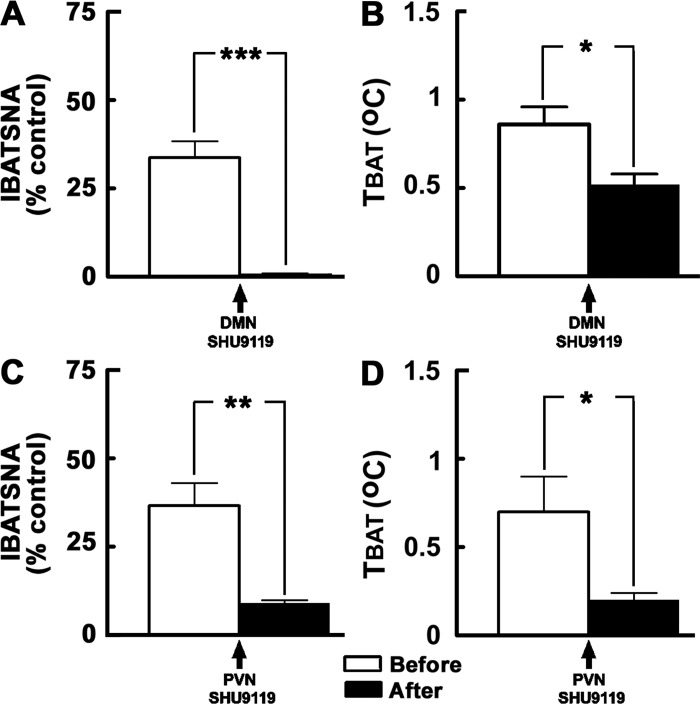
The results of this experiment are shown in Fig. 4, A and B (n = 5). Simulation of ARCN by microinjections of NMDA (10 mM) into the ARCN elicited increases in IBATSNA (33.7 ± 4.7%) (Fig. 4A) and TBAT (0.9 ± 0.1°C) (Fig. 4B). The ipsilateral DMN was identified in the same animals by microinjections of NMDA (10 mM). Twenty minutes later, SHU9119 (2 mM) was microinjected into the same site in the ipsilateral DMN. This dose of SHU9119 was selected from one of our previous publications (23). Blockade of MC3/4Rs in the DMN by SHU9119 did not elicit significant changes in baseline IBATSNA and TBAT. Within 2–3 min, NMDA (10 mM) was microinjected again into the previously identified site in the ARCN. Blockade of MC3/4Rs in the ipsilateral DMN resulted in a significant attenuation of increases in IBATSNA and TBAT elicited by NMDA microinjections into the ARCN. The increases in IBATSNA elicited by ARCN stimulation before and after the blockade of MC3/4Rs in the ipsilateral DMN were 33.7 ± 4.7% and 0.8 ± 0.2% (Fig. 4A), respectively (P < 0.001), and the increases in TBAT elicited by ARCN stimulation before and after the blockade of MC3/4Rs in the ipsilateral DMN were 0.9 ± 0.1°C and 0.5 ± 0.1°C (Fig. 4B), respectively (P < 0.05).
Fig. 4.
Effect of blockade of MC3/4Rs in the DMN and PVN on ARCN responses. A and B: unilateral microinjections of NMDA (10 mM, 30 nl) into the ARCN elicited increases in integrated IBATSNA and TBAT (open bars). Twenty minutes later, ipsilateral DMN was identified by microinjections of NMDA, and after NMDA effects subsided, SHU9119 (2 mM) was microinjected into the DMN (arrow); blockade of MC3/4Rs in the DMN by SHU9119 did not elicit significant changes in IBATSNA and TBAT (not shown). NMDA was again microinjected into the ARCN within 2 or 3 min; increases in IBATSNA and TBAT were significantly attenuated by MC3/4R blockade in the DMN (black bars). In the same animals, microinjections of aCSF (50 nl) into the DMN did not alter significantly NMDA-induced responses in IBATSNA and TBAT (not shown). C and D: procedures used were identical to those mentioned in A and B, except that SHU9119 and aCSF were microinjected into the PVN previously identified by microinjections of NMDA. Microinjections of SHU9119, but not microinjections of aCSF, into the PVN significantly attenuated increases in IBATSNA and TBAT elicited by ARCN stimulation. *P < 0.05; **P < 0.01; ***P < 0.001.
In a separate group of rats (n = 4), increases in IBATSNA elicited by microinjections of α-MSH (2 mM) into the DMN were blocked by prior microinjections of SHU9119 (2 mM) into the DMN; the increases in IBATSNA elicited by α-MSH before and after the blockade of MC3/4Rs in the DMN were 13.9 ± 1.8% and 4.2 ± 2.9%, respectively (P < 0.05). The dose of α-MSH used in this experiment was selected from a previous publication (20). This experiment showed that the dose of SHU9119 (2 mM) used in this study was sufficient to block MC3/4Rs in the DMN.
Effect of MCR blockade in the PVN.
The results of this experiment are shown in Fig. 4, C and D (n = 5). Simulation of ARCN by microinjections of NMDA (10 mM) into the ARCN elicited increases in IBATSNA (36.6 ± 6.4%) (Fig. 4C) and TBAT (0.7 ± 0.2°C) (Fig. 4D). The ipsilateral PVN was identified in the same animal by microinjections of NMDA (10 mM). Twenty minutes later, SHU9119 (2 mM) was microinjected into the same site in the ipsilateral PVN. Blockade of MC3/4Rs in the PVN by SHU9119 did not result in significant changes in baseline IBATSNA and TBAT. Within 2–3 min, NMDA (10 mM) was microinjected again into the previously identified site in the ARCN. Blockade of MC3/4Rs in the ipsilateral PVN resulted in a significant attenuation of increases in IBATSNA and TBAT elicited by NMDA microinjections into the ARCN. The increases in IBATSNA elicited by ARCN stimulation before and after the blockade of MC3/4Rs in the ipsilateral PVN were 36.6 ± 6.4% and 9 ± 0.8% (Fig. 4C), respectively (P < 0.01) and the increases in TBAT elicited by ARCN stimulation before and after the blockade of MC3/4Rs in the ipsilateral PVN were 0.7 ± 0.2°C and 0.2 ± 0.1°C (Fig. 4D), respectively (P < 0.05).
Effects on core body temperature.
Microinjections of NMDA into the ARCN, DMN, and PVN did not elicit significant changes in core body temperature. In the DMN and PVN, microinjections of aCSF, muscimol, and antagonists for iGLURs and MC3/4Rs did not evoke significant changes in baseline Tcore and did not alter Tcore values during ARCN stimulation by NMDA (Table 1). Although the skin cooling test that was done in the beginning of the experiment decreased Tcore (1.16 ± 0.1°C), ARCN stimulation was started only when core temperature returned to basal values on rewarming the animal.
Table 1.
Effect of ARCN stimulation on Tcore
| ARCN: NMDA Microinjections |
||
|---|---|---|
| Before, °C | After, °C | |
| DMN Microinjections | ||
| aCSF | 36.56 ± 0.5 | 36.94 ± 0.3 |
| Muscimol | 36.65 ± 0.5 | 37.02 ± 0.3 |
| d-AP7+NBQX | 36.58 ± 0.6 | 37.03 ± 0.3 |
| SHU9119 | 36.63 ± 0.5 | 37.06 ± 0.3 |
| Before, °C | After, °C | |
|---|---|---|
| PVN Microinjections | ||
| aCSF | 37.34 ± 0.2 | 37.49 ± 0.1 |
| Muscimol | 36.52 ± 0.4 | 36.95 ± 0.3 |
| d-AP7+NBQX | 36.88 ± 0.5 | 37.27 ± 0.2 |
| SHU9119 | 37.43 ± 0.1 | 37.52 ± 0.1 |
Effects of ARCN stimulation on Tcore (°C). Microinjections of artificial cerebrospinal fluid (aCSF), muscimol, ionotropic glutamate receptors (iGLUR) antagonists, and melanocortin 3/4 receptor (MC3/4R) antagonist into the dorsomedial nucleus (DMN) or the paraventricular nucleus (PVN) did not significantly alter Tcore during stimulation of arcuate nucleus (ARCN) by N-methyl-d-aspartic acid (NMDA). AP7, D(-)-2-amino-7-phosphono-heptanoic acid; NBQX, 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo-[f]quinoxaline-7-sulfonamide disodium; SHU9119, Ac-Nle-cyclo(-Asp-His-d-2-Nal-Arg-Trp-Lys)-NH2.
Histological verification of microinjection sites.
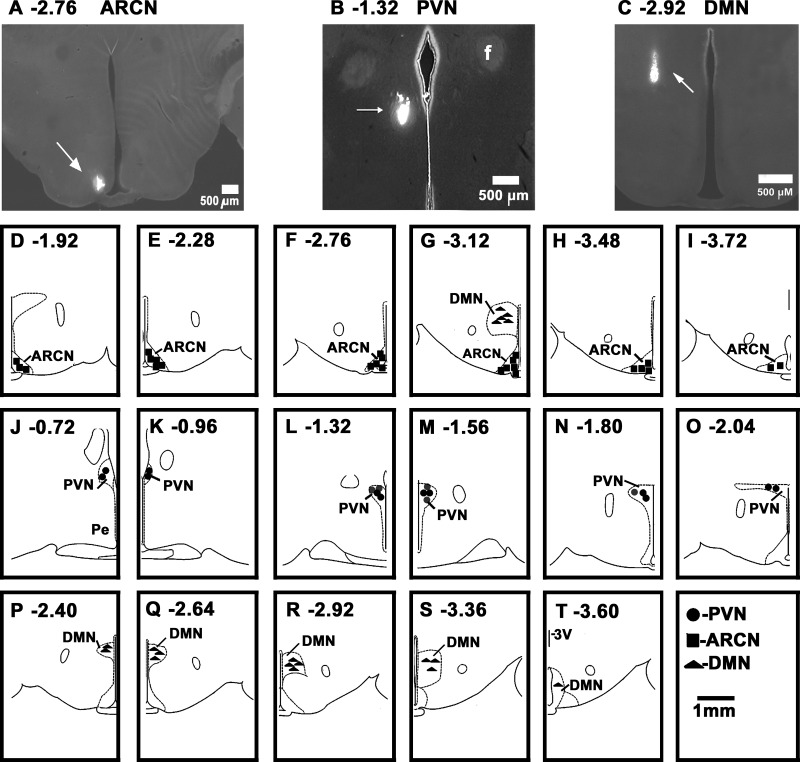
A typical microinjection site in the ARCN (2.76 caudal to the bregma) is shown in Fig. 5A; the center of the Lumafluor spot was 0.5 mm lateral to the midline and 9.8 mm deep from the dura. The diagrams showing microinjection sites in the ARCN at different rostrocaudal levels (1.92 to 3.72 caudal to the bregma) are depicted in Fig. 5, D–I. In these diagrams, each symbol represents a microinjection site in one animal.
Fig. 5.
Histological identification of microinjection sites. A: coronal section at a level 2.76 mm caudal to the bregma showing a Lumafluor microinjection site in the ARCN (arrow); the center of the spot was 0.5 mm lateral to the midline and 9.8 mm deep from the dura. B: coronal section at a level 1.32 mm caudal to the bregma showing a Lumafluor microinjection site in the PVN (arrow); the center of the spot was 0.4 mm lateral to the midline and 7.7 mm deep from the dura. C: coronal section at a level 2.92 mm caudal to the bregma showing a Lumafluor microinjection site in the DMN; the center of the spot was 0.7 mm lateral to the midline and 8.7 mm deep from the dura. A–C: bar = 500 μm each. D–I: drawings of coronal sections 1.92–3.72 mm caudal to the bregma showing the ARCN microinjection sites as dark squares. G: DMN microinjection sites (dark triangles) at this level. J–O: drawings of coronal sections 0.72–2.04 mm caudal to the bregma, showing the PVN microinjection sites as dark circles. P–T: drawings of coronal sections 2.40–3.60 mm caudal to the bregma showing the DMN microinjection sites as dark triangles. D–T: scale bar = 1 mm each; each symbol represents a site in one animal. f, fornix; ARCN, arcuate nucleus; DMN, dorsomedial nucleus; PVN, paraventricular nucleus.
A typical microinjection site in the PVN is shown in Fig. 5B; the Lumafluor spot was at a level 1.32 caudal to the bregma, and the center of the spot was 0.4 mm lateral to the midline and 7.7 mm deep from the dura. Diagrams showing other microinjection sites in the PVN, at different rostrocaudal levels (0.72 to 2.04 mm caudal to the bregma) are presented in Fig. 5, J–O.
A typical microinjection Lumafluor site in the DMN is shown in Fig. 5C; the spot was at a level 2.92 caudal to the bregma, and the center of the spot was 0.7 mm lateral to the midline and 8.7 mm deep from the dura. Diagrams showing other microinjection sites in the DMN, at different rostrocaudal levels (2.40 to 3.60 mm caudal to the bregma) are presented in Fig. 5, G and P–T.
In the diagrams, all sites are not shown because of overlapping. The rats in which the diffusion sphere of the marker was not within the boundaries of the desired nucleus (ARCN, DMN, and PVN), as shown in a standard atlas (43), were not included in this study.
Identification of projections from the ARCN to the DMN.
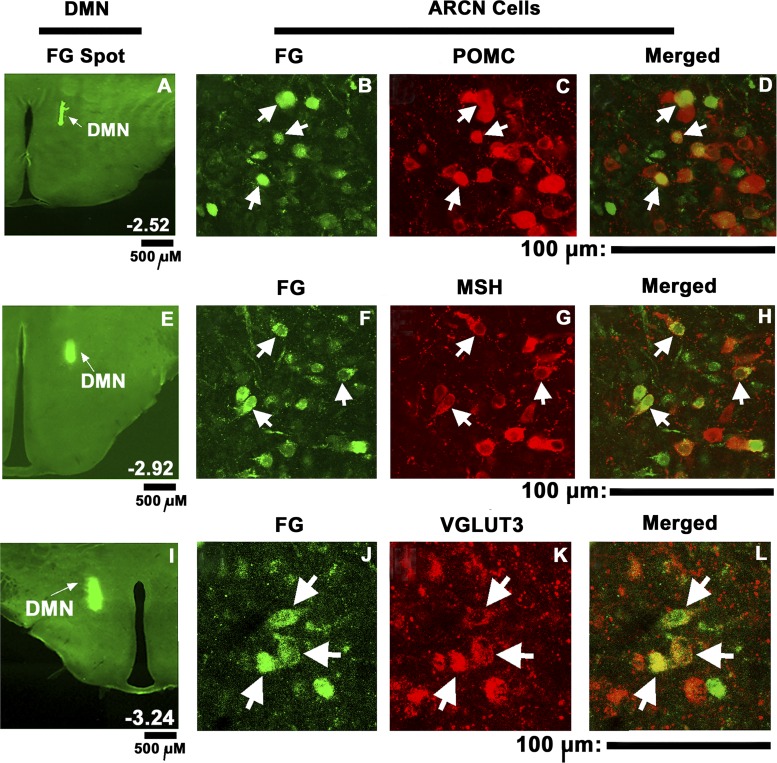
Microinjections of FG (5 nl) were placed in the middle segment of the DMN to allow spread of FG into most of this nucleus. Unilateral microinjections of FG into the DMN (n = 4) (Fig. 6, A, E, and I) resulted in retrograde labeling of ARCN cells bilaterally with ipsilateral preponderance (Fig. 6 B, F, and J). Neurons staining for POMC (Fig. 6C), α-MSH (Fig. 6G) and VGLUT3 (Fig. 6K) were present in the ARCN. Merged images (Fig. 6, D, H and L) showed that some of the POMC, α-MSH, and VGLUT3 containing ARCN neurons were labeled with FG retrogradely transported from the DMN, indicating that these neurons were directly projecting from the ARCN to the DMN. The percentages of retrogradely FG-labeled cells in the ARCN that contained POMC, α-MSH, and VGLUT3 were 39.95%, 41.4%, and 32.01%, respectively.
Fig. 6.
Retrograde tracing of ARCN projections to the DMN and immunohistochemistry. Fluoro-Gold microinjection site in the DMN (A, E, I). Subsequently, retrogradely labeled cells were observed in ipsilateral (B, F, J) and contralateral (not shown) ARCN. Immunohistochemistry showed cells staining for POMC (C), α-MSH (G), and VGLUT3 (K) in the ipsilateral ARCN. Merger of images in B and C showed that some ARCN cells retrogradely labeled with FG contained POMC (D, arrows). Merged images of F and G showed that some ARCN cells retrogradely labeled with FG contained α-MSH (H; arrows). Merger of images in J and K showed that some ARCN cells retrogradely labeled with FG contained VGLUT3 (L; arrows). Images of sections shown in A–D, E–H, and I–L are from different rats. FG, Fluoro-Gold; MSH, α-melanocyte stimulating hormone; POMC, proopiomelanocortin; VGLUT3, vesicular glutamate transporter-3. A, E and I: scale bars = 500 μm each; for other panels: scale bars = 100 μm each.
Direct projections from the ARCN to the PVN have been previously reported by us (22, 23, 47) and others (4, 5, 48). Direct projections from the ARCN neurons containing POMC and α-MSH to the PVN have also been previously reported (23) and a population of POMC neurons has been reported to be glutamatergic (25, 31).
DISCUSSION
In this article, we demonstrate that microinjections of NMDA into the ARCN elicit increases in IBATSNA and TBAT (BAT thermogenesis). Microinjections of an endogenous peptide (leptin) into the ARCN have also been reported to elicit increases IBATSNA and TBAT (44). Increases in IBATSNA and TBAT in response to ARCN stimulation are consistent with the reports in which cold exposure increased c-Fos expression in the ARCN (10). Anatomical basis for eliciting increases in IBATSNA and TBAT in response to ARCN stimulation is provided by retrograde trans-synaptic tracing studies using pseudorabies virus, which revealed a polysynaptic anatomical link of the ARCN to IBAT (10, 41). Direct projections from the ARCN to the PVN have been previously reported by us (22, 23, 47) and others (4, 5, 48). Direct projections from the ARCN to the DMN have also been reported in the literature (8, 14, 26, this study). The presence of direct projections from the ARCN to the DMN and PVN suggested that increases in IBATSNA and TBAT elicited by ARCN stimulation ma y be mediated by these projections as discussed in the following paragraphs.
A skin cooling test was done to ensure that BAT nerve was responding to peripheral cold stimulus (37). Although activation of peripheral nociceptors by cold stimulus may contribute to the increase in BAT nerve activity, this possibility did not affect our results because the skin cooling test was done only once in the beginning of the experiment, and sufficient time (at least 30 min) was allowed for complete recovery of IBATSNA and TBAT to baseline levels.
The increases in IBATSNA and TBAT elicited by ARCN stimulation were mediated partially via the DMN. This conclusion was based on our observation that inhibition of neurons located in the ipsilateral DMN by microinjections of muscimol attenuated increases in IBATSNA and TBAT elicited by ARCN stimulation; the dose of muscimol, which was used as a pharmacological tool to inhibit neurons, was selected from one of our previous reports (3). This observation is consistent with the well-established role of DMN in the control of BAT thermogenesis (18, 32, 34). The DMN contains neurons that are synaptically connected to BAT (6, 10, 11) via the rostral ventromedial medulla (40, 41, 46, 61). DMN neurons are activated by cold exposure or administration of endotoxin (61). We extended this information by demonstrating that the neurotransmitters in the projections from the ARCN to the DMN may be glutamate and α-MSH because increases in IBATSNA and TBAT elicited by ARCN stimulation were attenuated by blockade of iGLURs and MC3/4Rs in the ipsilateral DMN. The blockade of iGLURs was accomplished by microinjections of D-AP7 and NBQX, and the blockade of MC3/4Rs was achieved by microinjections of SHU9119. The doses of these iGLUR antagonists and SHU9119 were selected from one of our previous publications (23). It is known that subpopulations of POMC-containing neurons in the ARCN are glutamatergic (25, 31), Neurons in the ARCN are known to project to DMN (8, 14, 26, this study). Neurons containing POMC and α-MSH are present in the ARCN (21, 25, 31, 51, 52, this study), and a subpopulation of POMC-containing neurons is glutamatergic (25, 31). Accordingly, our retrograde tracing experiments showed that some of the POMC-, α-MSH-, and VGLUT3-containing neurons in the ARCN project to the DMN. VGLUT3 is one of the markers for glutamatergic neurons (42). Stimulation of ARCN neurons by NMDA may result in the release of glutamate and α-MSH in the DMN via direct projections. Glutamate and α-MSH would then stimulate DMN neurons via iGLURs and MC3/4Rs, respectively, and elicit increases in IBATSNA and TBAT. The presence of excitatory amino acid receptors (EAARs) (29) and MC3/4Rs (24, 35) in the DMN is consistent with this contention. Increases in IBATSNA and TBAT elicited by microinjections of prostaglandin E2 in the hypothalamic medial preoptic area are attenuated by blockade of EAARs in the DMN, suggesting that DMN neurons receive a glutamatergic input, but the source of this input is not known (29). In this article, we have demonstrated by retrograde tract tracing and aforementioned pharmacological experiments that one of the sources of the glutamatergic projection to the DMN is the ARCN.
Increases in IBATSNA and TBAT elicited by unilateral ARCN stimulation were attenuated by inhibition of neurons in the ipsilateral PVN by muscimol. These observations suggested that increases in IBATSNA and TBAT elicited by ARCN were partially mediated via the PVN. The role played by the PVN in mediating increases in IBATSNA in our study is consistent with the reports indicating that 1) the PVN is synaptically connected with BAT (6, 10, 41, 59) and 2) increases in TBAT elicited by microinjections of glutamate into the PVN were mediated via activation of sympathetic innervation to the BAT because systemic administration of chlorisondamine (ganglion blocker) and propranolol (β-adrenergic receptor antagonist) prevented the TBAT activation induced by PVN stimulation (1).
In our study, microinjections of NMDA (10 mM, 50 nl) into the PVN elicited increases in IBATSNA and TBAT. However, Madden and Morrison (30) reported that microinjections of smaller concentrations of NMDA (0.2 mM, 60 nl) into the PVN did not increase IBATSNA and TBAT.
Blockade of iGLURs in the PVN attenuated the increases in IBATSNA and TBAT elicited by ARCN stimulation in our study, suggesting that iGLURs located in the PVN partially mediate these responses. PVN receives glutamatergic projections from the ARCN (16), and numerous glutamate-immunoreactive synapses (58) and NMDA receptors (19) are present in the PVN. Stimulation of ARCN neurons by NMDA may release glutamate in the PVN via direct projections (23, 47, 50), which, in turn, may stimulate PVN neurons and elicit increases in IBATSNA and TBAT.
Increases in IBATSNA and TBAT elicited by ARCN stimulation are partially mediated via MC3/4Rs located in the PVN. This conclusion is based on our results in which blockade of MC3/4Rs by SHU9119 in the PVN attenuated the increases in IBATSNA and TBAT elicited by ARCN stimulation. ARCN neurons have been reported to contain α-MSH (21, 51, 52, this study). Stimulation of ARCN neurons by NMDA may release α-MSH in the PVN via direct projections (22, 23, 47, 50), which, in turn, may stimulate PVN neurons and elicit increases in IBATSNA and TBAT. Consistent with this conclusion are the following reports: 1) MC4Rs are located on the PVN neurons that are synaptically connected to BAT (55), 2) microinjection of melanocortin receptor agonists into the PVN increased TBAT (53, 55), and 3) activation of MC3/4Rs in the PVN increased sympathetic activity, although its effect on IBATSNA was not studied specifically (27).
Following the ARCN stimulation, activation of PVN and DMN neurons is likely to mediate increases in IBATSNA and TBAT via the projections of these nuclei to the rRPa where the BAT sympathetic premotor neurons are located (32). The presence of a descending glutamatergic input from the DMN to the rRPa supports this contention (12). Among other inputs, rRPa premotor sympathetic neurons receive a potent glutamatergic input (32). Increases in IBATSNA and TBAT are elicited by glutamate receptor activation in the rRPa (12). It has been suggested that activation of VGLUT3-expressing neurons in the rRPa increases IBATSNA and TBAT via glutamatergic neurotransmission in the intermediolateral cell column of the spinal cord (36).
In conclusion, chemical stimulation of the ARCN by microinjections of NMDA elicited increases in IBATSNA and TBAT, which were mediated via direct projections from the ARCN to the DMN and PVN. MC3/4Rs and iGLURs in the DMN and PVN mediated the increases in IBATSNA and TBAT elicited by the ARCN stimulation. Retrograde tracing combined with immunohistochemistry showed that ARCN neurons containing POMC, α-MSH, and VGLUT3 project directly to the DMN; similar projections from the ARCN to the PVN have been reported by us (22, 23, 47) and others (4, 5, 48).
GRANTS
This study was supported in part by National Institutes of Health Grants HL-024347 and HL-076248 awarded to Dr. H. N. Sapru.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
V.C.C. and H.N.S. conception and design of research; V.C.C. and K.K. performed experiments; V.C.C., K.K., and H.N.S. analyzed data; V.C.C., K.K., and H.N.S. interpreted results of experiments; V.C.C., K.K., and H.N.S. prepared figures; V.C.C. and H.N.S. drafted manuscript; V.C.C., K.K., and H.N.S. edited and revised manuscript; V.C.C., K.K., and H.N.S. approved final version of manuscript.
REFERENCES
- 1.Amir S. Stimulation of the paraventricular nucleus with glutamate activates interscapular brown adipose tissue thermogenesis in rats. Brain Res 508: 152–155, 1990. [DOI] [PubMed] [Google Scholar]
- 2.Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci 14: 351–355, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arakawa H, Chitravanshi VC, Sapru HN. Hypothalamic arcuate nucleus: a new site of cardiovascular action of angiotensin-(1–12) and angiotensin II. Am J Physiol Heart Circ Physiol 300: H951–H960, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bai FL, Yamano M, Shiotani Y, Emson PC, Smith AD, Powell JF, Tohyama M. An arcuato-paraventricular and -dorsomedial hypothalamic neuropeptide Y-containing system which lacks noradrenaline in the rat. Brain Res 331: 172–175, 1985. [DOI] [PubMed] [Google Scholar]
- 5.Baker RA, Herkenham M. Arcuate nucleus neurons that project to the hypothalamic paraventricular nucleus: neuropeptidergic identity and consequences of adrenalectomy on mRNA levels in the rat. J Comp Neurol 358: 518–530, 1995. [DOI] [PubMed] [Google Scholar]
- 6.Bamshad M, Song CK, Bartness TJ. CNS origins of the sympathetic nervous system outflow to brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 276: R1569–R1578, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Betz MJ, Enerback S. Human brown adipose tissue: What we have learned so far. Diabetes 64: 2352–2360, 2015. [DOI] [PubMed] [Google Scholar]
- 8.Bouret SG, Draper SJ, Simerly RB. Formation of projection pathways from the arcuate nucleus of the hypothalamus to hypothalamic regions implicated in the neural control of feeding behavior in mice. J Neurosci 24: 2797–2805, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 84: 277–359, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Cano G, Passerin AM, Schiltz JC, Card JP, Morrison SF, Sved AF. Anatomical substrates for the central control of sympathetic outflow to interscapular adipose tissue during cold exposure. J Comp Neurol 460: 303–326, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Cao WH, Fan W, Morrison SF. Medullary pathways mediating specific sympathetic responses to activation of dorsomedial hypothalamus. Neuroscience 126: 229–240, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Cao WH, Morrison SF. Glutamate receptors in the raphe pallidus mediate brown adipose tissue thermogenesis evoked by activation of dorsomedial hypothalamic neurons. Neuropharmacology 51: 426–437, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Chitravanshi VC, Kawabe K, Sapru HN. GABA and glycine receptors in the nucleus ambiguus mediate tachycardia elicited by chemical stimulation of the hypothalamic arcuate nucleus. Am J Physiol Heart Circ Physiol 309: H174–H184, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chronwall BM. Anatomy and physiology of the neuroendocrine arcuate nucleus. Peptides 6 (Suppl 2): 1–11, 1985. [DOI] [PubMed] [Google Scholar]
- 15.Ciriello J, McMurray JC, Babic T, deOliveira CVR. Collateral axonal projections from hypothalamic hypocretin neurons to cardiovascular sites in nucleus ambiguus and nucleus tractus solitarius. Brain Res 991: 133–141, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Csáki A, Kocsis K, Halász B, Kiss J. Localization of glutamatergic/aspartatergic neurons projecting to the hypothalamic paraventricular nucleus studied by retrograde transport of [3H]d-aspartate autoradiography. Neuroscience 101: 637–655, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Dampney RA. Arcuate nucleus—a gateway for insulin's action on sympathetic activity. J Physiol 589: 2109–2110, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DiMicco JA, Zaretsky DV. The dorsomedial hypothalamus: a new player in thermoregulation. Am J Physiol Regul Integr Comp Physiol 292: R47–R63, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Herman JP, Eyigor O, Ziegler DR, Jennes L. Expression of ionotropic glutamate receptor subunit mRNAs in the hypothalamic paraventricular nucleus of the rat. J Comp Neurol 422: 352–362, 2000. [PubMed] [Google Scholar]
- 20.Iwasa M, Kawabe K, Sapru HN. Activation of melanocortin receptors in the intermediololateral cell column of the upper thoracic cord elicits tachycardia in the rat. Am J Physiol Heart Circ Physiol 305: H885–H893, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobowitz DM, O'Donohue TL. Alpha-melanocyte stimulating hormone: immunohistochemical identification and mapping in neurons of rat brain. Proc Natl Acad Sci USA 75: 6300–6304, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawabe T, Kawabe K, Sapru HN. Cardiovascular responses to chemical stimulation of the hypothalamic arcuate nucleus in the rat: Role of the hypothalamic paraventricular nucleus. PLoS One 7: e45180, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawabe T, Kawabe K, Sapru HN. Effect of barodenervation on cardiovascular responses elicited from the hypothalamic arcuate nucleus of the rat. PLoS One 7: e53111, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kishi T, Aschkenasi CJ, Lee CE, Mountjoy KG, Saper CB, Elmquist JK. Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. J Comp Neurol 457: 213–235, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Kiss J, Csaba Z, Csáki A, Halász B. Glutamatergic innervation of neuropeptide Y and pro-opiomelanocortin-containing neurons in the hypothalamic arcuate nucleus of the rat. Eur J Neurosci 21: 2111–2119, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Krajewski SJ, Burke MC, Anderson MJ, McMullen NT, Rance NE. Forebrain projections of arcuate neurokinin B neurons demonstrated by anterograde tract-tracing and monosodium glutamate lesions in the rat. Neuroscience 166: 680–697, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li P, Cui BP, Zhang LL, Sun HJ, Liu TY, Zhu GQ. Melanocortin 3/4 receptors in paraventricular nucleus modulates sympathetic outflow and blood pressure. Exp Physiol 98: 435–443, 2013. [DOI] [PubMed] [Google Scholar]
- 28.Luong LN, Carrive P. Orexin microinjection in the medullary raphe increases heart rate and arterial pressure but does not reduce tail skin blood flow in the awake rat. Neuroscience 202: 209–217, 2012. [DOI] [PubMed] [Google Scholar]
- 29.Madden CJ, Morrison SF. Excitatory amino acid receptors in the dorsomedial hypothalamus mediate prostaglandin-evoked thermogenesis in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 286: R320–R325, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Madden CJ, Morrison SF. Neurons in the paraventricular nucleus of the hypothalamus inhibit sympathetic outflow to brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 296: R831–R843, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meister B. Neurotransmitters in key neurons of the hypothalamus that regulate feeding behavior and body weight. Physiol Behav 92: 263–271, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Morrison SF, Madden CJ. Central nervous system regulation of brown adipose tissue. Compr Physiol 4: 1677–1713, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morrison SF, Madden CJ, Tupone D. Central control of brown adipose tissue thermogenesis. Front Endocrinol 3: 1–19, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morrison SF, Madden CJ, Tupone D. Central neural regulation of brown adipose tissue thermogenesis and energy expenditure. Cell Metab 19: 741–756, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, Cone RD. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol Endocrinol 8: 1298–1308, 1994. [DOI] [PubMed] [Google Scholar]
- 36.Nakamura K, Matsumura K, Kobayashi S, Kaneko T. Sympathetic premotor neurons mediating thermoregulatory functions. Neurosci Res 51: 1–8, 2005. [DOI] [PubMed] [Google Scholar]
- 37.Nakamura K, Morrison SF. Central efferent pathways mediating skin cooling-evoked sympathetic thermogenesis in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 292: R127–R136, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakamura K, Morrison SF. A thermosensory pathway mediating heat-defense responses. Proc Natl Acad Sci USA 107: 8848–8853, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakamura T, Bhatt S, Sapru HN. Cardiovascular responses to hypothalamic arcuate nucleus stimulation in the rat: Role of sympathetic and vagal efferents. Hypertension 54: 1369–1375, 2009. [DOI] [PubMed] [Google Scholar]
- 40.Nakamura Y, Nakamura K, Matsumura K, Kobayashi S, Kaneko T, Morrison SF. Direct pyrogenic input from prostaglandin EP3 receptor-expressing preoptic neurons to the dorsomedial hypothalamus. Eur J Neurosci 22: 3137–3146, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oldfield BJ, Giles ME, Watson A, Anderson C, Colvill LM, McKinley MJ. The neurochemical characterisation of hypothalamic pathways projecting polysynaptically to brown adipose tissue in the rat. Neuroscience 110: 515–526, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Oliveira AL, Hydling F, Olsson E, Shi T, Edwards RH, Fujiyama F, Kaneko T, Hokfelt T, Cullheim S, Meister B. Cellular localization of three vesicular glutamate transporter mRNAs and proteins in rat spinal cord and dorsal root ganglia. Synapse 50: 117–129, 2003. [DOI] [PubMed] [Google Scholar]
- 43.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates, 6th ed., San Diego, CA: Academic Press, 2007. [Google Scholar]
- 44.Rahmouni K, Morgan DA. Hypothalamic arcuate nucleus mediates the sympathetic and arterial pressure responses to leptin. Hypertension 49: 647–652, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Rousset S, ves-Guerra MC, Mozo J, Miroux B, Cassard-Doulcier AM, Bouillaud F, Ricquier D. The biology of mitochondrial uncoupling proteins. Diabetes 53 (Suppl 1): S130–S135, 2004. [DOI] [PubMed] [Google Scholar]
- 46.Samuels BC, Zaretsky DV, DiMicco JA. Dorsomedial hypothalamic sites where disinhibition evokes tachycardia correlate with location of raphe-projecting neurons. Am J Physiol Regul Integr Comp Physiol 287: R472–R478, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Sapru HN. Role of the hypothalamic arcuate nucleus in cardiovascular regulation. Auton Neurosci 175: 38–50, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sawchenko PE. Toward a new neurobiology of energy balance, appetite, and obesity: the anatomists weigh in. J Comp Neurol 402: 435–441, 1998. [PubMed] [Google Scholar]
- 49.Shimazu T. Central nervous system regulation of liver and adipose tissue metabolism. Diabetologia (20 Suppl): 343–356, 1981. [PubMed] [Google Scholar]
- 50.Sim LJ, Joseph SA. Arcuate nucleus projections to brainstem regions which modulate nociception. J Chem Neuroanat 4: 97–109, 1991. [DOI] [PubMed] [Google Scholar]
- 51.Simpson KA, Martin NM, Bloom SR. Hypothalamic regulation of food intake and clinical therapeutic applications. Arq Bras Endocrinol Metabol 53: 120–128, 2009. [DOI] [PubMed] [Google Scholar]
- 52.Singru PS, Sanchez E, Fekete C, Lechan RM. Importance of melanocortin signaling in refeeding-induced neuronal activation and satiety. Endocrinology 148: 638–646, 2007. [DOI] [PubMed] [Google Scholar]
- 53.Skibicka KP, Grill HJ. Hypothalamic and hindbrain melanocortin receptors contribute to the feeding, thermogenic, and cardiovascular action of melanocortins. Endocrinology 150: 5351–5361, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sohn JW. Network of hypothalamic neurons that control appetite. BMB Rep 48: 229–233, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Song CK, Vaughan CH, Keen-Rhinehart E, Harris RB, Richard D, Bartness TJ. Melanocortin-4 receptor mRNA expressed in sympathetic outflow neurons to brown adipose tissue: Neuroanatomical and functional evidence. Am J Physiol Regul Integr Comp Physiol 295: R417–R428, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tupone D, Madden CJ, Cano G, Morrison SF. An orexinergic projection from perifornical hypothalamus to raphe pallidus increases rat brown adipose tissue thermogenesis. J Neurosci 31: 15,944–15,955, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tupone D, Madden CJ, Morrison SF. Autonomic regulation of brown adipose tissue thermogenesis in health and disease: potential clinical applications for altering BAT thermogenesis. Front Neurosci 8: 14, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van den Pol AN. Glutamate and aspartate immunoreactivity in hypothalamic presynaptic axons. J Neurosci 11: 2087–2101, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yoshida K, Nakamura K, Matsumura K, Kanosue K, Konig M, Thiel HJ, Boldogkoi Z, Toth I, Roth J, Gerstberger R, Hubschle T. Neurons of the rat preoptic area and the raphe pallidus nucleus innervating the brown adipose tissue express the prostaglandin E receptor subtype EP3. Eur J Neurosci 18: 1848–1860, 2003. [DOI] [PubMed] [Google Scholar]
- 60.Zhan C, Zhou J, Feng Q, Zhang JE, Lin S, Bao J, Wu P, Luo M. Acute and long-term suppression of feeding behavior by POMC neurons in the brainstem and hypothalamus, respectively. J Neurosci 33: 3624–3632, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang Y, Kerman IA, Laque A, Nguyen P, Faouzi M, Louis GW, Jones JC, Rhodes C, Munzberg H. Leptin-receptor-expressing neurons in the dorsomedial hypothalamus and median preoptic area regulate sympathetic brown adipose tissue circuits. J Neurosci 31: 1873–1884, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]