Abstract
Heat therapy has been shown to promote capillary growth in skeletal muscle and in the heart in several animal models, but the effects of this therapy on angiogenic signaling in humans are unknown. We evaluated the acute effect of lower body heating (LBH) and unilateral thigh heating (TH) on the expression of angiogenic regulators and heat shock proteins (HSPs) in healthy young individuals. Exposure to LBH (n = 18) increased core temperature (Tc) from 36.9 ± 0.1 to 37.4 ± 0.1°C (P < 0.01) and average leg skin temperature (Tleg) from 33.1 ± 0.1 to 39.6 ± 0.1°C (P < 0.01), but did not alter the levels of circulating angiogenic cytokines and bone marrow-derived proangiogenic cells (CD34+CD133+). In skeletal muscle, the change in mRNA expression from baseline of vascular endothelial growth factor (VEGF), angiopoietin 2 (ANGPT2), chemokines CCL2 and CX3CL1, platelet factor-4 (PF4), and several members of the HSP family was higher 30 min after the intervention in the individuals exposed to LBH (n = 11) compared with the control group (n = 12). LBH also reduced the expression of transcription factor FOXO1 (P = 0.03). Exposure to TH (n = 14) increased Tleg from 32.8 ± 0.2 to 40.3 ± 0.1°C (P < 0.05) but Tc remained unaltered (36.8 ± 0.1°C at baseline and 36.9 ± 0.1°C at 90 min). This intervention upregulated the expression of VEGF, ANGPT1, ANGPT2, CCL2, and HSPs in skeletal muscle but did not affect the levels of CX3CL1, FOXO-1, and PF4. These findings suggest that both LBH and TH increase the expression of factors associated with capillary growth in human skeletal muscle.
Keywords: angiogenesis, skeletal muscle, heat therapy, blood flow
skeletal muscle capillary rarefaction is a common feature and a significant contributor to exercise intolerance in several chronic disease states, including peripheral artery disease (PAD) (62), chronic heart failure (CHF) (14), and chronic obstructive pulmonary disease (COPD) (65). Emerging evidence also indicates that a progressive reduction in skeletal muscle microvessel density plays an important role in the development and progression of metabolic syndrome (17). Promoting capillary growth is, therefore, a major therapeutic goal to restore skeletal muscle function and exercise capacity in these populations. Surprisingly, few therapeutic strategies are known to effectively activate angiogenic signaling and increase skeletal muscle capillarization in humans. Exercise training is undoubtedly one of the most potent angiogenic therapies (18), but few patients engage and adhere to structured exercise programs (47, 61). Further, this option is challenging, or not amenable, for patients with severe disease and restricted locomotion. Gene and cell therapy are promising alternatives, but the results of most therapeutic angiogenesis trials to date have been largely disappointing (2). An urgent need remains for novel, more accessible strategies that stimulate angiogenesis in human skeletal muscle.
Heat therapy is a promising strategy that has recently been successfully used to treat patients with CHF (31, 49), severe COPD (76), and PAD (66, 74). Mounting evidence indicates that repeated exposure to whole body heat stress reduces the clinical symptoms and improves exercise tolerance in patients with these conditions (31, 49, 66, 76). The exact mechanisms underlying these documented clinical benefits are unclear, but several pieces of evidence indicate that heat therapy might work, in part, by promoting angiogenesis. First, whole body heat stress induced by far-infrared dry sauna or a heating blanket promotes the expression of the proangiogenic mediator vascular endothelial growth factor (VEGF) and increases capillary density in the myocardium in healthy (21) and hypertensive (28) rats, as well as in a model of myocardial infarction (69). Second, repeated treatment with far infrared dry sauna increases skeletal muscle capillary density in a mouse model of PAD (1, 40) and diabetes (26) and in rats treated with glucocorticoids (43). This angiogenic response in the heart and in skeletal muscle is closely coupled with increased expression of heat shock proteins (HSPs), molecular chaperones that are known to modulate the angiogenic process (40, 67, 71). Third, endothelial cells exposed to mild heat stress have an enhanced capacity for vascular tube formation, which is indicative of angiogenesis activity (60). Fourth, sauna therapy induces an increase in the number of circulating CD34+ progenitor cells (49, 66), which promote vascular growth and repair (36). Despite this compelling evidence derived primarily from cell culture and animal studies, the impact of systemic and local heat stress on angiogenic signaling in humans remains to be determined.
Skeletal muscle angiogenesis stems from the cumulative effects of transient changes in the abundance of proangiogenic mediators coupled with the inhibition of angiostatic factors (34, 51). Defining the time course and magnitude of changes in the transcriptional levels of these factors in response to a single stimulus is, therefore, critical toward understanding how the remodeling process is initiated and coordinated (15, 57). The goal of the present study was to determine the effects of a single 90-min session of heat therapy on systemic and skeletal muscle levels of key angiogenic regulators in humans. Water-circulating trousers were used to create two distinct experimental paradigms: 1) lower body heating (LBH), which induced an increase in body core temperature and significant changes in systemic hemodynamics, and 2) unilateral thigh heating (TH), in which the heating stimulus was confined to one thigh, and body core temperature remained unaltered. These distinct experimental approaches allowed us to examine the potential contribution of systemic responses to heat stress to local changes in the expression of angiogenic genes in skeletal muscle. Systemic heat stress has been shown to induce physiological responses that can impact skeletal muscle angiogenesis, including activation of the sympathetic nervous system (7) and increased levels of bone marrow-derived proangiogenic cells (CD34+CD133+) (49, 66). Therefore, we hypothesized that LBH would increase the circulating levels of proangiogenic cytokines and cells and promote an increase in the mRNA level of angiogenic regulators and HSPs in skeletal muscle. Because a systemic angiogenic response would not occur during TH, we hypothesized the changes in the expression of proangiogenic factors in skeletal muscle following this protocol would be smaller compared with LBH.
METHODS
Participants
Fifty-five young, normally active individuals (Table 1) were recruited to participate in three separate studies (protocol 1, n = 18; protocol 2, n = 23; and protocol 3, n = 14). Participants were asked to fill out a medical history questionnaire. Individuals were excluded if they were obese (BMI > 30 kg/m2), used tobacco products, were diabetic, were taking any medication other than birth control, participated in any kind of supervised physical activity or exercised for more than 3 days/wk. Female participants were tested during the early follicular phase of their menstrual cycle (days 1–7) or during the placebo phase if they were taking oral contraceptives. The Institutional Review Board at Purdue University approved all experimental procedures, and verbal and written consent were obtained from all participants.
Table 1.
Subject characteristics
|
Protocol 2 |
||||
|---|---|---|---|---|
| Protocol 1 | Control (33°C) | LBH (48°C) | Protocol 3 | |
| Number of subjects (male/female) | 18 (12/6) | 12 (8/4) | 11 (6/5) | 14 (12/2) |
| Age, yr | 22 ± 1.1 | 21 ± 0.6 | 24 ± 1.7 | 21 ± 0.8 |
| Body weight, kg | 70.3 ± 2.9 | 72.4 ± 4.6 | 70.7 ± 4.3 | 73.4 ± 3.0 |
| Height, cm | 172.2 ± 1.5 | 171.0 ± 2.5 | 173.5 ± 4.0 | 175.0 ± 2.4 |
| BMI, kg/m2 | 23.7 ± 0.9 | 24.6 ± 1.2 | 23.3 ± 0.9 | 23.9 ± 0.8 |
Values are expressed as mean ± SE.
LBH, lower body heating; BMI, body mass index.
Instrumentation and Experimental Protocols
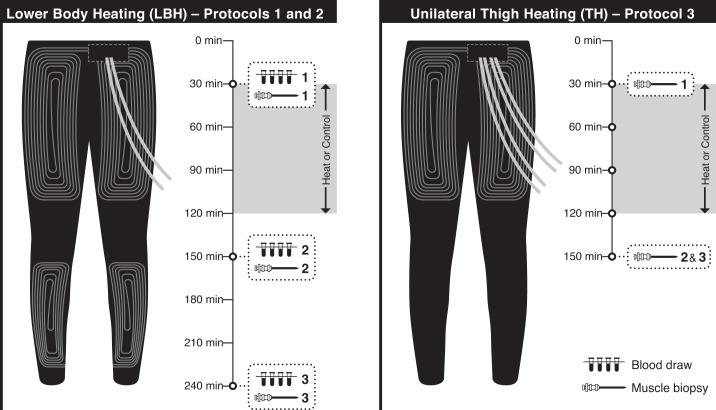
Three separate protocols were conducted to determine the angiogenic responses to LBH (protocols 1 and 2) and TH (protocol 3). The experimental design with time points for blood draws and muscle biopsies for each protocol is depicted in Fig. 1. All experimental sessions were completed in the morning in a temperature-controlled room (average temperature of 24.2 ± 0.2°C). Participants were asked to avoid caffeine and alcoholic beverages for 12 h and intense exercise for at least 24 h prior to the experimental sessions. Participants were also asked to abstain from food for at least 1 h prior to each laboratory visit.
Fig. 1.
Illustration of the water-circulating garments used for heat treatment and timelines for the experimental protocols. Left: lower body heating (LBH) was achieved by circulating hot water through tube-lined pants that covered the legs, thighs, and buttocks. This approach was used on protocols 1 and 2. Blood samples (protocol 1) and muscle biopsies (protocol 2) were taken prior to and 30 and 120 min following exposure to LBH or a control intervention. Right: a custom garment with tubing around the thigh was used for local heat treatment. This garment has a separate tubing circuit for each limb, which allows for one thigh to be exposed to heat treatment while the contralateral thigh serves as a control. This approach was used in protocol 3. Muscle biopsies were taken prior to and 30 min after the interventions.
The purpose of protocol 1 was to determine the effect of LBH on the circulating levels of angiogenic, inflammatory, and vasoactive mediators, as well as the number of circulating proangiogenic cells. Participants completed two experimental sessions, at least 72 h apart, in a randomized cross-over design. Subjects were given a wireless telemetry pill (HQ, Palmetto, FL) for core temperature monitoring during the experiments and were instructed to ingest it the night before the experiment (∼7–9 h before the experimental sessions) (82). Upon arrival at the laboratory, subject body weight and height were recorded, and four thermocouples (MLT422; ADInstruments, Colorado Springs, CO) were taped to the calf and thigh for measurement of mean leg skin temperature. The medial aspect of the calf on both legs was gently shaved, and laser-Doppler flow probes (VP12; Moor Instruments, Axminster, UK) were placed inside local heaters (VHP1; Moor Instruments) and were attached to the skin for the measurement of skin red blood cell flux (moorVMS- LDF2; Moor Instruments). Heart rate (HR) was monitored via a three-lead electrocardiogram (FE132; Bio Amp, ADInstruments). Systolic and diastolic blood pressures (BP) were measured from the left arm using an automated device (Tango+, Suntech Medical, Morrisville, NC). An intravenous catheter was placed in an antecubital vein of the right arm for blood sampling. Participants were asked to put on water-circulating trousers on top of shorts or underwear (Med-Eng, Ottawa, Canada). This garment is made of a tight-fitting elastic fabric, with an extensive network of medical-grade polyvinyl chloride tubing sewn onto the fabric and was designed to cover the calves, thighs, and buttocks (Fig. 1, left). In addition, to minimize heat loss during the interventions, participants also wore polyvinyl chloride pants and had their legs covered with a thermal foil blanket. After instrumentation, participants rested quietly for 30 min in the supine position, and a baseline blood sample was taken. Next, the water-circulating garment was connected to a heated bath circulator (Sahara S21, Thermo Scientific). In the LBH session, water at 48°C was perfused through the garment for 90 min with a goal to increase leg skin temperature to ∼39.5–40°C (24). In the control intervention, water at 33°C was circulated through the garment to maintain leg skin temperature at baseline levels. At the end of the intervention period, the garment was disconnected from the water circulator, and the participants remained supine for another 2 h. Blood samples were taken 30 and 120 min following the completion of the trials (Fig. 1, left). In the last 20 min of the protocol, the temperature of the local skin heaters placed on the calf was increased to 43°C at a rate of 0.1°C/s to promote maximal increases in skin blood flow and allow for the calculation of maximal cutaneous vascular conductance (CVCmax) (11). Leg skin temperature, cutaneous red blood cell flux, and HR were recorded continuously, while systolic and diastolic BP and core body temperature were recorded every 5 min for the entire duration of the protocol.
In protocol 2, enrolled participants were randomly allocated to either a group exposed to LBH (n = 11) or to a control group (n = 12). The purpose of this protocol was to determine the effect of LBH on the mRNA expression of angiogenic mediators in skeletal muscle. Participants were instrumented as described above in protocol 1 (Fig. 1, left). After 30 min of rest in the supine position, the thighs were exposed, and a biopsy was taken from the vastus lateralis muscle of a randomly selected thigh using a 5-mm Bergstrom biopsy needle (Pelomi Medical, Albruslund, Denmark), as described previously (19, 20, 64). Next, the water-circulating garment was perfused with either 48°C water (LBH group) or 33°C water (control group) for 90 min. Two additional muscle biopsies were taken 30 and 120 min after the end of the intervention (Fig. 1, left). The second biopsy was taken from the opposite leg used for the first biopsy, and the third biopsy was taken from a site at least ∼3 cm away from the first biopsy site.
In protocol 3, a custom-made garment was used to heat one thigh, while the opposite thigh served as a control (Fig. 1, right). The goal of this experiment was to investigate the effects of local heating, which does not evoke systemic responses, such as changes in core body temperature, on the mRNA expression of angiogenic mediators in the vastus lateralis muscle. Participants were asked to complete two experimental sessions. On the first session, two skin thermocouples were taped to each thigh for measurement of skin temperature, and participants were asked to put on the custom water-circulating garment and polyvinyl chloride pants and had their legs covered with a thermal foil blanket. A baseline muscle biopsy was taken after 30 min of rest in the supine position from one randomly selected thigh. Next, the garment was connected to the water circulators. In the thigh assigned to receive the heat treatment, hot water (48–52°C) was circulated through the garment for 90 min to increase skin temperature to ∼39–40°C as in protocols 1 and 2. In the opposite thigh, skin temperature was maintained at 33°C for the entire duration of the protocol. Thirty minutes after the end of the treatment, one muscle biopsy was taken from both the control and heated thighs, as described above (Fig. 1, right). The biopsies were only taken at 30 min after the intervention in this protocol because most of the changes in mRNA levels occurred at this time point in protocol 2. The second session was conducted at least 1 wk after session 1. The purpose of this session was to characterize the physiological responses to TH. Subjects were instructed to ingest the wireless telemetry pill (HQ, Palmetto, FL) for core temperature monitoring, as described for protocol 1. Upon arrival, subject weight and height were recorded, and skin thermocouples were taped to the thigh for the measurement of skin temperature. Participants were then fitted with the custom garment and polyvinyl chloride pants, as detailed above. A blood pressure cuff was wrapped around the left arm for blood pressure monitoring during the protocol using an automated device (Tango+, Suntech Medical, Morrisville, NC). After instrumentation, subjects were allowed to rest quietly for 30 min in the supine position. Next, the groin area was exposed, and the common femoral artery was imaged on both legs using a 10-MHz multifrequency linear probe attached to a high-resolution ultrasound machine (T3000; Terason, Burlington, MA). Diameter and velocity signals were acquired simultaneously in a duplex mode and were corrected with an insonation angle of 60°. Images were recorded for 1 min in each leg using screen capture software (Camtasia Studio, TechSmith). The garment was then connected to the water circulators for application of local heating and control treatments, as described above. Diameter and blood flow recordings were made every 30 min during the intervention, and once 30 min after the end of the heating protocol. Blood pressure and core body temperature were measured every 5 min, while skin temperature was recorded continuously for the entire duration of the protocol.
Measurements
Circulating factors.
Venous blood samples were collected in serum-separating tubes (BD Vacutainer SST plus), allowed to clot for 30 min at room temperature, centrifuged for 15 min at 1,200 rpms, aliquoted, and stored at −80°C until analysis. A custom Milliplex assay kit (EMD Millipore, Billerica, MA) was used to determine the concentrations of several angiogenic and inflammatory mediators, including granulocyte colony stimulating factor (G-CSF), granulocyte macrophage stimulating factor (GM-CSF), interleukin 6 (IL-6), interleukin 8 (IL-8), chemokine (C-C motif) ligand 2 (CCL2), tumor necrosis factor alpha (TNF-α), vascular endothelial growth factor (VEGF), fibroblast growth factor 2 (FGF-2), and chemokine (C-X3-C motif) ligand 1 (CX3CL1). The assay was performed following the manufacturer's protocol using the Bio-Plex 200 System (Bio-Rad, Hercules, CA) at the Bio-Plex Core Facility of Indiana University. The serum concentration of endothelin-1 was determined using an ELISA kit (R&D Systems, Minneapolis, MN), according to manufacturer's instructions.
Circulating proangiogenic cells.
A multiparametric flow cytometry method (45, 46) was used to identify circulating CD34+CD133+ cells. Briefly, venous blood samples were collected in a cell preparation tube with sodium heparin (BD Vacutainer CPT tubes) and taken to the Flow Cytometry and Cell Separation Facility (Bindley Bioscience Center, Purdue University). Samples were centrifuged within 2 h of collection for 15 min at 1,500 RCF, as suggested by the manufacturer. Mononuclear cells were isolated, washed in PBS with 0.5% BSA, and stained with antibodies against cell surface antigens, including anti-CD34-PE (BioLegend, San Diego, CA; no. 343506), anti-CD31-FITC (BioLegend, no. 303104), anti-CD45-BV510 (BioLegend, no. 304014), and anti-CD133-APC (Miltenyi, no. 130-090-826). In addition, to exclude dead/apoptotic cells, red blood cells, and monocytes, mononuclear cells were costained with Live/Dead stain-violet (no. L34955; Life Technologies, Carlsbad, CA), anti-CD235a-Pacific Blue (BioLegend, no. 306612), and anti-CD14-BUV395 (BD Biosciences, no. 563561), respectively. The cells were initially stained with Live/Dead stain for 30 min at 4°C, washed once with PBS/BSA, incubated with antibodies for 20 min at 4°C, washed twice in PBS/BSA, and analyzed using a BD FACSAria III Cell Sorter. At least 1,000,000 events were collected for each sample. The data were analyzed using Flowjo v9.4.3 (Treestar).
Skeletal muscle gene expression.
Biopsy samples were initially placed in a microcentrifuge tube containing 1 ml of RNAlater (Ambion, Carlsbad, CA). The sample was kept at 4°C overnight and then stored at −80°C until analysis. Approximately 30 mg of tissue was used for RNA extraction using the TRIzol Reagent (Invitrogen, Life Technologies), according to manufacturer's instructions. The quality and concentration of total RNA were measured by a spectrophotometer (Nanodrop 3000, ThermoFisher), as described previously (84). cDNA was prepared using the RT2 first strand kit following the manufacturer's instructions (Qiagen, Valencia, CA). In protocol 2, the expression of 27 genes related to angiogenesis and inflammation, as well as members of the HSP family, was determined using a custom RT2 Profiler PCR array kit (Qiagen) and the Roche LightCycler 480 PCR System (Roche Diagnostics, Indianapolis, IN). The three samples from each subject (baseline, 30 min and 120 min postintervention) were loaded in the same array plate. The list of genes analyzed is shown in Table 2 and includes the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH), as well as a reverse-transcription control (RTC), a positive PCR control (PPC), and a human genomic DNA contamination control. Data were analyzed using the GeneGlove Data Analyses Center (Qiagen, Valencia, CA). GAPDH was used to normalize cycle threshold (Ct) values. The comparative Ct method was used to calculate the changes in gene expression of each target mRNA relative to the baseline sample (33). A similar strategy was used to analyze the samples obtained in protocol 3, with the exception that the custom array had only 13 select genes, in addition to GAPDH, a reverse transcription control, and a human genomic DNA contamination control (Table 3).
Table 2.
List of genes included in the custom PCR array used in protocol 2
| Gene Symbol | Official Full Name |
|---|---|
| VEGFA | Vascular endothelial growth factor A |
| NOS3 | Nitric oxide synthase 3 |
| PPARGC1A | Peroxisome proliferator-activated receptor gamma, coactivator 1 alpha |
| CCL2 | Chemokine (C-C motif) ligand 2 |
| MMP9 | Matrix metallopeptidase 9 |
| MMP2 | Matrix metallopeptidase 2 |
| TIMP1 | TIMP metallopeptidase inhibitor 1 |
| ANGPT2 | Angiopoietin 2 |
| ANGPT1 | Angiopoietin 1 |
| TEK | TEK tyrosine kinase, endothelial |
| THBS1 | Thrombospondin 1 |
| PF4 | Platelet factor 4 |
| CXCL12 | Chemokine (C-X-C motif) ligand 12 |
| NCL | Nucleolin |
| FOXO1 | Forkhead box O1 |
| FOXO3 | Forkhead box O3 |
| IL6 | Interleukin 6 |
| IL8 | Interleukin 8 |
| TNF | Tumor necrosis factor |
| CX3CL1 | Chemokine (C-X3-C motif) ligand 1 |
| HSP90AA1 | Heat shock protein 90-kDa alpha class A member 1 |
| HSP90AB1 | Heat shock protein 90-kDa alpha class B member 1 |
| HSPA1A | Heat shock 70-kDa protein 1A |
| HSPA1B | Heat shock 70-kDa protein 1B |
| HSPA8 | Heat shock 70-kDa protein 8 |
| HSPD1 | Heat shock 60-kDa protein 1 |
| HSPB1 | Heat shock 27-kDa protein 1 |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| PPC | Positive PCR control |
| RTC | Reverse transcription control |
| HGDC | Human genomic DNA contamination |
Table 3.
List of genes included in the custom PCR array used in protocol 3
| Gene Symbol | Official Full Name |
|---|---|
| VEGFA | Vascular endothelial growth factor A |
| CCL2 | Chemokine (C-C motif) ligand 2 |
| ANGPT2 | Angiopoietin 2 |
| ANGPT1 | Angiopoietin 1 |
| PF4 | Platelet factor 4 |
| FOXO1 | Forkhead box O1 |
| CX3CL1 | Chemokine (C-X3-C motif) ligand 1 |
| HSP90AA1 | Heat shock protein 90-kDa alpha class A member 1 |
| HSP90AB1 | Heat shock protein 90-kDa alpha class B member 1 |
| HSPA1B | Heat shock 70-kDa protein 1B |
| HSPA8 | Heat shock 70-kDa protein 8 |
| HSPD1 | Heat shock 60-kDa protein 1 |
| HSPB1 | Heat shock 27-kDa protein 1 |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| RTC | Reverse transcription control |
| HGDC | Human genomic DNA contamination |
Data and Statistical Analysis
Leg skin temperature, cutaneous red blood cell flux, and HR were recorded at 40 Hz using a data acquisition system (PowerLab and LabChart, ADInstruments, Colorado Springs, CO), and the last 2 min of every 5-min bin was averaged for the entire protocol. Systolic and diastolic BP was recorded every 5 min, and mean arterial pressure was calculated as diastolic pressure plus one-third pulse pressure. Cutaneous vascular conductance (CVC) was calculated as red blood cell flux divided by mean arterial pressure and expressed as a percentage of CVCmax, as determined by the maximal value obtained during local heating at 43°C for 20 min.
Analysis of diameter and Doppler flow profile in the femoral artery in protocol 3 was performed using computerized edge-detection and wall-tracking software (FMD Studio, Quipu, Pisa, Italy). The Doppler flow analysis computed time-averaged anterograde (Vant) and retrograde (Vret) values of velocity (V), which were used for the calculation of positive and negative blood flows (BF) using the following equation: BF = (V × 3.14 × d2)/400, where d is the vessel diameter. Mean BF was computed as the algebraic sum of anterograde and retrograde BF. Anterograde and retrograde wall shear rates (SR) were calculated using the following equation: SR = 4 × V/d. The oscillatory shear index (OSI) was calculated as OSI = (SRret)/(SRant + SRret).
All statistical analyses were conducted using SAS (version 9.4; SAS Institute, Cary, NC), with results expressed as means ± SE. A two-way mixed-effect (with random individual effect and repeated measurements) ANOVA was used to compare the physiological responses and the levels of circulating factors and progenitor cells between trials in protocol 1, followed by Bonferroni post hoc comparisons when appropriate. In protocol 2, subject characteristics were compared between groups using unpaired t-tests. Because of nonnormal distribution, gene expression responses at each time point (30 min and 120 min) were compared between groups using the Wilcoxon Rank-Sum test. In protocol 3, a one-way repeated-measures ANOVA model was used to identify significant changes from baseline in heart rate, systolic and diastolic BP, and skin and core body temperature. A two-way mixed-effect ANOVA was employed to compare the blood flow parameters between the leg exposed to TH and the control leg. Post hoc analysis (Bonferroni) was performed when appropriate. As in protocol 2, gene expression responses had a nonnormal distribution in protocol 3, and differences between thighs were compared using the paired Wilcoxon rank test. To allow for a direct comparison between gene expression responses at 30 min postintervention between protocols 2 and 3, each variable was subjected to logarithm transformation. A t-test was then used to compare the changes in the expression of each gene between the two distinct interventions. For all analyses, P ≤ 0.05 was considered statistically significant.
RESULTS
Protocol 1
Physiological responses to LBH.
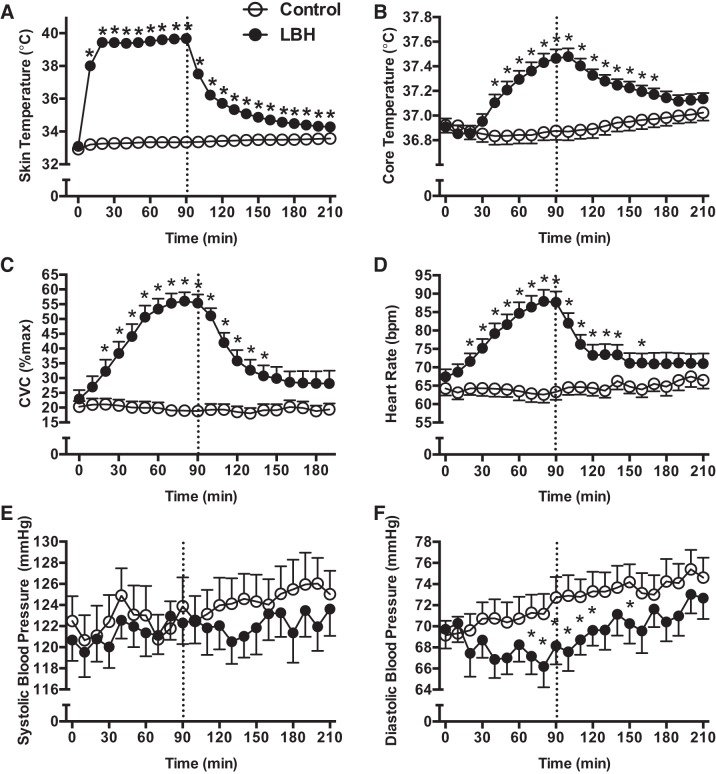
Figure 2 depicts the changes in skin and core temperatures, HR, systolic and diastolic blood pressure, and CVC, before, during, and after exposure to LBH or a control intervention. There were no baseline differences between trials for any of the physiological variables. As expected, exposure to LBH for 90 min induced marked increases from baseline in leg skin temperature (from 33.1 ± 0.1 to 39.6 ± 0.1°C, P < 0.01), CVC (from 22.9 ± 3.0 to 56 ± 3.0% max, P < 0.01), and HR (from 67 ± 2 to 87 ± 2 bpm, P < 0.01), as well as a modest increase in core temperature (from 36.9 ± 0.1 to 37.4 ± 0.1°C, P < 0.01). Systolic blood pressure was not affected by LBH, but diastolic blood pressure was significantly lower during the last 30 min of heating, and early in the recovery period after LBH compared with the control trial (P < 0.05, Fig. 2F).
Fig. 2.
Skin (A) and core temperature (B), CVC (C), HR (D), and systolic (E) and diastolic (F) blood pressure before, during, and after exposure to LBH (●) or a control intervention (○) for 90 min. Dashed line indicates the end of the intervention period. *P < 0.05 vs. Control.
Effect of LBH on circulating cytokines and bone marrow-derived proangiogenic cells.
The serum concentrations of angiogenic and inflammatory mediators and the percentage of proangiogenic cells (CD34+CD133+) measured prior to and 30 and 120 min following exposure to LBH or the control intervention are shown in Table 4. The concentration of three (IL-6, CX3CL1, and GM-CSF) out of the nine factors measured was at or below detection levels and, therefore, the data were excluded. There were no differences between trials for any of the measured growth factors, cytokines, or the percentage of CD34+CD133+ cells. The concentration of the potent vasoconstrictor ET-1 was significantly lower at 30 min following the intervention in the LBH trial compared with the control condition (P < 0.01) (Table 4).
Table 4.
Serum concentrations of angiogenic and inflammatory factors and the percentage of circulating proangiogenic cells (CD34+CD133+) before and 30 and 120 min following exposure to lower body heating or to a control intervention (protocol 1)
| Control (33°C) |
LBH (48°C) |
|||||
|---|---|---|---|---|---|---|
| Pre | 30 min post | 120 min post | Pre | 30 min post | 120 min post | |
| TNF-α, pg/ml | 13.2 ± 2.9 | 16.6 ± 3.8 | 16.2 ± 4.2 | 15.0 ± 3.3 | 17.5 ± 4.5 | 15.2 ± 3.1 |
| IL-8, pg/ml | 34.7 ± 9.6 | 39.6 ± 10.3 | 35.1 ± 8.7 | 33.4 ± 7.7 | 34.9 ± 8.5 | 38.9 ± 8.8 |
| VEGF, pg/ml | 656.6 ± 146.8 | 747.9 ± 165.8 | 737.1 ± 149.3 | 648.5 ± 123.6 | 704.8 ± 128.5 | 685.6 ± 135.4 |
| FGF2, pg/ml | 126.0 ± 39.6 | 139.9 ± 37.8 | 137.3 ± 34.6 | 115.6 ± 28.8 | 135.2 ± 35.2 | 136.2 ± 38.4 |
| G-CSF, pg/ml | 71.5 ± 22.8 | 81.6 ± 25.3 | 83.5 ± 27.1 | 63.1 ± 18.6 | 63.9 ± 16.7 | 70.8 ± 23.9 |
| CCL2, pg/ml | 666.1 ± 40.2 | 507.8 ± 36.0 | 504.1 ± 36.9 | 670.0 ± 48.1 | 511.2 ± 36.2 | 500.3 ± 35.9 |
| ET-1, pg/ml | 1.1 ± 0.06 | 1.5 ± 0.09 | 1.6 ± 0.1 | 1.2 ± 0.08 | 1.0 ± 0.08* | 1.4 ± 0.08 |
| CD34+CD133+, % live cells | 0.2 ± 0.03 | 0.19 ± 0.03 | 0.18 ± 0.03 | 0.21 ± 0.05 | 0.21 ± 0.04 | 0.19 ± 0.03 |
Values are expressed as means ± SE.
VEGF, vascular endothelial growth factor; FGF2, fibroblast growth factor 2; G-CSF, granulocyte colony-stimulating factor; CCL2, chemokine (C-C motif) ligand 2; ET-1, endothelin-1.
P < 0.05 vs. Control.
Protocol 2
Effect of LBH on the mRNA levels of angiogenic, inflammatory, and angiostatic mediators in skeletal muscle.
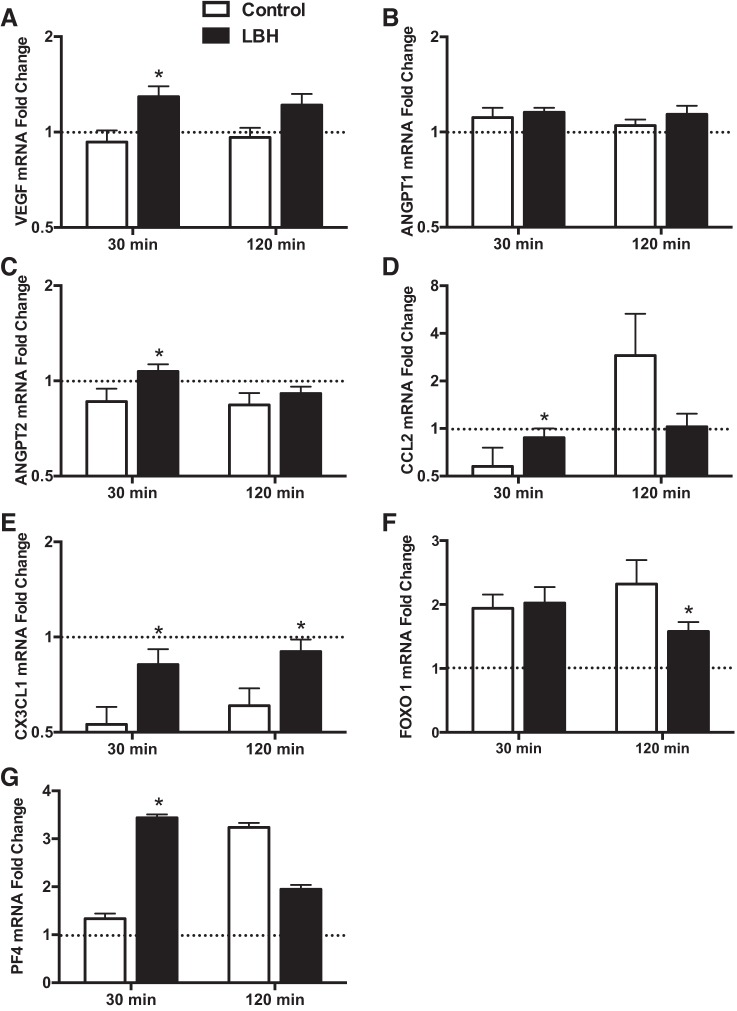
Changes in gene expression from baseline for select angiogenic, inflammatory, and angiostatic genes are shown in Fig. 3 and Table 5. The expression of three genes (IL-8, TNF, and MMP9) was near or below detection limits (i.e., Ct values ≥ 35 or not detectable), and the data were excluded. The mRNA expression of proangiogenic factors VEGF and angiopoietin 2 (ANGPT2) was increased at 30 min in the group exposed to LBH compared with the control group (P < 0.05) (Fig. 3). ANGPT1 expression was not significantly different between groups. The mRNA expression of chemokine CCL2 was higher in the LBH group at 30 min following the intervention (P = 0.02), while the levels of chemokine CX3CL1 were higher at both 30 and 120 min following LBH compared with the control group (P < 0.05). The expression of the angiostatic transcription factor FOXO1 was lower in the LBH group at 120 min following the end of the intervention period (P = 0.03). Conversely, the mRNA expression of the angiostatic factor platelet factor 4 (PF4) was increased at 30 min in the LBH group relative to the control group (P = 0.03). The fold changes in mRNA expression of NOS3, PPARGC1A, MMP2, TIMP1, TEK, THBS1, CXCL12, NCL, FOXO3, and IL-6 were not different between groups (Table 5).
Fig. 3.
Fold changes in skeletal muscle mRNA expression relative to the baseline sample of select angiogenic, inflammatory, and angiostatic mediators in the group exposed to LBH (solid bars; n = 11) and in the control group (open bars; n = 12). Biopsy samples were taken at baseline and 30 and 120 min following the completion of the interventions. The baseline sample was assigned a value of 1 and is represented as a dashed line. *P < 0.05 vs. Control.
Table 5.
Fold changes in skeletal muscle mRNA expression relative to the baseline sample of select angiogenic, inflammatory, and angiostatic mediators in the group exposed to LBH and in the control group (protocol 2)
| Control (33°C) (n = 12) |
LBH (48°C) (n = 11) |
|||
|---|---|---|---|---|
| 30 min | 120 min | 30 min | 120 min | |
| NOS3 | 1.07 ± 0.11 | 1.38 ± 0.25 | 1.16 ± 0.06 | 1.50 ± 0.14 |
| PPARGC1A | 1.18 ± 0.08 | 1.27 ± 0.16 | 1.32 ± 0.06 | 1.21 ± 0.03 |
| MMP2 | 0.99 ± 0.07 | 1.17 ± 0.12 | 0.98 ± 0.07 | 1.07 ± 0.08 |
| TIMP1 | 0.89 ± 0.09 | 1.18 ± 0.22 | 1.12 ± 0.11 | 0.97 ± 0.07 |
| TEK | 1.09 ± 0.11 | 1.30 ± 0.16 | 1.17 ± 0.05 | 1.23 ± 0.06 |
| CXCL12 | 0.98 ± 0.06 | 0.97 ± 0.06 | 0.98 ± 0.03 | 0.93 ± 0.03 |
| NCL | 1.05 ± 0.05 | 1.03 ± 0.04 | 1.09 ± 0.04 | 0.96 ± 0.03 |
| FOXO3 | 1.31 ± 0.06 | 1.34 ± 0.07 | 1.43 ± 0.11 | 1.28 ± 0.11 |
| IL6 | 0.73 ± 0.14 | 0.66 ± 0.14 | 0.65 ± 0.08 | 0.62 ± 0.06 |
| HSPA1A | 0.80 ± 0.05 | 0.73 ± 0.08 | 1.06 ± 0.10 | 0.93 ± 0.08 |
Biopsy samples were taken at baseline and 30 and 120 min following the completion of the interventions.
Effect of LBH on the mRNA levels of heat shock proteins.
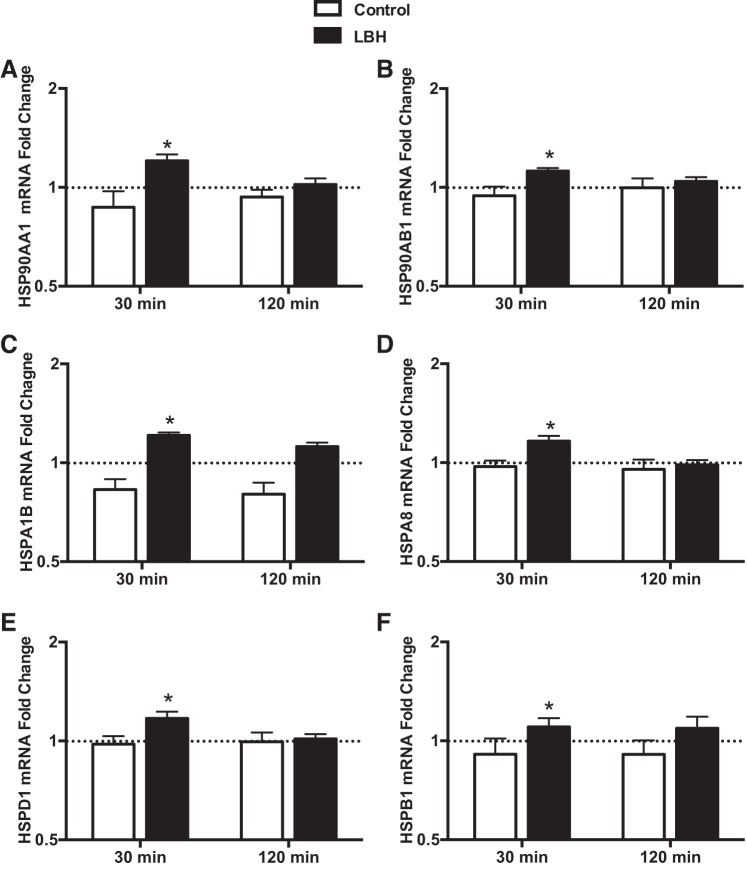
The relative mRNA expression of members of several HSP families is shown in Fig. 4. In agreement with what was observed for the angiogenic/inflammatory genes, increased expression of these factors in the LBH group was particularly evident at 30 min following the intervention. The mRNA levels of HSP90AA1 and HSP90AB1, members of the HSP90 family, were higher in the LBH group compared with control group at 30 min (P < 0.01; Fig. 4), but there were no differences between groups at 120 min. The expression of HSPA1A, also termed HSP72 and a member of the HSP70 family, was slightly higher in the LBH group at 30 min (fold change, Control: 0.8 ± 0.04 and LBH: 1.06 ± 0.09), but this difference did not reach statistical significance (P = 0.08; Table 5). The expression of HSPA1B and HSPA8, both members of the HSP70 family, was higher in the group exposed to LBH than in the control group at 30 min (P < 0.05; Fig. 4). Similarly, the mRNA levels of mitochondria-encoded HSPD1, a member of the chaperonin family, was higher at 30 min in the group exposed to heat treatment than in the group exposed to the sham intervention (P = 0.018; Fig. 4).
Fig. 4.
Fold changes in skeletal muscle mRNA expression relative to the baseline sample of select members of the HSP family in the group exposed to LBH (closed bars; n = 11) and in the control group (open bars; n = 12). Biopsy samples were taken at baseline and 30 and 120 min following the completion of the interventions. The baseline sample was assigned a value of 1 and is represented as a dashed line *P < 0.05 vs. Control.
Protocol 3
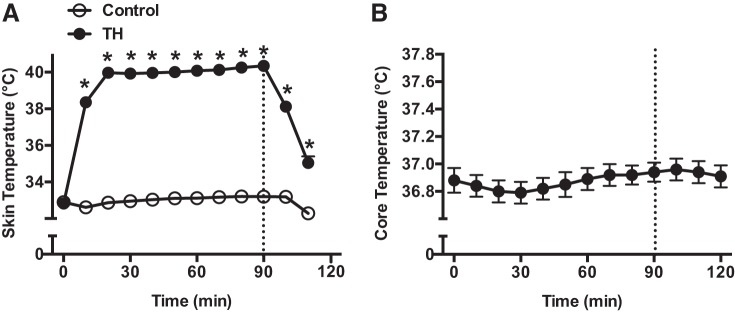
Physiological responses to TH.
Skin temperature and body core temperature responses to TH are shown on Fig. 5. By study design, skin temperature in the thigh exposed to local heating increased to ∼40°C during the treatment, while in the control thigh, the temperature was maintained at ∼33°C. Core temperature remained constant throughout the entire protocol. Systolic and diastolic BP and HR were also not significantly altered by TH treatment (data not shown). Compared with the control thigh, mean BF and anterograde SR in the femoral artery increased during TH, while retrograde SR and OSI were markedly reduced during the treatment (Table 6).
Fig. 5.
Skin temperature (A) and core temperature (B) before, during, and after exposure to thigh heating (TH) for 90 min. One thigh was heated (●), while the opposite thigh served a control (○). Dashed line indicates the end of the intervention period. *P < 0.05 vs. Control.
Table 6.
Flow profile and shear rate responses in the femoral artery to TH application in protocol 3
| Baseline |
30 min |
60 min |
90 min |
30 min post |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | H | C | H | C | H | C | H | C | H | |
| d (cm) | 0.66 ± 0.02 | 0.68 ± 0.02 | 0.69 ± 0.02 | 0.70 ± 0.02 | 0.68 ± 0.02 | 0.71 ± 0.02 | 0.69 ± 0.02 | 0.71 ± 0.02 | 0.68 ± 0.02 | 0.72 ± 0.03 |
| Vmean, cm/s | 8.6 ± 0.9 | 7.9 ± 0.9 | 11.2 ± 1.6 | 15.7 ± 1.8ϕ* | 12.7 ± 1.3ϕ | 17.8 ± 1.6ϕ* | 14.5 ± 1.7ϕ | 17.3 ± 1.5ϕ* | 12.8 ± 1.6ϕ | 13.1 ± 1.4ϕ |
| Vant, cm/s | 14.9 ± 1.0 | 13.9 ± 0.8 | 17.1 ± 1.4 | 19.9 ± 1.7ϕ* | 17.7 ± 1.3 | 21.3 ± 1.6ϕ* | 19.2 ± 1.5ϕ | 21.2 ± 1.3ϕ | 17.5 ± 1.4 | 17.9 ± 1.2ϕ |
| Vret (cm/s) | 6.3 ± 0.7 | 6.07 ± 0.6 | 6.0 ± 0.7 | 4.3 ± 0.3 ϕ* | 4.9 ± 0.5ϕ | 3.5 ± 0.4 ϕ* | 4.7 ± 0.5ϕ | 3.9 ± 0.5ϕ | 4.7 ± 0.68ϕ | 4.8 ± 0.7 |
| BF, ml/min | 179 ± 21 | 174 ± 24 | 251 ± 39 | 375 ± 58* | 283 ± 39 | 434 ± 57* | 321 ± 35 | 413 ± 48* | 286 ± 40 | 322 ± 40 |
| SRmean, s−1 | 52.5 ± 5.4 | 47.0 ± 5.3 | 65.6 ± 9.5 | 90.1 ± 10.0 ϕ* | 75.2 ± 7.4ϕ | 100.4 ± 8.4ϕ* | 85.8 ± 11.0ϕ | 98.7 ± 8.6ϕ | 75.1 ± 9.1ϕ | 74.8 ± 8.5ϕ |
| SRant, s−1 | 90.6 ± 6.1 | 82.9 ± 5.1 | 100.5 ± 8.7 | 114 ± 9.2 ϕ* | 104.6 ± 7.2 | 120 ± 7.5ϕ* | 113.1 ± 10ϕ | 121.1 ± 7.7ϕ | 102.8 ± 8.6 | 101.5 ± 7.5ϕ |
| SRret, s−1 | 38.2 ± 3.8 | 36.0 ± 3.5 | 34.9 ± 4.2 | 24.8 ± 2.1ϕ* | 29.3 ± 3.2ϕ | 19.8 ± 2.7ϕ* | 27.3 ± 2.7ϕ | 22.5 ± 3ϕ | 27.6 ± 3.8ϕ | 26.7 ± 3.8ϕ |
| OSI | 0.29 ± 0.02 | 0.30 ± 0.02 | 0.26 ± 0.02 | 0.19 ± 0.02ϕ* | 0.22 ± 0.02ϕ | 0.14 ± 0.02ϕ* | 0.20 ± 0.02ϕ | 0.16 ± 0.02ϕ* | 0.21 ± 0.03ϕ | 0.21 ± 0.03ϕ |
Values are expresed as means ± SE.
C, control thigh; H, heated thigh; d, diameter; Vmean, time-averaged mean velocity; Vant, time-averaged anterograde velocity; Vret, time-averaged retrograde velocity; SRmean, mean shear rate; SRant, anterograde shear rate; SRret, retrograde shear rate; OSI, oscillatory shear index.
P < 0.05 vs. baseline.
P < 0.05 vs. Control.
Effect of TH on the mRNA levels of angiogenic, inflammatory, and angiostatic mediators in skeletal muscle.
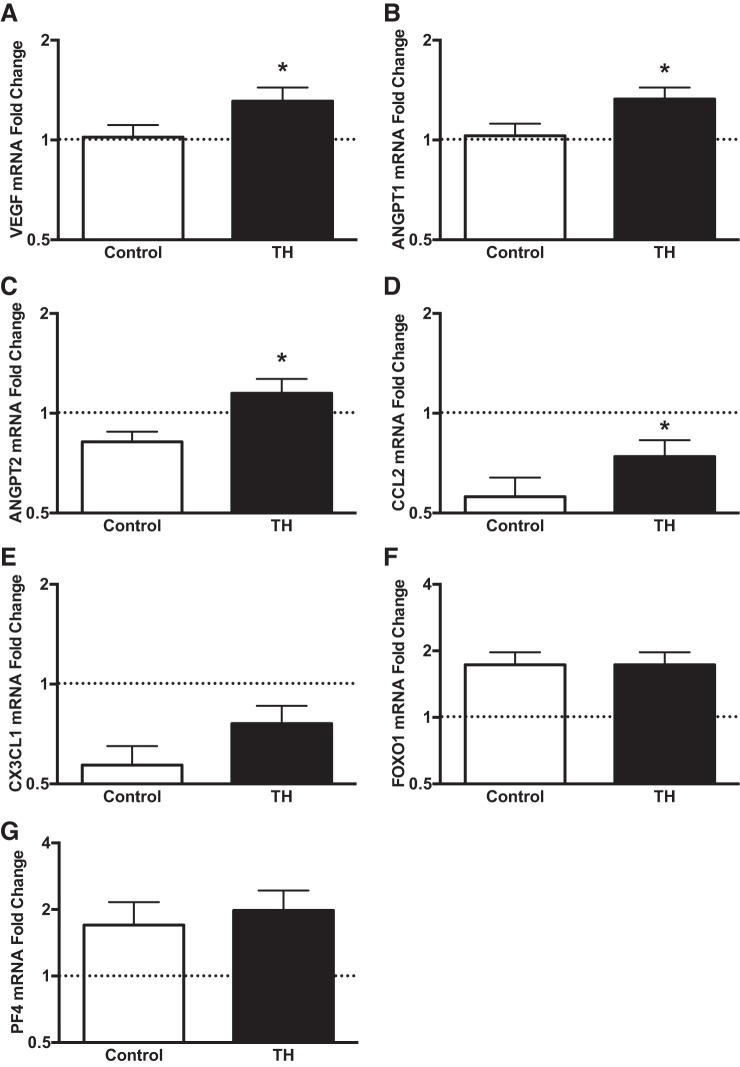
Changes in gene expression from baseline for select angiogenic genes following local heat treatment are shown in Fig. 6. In congruence with the findings from protocol 2, TH led to increased mRNA expression of VEGF, ANGPT2, and CCL2 relative to the control thigh (P < 005). In contrast to LBH, localized heating increased the levels of ANGPT1 (P < 0.05) but did not affect the expression of CX3CL1 (P = 0.16), FOXO1 (P = 0.85), and PF4 (P = 0.37).
Fig. 6.
Fold changes in skeletal muscle mRNA expression relative to the baseline sample of select angiogenic, inflammatory, and angiostatic mediators following exposure to TH. Biopsy samples were taken at baseline and 30 min following the completion of the intervention. The baseline sample was assigned a value of 1 and is represented as a dashed line. *P < 0.05 vs. Control.
Effect of TH on the mRNA levels of heat shock proteins.
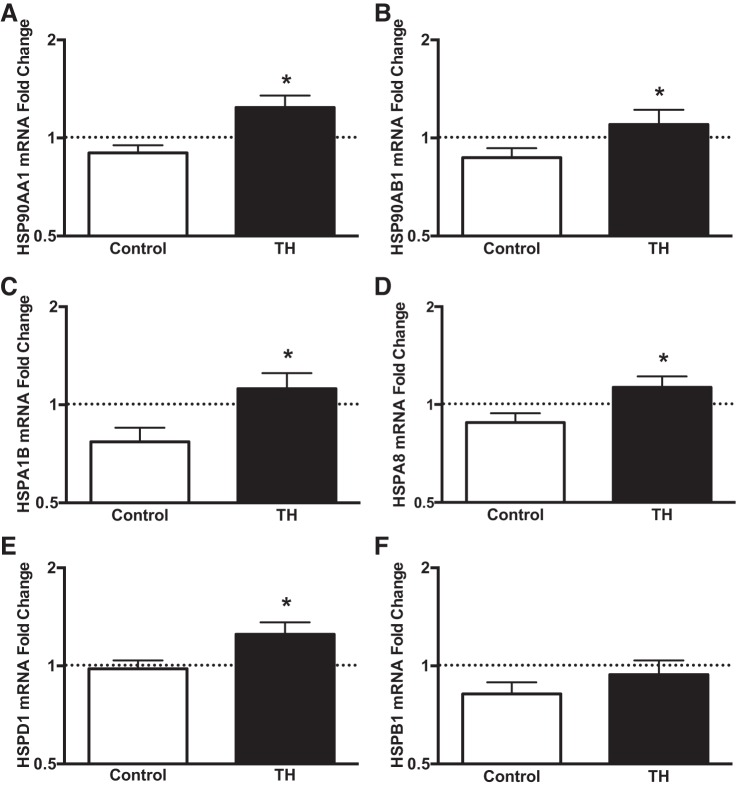
In close agreement with the changes induced by LBH, TH increased the expression of members of the HSP90 family (HSP90AA1 and HSP90AB1), HSP70 family (HSPA1B and HSPA8), and the chaperonin family (HSPD1) (Fig. 7).
Fig. 7.
Fold changes in skeletal muscle mRNA expression relative to the baseline sample of select members of the heat shock protein (HSP) family following exposure to TH. Biopsy samples were taken at baseline and 30 min following the completion of the intervention. The baseline sample was assigned a value of 1 and is represented as a dashed line. *P < 0.05 vs. Control.
Comparison of skeletal muscle gene expression responses between LBH and TH.
The comparison between the LBH and TH protocols for the changes in the expression of select angiogenic, inflammatory, and angiostatic factors and heat shock proteins 30 min after the interventions is shown in Table 7. Contrary to our initial hypothesis, the magnitude of change in the expression of these factors was not different between the two interventions.
Table 7.
Comparison of fold changes in skeletal muscle mRNA expression relative to the baseline sample of select angiogenic, inflammatory and angiostatic mediators and heat shock proteins between the LBH and TH protocols
| Protocol 2 (LBH) |
Protocol 3 (TH) |
|||
|---|---|---|---|---|
| C | LBH | H-C | P | |
| VEGFA | −0.07 ± 0.08 | 0.29 ± 0.10 | 0.30 ± 0.10 | 0.479 |
| CCL2 | −0.42 ± 0.18 | −0.13 ± 0.13 | 0.20 ± 0.10 | 0.362 |
| ANGPT2 | −0.14 ± 0.08 | 0.07 ± 0.06 | 0.33 ± 0.11 | 0.685 |
| ANGPT1 | 0.11 ± 0.08 | 0.15 ± 0.04 | 0.30 ± 0.12 | 0.118 |
| PF4 | 0.34 ± 0.53 | 2.44 ± 1.59 | 0.74 ± 0.55 | 0.286 |
| FOXO1 | 0.94 ± 0.21 | 1.02 ± 0.25 | 0.01 ± 0.11 | 0.700 |
| CX3CL1 | −0.47 ± 0.07 | −0.18 ± 0.10 | 0.19 ± 0.09 | 0.462 |
| HSP90AA1 | −0.13 ± 0.10 | 0.21 ± 0.05 | 0.33 ± 0.08 | 0.383 |
| HSP90AB1 | −0.06 ± 0.06 | 0.12 ± 0.02 | 0.23 ± 0.08 | 0.901 |
| HSPA1B | −0.17 ± 0.06 | 0.21 ± 0.11 | 0.35 ± 0.10 | 0.985 |
| HSPA8 | −0.03 ± 0.04 | 0.16 ± 0.04 | 0.25 ± 0.08 | 0.564 |
| HSPD1 | −0.02 ± 0.06 | 0.17 ± 0.06 | 0.27 ± 0.10 | 0.826 |
| HSPB1 | −0.09 ± 0.11 | 0.08 ± 0.07 | 0.12 ± 0.07 | 0.555 |
Values are expressed as means ± SE.
C, control; LBH, lower body heating; TH, unilateral thigh heating; H-C, difference in mRNA fold change between the heated thigh and the control thigh; P, P value for the comparisons between LBH and TH.
DISCUSSION
In the present study, we employed two experimental paradigms to determine the effect of a single session of heat therapy on the expression of angiogenic mediators and HSPs in humans. Mild systemic heat stress was induced by circulating 48°C water through a garment designed to cover the legs, thighs, and buttocks. This strategy increased core body temperature by ∼0.6°C and reduced diastolic BP and the serum concentration of the potent vasoconstrictor ET-1. Despite inducing these systemic effects, LBH did not affect the circulating levels of several cytokines or bone marrow-derived proangiogenic cells (CD34+CD133+). Conversely, in skeletal muscle, LBH evoked a transient increase in the mRNA expression of several important angiogenic factors, including VEGF and HSPs. To explore the mechanisms underlying this response, we designed a custom water-circulating garment that allowed for localized heat application to one thigh, while the contralateral thigh served as a control (Fig. 1, right). In this model, body core temperature and systemic hemodynamics were not significantly altered, while femoral blood flow in the heated thigh increased markedly compared with the control thigh. In close agreement with the responses induced by LBH, local heating also upregulated the expression of angiogenic factors and HSPs in skeletal muscle. These findings suggest that the acute angiogenic response to LBH stems largely from local mechanisms and is not influenced by the systemic responses induced by this treatment. Overall, this study reveals that heat therapy is a simple, noninvasive strategy to activate local angiogenic signaling and possibly promote vascular growth in skeletal muscle.
Effects of Heat Therapy on Circulating Angiogenic Factors and Proangiogenic Cells
The reported increase in circulating levels of bone marrow-derived CD34+ cells in patients with PAD (66) and CHF (49) after treatment with sauna therapy prompted us to investigate the acute effects of LBH on the numbers of these cells, as well as on the concentrations of angiogenic cytokines that participate in the recruitment and mobilization of progenitor cells. The angiogenic potential of CD34+ cells has been well documented in numerous clinical and preclinical studies (36) and appears to be related to the ability of these cells to produce and secrete angiogenic cytokines (37). Using a multiparametric flow cytometry protocol, we identified and quantified the number of cells that coexpress CD34 and the primitive stem cell marker CD133 (83). Contrary to our hypothesis and the aforementioned sauna studies (49, 66), a single session of LBH did not affect the levels of circulating cytokines and proangiogenic cells (Table 2). Combined, these findings indicate that acute LBH does not evoke a systemic angiogenic response in healthy young participants. Because sauna therapy typically induces larger changes in core temperature (∼1–1.2°C) (73) than what we observed in the present study (∼0.6°C), one potential explanation for the lack of effect of LBH on the systemic levels of these proangiogenic mediators is that the heat stress level was not sufficient to trigger the production and/or release of cytokines and promote the recruitment of progenitor cells. Another possibility is that 2 h was not long enough after LBH to induce changes in the levels of these circulating proangiogenic factors and cells. In addition, it is conceivable that multiple or longer sessions of LBH are necessary to effectively activate these angiogenic mediators.
Skeletal Muscle Angiogenic Signaling
Several animal studies demonstrated unequivocally that heat therapy promotes angiogenesis in the heart (21, 28, 69) and in skeletal muscle (1, 40, 43), but the molecular mechanisms underlying this adaptation remain poorly defined. One attractive candidate is VEGF, a potent angiogenic inducer that acts by promoting proliferation and enhancing migration and invasion of endothelial cells (5). Increased capillarization in the myocardium following whole body heat stress in rats is, indeed, associated with a marked upregulation of VEGF expression (21, 28, 69). In the present study, an increase in VEGF mRNA expression in skeletal muscle was observed following both LBH and TH, demonstrating that this central proangiogenic factor might also mediate the angiogenic response to heat therapy in skeletal muscle. VEGF regulates basal and exercise training-induced increases in skeletal muscle capillarization (80), and muscle-specific deletion of this molecule leads to capillary rarefaction (3, 52) and nearly abolishes exercise-induced capillary growth (53).
In addition to VEGF, heat therapy activates other key players involved in the angiogenic cascade. ANGPT1 and ANGPT2 are important proangiogenic factors that act as ligands for Tie-2 receptors in endothelial cells (16). Although the importance of angiopoietin signaling in the vasculature is well defined, the cellular origins and functional roles of these two factors in skeletal muscle have only recently received attention. ANGPT1 is the principal angiopoietin produced by skeletal myoblasts and myotubes and is an important regulator of myogenesis and angiogenesis (38, 42). Conversely, ANGPT2 has no effect on myoblast proliferation and migration and exerts a relatively weak and context-dependent effect on angiogenesis (41). Mofarrahi et al. (42) recently showed that overexpression of ANGPT1 following muscle injury in mice promoted capillary growth and accelerated the recovery of contractile performance. In this scenario, it is noteworthy that TH induced the expression of ANGPT1 in skeletal muscle (Fig. 6). This novel finding might help explain why repeated heat therapy application facilitates regeneration of injured skeletal muscle (50, 72).
Individuals exposed to LBH had higher mRNA levels of CCL2 and CX3CL1 relative to the control group (Fig. 3). CCL2 expression was also higher in the heated thigh compared with the control thigh on protocol 3 (Fig. 6). This is an important finding because these myokines/chemokines are involved in the attraction of monocytes/macrophages, which are critical for skeletal muscle remodeling (8). Impaired CCL2 signaling via deletion of its receptor, CCR2, delays angiogenesis and reduces VEGF levels following skeletal muscle injury in mice (48). Recently, Strömberg et al. (70) demonstrated that CX3CL1 stimulation of primary human myoblasts and myotubes promoted marked increases in the expression of proangiogenic factors and chemotactic mediators. These observations, coupled with the notion that the expression of both factors increases markedly in skeletal muscle following an acute bout of exercise in humans (6), indicate that these chemoattractants promote a microenvironment that facilitates angiogenesis and muscle repair (70).
The expression levels of the transcription factor FOXO1 were reduced 2 h after the intervention in the group treated with LBH. FoxO1 regulates the transcription of several angiostatic factors and acts to restrain angiogenesis in skeletal muscle (63, 68). A reduction in the expression of this factor might, therefore, allow for the initiation of the angiogenic cascade. Altogether, these findings indicate that both LBH and TH promote the expression of some pivotal factors that mediate capillary growth in skeletal muscle. It is conceivable that these acute responses might translate over time to increased capillary supply in skeletal muscle after repeated treatment. Indeed, evidence derived from exercise training studies indicates that skeletal muscle angiogenesis is preceded by repeated transient mRNA bursts of growth factors and proinflammatory mediators (23, 34). Nonetheless, it is important to highlight that the changes in the expression of some factors in the present study were transient and relatively small. Whether protein levels of these proangiogenic factors are also altered by acute heat therapy remains to be determined. Additional studies are needed to explore the long-term effects of repeated heat therapy on skeletal muscle capillarization in healthy and diseased populations.
One important observation of the current study was that the expression of some genes were altered in the control group on protocol 2 and in the thigh exposed to the control intervention on protocol 3. This response was particularly evident for CCL2 and CX3CL1, which were consistently downregulated following the control intervention on both protocols (Figs. 3 and 6). This response might be potentially explained by circadian oscillations in gene expression (56), changes in metabolic status (79), or forced physical inactivity for a long period of time. In agreement with our findings, prior studies have demonstrated that the mRNA expression of CX3CL1 tends to decrease slightly over time in resting/nonexercising skeletal muscle (6, 70). Intriguingly, however, the expression of CCL2 has been reported to increase, albeit not significantly, in resting muscle 2–4 h following a baseline biopsy (6, 70, 78). These changes in the expression of key myokines illustrate the critical importance of incorporating a control group in long experiments to account for time-dependent variations in skeletal muscle gene expression in humans.
Heat Shock Proteins and Angiogenesis
Most of the salutary effects of heat stress on skeletal muscle are thought to be mediated, in part, by HSPs (25). This highly conserved family of proteins function as molecular chaperones, and HSPs are involved in multiple cellular activities, including angiogenesis signaling (58, 67, 71). For instance, members of the HSP70 and HSP90 families have been reported to modulate angiogenesis in skeletal muscle (58, 67). Pharmacological blockade of HSP70s impairs endothelial cell migration and tube formation in vitro and abrogates capillary growth in a model of peripheral arterial insufficiency (67). Similarly, administration of HSP90 inhibitor 17-DMAG suppressed the angiogenic response to repeated dry infrared sauna therapy in mice with hindlimb ischemia (40). The HSP90 inhibitor 17-AAG has also been shown to impair migration and formation of capillary-like tubes in cultured human umbilical vein endothelial cells (HUVEC) (71). Given the importance of these factors for angiogenesis, it is surprising that few studies have determined the impact of passive heat stress on the expression of HSPs in skeletal muscle in humans. Morton et al. (44) showed that leg immersion in water at 45°C for 1 h had no effect on muscle protein content of HSPs in healthy young volunteers (44). Vardiman et al. (77) also found that a single session of local leg heating with fluidotherapy did not alter the content of HSP70 and HSP27p in skeletal muscle. In contrast, Touchberry et al. (75) reported that local heating with diathermy and hot packs increase the skeletal muscle content of HSP70 and HSP27p in female but not male subjects. Our study focused solely on the immediate transcriptional responses of several members of distinct HSP families. We report that both LBH and TH promote a rapid and very consistent upregulation of the mRNA expression of HSPs in human skeletal muscle, including members of the HSP90 and HSP70 families (Figs. 4 and 7). As these proteins have been shown to modulate angiogenesis in skeletal muscle, it is conceivable that the angiogenic response to heat therapy is regulated and potentially dependent on heat-induced activation of HSPs.
Potential Mechanisms
One hypothesis of the present study was that the skeletal muscle angiogenic response to LBH would be greater than that induced by TH, because in addition to activating local proangiogenic signals, systemic heat stress promotes physiological responses that can influence angiogenesis. For example, as discussed above, whole body heat stress has been shown to trigger the release and recruitment of bone marrow-derived proangiogenic cells (49, 66). Further, whole body heat stress evokes a marked increase in sympathoadrenal activity and an associated elevation in circulating levels of catecholamines (27). Catecholamines have been previously shown to contribute to skeletal muscle angiogenesis in a model of peripheral vascular insufficiency (7). Contrary to our initial predictions, exposure to LBH did not alter the levels of cytokines and proangiogenic cells and, most importantly, the increase in mRNA expression of angiogenic factors in skeletal muscle was remarkably similar between LBH and TH (Table 7). These findings suggest that the angiogenic response to both strategies derive from the activation of local signals capable of initiating the angiogenic cascade. One such mechanism would be the increase in skeletal muscle blood flow and shear stress that occurs during heat therapy application. Increased shear stress is known to induce the expression of several proangiogenic genes and provoke remodeling of the skeletal muscle microvasculature (4). The observed progressive increase in blood flow and shear rates in the femoral artery during TH (Table 6) is consistent with other recent reports (9, 10). Of note, the magnitude of change in thigh blood flow is remarkably similar during isolated leg and moderate whole body heating, which indicates that this hyperemic response is controlled by local mechanisms (9, 10). Until recently, the prevailing view was that these increases in bulk limb blood flow in response to heat stress were solely the result of changes in skin blood flow (see Ref. 12 for review). However, following the seminal report of Keller et al. (29), numerous studies have provided evidence that skeletal muscle blood flow also increases during local hyperthermia (10, 24, 55). For example, Heinonen et al. (24) showed using positron emission tomography that leg heating increases blood flow in the gastrocnemius muscle by ∼1 ml·min−1·100 g−1 (24). Although this change is considered small compared with the hyperemic responses induced by interventions such as exercise (12), it is fair to speculate that sustained increases in local flow during 90 min of heat therapy application and in the recovery period might induce a local change in the expression of angiogenic factors.
On the other hand, emerging evidence indicates that heat stress can promote angiogenesis independent of changes in blood flow. Rattan et al. (60) first showed that preexposure of HUVEC and human dermal microvascular endothelial cells to mild heat stress improved their ability to form capillary-like tubes. More recently, Li et al. (32) demonstrated that mild heat stress increased the expression of VEGF and angiopoietins and enhanced the formation of microvessel-like structures in a coculture system of outgrowth endothelial cells and osteoblasts. These authors speculate that this potent angiogenic response is related to the aforementioned heat-induced expression of HSPs (59), which are known to independently regulate angiogenesis in cultured cells (71) and in animal models (40, 67).
Clinical Implications
Heat therapy can be applied using several different modalities, including dry sauna (39), immersion in warm water (54), perfusion of hot water through a tube-lined garment (12), and other methods that are commonly used in rehabilitation settings, such as diathermy (75) and fluidotherapy (77). The use of liquid-circulating garments is attractive because this approach is simple, inexpensive, portable, and amenable for home-based application, which makes this option particularly suitable for patients with limited mobility. Although we choose to apply the treatment to the lower limbs, it is important to highlight that these garments can be customized to cover just about every region of the body. For instance, tube-lined suits that cover the whole body allow for a higher rate of heating and more significant changes in body core temperature than observed in the current study (12). This modality has been extensively employed to investigate thermoregulatory responses in older individuals (22), as well as in patients with hypertension (30), Type 2 diabetes (81), and chronic heart failure (13). It is conceivable that the higher magnitude of heat stress attained by this method could induce responses that are different than the ones evoked by targeting just the lower limbs.
On the other hand, local heating methods, such as the TH protocol used herein, afford the possibility of inducing substantial increases in tissue temperature, while maintaining body temperature stable. These strategies might be more appealing for patients that do not tolerate or have contraindications to whole body heat stress. Regardless of whether heat treatment is applied locally or systemically, the findings from the present study indicate that this therapeutic approach may serve as a practical tool to stimulate angiogenic signaling and promote vascular growth in skeletal muscle.
Limitations
One potential limitation of our experimental design was that subjects were not allowed to drink water during the experiment, and it is possible that sweat-induced dehydration during the LBH protocol might have contributed to the observed changes in skeletal muscle gene expression. However, this confounding effect seems unlikely given that changes in the expression of angiogenic factors and heat shock proteins were remarkably similar between the LBH and TH protocols (Table 7), even though there is negligible dehydration during localized limb heating. In addition, Logan-Sprenger et al. (35) recently reported that mild dehydration induced by overnight fluid restriction had no significant impact on the expression of HSP72 in skeletal muscle in humans. Taken together, these observations indicate that mild dehydration does not seem to impact the expression of angiogenic factors and heat shock proteins in skeletal muscle. It remains to be determined whether more severe levels of dehydration can affect the expression of these factors in humans.
Perspectives and Significance
Our results show that heat therapy, applied either to both legs or locally to one thigh of healthy young individuals, promotes the expression of key angiogenic mediators in skeletal muscle. These findings set the stage for future studies to test the hypothesis that repeated exposure to this therapy can lead to increased capillarization in healthy and diseased skeletal muscle. If this hypothesis is confirmed, heat therapy may become a practical, noninvasive therapeutic tool to reverse the rarefaction of the skeletal muscle capillary network that is commonly observed in individuals with chronic diseases such as COPD, PAD, and CHF.
GRANTS
This work was supported, in part, by a seed funding mechanism for facility usage from the College of Health and Human Sciences at Purdue University.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.M.K., K.K., D.N., Y.N., A.N.B., T.P.G., and B.T.R. performed experiments; A.M.K., K.K., D.N., Y.N., A.N.B., S.K., Q.S., and B.T.R. analyzed data; A.M.K., K.K., D.N., A.N.B., Q.S., T.P.G., and B.T.R. interpreted results of experiments; A.M.K., K.K., D.N., Y.N., A.N.B., B.J.W., S.K., J.S., Q.S., T.P.G., and B.T.R. approved final version of manuscript; K.K. and B.T.R. prepared figures; K.K., B.J.W., S.K., J.S., T.P.G., and B.T.R. edited and revised manuscript; B.J.W. and B.T.R. conception and design of research; B.T.R. drafted manuscript.
ACKNOWLEDGMENTS
We thank Jeff Woodliff from the Bindley Bioscience Center Flow Cytometry and Cell Separation Facility at Purdue for performing the measurement and analysis of circulating progenitor cells and Douglas Maish for assisting with catheter placement.
REFERENCES
- 1.Akasaki Y, Miyata M, Eto H, Shirasawa T, Hamada N, Ikeda Y, Biro S, Otsuji Y, Tei C. Repeated thermal therapy up-regulates endothelial nitric oxide synthase and augments angiogenesis in a mouse model of hindlimb ischemia. Circ J 70: 463–470, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Annex BH. Therapeutic angiogenesis for critical limb ischaemia. Nat Rev Cardiol 10: 387–396, 2013. [DOI] [PubMed] [Google Scholar]
- 3.Bonner JS, Lantier L, Hasenour CM, James FD, Bracy DP, Wasserman DH. Muscle-specific vascular endothelial growth factor deletion induces muscle capillary rarefaction creating muscle insulin resistance. Diabetes 62: 572–580, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown MD, Hudlicka O. Modulation of physiological angiogenesis in skeletal muscle by mechanical forces: involvement of VEGF and metalloproteinases. Angiogenesis 6: 1–14, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Byrne AM, Bouchier-Hayes DJ, Harmey JH. Angiogenic and cell survival functions of vascular endothelial growth factor (VEGF). J Cell Mol Med 9: 777–794, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catoire M, Mensink M, Kalkhoven E, Schrauwen P, Kersten S. Identification of human exercise-induced myokines using secretome analysis. Physiol Genomics 46: 256–267, 2014. [DOI] [PubMed] [Google Scholar]
- 7.Chalothorn D, Zhang H, Clayton JA, Thomas SA, Faber JE. Catecholamines augment collateral vessel growth and angiogenesis in hindlimb ischemia. Am J Physiol Heart Circ Physiol 289: H947–H959, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Chazaud B, Sonnet C, Lafuste P, Bassez G, Rimaniol AC, Poron F, Authier FJ, Dreyfus PA, Gherardi RK. Satellite cells attract monocytes and use macrophages as a support to escape apoptosis and enhance muscle growth. J Cell Biol 163: 1133–1143, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiesa ST, Trangmar SJ, Gonzalez-Alonso J. Temperature and blood flow distribution in the human leg during passive heat stress. J Appl Physiol 120:1047–1058, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiesa ST, Trangmar SJ, Kalsi KK, Rakobowchuk M, Banker DS, Lotlikar MD, Ali L, Gonzalez-Alonso J. Local temperature-sensitive mechanisms are important mediators of limb tissue hyperemia in the heat-stressed human at rest and during small muscle mass exercise. Am J Physiol Heart Circ Physiol 309: H369–H380, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cracowski JL, Minson CT, Salvat-Melis M, Halliwill JR. Methodological issues in the assessment of skin microvascular endothelial function in humans. Trends Pharmacol Sci 27: 503–508, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Crandall CG, Wilson TE. Human cardiovascular responses to passive heat stress. Compr Physiol 5: 17–43, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cui J, Arbab-Zadeh A, Prasad A, Durand S, Levine BD, Crandall CG. Effects of heat stress on thermoregulatory responses in congestive heart failure patients. Circulation 112: 2286–2292, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Duscha BD, Kraus WE, Keteyian SJ, Sullivan MJ, Green HJ, Schachat FH, Pippen AM, Brawner CA, Blank JM, Annex BH. Capillary density of skeletal muscle: a contributing mechanism for exercise intolerance in class II-III chronic heart failure independent of other peripheral alterations. J Am Coll Cardiol 33: 1956–1963, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Egan B, O'Connor PL, Zierath JR, O'Gorman DJ. Time course analysis reveals gene-specific transcript and protein kinetics of adaptation to short-term aerobic exercise training in human skeletal muscle. PloS One 8: e74098, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fagiani E, Christofori G. Angiopoietins in angiogenesis. Cancer Lett 328: 18–26, 2013. [DOI] [PubMed] [Google Scholar]
- 17.Frisbee JC, Goodwill AG, Frisbee SJ, Butcher JT, Brock RW, Olfert IM, DeVallance ER, Chantler PD. Distinct temporal phases of microvascular rarefaction in skeletal muscle of obese Zucker rats. Am J Physiol Heart Circ Physiol 307: H1714–H1728, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gavin TP. Basal and exercise-induced regulation of skeletal muscle capillarization. Exerc Sport Sci Rev 37: 86–92, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gavin TP, Drew JL, Kubik CJ, Pofahl WE, Hickner RC. Acute resistance exercise increases skeletal muscle angiogenic growth factor expression. Acta Physiol (Oxf) 191: 139–146, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Gavin TP, Robinson CB, Yeager RC, England JA, Nifong LW, Hickner RC. Angiogenic growth factor response to acute systemic exercise in human skeletal muscle. J Appl Physiol 96: 19–24, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Gong B, Asimakis GK, Chen Z, Albrecht TB, Boor PJ, Pappas TC, Bell B, Motamedi M. Whole-body hyperthermia induces up-regulation of vascular endothelial growth factor accompanied by neovascularization in cardiac tissue. Life Sci 79: 1781–1788, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Greaney JL, Stanhewicz AE, Proctor DN, Alexander LM, Kenney WL. Impairments in central cardiovascular function contribute to attenuated reflex vasodilation in aged skin. J Appl Physiol 119: 1411–1420, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gustafsson T, Rundqvist H, Norrbom J, Rullman E, Jansson E, Sundberg CJ. The influence of physical training on the angiopoietin and VEGF-A systems in human skeletal muscle. J Appl Physiol 103: 1012–1020, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Heinonen I, Brothers RM, Kemppainen J, Knuuti J, Kalliokoski KK, Crandall CG. Local heating, but not indirect whole body heating, increases human skeletal muscle blood flow. J Appl Physiol 111: 818–824, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horowitz M, Kodesh E. Molecular signals that shape the integrative responses of the heat-acclimated phenotype. Med Sci Sports Exerc 42: 2164–2172, 2010. [DOI] [PubMed] [Google Scholar]
- 26.Huang PH, Chen JW, Lin CP, Chen YH, Wang CH, Leu HB, Lin SJ. Far infra-red therapy promotes ischemia-induced angiogenesis in diabetic mice and restores high glucose-suppressed endothelial progenitor cell functions. Cardiovasc Diabetol 11: 99, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iguchi M, Littmann AE, Chang SH, Wester LA, Knipper JS, Shields RK. Heat stress and cardiovascular, hormonal, and heat shock proteins in humans. J Athletic Train 47: 184–190, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ihori H, Nozawa T, Sobajima M, Shida T, Fukui Y, Fujii N, Inoue H. Waon therapy attenuates cardiac hypertrophy and promotes myocardial capillary growth in hypertensive rats: a comparative study with fluvastatin. Heart Vessels 1–9, 2015. [DOI] [PubMed]
- 29.Keller DM, Sander M, Stallknecht B, Crandall CG. α-Adrenergic vasoconstrictor responsiveness is preserved in the heated human leg. J Physiol 588: 3799–3808, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kellogg DL Jr, Morris SR, Rodriguez SB, Liu Y, Grossmann M, Stagni G, Shepherd AM. Thermoregulatory reflexes and cutaneous active vasodilation during heat stress in hypertensive humans. J Appl Physiol 85: 175–180, 1998. [DOI] [PubMed] [Google Scholar]
- 31.Kihara T, Biro S, Imamura M, Yoshifuku S, Takasaki K, Ikeda Y, Otuji Y, Minagoe S, Toyama Y, Tei C. Repeated sauna treatment improves vascular endothelial and cardiac function in patients with chronic heart failure. J Am Coll Cardiol 39: 754–759, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Li M, Fuchs S, Bose T, Schmidt H, Hofmann A, Tonak M, Unger R, Kirkpatrick CJ. Mild heat stress enhances angiogenesis in a co-culture system consisting of primary human osteoblasts and outgrowth endothelial cells. Tissue Eng Methods 20: 328–339, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Δ ΔC(T)) method. Methods 25: 402–408, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Lloyd PG, Prior BM, Yang HT, Terjung RL. Angiogenic growth factor expression in rat skeletal muscle in response to exercise training. Am J Physiol Heart Circ Physiol 284: H1668–H1678, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Logan-Sprenger HM, Heigenhauser GJ, Jones GL, Spriet LL. The effect of dehydration on muscle metabolism and time trial performance during prolonged cycling in males. Physiol Rep 3: pii: e12483, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mackie AR, Losordo DW. CD34-positive stem cells: in the treatment of heart and vascular disease in human beings. Tex Heart Inst J 38: 474–485, 2011. [PMC free article] [PubMed] [Google Scholar]
- 37.Majka M, Janowska-Wieczorek A, Ratajczak J, Ehrenman K, Pietrzkowski Z, Kowalska MA, Gewirtz AM, Emerson SG, Ratajczak MZ. Numerous growth factors, cytokines, and chemokines are secreted by human CD34+ cells, myeloblasts, erythroblasts, and megakaryoblasts and regulate normal hematopoiesis in an autocrine/paracrine manner. Blood 97: 3075–3085, 2001. [DOI] [PubMed] [Google Scholar]
- 38.McClung JM, Reinardy JL, Mueller SB, McCord TJ, Kontos CD, Brown DA, Hussain SN, Schmidt CA, Ryan TE, Green TD. Muscle cell derived angiopoietin-1 contributes to both myogenesis and angiogenesis in the ischemic environment. Front Physiol 6: 161, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miyata M, Tei C. Waon therapy for cardiovascular disease: innovative therapy for the 21st century. Circ J 74: 617–621, 2010. [DOI] [PubMed] [Google Scholar]
- 40.Miyauchi T, Miyata M, Ikeda Y, Akasaki Y, Hamada N, Shirasawa T, Furusho Y, Tei C. Waon therapy upregulates Hsp90 and leads to angiogenesis through the Akt-endothelial nitric oxide synthase pathway in mouse hindlimb ischemia. Circ J 76: 1712–1721, 2012. [DOI] [PubMed] [Google Scholar]
- 41.Mofarrahi M, Hussain SN. Expression and functional roles of angiopoietin-2 in skeletal muscles. PloS One 6: e22882, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mofarrahi M, McClung JM, Kontos CD, Davis EC, Tappuni B, Moroz N, Pickett AE, Huck L, Harel S, Danialou G, Hussain SN. Angiopoietin-1 enhances skeletal muscle regeneration in mice. Am J Physiol Regul Integr Comp Physiol 308: R576–R589, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morimoto Y, Kondo Y, Kataoka H, Honda Y, Kozu R, Sakamoto J, Nakano J, Origuchi T, Yoshimura T, Okita M. Heat treatment inhibits skeletal muscle atrophy of glucocorticoid-induced myopathy in rats. Physiol Res 64: 897–905, 2015. [DOI] [PubMed] [Google Scholar]
- 44.Morton JP, Maclaren DP, Cable NT, Campbell IT, Evans L, Bongers T, Griffiths RD, Kayani AC, McArdle A, Drust B. Elevated core and muscle temperature to levels comparable to exercise do not increase heat shock protein content of skeletal muscle of physically active men. Acta Physiol (Oxf) 190: 319–327, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Mund JA, Estes ML, Yoder MC, Ingram DA Jr, Case J. Flow cytometric identification and functional characterization of immature and mature circulating endothelial cells. Arterioscler Thromb Vasc Biol 32: 1045–1053, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mund JA, Shannon H, Sinn AL, Cai S, Wang H, Pradhan KR, Pollok KE, Case J. Human proangiogenic circulating hematopoietic stem and progenitor cells promote tumor growth in an orthotopic melanoma xenograft model. Angiogenesis 16: 953–962, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nieuwenhuis MM, Jaarsma T, van Veldhuisen DJ, Postmus D, van der Wal MH. Long-term compliance with nonpharmacologic treatment of patients with heart failure. Am J Cardiol 110: 392–397, 2012. [DOI] [PubMed] [Google Scholar]
- 48.Ochoa O, Sun D, Reyes-Reyna SM, Waite LL, Michalek JE, McManus LM, Shireman PK. Delayed angiogenesis and VEGF production in CCR2−/− mice during impaired skeletal muscle regeneration. Am J Physiol Regul Integr Comp Physiol 293: R651–R661, 2007. [DOI] [PubMed] [Google Scholar]
- 49.Ohori T, Nozawa T, Ihori H, Shida T, Sobajima M, Matsuki A, Yasumura S, Inoue H. Effect of repeated sauna treatment on exercise tolerance and endothelial function in patients with chronic heart failure. Am J Cardiol 109: 100–104, 2012. [DOI] [PubMed] [Google Scholar]
- 50.Oishi Y, Hayashida M, Tsukiashi S, Taniguchi K, Kami K, Roy RR, Ohira Y. Heat stress increases myonuclear number and fiber size via satellite cell activation in rat regenerating soleus fibers. J Appl Physiol 107: 1612–1621, 2009. [DOI] [PubMed] [Google Scholar]
- 51.Olfert IM, Birot O. Importance of anti-angiogenic factors in the regulation of skeletal muscle angiogenesis. Microcirculation 18: 316–330, 2011. [DOI] [PubMed] [Google Scholar]
- 52.Olfert IM, Howlett RA, Tang K, Dalton ND, Gu Y, Peterson KL, Wagner PD, Breen EC. Muscle-specific VEGF deficiency greatly reduces exercise endurance in mice. J Physiol 587: 1755–1767, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Olfert IM, Howlett RA, Wagner PD, Breen EC. Myocyte vascular endothelial growth factor is required for exercise-induced skeletal muscle angiogenesis. Am J Physiol Regul Integr Comp Physiol 299: R1059–R1067, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oyama J, Kudo Y, Maeda T, Node K, Makino N. Hyperthermia by bathing in a hot spring improves cardiovascular functions and reduces the production of inflammatory cytokines in patients with chronic heart failure. Heart Vessels 28: 173–178, 2013. [DOI] [PubMed] [Google Scholar]
- 55.Pearson J, Low DA, Stohr E, Kalsi K, Ali L, Barker H, Gonzalez-Alonso J. Hemodynamic responses to heat stress in the resting and exercising human leg: insight into the effect of temperature on skeletal muscle blood flow. Am J Physiol Regul Integr Comp Physiol 300: R663–R673, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perrin L, Loizides-Mangold U, Skarupelova S, Pulimeno P, Chanon S, Robert M, Bouzakri K, Modoux C, Roux-Lombard P, Vidal H, Lefai E, Dibner C. Human skeletal myotubes display a cell-autonomous circadian clock implicated in basal myokine secretion. Mol Metab 4: 834–845, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perry CG, Lally J, Holloway GP, Heigenhauser GJ, Bonen A, Spriet LL. Repeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscle. J Physiol 588: 4795–4810, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pfosser A, Thalgott M, Buttner K, Brouet A, Feron O, Boekstegers P, Kupatt C. Liposomal Hsp90 cDNA induces neovascularization via nitric oxide in chronic ischemia. Cardiovasc Res 65: 728–736, 2005. [DOI] [PubMed] [Google Scholar]
- 59.Rattan SI, Fernandes RA, Demirovic D, Dymek B, Lima CF. Heat stress and hormetin-induced hormesis in human cells: effects on aging, wound healing, angiogenesis, and differentiation. Dose-Response 7: 90–103, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rattan SI, Sejersen H, Fernandes RA, Luo W. Stress-mediated hormetic modulation of aging, wound healing, and angiogenesis in human cells. Ann NY Acad Sci 1119: 112–121, 2007. [DOI] [PubMed] [Google Scholar]
- 61.Regensteiner JG. Exercise rehabilitation for the patient with intermittent claudication: a highly effective yet underutilized treatment. Curr Drug Targets Cardiovasc Haematol Disord 4: 233–239, 2004. [DOI] [PubMed] [Google Scholar]
- 62.Robbins JL, Jones WS, Duscha BD, Allen JD, Kraus WE, Regensteiner JG, Hiatt WR, Annex BH. Relationship between leg muscle capillary density and peak hyperemic blood flow with endurance capacity in peripheral artery disease. J Appl Physiol 111: 81–86, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roudier E, Milkiewicz M, Birot O, Slopack D, Montelius A, Gustafsson T, Paik JH, DePinho RA, Casale GP, Pipinos II, Haas TL. Endothelial FoxO1 is an intrinsic regulator of thrombospondin 1 expression that restrains angiogenesis in ischemic muscle. Angiogenesis 16: 759–772, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ryan NA, Zwetsloot KA, Westerkamp LM, Hickner RC, Pofahl WE, Gavin TP. Lower skeletal muscle capillarization and VEGF expression in aged vs. young men. J Appl Physiol 100: 178–185, 2006. [DOI] [PubMed] [Google Scholar]
- 65.Saey D, Michaud A, Couillard A, Cote CH, Mador MJ, LeBlanc P, Jobin J, Maltais F. Contractile fatigue, muscle morphometry, and blood lactate in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 171: 1109–1115, 2005. [DOI] [PubMed] [Google Scholar]
- 66.Shinsato T, Miyata M, Kubozono T, Ikeda Y, Fujita S, Kuwahata S, Akasaki Y, Hamasaki S, Fujiwara H, Tei C. Waon therapy mobilizes CD34+ cells and improves peripheral arterial disease. J Cardiol 56: 361–366, 2010. [DOI] [PubMed] [Google Scholar]
- 67.Shiota M, Kusakabe H, Izumi Y, Hikita Y, Nakao T, Funae Y, Miura K, Iwao H. Heat shock cognate protein 70 is essential for Akt signaling in endothelial function. Arterioscler Thromb Vasc Biol 30: 491–497, 2010. [DOI] [PubMed] [Google Scholar]
- 68.Slopack D, Roudier E, Liu ST, Nwadozi E, Birot O, Haas TL. Forkhead BoxO transcription factors restrain exercise-induced angiogenesis. J Physiol 592: 4069–4082, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sobajima M, Nozawa T, Shida T, Ohori T, Suzuki T, Matsuki A, Inoue H. Repeated sauna therapy attenuates ventricular remodeling after myocardial infarction in rats by increasing coronary vascularity of noninfarcted myocardium. Am J Physiol Heart Circ Physiol 301: H548–H554, 2011. [DOI] [PubMed] [Google Scholar]
- 70.Stromberg A, Olsson K, Dijksterhuis JP, Rullman E, Schulte G, Gustafsson T. CX3CL1-a macrophage chemoattractant induced by a single bout of exercise in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 310: R297–R304, 2016. [DOI] [PubMed] [Google Scholar]
- 71.Sun J, Liao JK. Induction of angiogenesis by heat shock protein 90 mediated by protein kinase Akt and endothelial nitric oxide synthase. Arterioscler Thromb Vasc Biol 24: 2238–2244, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Takeuchi K, Hatade T, Wakamiya S, Fujita N, Arakawa T, Miki A. Heat stress promotes skeletal muscle regeneration after crush injury in rats. Acta Histochem 116: 327–334, 2014. [DOI] [PubMed] [Google Scholar]
- 73.Tei C, Horikiri Y, Park JC, Jeong JW, Chang KS, Toyama Y, Tanaka N. Acute hemodynamic improvement by thermal vasodilation in congestive heart failure. Circulation 91: 2582–2590, 1995. [DOI] [PubMed] [Google Scholar]
- 74.Tei C, Shinsato T, Miyata M, Kihara T, Hamasaki S. Waon therapy improves peripheral arterial disease. J Am Coll Cardiol 50: 2169–2171, 2007. [DOI] [PubMed] [Google Scholar]
- 75.Touchberry C, Le T, Richmond S, Prewitt M, Beck D, Carr D, Vardiman P, Gallagher P. Diathermy treatment increases heat shock protein expression in female, but not male skeletal muscle. Eur J Appl Physiol 102: 319–323, 2008. [DOI] [PubMed] [Google Scholar]
- 76.Umehara M, Yamaguchi A, Itakura S, Suenaga M, Sakaki Y, Nakashiki K, Miyata M, Tei C. Repeated waon therapy improves pulmonary hypertension during exercise in patients with severe chronic obstructive pulmonary disease. J Cardiol 51: 106–113, 2008. [DOI] [PubMed] [Google Scholar]
- 77.Vardiman JP, Jefferies L, Touchberry C, Gallagher P. Intramuscular heating through fluidotherapy and heat shock protein response. J Athletic Train 48: 353–361, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vella L, Caldow MK, Larsen AE, Tassoni D, Della Gatta PA, Gran P, Russell AP, Cameron-Smith D. Resistance exercise increases NF-κB activity in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 302: R667–R673, 2012. [DOI] [PubMed] [Google Scholar]
- 79.Vissing K, Andersen JL, Schjerling P. Are exercise-induced genes induced by exercise? FASEB J 19: 94–96, 2005. [DOI] [PubMed] [Google Scholar]
- 80.Wagner PD. The critical role of VEGF in skeletal muscle angiogenesis and blood flow. Biochem Soc Trans 39: 1556–1559, 2011. [DOI] [PubMed] [Google Scholar]
- 81.Wick DE, Roberts SK, Basu A, Sandroni P, Fealey RD, Sletten D, Charkoudian N. Delayed threshold for active cutaneous vasodilation in patients with Type 2 diabetes mellitus. J Appl Physiol 100: 637–641, 2006. [DOI] [PubMed] [Google Scholar]
- 82.Wilkinson DM, Carter JM, Richmond VL, Blacker SD, Rayson MP. The effect of cool water ingestion on gastrointestinal pill temperature. Med Sci Sports Exerc 40: 523–528, 2008. [DOI] [PubMed] [Google Scholar]
- 83.Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, Olweus J, Kearney J, Buck DW. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood 90: 5002–5012, 1997. [PubMed] [Google Scholar]
- 84.Zhang P, Liang X, Shan T, Jiang Q, Deng C, Zheng R, Kuang S. mTOR is necessary for proper satellite cell activity and skeletal muscle regeneration. Biochem Biophys Res Commun 463: 102–108, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]