This research is noteworthy, in that it supports a new mechanism by which chemerin can act to modify blood pressure. This expands the repertoire of functions assigned to this adipokine and forces us to enlarge our view of the role adipokines play in blood pressure regulation.
Keywords: chemerin, ChemR23, electrical field stimulation, perivascular adipose tissue, vascular smooth muscle
Abstract
The adipokine chemerin causes arterial contraction and is implicated in blood pressure regulation, especially in obese subjects with elevated levels of circulating chemerin. Because chemerin is expressed in the perivascular adipose tissue (PVAT) that surrounds the sympathetic innervation of the blood vessel, we tested the hypothesis that chemerin (endogenous and exogenous) amplifies the sympathetic nervous system in mediating electrical field-stimulated (EFS) contraction. The superior mesenteric artery, with or without PVAT and with endothelium and sympathetic nerve intact, was mounted into isolated tissue baths and used for isometric contraction and stimulation. Immunohistochemistry validated a robust expression of chemerin in the PVAT surrounding the superior mesenteric artery. EFS (0.3–20 Hz) caused a frequency-dependent contraction in isolated arteries that was reduced by the chemerin receptor ChemR23 antagonist CCX832 alone (100 nM; with, but not without, PVAT), but not by the inactive congener CCX826 (100 nM). Exogenous chemerin-9 (1 μM)-amplified EFS-induced contraction in arteries (with and without PVAT) was blocked by CCX832 and the α-adrenergic receptor antagonist prazosin. CCX832 did not directly inhibit, nor did chemerin directly amplify, norepinephrine-induced contraction. Whole mount immunohistochemical experiments support colocalization of ChemR23 with the sympathetic nerve marker tyrosine hydroxylase in superior mesenteric PVAT and, to a lesser extent, in arteries and veins. These studies support the idea that exogenous chemerin modifies sympathetic nerve-mediated contraction through ChemR23 and that ChemR23 may be endogenously activated. This is significant because of the well-appreciated role of the sympathetic nervous system in blood pressure control.
NEW & NOTEWORTHY
This research is noteworthy, in that it supports a new mechanism by which chemerin can act to modify blood pressure. This expands the repertoire of functions assigned to this adipokine and forces us to enlarge our view of the role adipokines play in blood pressure regulation.
because of its location, the perivascular adipose tissue (PVAT) has the potential to affect the function of the blood vessels it encases. This can occur by secretion of adipokines that directly affect vascular tone, such as the relaxant adiponectin (25). We recently discovered the protein chemerin in PVAT and demonstrated that a shorter chemerin agonist, chemerin-9, caused direct arterial contraction through activation of the best-characterized chemerin receptor, ChemR23 (3, 8, 27, 33, 38, 48). Chemerin is secreted from the liver and fat depots (10), functioning as an adipokine that regulates adipogenesis (12, 31, 32) and as an activator of inflammatory cells (13, 50, 52). Circulating levels of chemerin are positively associated with body mass index (1, 4, 9, 16, 36, 37, 40). Because of our long-term interest in understanding whether chemerin could play a role in obesity-associated hypertension, we turned our attention to a means by which arterial function could be modified, namely, activation of the sympathetic nervous system.
There is no question that the sympathetic nervous system exerts control over vascular tone and, thus, total peripheral resistance. Sympathetic varicosities are found in the adventitial-medial border of the blood vessel, and this means that the nerves must carry through the PVAT itself. These nerves are likely exposed to substances in PVAT such as chemerin (5). We tested the hypothesis that chemerin potentiates the actions of sympathetic nerves in isolated vasculature.
Our model is the male Sprague-Dawley rat superior mesenteric artery with PVAT and the endothelium and sympathetic nerve intact. This artery was chosen for several reasons. 1) The superior mesenteric artery is in the viscera, and it is visceral fat that is associated with the greatest cardiovascular risk (21, 46, 47). 2) The superior mesenteric artery controls a significant percentage of cardiac output. 3) The superior mesenteric artery is amenable for use in electrical field stimulation (EFS) protocols through which pharmacological manipulations can be made. We used chemerin-9 as a biologically active agonist of ChemR23 (51) and tyrosine hydroxylase (TH) as a marker of sympathetic nerves. This is logical, given that TH is the rate-limiting enzyme of catecholamine synthesis. These experiments will show, for the first time, the ability of chemerin and/or ChemR23 to potentiate the function of the adrenergic nerve, potentially by the presence of ChemR23 on sympathetic nerves both within PVAT and the vessels themselves.
MATERIALS AND METHODS
Animals
The Michigan State University Institutional Animal Care and Use Committee approved all protocols. Male Sprague-Dawley rats (225–300 g body wt; Charles River Laboratories) were used in this study.
Materials
Chemerin-9 was purchased from GenScript (Piscataway, NJ). CCX832 and CCX826 were provided by ChemoCentryx. Dimethylsulfoxide, norepinephrine hydrochloride, phenylephrine hydrochloride, and prazosin were purchased from Sigma Chemical (St. Louis, MO). Tetrodotoxin was obtained from Tocris (Minneapolis, MN).
Tissue Preparation
Rats were anesthetized with pentobarbital sodium (60–80 mg/kg ip), and the superior mesenteric artery was removed and placed in physiological salt solution containing (mM) 130 NaCl 130, 4.7 KCl, 1.8 KH2PO4, 1.7 MgSO4·7H2O, 14.8 NaHCO3, 5.5 dextrose, 0.03 CaNa2EDTA, and 1.6 CaCl2 (pH 7.2). Sections of the artery were cut into rings (2- to 3-mm rings for slide sections or 0.5- to 1-cm-wide tissue for whole mount) for immunohistochemistry or measurement of isometric contractile force. In immunohistochemical and most EFS experiments, the surrounding PVAT was left intact. Some superior mesenteric arteries were cleaned of PVAT before experimentation to investigate the potential interaction of chemerin-9 and CCX832 with the adrenergic receptor in arterial smooth muscle and the role of nerve/PVAT on ChemR23 dependence of EFS-induced contraction. Sections for whole mount experiments were cleaned of blood and excess PVAT, and vessels were exposed, with the PVAT that connected artery and vein retained.
Immunohistochemistry
Chemerin.
Slides containing sections of paraffin-embedded, formalin-fixed rat superior mesenteric artery + PVAT (8 μm thick) were dewaxed, and antigens were retrieved using unmasking solution (Vector Laboratories, Burlingame, CA) and subjected to a standard protocol. Slides were incubated with chemerin antibody (rabbit, 1:100 dilution; catalog no. H-002-52, Phoenix Pharmaceutical, Belmont, CA) in the appropriate 1.5% blocking serum. Slides were washed in phosphate-buffered saline and incubated with an anti-rabbit peroxidase-conjugated secondary antibody in 1.5% blocking serum for 30 min and then in Vectastain Elite ABC Reagent (Vector Laboratories) for 30 min. 3,3-Diaminobenzidine was applied for 1 min to all sections. The slides were counterstained with hematoxylin (15 s; Vector Laboratories). Images were captured on a Nikon Eclipse Ti microscope with MMI imaging software.
ChemR23 and TH.
Connected vessels (artery and vein) were perpendicularly cut in half (for paired primary antibody and no primary antibody experiments) and pinned into mini petri dishes to be fixed. Tissues, which contained the artery and vein connected by PVAT, were first incubated overnight with Zamboni's fixative at 4°C and then with 70% ethanol for 24 h and 50% ethanol for 48 h at 4°C. Tissues were washed four times with PBS with 0.1% Triton X-100 (PBS-TX) for 10 min and then incubated with 5% goat blocking serum (made with PBS-TX) for 1 h. Primary antibody was then added to one of each of the paired sections [50:50 TH (1:1,000 dilution; catalog no. AB152, EMD Millipore, Temecula, CA) and ChemR23 (1:25 dilution; catalog no. sc-398769, Santa Cruz Biotechnology, Dallas, TX) made in goat blocking serum] for 48 h at 4°C in a humidified chamber. Tissues were washed four times for 10 min with PBS-TX before secondary antibody was added [in PBS: 50:50 Alexa Fluor 488 goat anti-mouse (1:1,000 dilution) and Alexa Fluor 568 goat anti-rabbit (1:1,000 dilution); catalog nos. A11029 and A11036, respectively, Thermo Fisher, Eugene, OR] for 1 h and then washed four times for 10 min with PBS-TX. Tissues were transferred to slides and covered with 4′,6-diamidino-2-phenylindole (DAPI; ProLong Gold Antifade Mount with DAPI, catalog no. P-36931, Thermo Fisher Scientific, Carlsbad, CA), and a coverslip was applied and sealed with clear nail polish. Slides were left to dry for 1 day before imaging. Rat brain sections (region 4, Zyagen, San Diego, CA) were used as a positive control for TH.
Isolated Tissue Bath
Two parallel stainless steel hooks were introduced into the lumen of the artery. One segment was fixed within the warmed (37°C) and aerated (95% O2-5% CO2) tissue bath (30 ml). The other was connected to an isometric force transducer (model FT03, Grass Instruments, Quincy, MA), which was connected to PowerLab data acquisition hardware (ADInstruments, Colorado Springs, CO). Tissues were placed under optimal resting tension (1,200 mg; previously determined) and allowed to equilibrate for 1 h before an initial challenge with a maximal concentration (10−5 M) of phenylephrine (PE). After the initial PE challenge, tissues were washed until tone returned to baseline. Tissues were subjected to one of the following protocols.
Protocol 1: EFS
Superior mesenteric artery segments were mounted between two platinum electrodes (positioned within the tissue bath) connected to a stimulator (model S88, Grass Instruments), and an electrical stimulus was delivered according to the following protocol: 30 stimuli, 0.5-ms stimulus duration, 0.3- to 20-Hz frequency, and 14 V. Isometric contractile force that resulted from this stimulation was measured in an isolated tissue bath as outlined above. After initial challenge with PE, a 20-Hz maximal stimulus was delivered to each vascular segment to validate the presence of an activatable nerve. This 20-Hz contraction was ∼30% of the initial PE challenge in all groups. All subsequent data are expressed as a percentage of the initial 20-Hz maximal contraction. The fast sodium channel inhibitor tetrodotoxin (300 nM) was added to the tissue bath after a brief washout period following the initial 20-Hz stimulus to validate the neuronal origin of the stimulus. Because in preliminary experiments the α1-adrenergic receptor antagonist prazosin (50 nM) abolished the 20 Hz-induced contraction, the response we were studying was sympathetic in nature.
EFS was applied sequentially from low to high intensity, with ≥20 min of washing and recovery between each challenge and longer washing-and-recovery periods when antagonists were used. In some experiments, abbreviated frequency-response curves were constructed. In one series of experiments, only one midlevel (5-Hz) frequency was used. One of the following protocols was employed.
Protocol 1A.
The following experimental conditions were used on tissues with and without PVAT. A 5-min incubation with vehicle/agonists was performed after a 5-min incubation with antagonist: 1) vehicle, 2) chemerin-9 (1 μM), 3) the ChemR23 antagonist CCX832 (100 nM), or 4) chemerin-9 after CCX832 (100 nM). The full EFS range (0.3–20 Hz) was investigated. In separate experiments with and without PVAT, a longer 30-min incubation with vehicle (0.1% dimethylsulfoxide), the ChemR23 receptor antagonist CCX832, or the inactive congener of CCX832, CCX826 (100 nM), alone was used prior to construction of the frequency-response curve. More frequencies were used at the higher end of the EFS range (2–20 Hz), as this was the field of interest moving forward.
Protocol 1B.
Tissues were incubated with 0.1% methanol (prazosin vehicle) or prazosin (1 μM) for 1 h prior to incubation with water (chemerin vehicle) or chemerin-9 (1 μM) before challenge with a 5-Hz stimulus. This protocol tested the adrenergic receptor dependence of the chemerin-9-amplified EFS contraction.
Protocol 2: Basic Contractility
PVAT was completely removed from the superior mesenteric arteries, and arteries were mounted and established as described above, except for removal of the electrodes from the apparatus. After initial challenge with PE, arteries were washed and then exposed to vehicle or CCX832 for 1 h. Cumulative concentration-response curves to norepinephrine (NE) were constructed.
Data Analyses
For slide-based, non-whole mount immunohistochemistry experiments, sections from a minimum of four animals were used. Adjustments in brightness and contrast were made to the whole panel, not a portion of an image. All quantified results are presented as means ± SE. EFS data are reported as a percentage of an original 20-Hz-stimulated contraction. Non-EFS contractility results are reported as a percentage of the initial PE-induced contraction. A two-way ANOVA with Bonferroni's correction was used to determine statistical differences in EFS maximums when more than two groups were compared. In all cases, P < 0.05 was considered significant.
For whole mount imaging to investigate ChemR23 and TH colocalization, slides were imaged using the Olympus FluoView 1000 confocal laser scanning microscope at Michigan State University Center for Advanced Microscopy. Nuclei of all cells were stained blue with DAPI, which was excited with a 405-nm diode laser, and emission was recorded using a 425- to 475-nm band-pass filter. ChemR23 stained green with Alexa Fluor 488 (FITC) and was excited with a 488-nm argon laser, and emission was recorded using a 500- to 545-nm band-pass filter. TH stained red with Alexa Fluor 568 (TRITC) and was excited with a 559-nm solid state laser, and emission was recorded using a 575- to 675-nm band-pass filter. Photomultiplier tube detector sensitivities were optimized by setting minimum detection levels for each channel using sections without addition of primary antibody. Two series of confocal images (xyz) were taken on sections with primary antibody and one was taken on sections without primary antibody for each artery, PVAT, and vein. They were acquired through the depth (25–150 μm) of each tissue section, in 1- or 2-μm step increments, using a UPlanSApo ×20 objective (numerical aperture 0.75). One individual confocal image was selected for each positive tissue section series (2 series per tissue, 2 individual images). These individual confocal images were then paired with an individual confocal image without primary antibody from the same animal, with DAPI used to match the depths. These were grouped on Photoshop (Adobe Photoshop CC 2014) to create one grouped image. Color enhancement and adjustments were applied to the entire grouped image; therefore, images with and without primary antibody were identically affected. Positive results were determined as TRITC visualization for TH, FITC visualization for ChemR23, or yellow for colocalization of FITC and TRITC. These images were used in two different approaches to quantify TH and ChemR23 localization.
In a first approach to analyzing TH and ChemR23 colocalization, the frequency of overlapping signal (yellow) was determined using multiple display sources (6 different monitors from 3 different rooms to visualize the images). Positive results were determined by two different individuals, one of whom was blinded to experimental conditions, scoring the entire image using a binary scale (0 = no staining, 1 = staining). A one-way ANOVA with Bonferroni's correction was run on tissue-specific group values to determine statistical differences between TH and ChemR23 fluorophore pixel colocalization in different tissue types.
A second analysis of colocalization was conducted using FluoView FV1000 confocal software. Instead of selecting one individual confocal plane, we analyzed the signal from an entire z-series for the Mander's overlap coefficient (MOC). No region of interest was selected to allow analysis of the entire plane, and the entire depth of the series was processed for all conditions and tissues. Therefore, all cell types present were analyzed. There were more images in z-stacks collected from samples treated with primary antibody than samples that were not treated with primary antibody. More images collected meant that more negative pixels were also collected, which diluted the number of positive pixels in the primary series. This difference in positive-to-negative pixel ratio between tissue treated with primary antibody and tissue not treated with primary antibody lowers the MOC. Since areas considered “background” could not be consistently found throughout the different tissue types, none was subtracted or offset in these calculations. A two-way ANOVA with Bonferroni's correction was run to determine statistical differences in the MOC for ChemR23 in TH between different tissue types and between groups treated with primary antibody and groups not treated with primary antibody.
RESULTS
Chemerin Is Expressed in PVAT Around Superior Mesenteric Artery
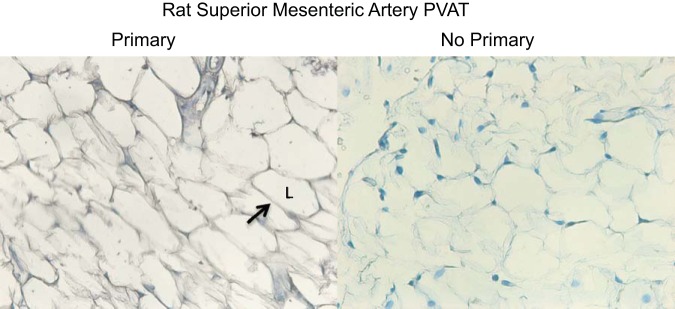
Figure 1 shows the immunohistochemical staining of the PVAT surrounding the superior mesenteric artery with an antibody against chemerin in the presence of the primary antibody (left) and without the primary antibody in the reaction (right). The cytosol of the adipose cells stained robustly for chemerin, which indicates that PVAT is an endogenous source of chemerin to the artery and confirms previous findings (48).
Fig. 1.
Immunohistochemical localization of chemerin to perivascular adipose tissue (PVAT) of rat superior mesenteric artery in experiments in which primary antibody directed against chemerin was present (left) and experiments in which the primary antibody was absent (right). Arrow points to an area of adipose cell cytoplasm that stains positively. L, lipid droplet. Images are representative of 4 different animals.
Exogenous Chemerin Amplifies EFS-Induced Contraction Through ChemR23
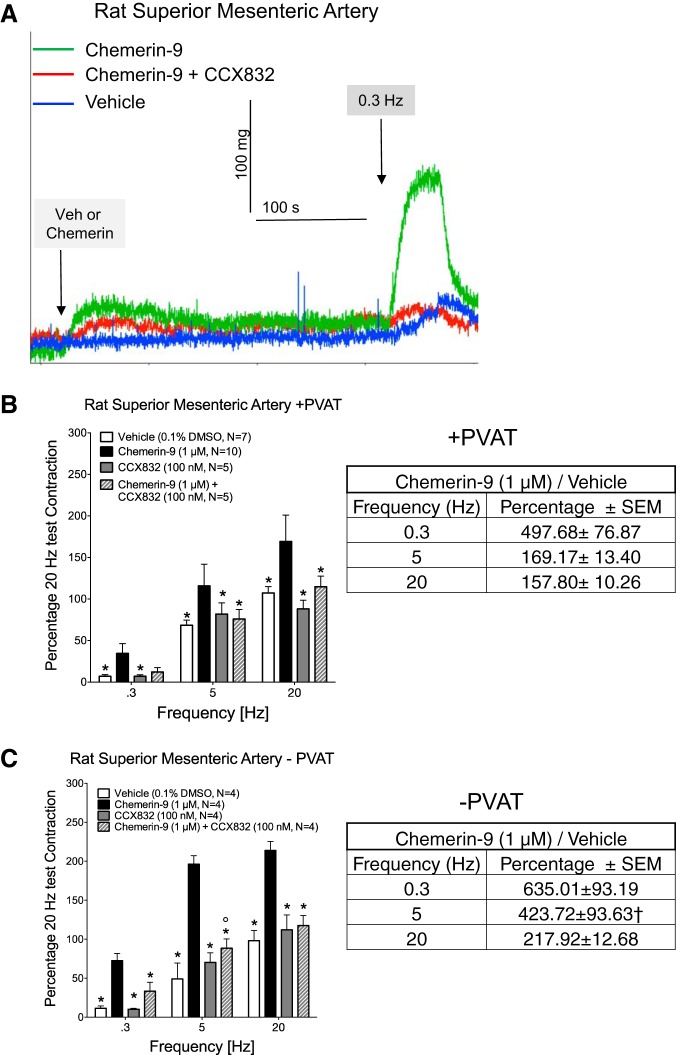
In Fig. 2, we examined the ability of chemerin-9 to amplify EFS-induced contraction. Figure 2A, shows representative traces of experimental results in the artery in the presence and absence of PVAT. All incubations of agonist and antagonists were short (5 min), with antagonists administered 5 min prior to addition of vehicle or agonist. Chemerin-9 caused a small contraction from baseline (average of 103.5 ± 1.56 mg overall in the absence and presence of PVAT combined, green line in Fig. 2A). The EFS-induced contraction was quantified by setting the baseline after the chemerin-9-induced contraction had plateaued; thereby the amplified contraction alone is shown by the solid black bars in Fig. 2, B and C. Exogenous chemerin-9 amplified EFS-induced contraction significantly at all frequencies in tissues with (Fig. 2B) and without (Fig. 2C) PVAT. Amplification appeared to be greater in the tissues without than with PVAT. The chemerin-9-amplified contraction as a percentage of their respective vehicle is shown in Fig. 2, B and C (right). A two-way ANOVA followed by Bonferroni's correction supported a significant increase in the 5-Hz group only.
Fig. 2.
Exogenous chemerin-9 amplifies electrical field stimulation (EFS)-induced contraction through the chemerin receptor ChemR23. Isolated superior mesenteric arteries in the presence (+) and absence (−) of perivascular adipose tissue (PVAT) were exposed to vehicle or the ChemR23 antagonist CCX832 for 5 min prior to addition of vehicle or chemerin-9. A: representative traces of experimental results in the artery in the presence of PVAT. B and C: results for tissues with and without PVAT. Left: means ± SE; n, number of animals. *Significantly different from chemerin-9; °significantly different from vehicle, by 2-way ANOVA (P < 0.05). Right: chemerin-9-amplified contraction as a percentage of vehicle per frequency in the presence and absence of PVAT. †Significantly different from +PVAT value.
Importantly, the ChemR23 receptor antagonist CCX832 blocked the amplification of EFS-induced contraction caused by exogenous chemerin-9, although blockade was only partial at the 5-Hz stimulus in tissues with PVAT (Fig. 2B). We investigated whether repeated exposure to CCX832 would diminish general contractility. Table 1 presents the magnitude of maximum PE-induced contraction at the beginning and end of the experiments shown in Fig. 2. The inability of repeated exposure to CCX832 to reduce PE-induced contraction suggests that CCX832 is not toxic or generally inhibitory to smooth muscle contraction. Finally, the amplified contraction caused by chemerin in arteries with PVAT was also dependent on the adrenergic receptor, given that the α-adrenergic receptor antagonist prazosin (1 μM) equally and significantly reduced a midrange (5-Hz) stimulation in naïve and chemerin-9-exposed groups (Fig. 3). This finding suggests that chemerin-9 may be directly interacting with the nerves that control NE release. Interestingly, at the 20-Hz stimulus in tissue with PVAT only, the 5-min incubation with CCX832 alone modestly reduced EFS-induced contraction (Fig. 2B), and this motivated us to carry out the following experiments.
Table 1.
Contraction to PE in rat superior mesenteric artery without and with PVAT before and after exposure to CCX832
| −PVAT |
+PVAT |
|||
|---|---|---|---|---|
| Initial contraction, mg | Final contraction, mg | Initial contraction, mg | Final contraction, mg | |
| Vehicle | 1,172 ± 28 | 1,294 ± 52 | 944 ± 46 | 1,191 ± 56 |
| Chemerin-9 | 909 ± 91 | 798 ± 162 | 823 ± 34 | 1,041 ± 21 |
| CCX832 | 1,149 ± 82 | 1,211 ± 88 | 913 ± 68 | 827 ± 26 |
| Chemerin-9 + CCX832 | 1,256 ± 57 | 1,408 ± 64 | 1,091 ± 112 | 994 ± 55 |
Values are means ± SE for number of animals reported in Fig. 2. Rat superior mesenteric artery without (−) and with (+) perivascular adipose tissue (PVAT) was stimulated with 10−5 M phenylephrine (PE) and exposed to chemerin-9 (1 μM), CCX832 (100 nM), or chemerin-9 + CCX832.
Fig. 3.
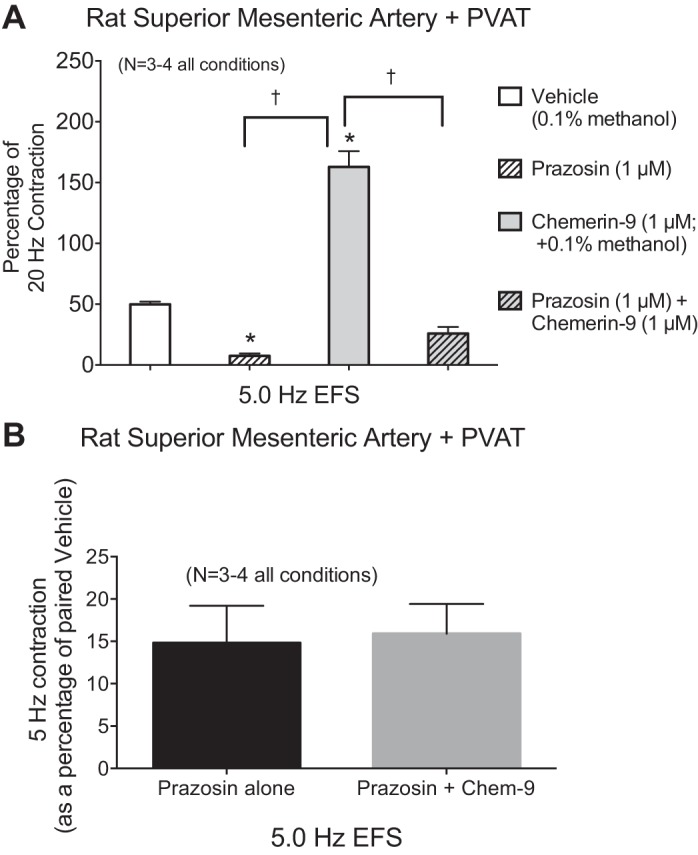
Chemerin-9-amplified EFS-induced contraction is dependent on α-adrenergic receptor function. A: the α1-adrenergic receptor antagonist prazosin reduced EFS (5.0 Hz)-induced contraction in the presence of vehicle (vehicle and prazosin) and chemerin-9 (chemerin-9 and chemerin-9 + prazosin) in the superior mesenteric artery with PVAT. B: reduction in contraction caused by prazosin was statistically similar for both experimental groups (e.g., tissues incubated without and with chemerin-9). Values are means ± SE; n, number of animals. *P < 0.05 vs. vehicle; †P < 0.05 between connected bars (by ANOVA).
Endogenous Chemerin May Facilitate EFS-Induced Contraction Through ChemR23
We incubated segments of artery with PVAT with vehicle, the same concentration of the ChemR23 antagonist CCX832, or the inactive congener of CCX832, CCX826, as used in Fig. 2 alone with tissues, for a longer period (30 min). In these experiments, CCX832 alone, but not the inactive congener CCX826, reduced EFS-induced contraction consistently (Fig. 4, A and C). Importantly, when we removed PVAT, a 30-min incubation with CCX832 (100 nM) did not reduce an abbreviated EFS-induced contractile curve on its own (Fig. 4B). These data suggest that endogenous chemerin may support EFS-induced contraction and/or that ChemR23 is (constitutively) active and supports nerve-mediated contraction.
Fig. 4.
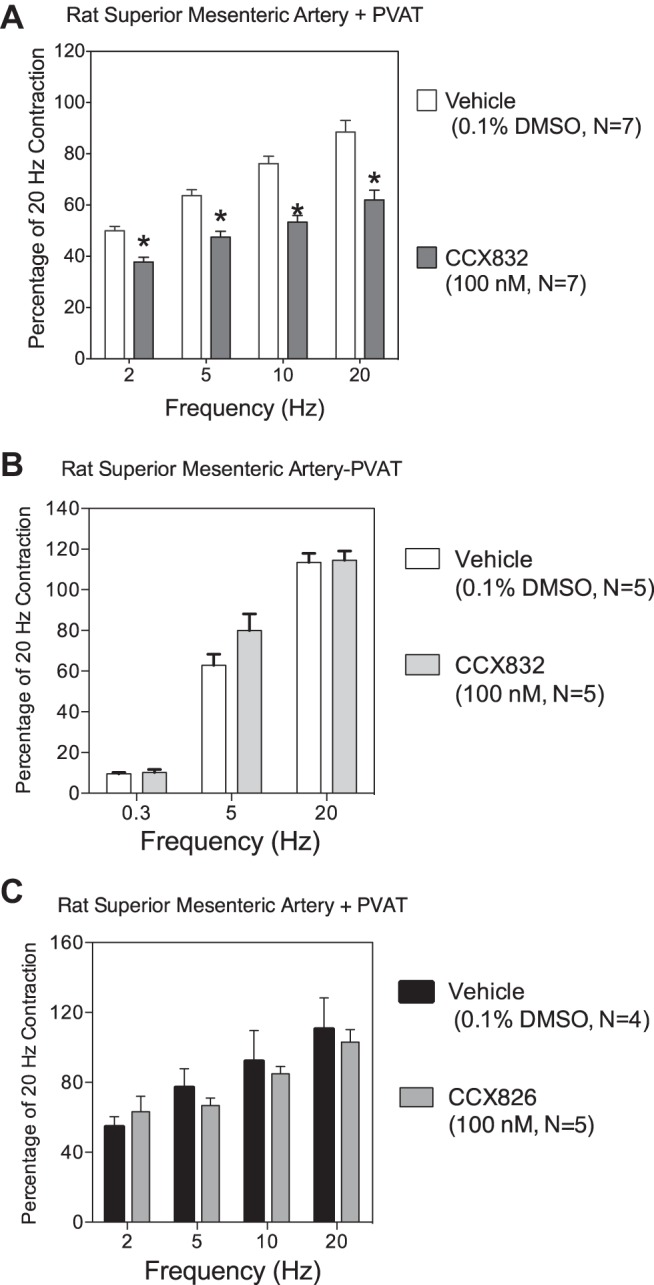
Endogenous activation of ChemR23 amplifies EFS-induced contraction. A and B: the ChemR23 receptor antagonist CCX832 alone reduced EFS-induced contraction in the presence, but not in the absence, of PVAT. C: the inactive congener CCX826 did not reduce EFS-induced contraction in arteries in the presence of PVAT after a 30-min incubation. Values are means ± SE; n, number of animals. *Statistically different from vehicle response within each frequency.
The same concentration of CCX832 that reduced exogenous chemerin-induced and basal EFS-induced contraction did not shift the NE-induced concentration-response curve (Fig. 5A). EC50 values for NE in causing contraction were not different between arteries incubated with vehicle and CCX832 [−log EC50 (M) = 7.35 ± 0.07 and 7.23 ± 0.06, respectively, P > 0.05]. Similarly, addition of exogenous chemerin (1 μM) for 5 min prior to NE additions did not directly modify NE-induced contraction [Fig. 5B; −log EC50 (M) vehicle = 7.50 ± 0.04 and 7.40 ± 0.05 for vehicle- and chemerin-9-incubated segments, respectively, P > 0.05].
Fig. 5.
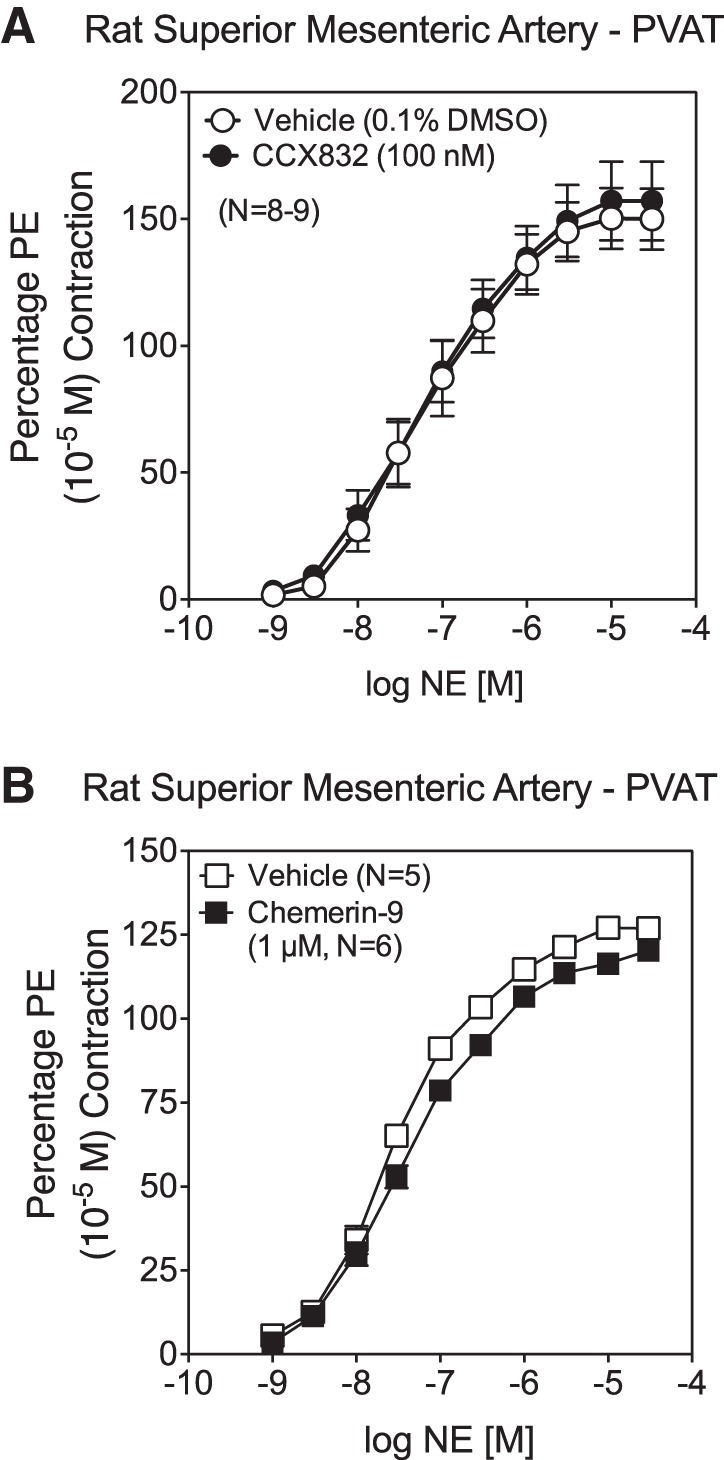
Neither CCX832 nor chemerin-9 directly affects adrenergic receptor function. A and B: concentration-response curve to norepinephrine (NE) in the isolated mesenteric artery in the absence of PVAT was not shifted, nor was magnitude altered, at a concentration of CCX832 or chemerin-9 that influenced EFS-induced contraction. Values are means ± SE; n, number of animals.
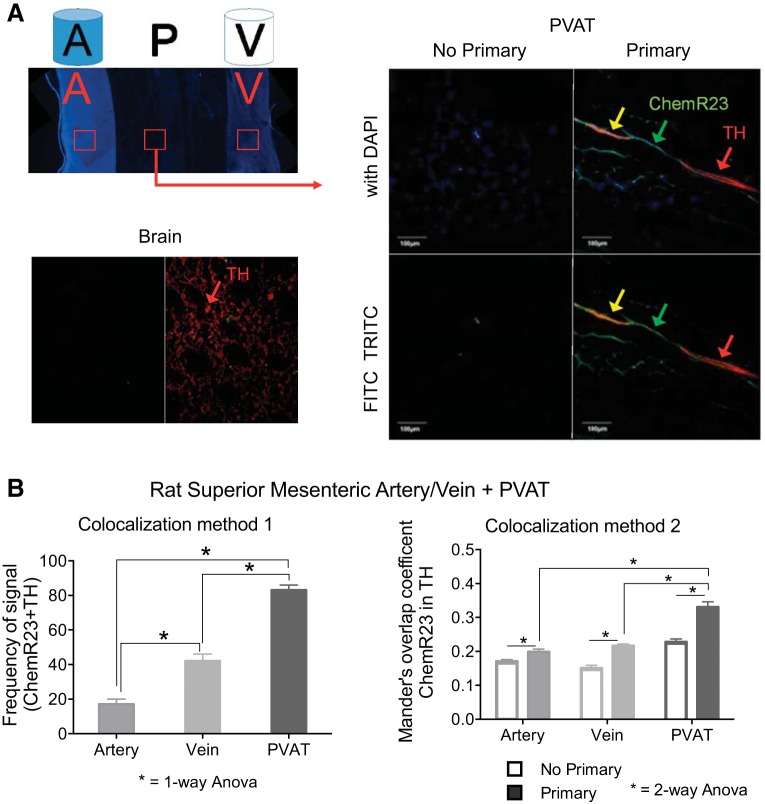
Finally, we investigated whether ChemR23 was present on the sympathetic nerves within and around the vessels, as this would provide one explanation for chemerin-amplified EFS-induced contraction. We used whole mounts of superior mesenteric arteries and veins connected by PVAT in fluorescence-based immunohistochemistry. The arteries, veins, and PVAT were visualized for detection of ChemR23, TH, and colocalization of these two proteins within these segments; tissues from six different animals were investigated. Figure 6A (top) depicts a cartoon of the vessels (artery and vein) and PVAT orientation within the whole mount tissues and, beneath it, a DAPI-stained image of the same whole mount tissue depicting where the confocal measures that generated the data in Fig. 6B were taken (red boxes). TH staining in the rat brain, shown beneath these images, demonstrates our ability to visualize this protein. In Fig. 6A, right, we focus on the PVAT that connects the artery and vein (red box with arrow leading to images taken in PVAT). In these images, we identify sections of TH-positive structures that also stain for ChemR23 (yellow; Fig. 6A, right).
Fig. 6.
Colocalization of ChemR23 and tyrosine hydroxylase (TH) in superior mesenteric artery, vein, and PVAT. A, left: cartoon depiction (top) and DAPI-stained (blue; bottom) low-magnification image of the whole mount tissue used to determine ChemR23 and TH colocalization in the artery (A), PVAT (P), and vein (V). Red boxes show 1 example of the regions used for the artery (A), PVAT (P), and vein (V) for confocal imaging and measures that generated data in B. Red box with arrow shows an example of the PVAT region (P) that was taken to generate the confocal colocalization (A, right). Immediately below the whole mount cartoon and image is a representative image of the rat brain coronal section without (left) and with (right) the TH antibody, used as a positive control. A, right: confocal image of superior mesenteric PVAT depicts positive signal from ChemR23 (green, FITC) and TH (red, TRITC) and colocalization (yellow). Images using filters for FITC and TRITC alone (bottom) are combined with DAPI (top). Arrows indicate a nerve as an area of interest. Scale bar = 100 μm. B: quantitation of colocalization using method 1 (left; observer-driven) and method 2 (right; Mander's overlap coefficient). Values are means ± SE for 6 different animals. *Statistical difference (P < 0.05) between connected bars.
Two different approaches were taken to test colocalization of TH and ChemR23 in these sections that encompassed the artery and vein with surrounding PVAT. First, we used individual scoring to determine frequency of FITC, TRITC, and colocalized color (yellow) in the PVAT, veins, and arteries. Two independent observers took these measures, and their results are shown in Fig. 6B, left; these observations focused on comparing regions of staining. In the PVAT, TH-positive nerves were present, ChemR23 was present in nerve and adipocytes, and colocalization was detected in 83 ± 3% of samples (representative image in Fig. 6A right; colocalization data reported in Fig. 6B, left). TH-positive nerves, independent of ChemR23 staining, also coursed through the individual artery and vein (not shown). Similarly, ChemR23 was independently present in the artery and vein in smooth muscle cells and nerves (not shown), but significantly less colocalization of ChemR23 with TH-positive nerves was observed in each of these vessels (Fig. 6B). Although TH and ChemR23 were detected and quantified independently in arteries, veins, and PVAT, only colocalization values are shown in Fig. 6B, left.
A second more formal approach entailed use of the whole signal from an entire z-series for analysis of the MOC. Figure 6B, right, shows the MOC of ChemR23 in TH, suggesting that, in the primary-antibody image vs. the no-primary-antibody image, as well as PVAT vs. artery or vein, there is an overall greater colocalization of ChemR23 with TH. These absolute values (<0.5) suggest that the colocalization is generally weak (55) but still presented as statistically significant (P < 0.05).
DISCUSSION
The present study shows, for the first time, the ability of the adipokine chemerin to enhance EFS-induced contraction. This finding is important, because it is one mechanism by which chemerin may influence blood pressure. This relationship is relevant, given that a growing number of epidemiological reports support a positive relationship between body mass index and circulating chemerin, as well as a positive relationship between circulating chemerin levels and risk for cardiovascular disease, including hypertension (1, 4, 9, 16, 36, 37, 40). Focus on the chemerin axis [presence of agonist, chemerin, and receptor (ChemR23)] in the arterial vasculature is supported by others. Kaur et al. (17) identified ChemR23 in human endothelial cells, and Kostopoulos et al. (19) confirmed chemerin and ChemR23 expression in human arteries and PVAT. More recently, infusion of chemerin into the mouse modestly elevated blood pressure (20), and chemerin has been suggested to reduce nitric oxide signaling in the rat aorta (29). Understanding the functions of chemerin is important, because circulating levels of chemerin can be reduced and health can be improved through a number of interventions that include bariatric surgery and exercise (7, 22, 24, 28, 30, 34, 39, 44). Other pathologies, such as arterial stiffness (54), type 2 diabetes (14, 49, 53), (obese) preeclampsia (41, 45), and polycystic ovary syndrome (15, 18), have been associated with elevated chemerin levels. Moreover, a recent study in humans suggests that high chemerin concentrations were associated with kidney dysfunction and may be predictive for other cardiovascular events (23). For these reasons, the present study was undertaken. To our understanding, this is one of the first times an adipokine receptor has been located to a sympathetic nerve in the periphery.
Does Chemerin Amplify EFS-Induced Arterial Contraction?
Although secreted in different isoforms, circulating chemerin appears to exert its biological effects through primarily one receptor, ChemR23. We investigated whether chemerin external to the artery could influence arterial function. Chemerin-9 was used as an agonist with recognized biological activity (51). Added exogenously to the tissue bath, chemerin-9 amplified EFS-induced contraction, increasing the contraction that resulted with stimulation. Chemerin-9-induced potentiation of EFS-stimulated contraction, when reported as a percentage of vehicle, was greater in the absence than in the presence of PVAT at 5-Hz stimulation and was modestly, but not significantly, elevated at other frequencies. While we have not specifically investigated the mechanisms behind this difference, there are two possible explanations for this observation. 1) The tissues with PVAT are already exposed to chemerin that is endogenous to the PVAT. This would explain why CCX832 alone in tissues with, but not without, PVAT showed a reduction in EFS-induced contraction. Addition of exogenous chemerin would have less effect in those tissues with PVAT than in those without PVAT. 2) With PVAT removal, we may have also removed a substance that reduces contractility. For example, adiponectin is well recognized to mediate what is normally thought of as the anticontractile effects of PVAT [e.g., with removal of PVAT, contractile curves shift leftward and/or upward (25)]. By removing adiponectin and/or other species, we may have clarified the actions of chemerin-9 in enhanced nerve-mediated contraction in arteries without PVAT. However, it is fair to state that the net positive or negative effects of PVAT on EFS-induced vasoconstriction are not yet clear, and our explanations are speculative.
Importantly, the chemerin-9-amplified contraction could be reduced by the ChemR23 receptor antagonist CCX832. This finding suggests that circulating chemerin has the ability to amplify the actions of the sympathetic nerve in a receptor-dependent manner. Importantly, prazosin inhibited the chemerin-9-induced amplified contraction. There is no evidence to suggest that prazosin can act as a ChemR23 antagonist; thus the most logical interpretation is that chemerin-9 is directly amplifying actions of the sympathetic nerve to cause a greater contraction. The mechanism by which chemerin-9 would do this (e.g., an increase in cytosolic calcium or an increase in release of NE) is speculative.
We also examined whether chemerin endogenous to the artery could also amplify EFS-induced contraction. We confirmed the finding that mesenteric arterial PVAT expresses chemerin. This particular fat is important to study, because it directly surrounds the artery and, thus, is a source of chemerin in close proximity to the artery. CCX832, which was first reported in 2013 as an original small-molecule ChemR23 antagonist (48), allows us to examine if there is endogenous activation of ChemR23. CCX826 provided a structure control for CCX832. CCX832, but not CCX826, reduced EFS-induced contraction at all frequencies tested in the absence of added chemerin. One could consider reduction of contraction in CCX832-incubated tissues as a potential sign of toxicity, but several findings suggest that this is not the case. 1) A longer incubation (1 h vs. 30 min) with the same concentration of CCX832 (100 nM) did not modify NE-induced contraction (Fig. 5). 2) The structurally related CCX826 did not inhibit contraction, supporting the idea that the pharmacophore (yet to be reported) is not cause for toxicity. 3) Tissue contraction in response to the α-adrenergic agonist PE after CCX832 was not reduced (Table 1).
To be fair, we cannot prove that it is endogenous chemerin released from PVAT that potentiates EFS-induced contraction. Rather, it is more fair to state that something within the preparation is supporting basal activation of ChemR23, because antagonism of this receptor reduced EFS-induced contraction. This raises interesting possibilities for what could activate ChemR23 and makes future experiments that study release of endogenous chemerin critical. Not only chemerin, but lipids such as resolvin E1, can activate ChemR23 (2).
The finding of ChemR23-TH costaining within the PVAT is consistent with the finding that removal of PVAT negated the ability of CCX832 to inhibit EFS-induced contraction. Furthermore, our two separate approaches toward quantifying colocalization of ChemR23 and TH are in agreement based on relative differences in colocalization of ChemR23 and TH between tissues. The blinded-observer tests were done with rigor, and positive/negative scoring by the two observers agreed, as evidenced by the low SE bars in Fig. 6B, left. However, the MOC values were relatively low for overlap of ChemR23 and TH in all tissues, considering that possible MOCs range from 0 to 1. Because the MOC approach considers the whole image, rather than just part of the image, the largely negative background (that could not be offset) dilutes all positive signal. Despite the low MOC values, the relative differences in colocalization of ChemR23 and TH between artery, vein, and PVAT are consistent between quantitation methods, suggesting a real phenomenon in two distinct ways. This work has provided the impetus for future studies that focus specifically on isolation of the sympathetic nerves (within and outside the vessel) for measurement of TH and ChemR23. Both approaches provide at least some confidence that ChemR23 can be found on a TH-positive structure. Notably, colocalization of TH and ChemR23 occurred within the vessels themselves, and this supports the continued ability of chemerin-9 to potentiate EFS-induced contraction in the arteries without PVAT.
We provide two possible explanations for why CCX832 alone would reduce EFS-induced contraction. In removing PVAT, we removed the agonist (chemerin) that would activate ChemR23 and amplify contraction. Alternatively, or in addition, by removing the PVAT, we remove some of the sympathetic nerve structures on which ChemR23 and TH colocalize and, thereby, remove the structure (ChemR23 on TH-positive nerves) that is activated by chemerin. Our experiments cannot discriminate between these two possibilities, and this will be a goal of future studies. For the first time, ChemR23 has been associated with TH-positive nerves in the periphery, and this finding adds to the small amount of literature investigating adipokines as modifiers of nerve activity. For example, adiponectin, when administered intracerebroventricularly to rats, reduced renal sympathetic nerve activity (43), and leptin activated the sympathetic nerves to white adipose tissue in the rat (35).
Chemerin and ChemR23 Do Not Directly Affect α-Adrenergic Receptor Function
Although the colocalization of ChemR23 and TH supports one mechanism by which ChemR23 could enhance EFS-induced contraction, we must consider whether chemerin or ChemR23 activation potentiates the actions of NE at the level of the adrenergic receptor. We addressed these issues in contractility experiments. Chemerin-9 itself did not cause a leftward or an upward shift in the NE concentration-response curve, and the ChemR23 antagonist CCX832 did not result in a rightward or a downward shift in NE-induced contraction. We previously reported that addition of an adrenergic agonist (PE) would amplify contraction to chemerin-9 (48). In this previous experiment, a half-maximal contraction to PE was established and then chemerin-9 was added. In the present experiments, chemerin-9 was added first and then NE was added. Addition of chemerin-9 did cause a small contraction (reported as 103.5 ± 1.56 mg), but when NE was added cumulatively thereafter, this addition did not shift the curve compared with tissues exposed to vehicle. The lack of a shift in the NE curve by chemerin-9 suggests that 1) processes have to be started by an α-adrenergic receptor before chemerin can exert an effect (in the presence of the endothelium) and/or 2) synergism requires that contraction reaches a certain magnitude. Contraction to chemerin-9 in the presence of the endothelium is perhaps 20% of that of a half-maximal PE/NE contraction and may not reach such a magnitude. Our experiments cannot distinguish between these possibilities, and the mechanism of synergism is beyond the scope of this study.
Relative to CCX832 selectivity and specificity, the concentration of CCX832 used is sufficient, given the high affinity of CCX832 for the rat ChemR23 [IC50 = 2 nM in radioligand-binding experiments (48)]. These findings support the idea that potentiation of EFS-induced contraction by chemerin does not occur at the level of the arterial smooth muscle or adrenergic receptor. This finding, combined with the fact that chemerin-9-amplified EFS contraction was adrenergic receptor-dependent, suggests the novel discovery of ChemR23 function in sympathetic nerves.
Limitations
We recognize limitations in this study. The accuracy of our MOC values are diluted by three factors that arise with selecting images containing regions of interest throughout the entire plane. 1) Background could not be offset consistently, because the majority of the images focused on areas containing no visible background (regions lacking measurable signal), and since this could not be calculated for all, it was not subtracted from any image to maintain consistency. 2) Because more images were collected in z-stacks from samples with primary antibody, more negative pixels were quantitated in primary- than no-primary-antibody samples. Therefore, the greater number of negative pixels taken into account alongside positive pixels weakened the positive-to-negative signal ratio used to calculate the MOC in primary antibody-treated images. 3) The nerve complex ran through different areas of the visualized plane, depending on the depth, and a focused region of interest could not be specified for calculations, which meant that all the gaps between nerves were included in the quantification. Our colocalization studies, although thorough, would benefit from future experimentation on the sympathetic nerve directly for ChemR23 expression and investigation of whether and how chemerin-9/chemerin can directly change sympathetic nerve activity.
We have not examined whether immune cells resident in PVAT and the artery might connect chemerin to the sympathetic nerve indirectly. One of the best-recognized actions of chemerin is stimulation of immune cell function (50, 52), and immune cells exist in PVAT (42). Our results are not inconsistent with the possibility that chemerin-9 stimulates immune cells, which, in turn, activate the nerve to potentiate contraction. Conversely, we have not considered the potential anti-inflammatory functions of chemerin peptides and/or activation of ChemR23 (6). We also need to sort out what may be individual/unique biological responsibilities of the many isoforms of chemerin and how they interact with the three recognized receptors of chemerin, ChemR23/CMKLR1 (chemokine-like receptor 1), GPCR 1 (GPR1), and chemokine receptor-like 2 (CCRL2), to effect a growing list of biological responsibilities attributed to chemerin (11, 26). Finally, one explanation for the ability of CCX832 alone to reduce a biological response is that ChemR23 has constitutive activity in our preparation. ChemR23 has not been reported to have such activity, but it remains something to which we must pay attention.
In summary, we demonstrate that the adipokine agonist chemerin is present in mesenteric arterial PVAT. Exogenous chemerin amplified EFS-induced arterial contraction, an action reduced by the ChemR23 antagonist CCX832. CCX832 alone reduced EFS-induced arterial contraction (in the presence of PVAT only). Colocalization of ChemR23 to TH-positive structures in PVAT suggests that direct action on the nerves is one way chemerin amplifies EFS-induced contraction. All these mechanisms are supportive of chemerin in promoting arterial constriction and blood pressure.
GRANTS
This work was funded by National Institutes of Health Grant HL-117847.
DISCLOSURES
T. Charvat, S. Punna, and A. Krasinksi are employed by ChemoCentryx.
AUTHOR CONTRIBUTIONS
E.S.D., B.M.W., and S.W.W. performed the experiments; E.S.D., B.M.W., and S.W.W. analyzed the data; E.S.D., B.M.W., and S.W.W. interpreted the results of the experiments; E.S.D. and S.W.W. prepared the figures; E.S.D. and S.W.W. drafted the manuscript; E.S.D., B.M.W., and S.W.W. edited and revised the manuscript; E.S.D., B.M.W., T.C., A.K., S.P., and S.W.W. approved the final version of the manuscript; S.W.W. developed the concept and designed the research.
ACKNOWLEDGMENTS
We acknowledge the expert technical help of Roxanne Fernandes in developing the whole mount preparation for TH and ChemR23 colocalization.
REFERENCES
- 1.Alfadda AA, Sallam RM, Chishti MA, Moustafa AS, Fatma S, Alomaim WS, Al-Naami MY, Bassas AF, Chrousos GP, Jo H. Differential patterns of serum concentration and adipose tissue expression of chemerin in obesity: adipose depot specificity and gender dimorphism. Mol Cell 33: 591–596, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Back M, Powell WS, Dahlen SE, Drazen JM, Evans JF, Serhan CN, Shimizu T, Yokomizo T, Rovati GE. Update on leukotriene, lipoxin and oxoeicosanoid receptors: IUPHAR Review 7. Br J Pharmacol 171: 3551–3574, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bondue B, Wittamer V, Parmentier M. Chemerin and its receptors in leukocyte trafficking, inflammation and metabolism. Cytokine Growth Factor Rev 25: 331–338, 2011. [DOI] [PubMed] [Google Scholar]
- 4.Bozaoglu K, Bolton K, McMillan J, Zimmet P, Jowett J, Collier G, Walder K, Segal D. Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology 148: 4687–4694, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Bulloch JM, Daly CJ. Autonomic nerves and perivascular fat: interactive mechanisms. Pharmacol Ther 13: 61–73, 2014. [DOI] [PubMed] [Google Scholar]
- 6.Cash JL, Hart R, Russ A, Dixon JP, College WH, Doran J, Hendrick AG, Carlton MB, Greaves DR. Synthetic chemerin-derived peptides suppress inflammation through ChemR23. J Exp Med 205: 767–775, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chakaroun R, Raschpichler M, Kloting N, Oberbach A, Flehmig G, Kern M, Schon MR, Shang E, Lohmann T, Drebler M, Fasshauer M, Stumvoll M, Bluher M. Effects of weight loss and exercise on chemerin serum concentrations and adipose tissue expression in human adiposity. Metab Clin Exp 61: 706–714, 2012. [DOI] [PubMed] [Google Scholar]
- 8.Davenport AP, Alexander SP, Sharman JL, Pawson AJ, Benson HE, Monaghan AE, Liew WC, Mpamhanga CP, Bonner TI, Neubig RR, Pin JP, Spedding M, Harmar AJ. International Union of Basic and Clinical Pharmacology. LXXXVIII. G protein-coupled receptor list: recommendations for new pairings with cognate ligands. Pharmacol Rev 65: 967–986, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong B, Ji W, Zhang Y. Elevated serum chemerin levels are associated with the presence of coronary artery disease in patients with metabolic syndrome. Intern Med 50: 1093–1097, 2011. [DOI] [PubMed] [Google Scholar]
- 10.Ernst MC, Sinal CJ. Chemerin: at the crossroads of inflammation and obesity. Trends Endocrinol Metab 21: 660–666, 2010. [DOI] [PubMed] [Google Scholar]
- 11.Ferland DJ, Watts SW. Chemerin: a comprehensive review elucidating the need for cardiovascular research. Pharmacol Res 99: 351–361, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goralski KB, McCarthy TC, Hanniman EA, Zabel BA, Butcher EC, Parlee SD, Muruganandan S, Sinal CJ. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J Biol Chem 282: 28175–28188, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Hart R, Greaves DR. Chemerin contributes to inflammation by promoting macrophage adhesion to VCAM-1 and fibronectin through clustering of VLA-4 and VLA-5. J Immunol 185: 3728–3739, 2010. [DOI] [PubMed] [Google Scholar]
- 14.Hu W, Feng P. Elevated serum chemerin concentrations are associated with renal dysfunction in type 2 diabetic patients. Diabetes Res Clin Pract 91: 159–163, 2011. [DOI] [PubMed] [Google Scholar]
- 15.Huang R, Yue J, Sun Y, Zheng J, Tao T, Li S, Liu W. Increased serum chemerin concentrations in patients with polycystic ovary syndrome: relationship between insulin resistance and ovarian volume. Clin Chim Acta 450: 366–369, 2015. [DOI] [PubMed] [Google Scholar]
- 16.Jialal I, Devaraj S, Kaur H, Adams-Huet B, Bremer AA. Increased chemerin and decreased omentin-1 in both adipose tissue and plasma in nascent metabolic syndrome. J Clin Endocrinol Metab 98: E514–E517, 2013. [DOI] [PubMed] [Google Scholar]
- 17.Kaur J, Adya R, Tan BK, Chen J, Randeva HS. Identification of chemerin receptor (ChemR23) in human endothelial cells: chemerin-induced endothelial angiogenesis. Biochem Biophys Res Commun 391: 1762–1768, 2010. [DOI] [PubMed] [Google Scholar]
- 18.Kort DH, Kostolias A, Sullivan C, Lobo RA. Chemerin as a marker of body fat and insulin resistance in women with polycystic ovary syndrome. Gynecol Endocrinol 31: 152–155, 2015. [DOI] [PubMed] [Google Scholar]
- 19.Kostopoulos CG, Spioglou SG, Varakis JN, Apostolakis E, Papadaki HH. Chemerin and CMKLR1 expression in human arteries and periadventitial fat: a possible role for local chemerin in atherosclerosis? BMC Cardiovasc Disord 14: 56, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kunimoto H, Kazama K, Takai M, Oda M, Okada M, Yamawaki H. Chemerin promotes the proliferation and migration of vascular smooth muscle and increases mouse blood pressure. Am J Physiol Heart Circ Physiol 309: H1017–H1028, 2015. [DOI] [PubMed] [Google Scholar]
- 21.Lambert EA, Straznicky NE, Dixon JB, Lambert GW. Should the sympathetic nervous system be a target to improve cardiometabolic risk in obesity? Am J Physiol Heart Circ Physiol 309: H244–H258, 2015. [DOI] [PubMed] [Google Scholar]
- 22.Lee MK, Chu SH, Lee DC, An KY, Park JH, Kim DI, Kim J, Hong S, Im JA, Lee JW, Jeon JY. The association between chemerin and homeostasis assessment of insulin resistance at baseline and after weight reduction via lifestyle modifications in young obese adults. Clin Chim Acta 421: 109–115, 2013. [DOI] [PubMed] [Google Scholar]
- 23.Leiherer A, Muendlein A, Kinz E, Vonbank A, Rein P, Fraunberger P, Malin C, Saely CH, Drexel H. High plasma chemerin is associated with renal dysfunction and predictive for cardiovascular events: insights from phenotype and genotype characterization. Vascul Pharmacol 77: 60–68, 2016. [DOI] [PubMed] [Google Scholar]
- 24.Lloyd JW, Zerfass KM, Heckstall EM, Evans KA. Diet-induced increases in chemerin are attenuated by exercise and mediate the effect of diet on insulin and HOMA-IR. Ther Adv Endocrinol Metab 6: 189–198, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lynch FM, Withers SB, Yao E, Werner ME, Edwards G, Weston AH, Heagerty AM. Perivascular adipose tissue-derived adiponectin activates BKCa channels to induce anticontractile responses. Am J Physiol Heart Circ Physiol 304: H786–H795, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mattern A, Zellmann T, Beck-Sickinger AG. Processing, signaling and physiological function of chemerin. IUBMB Life 66: 19–26, 2014. [DOI] [PubMed] [Google Scholar]
- 27.Meder W, Wendland M, Busmann A, Kutzleb C, Spodsberg N, John H, Richter R, Schleuder D, Meyer M, Forssmann WG. Characterization of human circulating TIG2 as a ligand for the orphan receptor chemR23. FEBS Lett 555: 495–499, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Neuparth MJ, Proenca JB, Santos-Silva A, Coimbra S. The positive effect of moderate walking exercise on chemerin levels in Portuguese patients with type 2 diabetes mellitus. J Investig Med 62: 350–353, 2014. [DOI] [PubMed] [Google Scholar]
- 29.Neves KB, Lobato NS, Lopes RA, Filgueira FP, Zanotto CZ, Oliveira AM, Tostes RC. Chemerin reduces vascular nitric oxide/cGMP signaling in rat aorta: a link to vascular dysfunction in obesity? Clin Sci (Lond) 127: 111–122, 2014. [DOI] [PubMed] [Google Scholar]
- 30.Ress C, Tschoner A, Engl J, Klaus A, Tilg H, Ebenbichler CF, Patsch JR, Kaser S. Effect of bariatric surgery on circulating chemerin levels. Eur J Clin Invest 40: 277–280, 2010. [DOI] [PubMed] [Google Scholar]
- 31.Roh SG, Song SH, Choi KC, Katoh K, Wittamer V, Parmentier M, Sasaki SI. Chemerin—a new adipokine that modulates adipogenesis via its own receptor. Biochem Biophys Res Commun 362: 1013–1018, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Rourke JL, Dranse HJ, Sinal CJ. Towards an integrative approach to understanding the role of chemerin in human health in disease. Obes Rev 14: 245–262, 2013. [DOI] [PubMed] [Google Scholar]
- 33.Samson M, Edinger AL, Stordeur P, Rucker J, Verhasselt V, Sharron M, Govaerts C, Mollereau C, Vassart G, Doms RW, Parmentier M. ChemR23, a putative chemoattractant receptor, is expressed in monocyte derived dendritic cells and macrophages and is a coreceptor for SIV and some primary HIV-1 strains. Eur J Immunol 28: 1689–1700, 1998. [DOI] [PubMed] [Google Scholar]
- 34.Sell H, Divoux A, Poitou C, Basdevant A, Bouillot JL, Bedossa P, Tordjman J, Eckel J, Clement K. Chemerin correlates with markers for fatty liver in morbidly obese patients and strongly decreases after weight loss induced by bariatric surgery. J Clin Endocrinol Metab 95: 2892–2896, 2010. [DOI] [PubMed] [Google Scholar]
- 35.Shen J, Tanida M, Niijima A, Nagai K. In vivo effects of leptin on autonomic nerve activity and lipolysis in rats. Neurosci Lett 416: 193–197, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Shin HY, Lee DC, Chu SH, Jeon JY, Lee MK, Im JA, Lee JW. Chemerin levels are positively correlated with abdominal visceral fat accumulation. Clin Endocrinol 77: 47–50, 2012. [DOI] [PubMed] [Google Scholar]
- 37.Sledzinski T, Korczynska J, Hallmann A, Kaska L, Proczko-Markuszewska M, Stefanaiak T, Sledzinski M, Swierczynski J. The increase in serum chemerin concentration is mainly associated with the increase of body mass index in obese, non-diabetic subjects. J Endocrinol Invest 36: 428–434, 2013. [DOI] [PubMed] [Google Scholar]
- 38.Song SH, Fukui K, Nakajima K, Kozakai T, Sasaki S, Roh SG, Katoh K. Cloning, expression analysis, and regulatory mechanisms of bovine chemerin and chemerin receptor. Domest Anim Endocrinol 39: 97–105, 2010. [DOI] [PubMed] [Google Scholar]
- 39.Stefanov T, Bluher M, Vekova A, Bonova I, Tzvetkov S, Kurktschiev D, Temelkova-Kurktschiev T. Circulating chemerin decreases in response to a combined strength and endurance training. Endocrine 45: 382–391, 2014. [DOI] [PubMed] [Google Scholar]
- 40.Stejskal D, Karpised M, Hanulova Z, Svestak M. Chemerin is an independent marker of the metabolic syndrome in a Caucasian population—a pilot study. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 152: 217–221, 2008. [DOI] [PubMed] [Google Scholar]
- 41.Stepan H, Philipp A, Roth I, Kralisch S, Jank A, Schaarschmidt W, Lossner U, Kratzsch J, Bluher M, Stumvoll M, Fasshauer M. Serum levels of the adipokine chemerin are increased in preeclampsia during the 6 months after pregnancy. Regul Pept 168: 69–72, 2011. [DOI] [PubMed] [Google Scholar]
- 42.Szasz T, Bornfim GF, Webb RC. The influence of perivascular adipose tissue on vascular homeostasis. Vasc Health Risk Manag 9: 105–116, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanida M, Shen J, Horii Y, Matsuda M, Kihara S, Funahashi T, Shimomura I, Sawai H, Fukuda Y, Matsuzawa Y, Nagai K. Effects of adiponectin on the renal sympathetic nerve activity and blood pressure in rats. Exp Biol Med (Maywood) 232: 390–397, 2007. [PubMed] [Google Scholar]
- 44.Terra X, Auguet T, Guiu-Jurado E, Berlanga A, Orellana-Gavalda JM, Hernandez M, Sabench F, Porras JA, Llutart J, Martinez S, Aguilar C, Del Castillo D, Richart C. Long-term changes in leptin, chemerin and ghrelin levels following different bariatric surgery procedures: Roux-en-Y gastric bypass and sleeve gastrectomy. Obes Surg 23: 1790–1798, 2013. [DOI] [PubMed] [Google Scholar]
- 45.Turgut A, Ozler A, Goruk NY, Tunc SY, Sak ME, Evsen MS, Evliyaoglu O, Gul T. Serum levels of adipokines, free fatty acids, and oxidative stress markers in obese and non-obese preeclamptic patients. Clin Exp Obstet Gynecol 42: 473–479, 2015. [PubMed] [Google Scholar]
- 46.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature 444: 875–880, 2006. [DOI] [PubMed] [Google Scholar]
- 47.Walker GE, Marzullo P, Ricotti R, Bona G, Prodam F. The apthophysiology of abdominal adipose tissue depots in health and disease. Horm Mol Biol Clin Investig 19: 57–74, 2014. [DOI] [PubMed] [Google Scholar]
- 48.Watts SW, Dorrance AM, Penfold ME, Rourke JL, Sinal CJ, Seitz B, Sullivan TJ, Charvat TT, Thompson JM, Burnett R, Fink GD. Chemerin connects fat to arterial contraction. Arterioscler Thromb Vasc Biol 33: 1320–1328, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weigert J, Neumeir M, Wanninger J, Filarsky M, Bauer S, Wiest R, Farkas S, Schere MN, Schaffler A, Aslanidis C, Scholmerich J, Buechler C. Systemic chemerin is related to inflammation rather than obesity in type 2 diabetes. Clin Endocrinol 72: 342–348, 2010. [DOI] [PubMed] [Google Scholar]
- 50.Wittamer V, Franssen JD, Vulcano M, Mirjolet JF, Le Poul E, Migeotte I, Brezillon S, Tyldesley R, Blanpain C, Detheux M, Mantovani A, Sozzani S, Vassart G, Parmentier M, Communi D. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J Exp Med 198: 977–985, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wittamer V, Gregoire F, Robberecht P, Vassart G, Communi D, Parmentier M. The C-terminal nonapeptide of mature chemerin activates the chemerin receptor with low nanomolar potency. J Biol Chem 279: 9956–9962, 2004. [DOI] [PubMed] [Google Scholar]
- 52.Wittamer V, Bondue B, Guillabert A, Vassart G, Parmentier M, Communi D. Neutrophil-mediated maturation of chemerin: a link between innate and adaptive immunity. J Immunol 175: 487–483, 2005. [DOI] [PubMed] [Google Scholar]
- 53.Yang M, Yang G, Dong J, Liu Y, Zong H, Liu H, Boden G, Li L. Elevated plasma levels of chemerin in newly diagnosed type 2 diabetes mellitus with hypertension. J Investig Med 58: 883–886, 2010. [DOI] [PubMed] [Google Scholar]
- 54.Yoo HJ, Choi HY, Yang SJ, Kim HE, Seo JA, Kim SG, Kim NH, Choi KM, Choi DS, Baik SH. Circulating chemerin level is independently correlated with arterial stiffness. J Atheroscler Thromb 19: 59–66, 2012. [DOI] [PubMed] [Google Scholar]
- 55.Zinchuk V, Wu Y, Grossenbacher-Zinchuk O. Bridging the gap between qualitative and quantitative colocalization results in fluorescence microscopy studies. Sci Rep 3: 1365, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]