Abstract
Early-life experience (ELE) can significantly affect life-long health and disease, including cardiovascular function. Specific dimensions of emotionality also modify risk of disease, and aggressive traits along with social inhibition have been established as independent vulnerability factors for the progression of cardiovascular disease. Yet, the biological mechanisms mediating these associations remain poorly understood. The present study utilized the inherently stress-susceptible and socially inhibited Wistar-Kyoto rats to determine the potential influences of ELE and trait aggression (TA) on cardiovascular parameters throughout the lifespan. Pups were exposed to maternal separation (MS), consisting of daily 3-h separations of the entire litter from postnatal day (P)1 to P14. The rats were weaned at P21, and as adults were instrumented for chronic radiotelemetry recordings of blood pressure and heart rate (HR). Adult aggressive behavior was assessed using the resident-intruder test, which demonstrated that TA was independent of MS exposure. MS-exposed animals (irrespective of TA) had significantly lower resting HR accompanied by increases in HR variability. No effects of MS on resting blood pressure were detected. In contrast, TA correlated with increased resting mean, systolic, and diastolic arterial pressures but had no effect on HR. TA rats (relative to nonaggressive animals) also manifested increased wall-to-lumen ratio in the thoracic aorta, increased sensitivity to phenylephrine-induced vascular contractility, and increased norepinephrine content in the heart. Together these data suggest that ELE and TA are independent factors that impact baseline cardiovascular function.
Keywords: emotionality, coping, maternal separation, heart rate, blood pressure
extensive evidence has implicated depression, anxiety, and chronic life stress as predisposing factors for the frequency and severity of heart disease (3, 63, 76). Similarly, distinct dimensions of emotionality have also been linked to cardiovascular disease (44). For example, individuals with type A personality (highly competitive and hostile) had increased risk of heart disease (28, 29). More recent work demonstrated that negative affect and social inhibition mediate much of the association between type A profile and heart disease (22, 23). The constellation of these two traits has been proposed to form type D (for “distressed”) personality profile of individuals who are likely to experience increased anger and manifest hostility and aggression (44). Both negative affectivity and social inhibition, the two components of type D personality, appear to be highly heritable, stable over time, and predict poor cardiovascular health (45).
Aggressive behaviors are evolutionary conserved traits that are required for survival in the face of competition from rivals over territory, food, and reproductive opportunities along with the necessity for self-preservation when faced with predator threat (50, 65). However, aggression is also associated with a host of emotional and physiological abnormalities. For example, highly aggressive mice exhibit numerous autonomic, endocrine, and neurobiological alterations (65), including cardiovascular disturbances and hypothalamic-pituitary-adrenal (HPA) axis dysfunction (10, 11, 100). Work in highly aggressive rats has shown impaired cardiovagal regulation, propensity for cardiac arrhythmias, and increased sympathoadrenal activation (12, 83, 84). These findings in rodents mirror human studies that demonstrate a strong relationship among aggression, hostility, and increased risk for cardiac disease as in type D personality (86).
Early-life experience (ELE) can have a major influence on life-long health and disease. Previous studies have shown that adverse ELE can have detrimental effects on a variety of behavioral, physiological, and endocrine parameters in adulthood in various species (18, 74, 87, 93, 94). One way to model adverse ELE in rodents is by separating pups from their dam on a daily basis during the first 2 wk of life. Extensive studies that have utilized this paradigm have demonstrated adverse behavioral and cardiovascular consequences following prolonged daily separations during the first 2 wk of life (52, 54, 55), while brief separations from the dam appear to be protective (27, 35, 72). Taken together these findings demonstrate the importance of ELE and hostile or aggressive temperament in cardiovascular regulation. However, to our knowledge these two factors have not been previously explored in a preclinical model.
In our previous work we demonstrated that the stress-susceptible Wistar-Koyoto (WKY) rats demonstrate high levels of depressive- and anxiety-like behaviors along with social withdrawal compared with other rat strains (64), thus recapitulating the negative affectivity and social isolation characteristic of the human type D personality. We also demonstrated that these rats respond in a unique fashion to prolonged maternal separation (MS) during the early postnatal period by increasing their social behaviors and diminishing depressive- and anxiety-like behaviors (73). In contrast, the genetically related Wistar rats exhibit the opposite response to MS by increasing their anxiety-like behavior and diminishing social interaction (73). Given this improvement in type D-like behavioral features (i.e., decreased depressive- and anxiety-like behavior and increased social interaction) of MS-exposed WKY rats, we hypothesized that this manipulation would also elicit protective cardiovascular changes. In addition, we aimed to determine the potential impact of ELE on trait aggression (TA) and the influence of this variable on baseline cardiovascular parameters. In the present study, we demonstrate that 1) TA is independent of MS exposure in WKY rats; 2) MS exposure correlates with lower resting heart rate (HR) and increased HR variability (HRV); and 3) by contrast, TA correlates with increased resting blood pressure, increased wall-to-lumen ratio of the thoracic aorta, increased sensitivity to phenylephrine-induced contractility in the thoracic aorta, and increased norepinephrine content in the heart.
METHODS
All animal handling and experimental procedures were approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee and conformed to the Guide for the Care and Use of Laboratory Animals (National Research Council 2011).
Animals.
Eight pairs of male and female WKY rats were purchased from Charles River Laboratories (Kingston, NY). Upon arrival, rats of the same sex were housed two to three per cage (size 43 × 21.5 × 24.5 cm made of clear acrylic) in a temperature-controlled animal housing facility with a 12:12-h light-dark cycle with the lights on at 6:00 AM. Following a 1-wk acclimatization period, male and female pairs were housed together to generate the pups used in the experiment. Over a period of 1 wk the cages were checked for the presence of sperm plugs, and once detected the male rat was removed from each cage.
From postnatal day(P)1 through P14, we initiated a separation procedure modeled after Plotsky and Meaney (72) and as previously described (14). Between P1–P14, newborn pups experienced either maternal separation (MS) or neonatal handling (NH). Numerous variations occur between laboratories in regards to maternal separation protocol and the comparison group to ascertain the effects of early-life stress. Separation paradigms include a single 24-h separation, or repeated daily separations lasting 1–6 h during the first 2–3 postnatal wk (19, 20, 48). In addition to these differences a variety of comparison groups have been used, including 1) nonhandled; 2) animal facility reared; or 3) briefly handled (3–15 min) pups (8, 35, 48, 51, 52, 54, 55, 72). Extensive literature demonstrates the contrasting effects of NH vs. MS in terms of HPA axis reactivity and stress-elicited behaviors in adulthood (8, 51, 59, 72); thus we decided to use these manipulations in the present study. The pups that experienced MS were separated as an entire litter for 180 min daily within the 3.5-h window period and occurred between 8:30 AM and 12:00 PM. The pups that experienced NH were handled in the same manner, except their daily separations were 15 min in duration. For each dam the entire litter was transferred to a different room in a small cage and placed on a heating pad (∼37°C), whereas the dam remained in the home cage. All littermates remained in close contact throughout the separation period and were returned to the home cage after the conclusion of separation. After the final separation on P14, litters remained undisturbed until weaning on P21 at which time male pups were separated, group housed (3 per cage), and utilized in subsequent behavioral and physiological studies. Rats with the same ELE (i.e., NH or MS) were housed together and were allowed to develop to adulthood undisturbed, except to be weighed each week and for the regular cage changes associated with animal housing until P60. Between P60 and P70, rats were tested on a battery of behavioral tests (in the order indicated): open field test (to assess anxiety-like behavior), forced swim test (to assess depressive-like behavior), and social interaction test (to assess social behaviors). Results of these behavioral experiments have been previously published (73).
The animals were then allowed to recover from the stress of behavior testing (over ∼2 wk), were surgically implanted with radiotelemetry probes (∼2 wk), and were allowed to recover from the stress of surgery (∼2 wk). Recovery was verified by the presence of reliable circadian fluctuations in blood pressure and HR. The study was designed to record baseline cardiovascular function and to also evaluate responses to acute stressors during intervening periods between baseline recordings. Data from baseline recordings is presented in the present article, while stress-elicited data will be presented in a separate article in the future.
First baseline recording was performed at P130, followed by a 7-wk intervening period until the second baseline recording at P179. It was during this 7-wk period that the rats were exposed to two acute stressors (i.e., daytime cage change and restraint). The rats were allowed to recover ∼2 wk between stressors and ∼2 wk from the time of the last stressor till the next baseline recording. Examination of our cardiovascular data revealed very similar baseline parameters [i.e., HR, mean arterial pressure (MAP), systolic blood pressure (SBP), diastolic blood pressure (DBP)] at P130 and P179, indicating full recovery from acute stressors. Afterwards, baseline recordings were obtained every 2 wk until P221, at which time we had to delay our experiment due to the unexpected construction in our animal facility. We obtained another recording at P277 before euthanasia to ensure stability of our baseline differences.
Radiotelemetry surgery.
Four weeks after the conclusion of the behavioral testing (∼P101-P109), n = 10 MS and n = 10 NH rats were randomly chosen and instrumented with cardiovascular radiotelemetry probes (PA-C40; DSI International, St. Paul, MN). Anesthesia was induced with 5% isoflurane and maintained at 2.0–2.5% in 1 l/min O2. Surgical plane of anesthesia was verified by the absence of limb withdraw to painful pinch stimuli. With the use of aseptic techniques blood pressure transducers (PA-C40; Data Sciences International) were implanted into the abdominal aorta and glued in place with surgical glue (Vetbond). The body of the telemetry device was sutured into the abdominal wall with silk suture, and the incision was closed with monofilament suture. Before the first incision rats were injected with carprofen (5 mg/kg sc) and buprenorphine (0.1 mg/kg sc) for pain control. Immediately following the surgery, rats recovered from anesthesia in a warm, clean cage with water provided in a Petri-dish. Afterwards, they were individually housed and recovered for a week before commencement of cardiovascular recordings. During this recovery period animals were checked daily for signs of distress, such as failure to gain weight, spiked coat, nasal discharge, and decreased water and food intake. They were injected with buprenorphine (0.1 mg/kg sc) if necessary, and topical triple antibiotic ointment was applied to the skin suture wound on a daily basis until complete recovery.
Data acquisition and analysis.
Blood pressure and activity counts were acquired using Dataquest ART 4.3 software (Data Sciences International). Continuous 24-h blood pressure recordings were made at specific time points during development: P130, P179, P193, P207, P221, and P277. Rats were kept undisturbed without human interference during these recording sessions. Blood pressure signal was sampled at 500 Hz; activity signal was based on transmitter signal strength changes that provided an approximate index of overall activity and was collected at 250 Hz.
MAP, SBP, and DBP along with HR were extracted from the blood pressure signal, which was sampled at 500 Hz. Baseline cardiovascular parameters and activity were analyzed separately for the 12-h dark/active period and the 12-h light/inactive period.
HRV analysis.
HRV was calculated from the continuous blood pressure recordings that were binned into 5-min continuous segments as recommended by the Task Force of the European Society of Cardiology the North American Society of Pacing Electrophysiology (1). A total of 288 segments were collected over 24 h. Data were analyzed separately for the dark and light cycles, with 144 segments for each 12-h period. HRV was calculated as standard deviation of interbeat-interval (SDNN) during each of the 5-min segments using Dataquest ART 4.3 software (Data Sciences International).
For the calculation of HRV in frequency domain analyses, four 30-min continuous blood pressure segments were used. Two segments were used from the light/inactive phase between 1) 06:00 AM and 12:00 PM, and 2) 12:00 PM and 6:00 PM, and the other two segments were used from the dark/active phase between 1) 6:00 PM and 12:00 AM, and 2) 12:00 AM and 06:00 AM. The spectral analysis of HRV was calculated using Hemolab Analyzer and batch processor software (http://haraldstauss.com/HaraldStaussScientific/hemolab/). Hemolab software utilizes the fast Fourier transform (FFT) method for spectral analysis of HRV. The boundaries for the frequency bands were selected as follows: total power (TP) 0.0–5.0 Hz, high frequency (HF) 1.0–2.4 Hz, low frequency (LF) 0.2–0.6 Hz, and very low frequency (VLF) 0.02–0.2 Hz.
Spontaneous baroreceptor reflex analysis.
DSI data files were imported into the Hemolab Analyzer software for spontaneous baroreceptor reflex sensitivity (sBRS) quantification. For this analysis, two recording segments were used during the light/inactive phase between 1) 06:00 AM and 12:00 PM, and 2) 12:00 PM and 6:00 PM. Two segments during the dark/active phase between 1) 6:00 PM and 12:00 AM, and 2) 12:00 AM and 06:00 AM were also used. Analysis segments that were 30 min in duration were selected during each of these four periods when activity counts were minimal and stable, indicating that animals were quietly resting. Blood pressure data within segment were visually inspected and artifacts were manually removed. Hemolab Analyzer software utilizes the sequence method to calculate sBRS as first described by Bertinieri et al. (4). The software determined baroreflex gain by identifying sequences of four or more heartbeats where blood pressure and the pulse interval changed in same direction. Baroreflex gain was then determined by averaging the slope of linear regressions of the individual sequences; only individual sequences with the correlation coefficient (i.e., r) ≥0.8 were included in this analysis. Aberrant BRS sequences due to noise were visually inspected and manually removed from the analysis.
Resident-intruder test.
Resident-intruder testing was performed between P263 and P266 and was based on previously published methodology (56). To increase their aggressive behavior, resident rats were housed in isolation for a prolonged period of time, which is a manipulation that reliably increases aggressive behaviors in rats and mice (56, 69). Specifically, these animals were single-housed after implantation with radiotelemetry probes at P101 and were tested on their responses to intruder conspecifics on P263. Intruder rats were age-matched, socially housed (2–3 per cage) WKY male rats that were placed in the resident's homecage for 10 min. Testing was performed in the early afternoon, when the resident was allowed to rest quietly in its homecage for 1–2 h before testing. At the beginning of the test phase, the intruder conspecific was placed into the resident homecage for 10 min. The encounter was recorded and digitized, and was subsequently scored by an experienced observer who was blinded to the treatment groups. Rats were closely observed to ensure that no physical injuries resulted from the encounter; despite aggressive displays by some of the rats, none of the rats needed to be separated because of potential for serious physical harm (e.g., bites, bleeding). Resident and intruder behavior was quantified in the social, nonsocial, and aggressive domains using published methodology (41). Several behaviors including social, nonsocial, offensive, and defensive behaviors were quantified for the resident (test) rats as well as intruder rats. Social exploration (i.e., exploration of the counterpart without any signs of aggression) and nonsocial exploration (exploration of the cage) were scored for resident rats as well as intruder rats. Other behaviors that were quantified for resident rats were clinch attack (i.e., pinning the counterpart down into a submissive posture), ano-genital sniffing (i.e., sniffing genitalia of the counterpart as investigatory behavior), lateral threat (i.e., lateral movement toward the counterpart preceding an attack), rest/inactivity, and grooming behavior. Behaviors that were quantified for intruder rats included submissive posture (prolonged supine position following attack), move away (moving away from resident rats following threat), rearing (i.e., assumption of an upright posture using only the hind limbs), upright posture (i.e., standing on hindlimbs when interacting with the resident rats), and freezing (i.e., prolonged duration of immobility) behaviors.
Tissue and blood collection.
At the end of the experiment (between P342 and P355), between 8:00 AM and 12:00 PM, rats were anesthetized with 5% isoflurane (maintained at 2.0–2.5%) in O2 delivered at 1.0 l/min. Following thoracotomy, blood was collected from the left ventricle by a puncture with a large gauge needle into EDTA-coated tubes. Whole blood was then centrifuged at 2,000 g for 15 min to collect plasma, which was stored at −80°C until further analyses. Following the blood collection, rats were rapidly decapitated using a sharp guillotine, and a 1- to 2-cm segment of the thoracic aorta was harvested and stored in the Krebs-Henseleit buffer for the vessel-response study that was conducted later that same day. The left ventricle was freshly dissected from the heart and then weighed before being flash frozen at −30°C in isopentane. Tissue samples were then stored at −80°C until further processing.
Vascular reactivity.
Isometric tension was measured in isolated thoracic aortic ring segments of WKY rats. Rat aortas were excised and placed in Krebs-Henseleit buffer at 25°C. Subsequently, fat and adhering tissue was excised and cleansed from the aortic segments after the tissue harvest. The vessel was cut into individual ring segments (2–3 mm in width) and suspended from a force-displacement transducer in a tissue bath at 37°C. Ring segments were bathed in Krebs-Henseleit buffer of the following composition (mM): 118 NaCl, 4.6 KCl, 27.2 NaHCO3, 1.2 KH2PO4, 1.2 MgSO4, 1.75 CaCl2, 0.03 Na2EDTA, and 11.1 glucose. Buffer was maintained at 37°C and aerated with 95% O2-5% CO2. A passive load of 2 g was applied to all ring segments and maintained at this level throughout the experiment. At the beginning of each experiment, indomethacin-treated ring segments were depolarized with KCl (70 mM) to determine the maximal contractile capacity of the vessel. Rings were then thoroughly washed with Krebs-Henseleit buffer and allowed to equilibrate. In subsequent experiments, the contractile response of vessels was tested by cumulative addition of phenylephrine (PE: 1 × 10−9 to 3 × 10−6). Real-time data were collected for all experiments and downloaded to an IBM PC for analysis. Dose-response profiles were normalized by calculating the contractile response at each concentration of PE as a percentage of the response to 70 mM KCl. The effective concentration of PE eliciting 50% of the maximum response (EC50) was calculated and tested for differences between treatment groups.
Vascular structure.
Thoracic aortas were sectioned into 10- to 15-mm segments and fixed in 10% formalin at room temperature for structural studies. Tissue samples were dehydrated through a series of graded ethanols, defatted in xylene, paraffin embedded, and sectioned on the microtome at 20 μm. Sections were stained with hematoxylin and eosin using a standard protocol. Tissues were examined using an Olympus BX61 microscope (Olympus America, Center Valley, PA) outfitted with motorized stage (96S100LE; Ludl Electronic Products, Hawthorne, NY) and a cooled mono CCD camera (Orca R2; Hamamatsu, Middlesex, NJ). Tissue sections were digitized using CellSens software (Olympus America) under a ×10 objective. To calculate the wall-to-lumen ratio, we quantified the total media-intima area and the total area of the aortic lumen within a randomly selected section of the thoracic aorta.
Catecholamine quantification.
Norepinephrine content in the left ventricle and in plasma was quantified using HPLC by the Vanderbilt University's Neurochemistry Core (https://medschool.vanderbilt.edu/vbi-core-labs/neurochemistry-core; Nashville, TN).
Corticosterone measurement.
Plasma samples were diluted and circulating corticosterone levels were assayed using a commercially-available ELISA kit (Corticosterone EIA Kit, ADI-900-097; Enzo Life Sciences) according to manufacturer's instructions. Light absorbance was read with a multimode plate reader (Synergy HT; BioTek Instruments) at 405 nm. Data were analyzed using four-point sigmoidal algorithm provided in GraphPad Prism 6.0 software (GraphPad Software, LaJolla, CA).
Statistical analysis.
Pearson's χ2-squared test was utilized to evaluate the association between TA and ELE. Repeated-measures analysis using linear mixed models was performed to examine the associations between the outcome variables (HR, SDNN, sBRS, MAP, SBP, DBP, and activity), ELE, and TA over time, where ELE and TA were both included in the models as fixed factors. To control for the possible effects of litter, litter number from each animal was included as a covariate in these analyses. The interactions between ELE and TA were also tested. Since none of them reached significance (P < 0.05), the results were presented without the interaction term. An appropriate covariance structure was selected for the model based on initial assessments of the covariance estimates and the goodness-of-fit indexes. A likelihood ratio test was used to confirm the best fitted model with the most suitable covariance structure. Pearson correlation coefficient was used to analyze the correlations between thoracic wall-to-lumen ratio and SBP, and between thoracic wall-to-lumen ratio and DBP. Contractility of the thoracic aorta in response to exogenous phenylephrine application was analyzed using dose-response curves, which were used to extract logEC50 values for further analysis. Potential differences in the plasma corticosterone levels were evaluated with a two-way ANOVA with ELE and TA as fixed factors. All other comparisons were performed using the Student's t-test, or the Mann-Whitney U-test if the data did not follow a normal distribution. Normality of data was tested with the D'Agostino and Pearson omnibus test. A P < 0.05 was considered statistically significant in two-tailed statistical tests. All analyses were conducted using SPSS version 22.0 (IBM) and GraphPad Prism 6 (http://www.graphpad.com/). Results are presented as mean ± SE.
RESULTS
Resident-intruder test.
Upon introduction of the intruder rat into their homecages, resident rats actively explored the new animal, i.e., they actively approached, sniffed and in some cases attacked the intruder. At the same time, the intruder rats explored the novel environment of the new cage by moving around, rearing, sniffing, and to some extent exploring the resident rat. Only some of the resident rats exhibited clinch attack behavior, which is characteristic of the territorial aggressive behavior in rodents (66). Other residents did not exhibit clinch attack, though some manifested lateral threat behavior, and none of the intruder rats exhibited aggressive behaviors. All of the intruder animals that were attacked by residents adopted a submissive posture. One-half of MS (n = 5) and one-half of NH (n = 5) animals exhibited clinch attack and were classified as trait aggressive (TA). The remaining animals from the MS (n = 5) and NH (n = 5) groups did not show clinch attack and were classified as nonaggressive (NA), indicating that ELE did not impact expression of aggression (χ2 = 0, P = 1).
The latency to clinch attack by TA rats was 151.0 ± 22.0 s, with the average frequency of clinch attacks of 2.3 ± 0.4 and a duration of 7.1 ± 1.0 s per attack. TA rats also demonstrated decreased levels of nonsocial exploration [t(18) = 2.19, P = 0.042] along with increased duration of rest and inactivity [t(17) = 2.86, P = 0.011; Table 1]. Overall, TA rats exhibited significantly greater offensive score, calculated as a sum of clinch attack, lateral threat, upright posture and keep down behavior [t(18) = 3.11, P = 0.006; Table 1]. They also spent 22.1% of their time in offensive behaviors, while the NA rats did so 3.2% of the time (Fig. 1).
Table 1.
Behavioral characterization of resident and intruder rats
| Resident Rats |
Intruder Rats |
|||||
|---|---|---|---|---|---|---|
| Behavior Duration, s | TA | NA | P | TA | NA | P |
| Social exploration | 170.2 ± 21 | 167.1 ± 16 | 0.91 | 75.9 ± 16 | 47.6 ± 11 | 0.15 |
| Nonsocial exploration | 130.2 ± 24* | 211.9 ± 33* | 0.042* | 104.1 ± 25* | 219.8 ± 35* | 0.015* |
| Rest inactivity | 89.5 ± 25* | 13.0 ± 5* | 0.011* | — | — | — |
| Grooming | 55.4 ± 20 | 119.0 ± 30 | 0.091 | — | — | — |
| Offensive score | 126.0 ± 34* | 17.6 ± 7* | 0.006* | |||
| Submissive posture | — | — | — | 183.6 ± 50* | 1.7 ± 1* | <0.0001* |
| Move away | — | — | — | 5.4 ± 2* | 0.6 ± 0.4* | 0.036* |
| Rearing | — | — | — | 14.0 ± 5 | 31.8 ± 8 | 0.063 |
| Upright posture | — | — | — | 16.9 ± 6 | 6.8 ± 2 | 0.19 |
| Freezing | — | — | — | 156.3 ± 44 | 187.8 ± 43 | 0.62 |
Resident rats were classified as trait aggressive (TA) if they displayed clinch attack; if not, they were classified as nonaggressive (NA). Behaviors of these 2 types of residents and of their corresponding intruders were quantified separately. For resident rats, social exploration score also included ano-genital sniffing, which was not quantified in the intruder rats. Offensive score was also calculated for the residents, and was the sum of clinch attack, lateral threat, upright posture, and keep down behavior. Data are presented as mean ± SE.
P < 0.05, statistically significant differences.
Fig. 1.
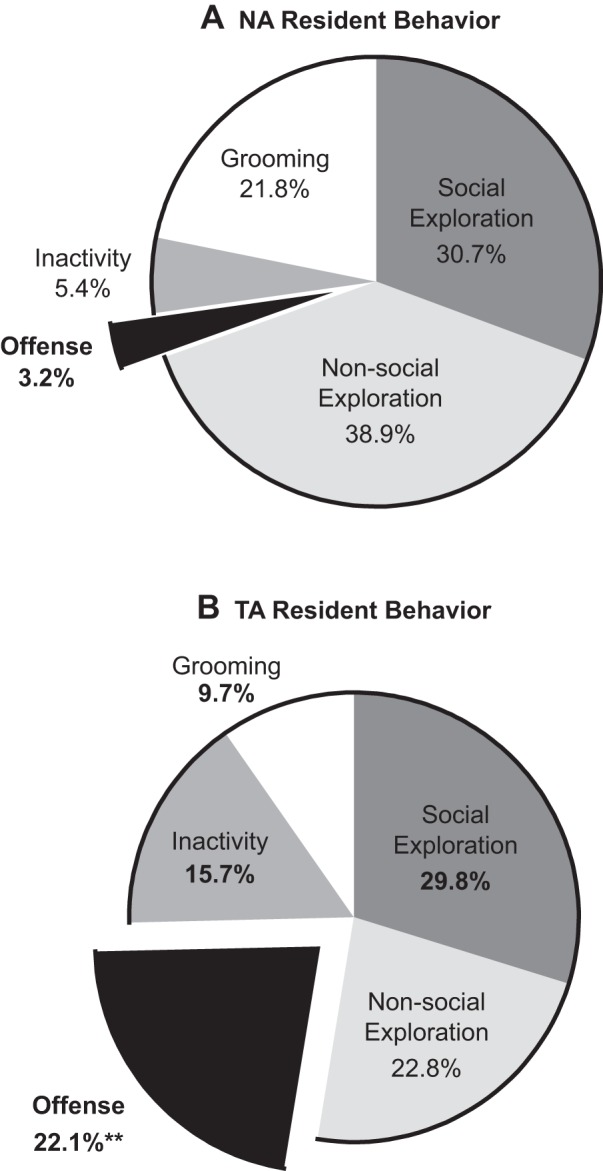
Mean duration (percentage of time) spent in five behavioral categories of nonaggressive (NA; A) and trait aggressive (TA; B) rats; n = 10 per group. **P < 0.01 vs. NA group.
The intruder rats paired with TA residents spent significantly more time in submissive posture (U = 4, P < 0.0001), longer duration in move away behavior (U = 17.5, P = 0.036), and less time in nonsocial exploration [t(18) = 2.69, P = 0.015; Table 1].
Resting HR.
Dependent variables presented in Tables 2 and 3 were analyzed by treating ELE (MS vs. NH) and aggression (TA vs. NA) as fixed factors and age as a repeated measure with litter as a covariate using linear mixed model analysis. Data are presented separately to highlight the effects of ELE and TA.
Table 2.
Summary of cardiovascular and activity measurements
| Early Life Experience |
Trait Aggression |
|||||
|---|---|---|---|---|---|---|
| Dependent Variable,1 unit | NH | MS | P value | TA | NA | P value |
| HR (light), beats/min | 286.5 ± 1.83 | 278.2 ± 1.49 | 0.03* | 282.6 ± 1.73 | 282.1 ± 1.76 | 0.41 |
| HR (dark), beats/min | 325.1 ± 2.06* | 315.5 ± 1.92* | 0.005* | 323.7 ± 2.10 | 317.0 ± 1.99 | 0.07 |
| SDNN (light), s | 0.014 ± 0.0003* | 0.016 ± 0.0003* | 0.03* | 0.015 ± 0.0003 | 0.015 ± 0.0004 | 0.87 |
| SDNN (dark), s | 0.014 ± 0.0003* | 0.016 ± 0.0003* | 0.03* | 0.015 ± 0.0003 | 0.015 ± 0.0004 | 0.09 |
| sBRS (light),2 ms/mmHg | 2.64 ± 0.15 | 3.17 ± 0.12 | 0.05 | 2.67 ± 0.13 | 3.14 ± 0.14 | 0.08 |
| sBRS (dark), ms/mmHg | 2.61 ± 0.15 | 3.03 ± 0.13 | 0.11 | 2.65 ± 0.13 | 3.00 ± 0.15 | 0.51 |
| MAP (light), mmHg | 106.1 ± 0.97 | 105.0 ± 0.90 | 0.19 | 108.8 ± 0.67* | 102.4 ± 0.99* | 0.004* |
| MAP (dark), mmHg | 107.9 ± 0.92 | 107.4 ± 0.89 | 0.13 | 110.9 ± 0.64* | 104.4 ± 0.93* | 0.001* |
| SBP (light), mmHg | 129.0 ± 0.99 | 127.3 ± 0.97 | 0.24 | 131.4 ± 0.81* | 125.0 ± 0.97* | 0.02* |
| SBP (dark), mmHg | 131.2 ± 0.97 | 129.9 ± 0.94 | 0.90 | 134.0 ± 0.77* | 127.1 ± 0.91* | 0.001* |
| DBP (light), mmHg | 86.5 ± 1.10 | 85.4 ± 0.95 | 0.30 | 89.2 ± 0.72* | 82.7 ± 1.12* | 0.02* |
| DBP (dark), mmHg | 88.2 ± 1.02 | 87.6 ± 0.96 | 0.08 | 90.9 ± 0.70* | 84.8 ± 1.09* | 0.001* |
| Activity (light), AU | 0.63 ± 0.02* | 0.51 ± 0.02* | <0.0001* | 0.58 ± 0.02 | 0.57 ± 0.02 | 0.59 |
| Activity (dark), AU | 1.88 ± 0.04 | 1.86 ± 0.04 | 0.72 | 2.0 ± 0.04 | 1.8 ± 0.03 | 0.06 |
Data are presented as mean ± SE. Data were analyzed using linear mixed modeling to evaluate main effects of early-life experience (NH vs. MS) and aggression (TA vs. NA). MS, maternal separation; NA, nonaggressive; NH, neonatal handling; TA, trait aggressive; HR, heart rate; SDNN, standard deviation of interbeat-interval; sBRS, spontaneous baroreceptor reflex sensitivity; MAP, mean arterial pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; AU, arbitrary units.
Modeling based on unstructured covariance structure
modeling based on compound symmetry covariance structure.
P < 0.05, statistical significance.
Table 3.
Summary of heart rate variability analyses in frequency domain
| Early Life Experience |
Trait Aggression |
|||||
|---|---|---|---|---|---|---|
| Dependent Variable1 (Frequency)-Phase, unit | NH | MS | P value | TA | NA | P value |
| TP (0-5.0 Hz)-light, ms2 | 282.8 ± 14.4* | 377.4 ± 18.4* | 0.021* | 329.1 ± 15.3 | 331.0 ± 19.7 | 0.45 |
| TP (0-5 Hz)-dark, ms2 | 437.1 ± 18.1* | 526.2 ± 23.5* | 0.002* | 480.4 ± 17.4 | 482.2 ± 25.4 | 0.63 |
| VLF2 (0.02–0.2 Hz)-light, ms2 | 138.3 ± 6.9 | 187.7 ± 9.0 | 0.12 | 155.2 ± 7.2 | 170.8 ± 9.8 | 0.50 |
| VLF2 (0.02–0.2 Hz)-dark, ms2 | 205.1 ± 9.4 | 256.9 ± 12.2 | 0.22 | 226.8 ± 9.5 | 234.8 ± 13.0 | 0.82 |
| LF2 (0.2–0.6 Hz)-light, ms2 | 10.1 ± 1.2 | 10.0 ± 0.8 | 0.66 | 10.5 ± 1.1 | 9.6 ± 0.9 | 0.53 |
| LF (0.2–0.6 Hz)-dark, ms2 | 15.8 ± 0.9 | 15.1 ± 0.8 | 0.73 | 15.2 ± 0.8 | 15.7 ± 1.0 | 0.37 |
| HF (1–2.4 Hz)-light, ms2 | 17.7 ± 4.1 | 11.4 ± 0.8 | 0.98 | 18.2 ± 4.1 | 10.9 ± 0.9 | 0.22 |
| HF (1–2.4 Hz)-dark, ms2 | 25.5 ± 3.7 | 15.6 ± 0.9 | 0.35 | 24.1 ± 3.5 | 17.1 ± 1.7 | 0.26 |
| LF/HF2 (ratio)-light, % | 80.7 ± 3.4 | 93.2 ± 3.3 | 0.20 | 82.9 ± 3.6 | 91.0 ± 3.1 | 0.40 |
| LF/HF (ratio)-dark, % | 89.7 ± 3.9 | 102.0 ± 3.4 | 0.13 | 89.8 ± 4.2 | 101.8 ± 3.1 | 0.09 |
Data are presented as mean ± SE; numbers in parentheses in the left column represent frequency ranges that were analyzed. Data were analyzed using linear mixed modeling to evaluate main effects of early-life experience (NH vs. MS) and aggression (TA vs. NA). HF, high frequency; LF, low frequency; MS, maternal separation; NA, nonaggressive; NH, neonatal handling; TA, trait aggressive; TP, total power; VLF, very low frequency.
Modeling based on unstructured covariance structure
modeling based on compound symmetry covariance structure.
P < 0.05, significant differences.
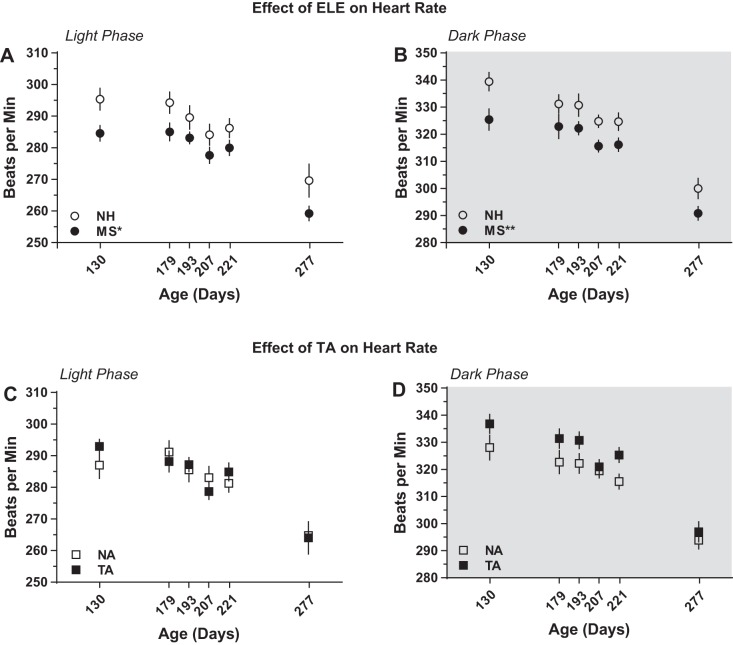
MS-exposed rats showed lower HR throughout their life both in the light (inactive) and dark (active) phases of the 24-h cycle. In the light phase, there was a significant main effect of ELE [F(1,63.2) = 4.86, P = 0.031; Fig. 2A], with MS rats having an average HR of 278.2 ± 1.49 vs. 286.5 ± 1.83 beats/min for the NH group (P < 0.05; Table 2). Significant main effect of ELE [F(1,17.1) = 10.3, P = 0.005; Fig. 2B] was also observed during the dark phase, with mean values of 315.5 ± 1.92 beats/min for the MS group and 325.1 ± 2.06 beats/min for NH (P < 0.01; Table 2). In contrast, no effect of aggression on resting HR was detected either in the light [F(1,24.2) = 0.69, P = 0.41; Fig. 2C] or in the dark [F(1,17.1) = 3.67, P = 0.072; Fig. 2D] phase of the 24 h cycle.
Fig. 2.
Early-life experience (ELE) significantly affects resting heart rate. Cardiovascular recordings were made at postnatal days 130, 179, 193, 221, and 277 via chronic indwelling radiotelemetry probes. A main effect of early-life experience was observed, both in the light (A) and the dark (B) phases of the 24-h cycle. In contrast, no effect of trait aggression was detected either during the light (C) or during the dark (B) phase. MS, maternal separation; NA, nonaggressive; NH, neonatal handling; TA, trait aggressive. *P < 0.05 vs. NH; **P < 0.01 vs. NH; n = 10 per group.
HRV and BRS.
MS-exposed rats showed increased SDNN throughout life both in the light and in the dark phase. In the light phase, there was a significant main effect of ELE [F(1,17.0) = 5.53, P = 0.031; Fig. 3A] but not of aggression [F(1,17.0) = 0.028, P = 0.87; Table 2]. Significant main effect of ELE [F(1,30.9) = 4.88, P = 0.035; Fig. 3B], but not of aggression [F(1,7.7) = 3.72, P = 0.09; Table 2], was also observed during the dark phase.
Fig. 3.
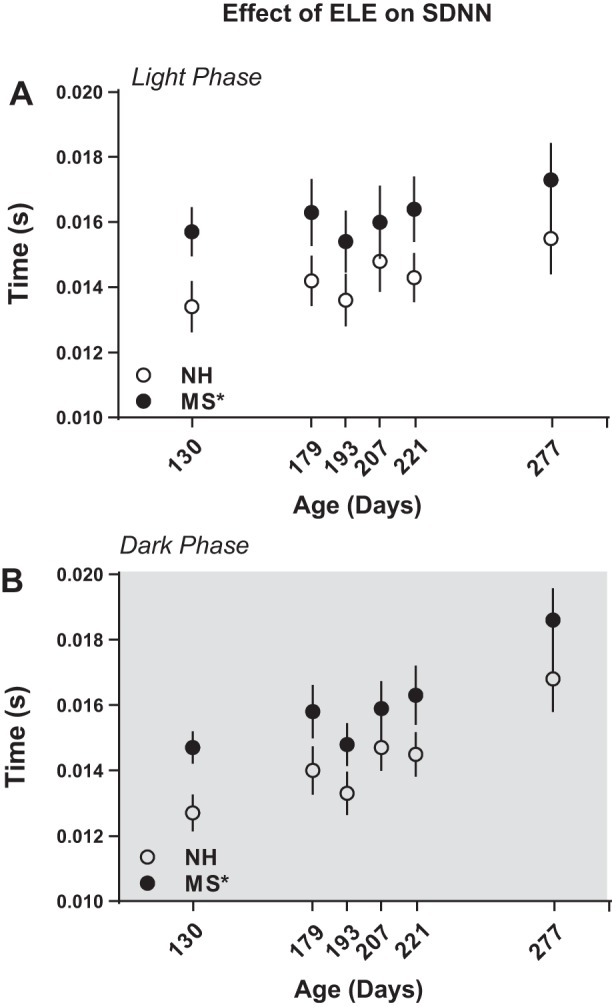
Maternal separation increases baseline heart rate variability (HRV) throughout life. HRV was calculated in the time domain using standard deviation of interbeat-interval (SDNN) during the light (A) and the dark (B) phase at postnatal days 130, 179, 193, 221, and 277. *P < 0.05 vs. NH; n = 10 per group.
We followed up on the SDNN analysis to the analysis of HRV in the frequency domain. This approach revealed an increase in TP in the MS rats in the light [F(1,17.0) = 6.44, P = 0.021] and in the dark [F(1,17.2) = 13.49, P = 0.002] phases (Table 3). No effect of aggression on TP was detected either in the light [F(1,8.4) = 0.62, P = 0.453] or in the dark [F(1,17.2) = 0.247, P = 0.625; Table 3]. Group differences within the individual frequency bands did not reach statistical significance for either ELE or TA in either the light or the dark phase (Table 3). Likewise, no differences in the LF/HF power ratios were detected (Table 3).
MS-exposed rats also exhibited increases in sBRS. In the light phase, there was a trend for the main effect of ELE [F(1,17.7) = 4.28, P = 0.054; Table 2] and also a trend towards significance for an increase in sBRS in NA rats [F(1,17.7) = 3.44, P = 0.08; Table 2]. In the dark phase a trend toward significance was also detected for ELE [F(1,0.92) = 43.4, P = 0.11; Table 2], but not for aggression [F(1,12.68) = 0.47, P = 0.51; Table 2].
Baseline blood pressure parameters.
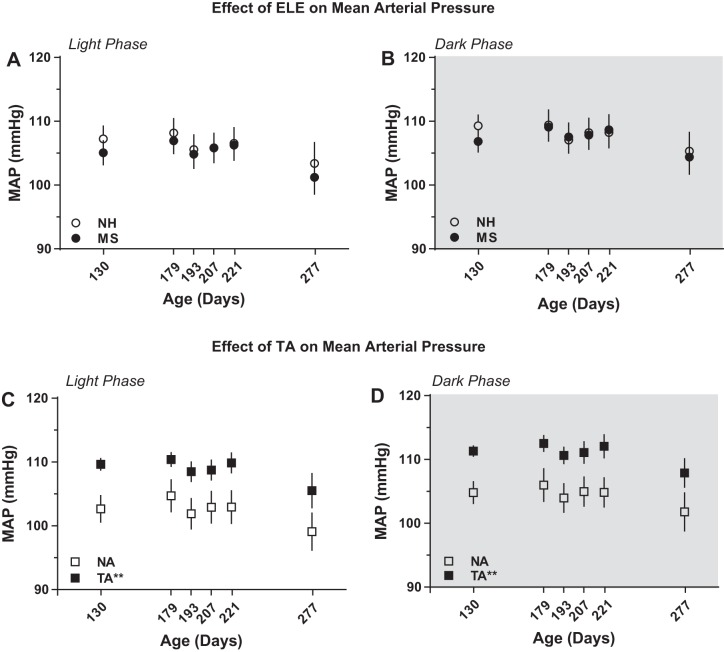
In contrast to resting HR, no effects of ELE on resting MAP were detected during either the light [F(1,18.6) = 1.83, P = 0.19; Fig. 4A] or the dark [F(1,11.1) = 2.75, P = 0.13; Fig. 4B] phase. However, there was a significant effect of aggression during both the light [F(1,18.6) = 10.75, P = 0.004; Fig. 4C] and the dark [F(1,11.1) = 18.1, P = 0.001; Fig. 4D; Table 2] phase, with TA rats showing ∼6–7 mmHg higher MAP than the NA rats.
Fig. 4.
Trait aggression, but not ELE, impacts resting mean arterial blood pressure (MAP). MAP levels did not differ between neonatally handled (NH) or maternally separated (MS) rats either during the light (A) or the dark (B) phase. In contrast, rats classified as trait aggressive (TA) showed significant elevations of MAP during the light (C) and the dark (D) phase compared with the nonaggressive (NA) rats. **P < 0.01 vs. NA; n = 10 per group.
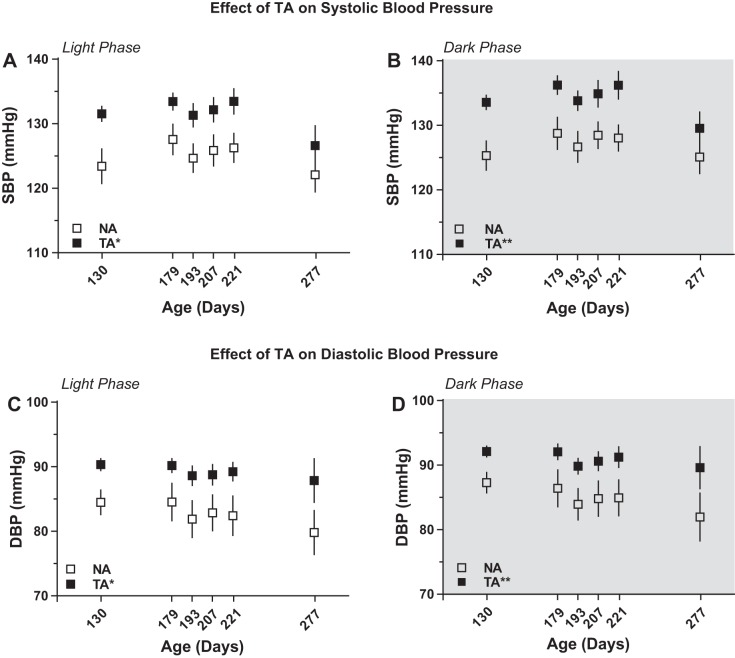
There was significant main effect of aggression on SBP in the light [F(1,30.7) = 6.08, P = 0.019; Fig. 5A] and in the dark [F(1,17.0) = 16.77, P = 0.001; Fig. 5B] phase (Table 2). Similar to SBP, there was a significant main effect of aggression on DBP both during the light [F(1,17.0) = 6.8, P = 0.019; Fig. 5C] and dark phases [F(1,17.0) = 18.3, P = 0.001; Fig. 5D] of the 24-h cycle (Table 2). Similar to MAP, these differences were ∼6–7 mmHg higher SBP and DBP in the TA rats.
Fig. 5.
Trait aggression correlates with increased resting systolic (SBP) and diastolic (DBP) blood pressures. Trait aggressive (TA) rats exhibited increases in SBP during the light (A) and the dark (B) phase compared with the nonaggressive (NA) rats; n = 10 per group. TA rats also manifested increases in DBP during the light (C) and the dark (D) phase across the lifespan. *P < 0.05; **P < 0.01 vs. NA; n = 10 per group.
Baseline activity.
When examining overall activity levels, there was a significant main effect of ELE [F(1,17.0) = 20.6, P < 0.0001], but not of aggression [F(1,17.0) = 0.3, P = 0.59], during the light phase, with NH rats showing higher activity levels (0.63 ± 0.02 AU) compared with MS rats (0.51 ± 0.02 AU; Table 2). No significant effects of TA [F(1,15.2) = 4.33, P = 0.055] or ELE [F(1,29.8) = 0.13, P = 0.72; Table 2] on activity in the dark phase were detected.
Vascular structure.
Our radiotelemetry data indicated significant effects of aggression on resting levels of MAP, SBP, and DBP across both light and dark phases of the 24-h cycle. Based on these observations, we hypothesized that these group differences would correlate with alterations in the vascular structure and function.
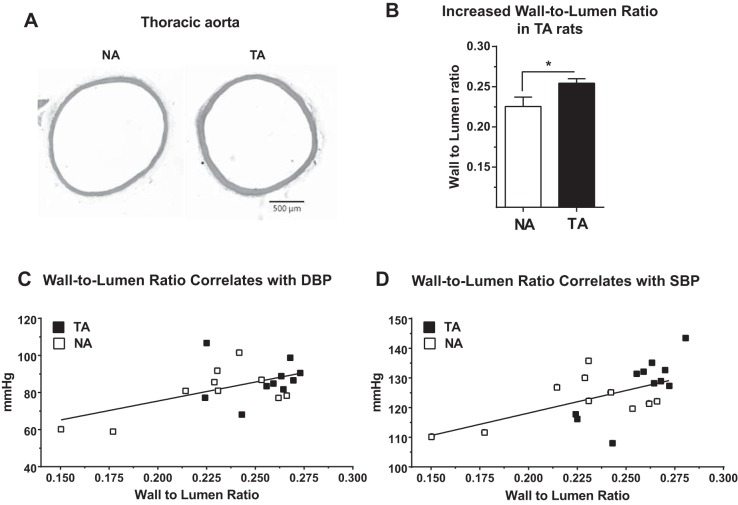
To examine potential alterations in vascular structure, we cut paraffin-embedded transverse sections of the thoracic aorta and then stained them with hematoxylin and eosin. Visual inspection of the specimens suggested increased vascular wall thickness in TA rats (Fig. 6A). Quantitative analyses revealed a significant increase in the wall-to-lumen ratio in the thoracic aorta of TA rats compared with their NA counterparts [t(18) = 2.24, P = 0.038; Fig. 6B]. suggestive of arterial thickening in TA rats. Furthermore, wall-to-lumen ratio was correlated with both resting DBP (24-h average at P277; r = 0.54, P = 0.014; Fig. 6C) and SBP (24-h average at P277; r = 0.58, P = 0.0065; Fig. 6D) with an apparent segregation of the TA data points toward higher wall-to-lumen ratios and increased DBP and SBP.
Fig. 6.
Trait aggression (TA) correlates with vascular remodeling. Thoracic aortas from TA rats appeared to have increased wall thickness compared with those from nonaggressive (NA) rats (A). Wall-to-lumen ratio was significantly increased in the TA rats (B), and this ratio was significantly and directly correlated with resting diastolic (DBP; r = 0.54, P = 0.014; C) and systolic (SBP; r = 0.58, P = 0.0065; D) blood pressures. *P < 0.05; n = 10 per group.
Vascular reactivity.
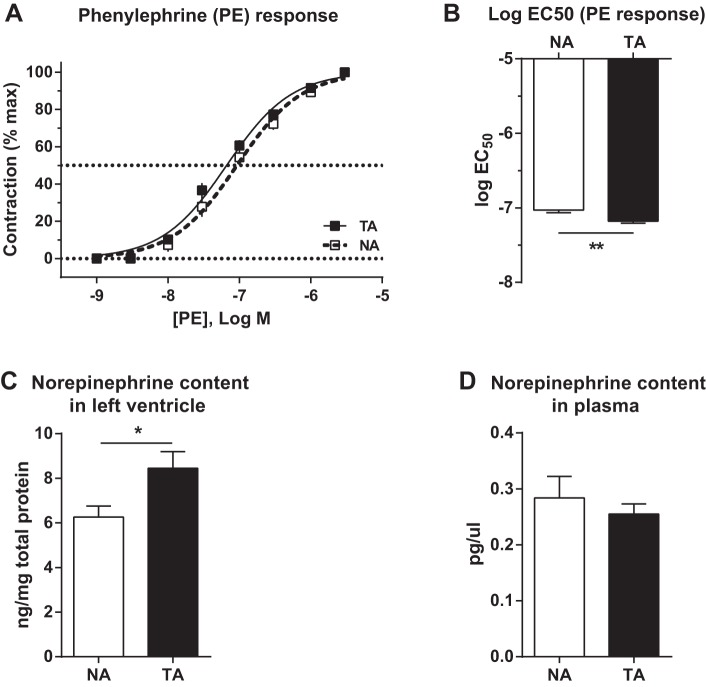
To determine whether increased wall-to-lumen ratio in TA rats was accompanied by differences in vascular reactivity, freshly dissected thoracic aortae were tested for their contractile response to PE, an α1-adrenergic receptor agonist. Examination of contractile responses across a wide range of PE concentrations revealed a leftward shift of the dose-response curve in TA rats (Fig. 7A), which corresponded with a significant decrease of the logEC50 in TA rats [t(17) = 3.4, P = 0.0034; Fig. 7B]. No differences in the maximal response induced by PE were detected: TA: 147.2 ± 14.26% contraction (70 mM KCl) vs. NA: 136.8 ± 13.49% contraction (70 mM KCl) [t(17) = 0.523, P = 0.61]. Likewise, no differences in KCl-induced contractile responses were detected: TA: 3.22 ± 0.25 mN vs. NA: 3.10 ± 0.28 mN [t(18) = 0.344; P = 0.74].
Fig. 7.
Trait aggression correlates with increased sensitivity to phenylephrine (PE) along with increased norepinephrine content in the left ventricle of the heart. There was a modest leftward shift of the PE dose-response curve in aortas extracted from trait aggressive (TA) rats (A). This corresponded with a significant decrease in the logEC50 in the TA rats (B); n = 9–10 per group. These changes were accompanied by an increase in the content of norepinephrine in the left ventricle (C), but not in the plasma (D), of TA rats. *P < 0.05; **P < 0.01; n = 10 per group.
To determine whether vascular reactivity differences between TA and NA rats may be accompanied by altered levels of norepinephrine in the periphery, we quantified norepinephrine content in plasma and peripheral organs. This analysis revealed a significant increase of norepinephrine content in the left ventricle of TA rats [t(18) = 2.44, P = 0.025; Fig. 7], but no differences in circulating plasma levels [t(18) = 0.68, P = 0.5; Fig. 7D]. No effects of TA on the weight of the heart [NA: 0.35 ± 0.03 g/100 g body weight (BW); TA: 0.31 ± 0.01 g/100 g BW; P > 0.1] or of the left ventricle (NA: 0.25 ± 0.02 g/100 g BW; TA: 0.23 ± 0.01 g/100 g BW; P > 0.1) were detected.
Corticosterone levels.
To determine potential differences in the HPA function, corticosterone was measured in the plasma samples. Two-way ANOVA failed to uncover significant main effects of ELE [NH: 66.00 ± 12.08 ng/ml, MS: 75.69 ± 11.46 ng/ml; F(1,16) = 0.31, P = 0.59] or of TA [NA: 71.51 ± 12.06 ng/ml, TA: 70.18 ± 11.70 ng/ml; F(1,16) = 0.006; P = 0.94].
DISCUSSION
Data presented in this study suggest that ELE and TA are independent factors that impact the cardiovascular system in distinct ways. Specifically, our data indicate that ELE can affect resting HR and HRV throughout life. MS-exposed rats had decreased HR along with increased SDNN (i.e., time domain HRV) and TP (frequency domain HRV) up to 9 mo of age (the longest time point in our study). By contrast, ELE did not significantly affect blood pressure parameters, which were strongly influenced by aggression. TA rats exhibited significantly elevated MAP, SBP, and DBP throughout the 24-h cycle (Table 2). The higher levels of these parameters in TA animals appeared to be independent of activity, because no statistically significant differences in activity were detected in either the light or the dark phase (Table 2). In contrast to ELE, HR variables (i.e., HR, SDNN, and TP) were not influenced by TA (Tables 2 and 3). These cardiovascular differences did not appear to be activity dependent, because significant effects of ELE on HR, SDNN, and TP were observed both during the dark and the light phases, while activity differed between NH and MS rats only during the light phase (Table 2). Likewise, TA rats exhibited higher MAP, SBP, and DBP levels throughout the 24-h cycle, while no statistically significant differences in their activity were detected. We previously reported that compared with their NH-exposed counterparts, MS-exposed WKY rats manifest decreases in depressive- and anxiety-like behaviors (73). The present data extend these observations and demonstrate adaptive cardiovascular effects of MS in WKY rats.
The present study did not detect an influence of ELE on the emergence of TA; exactly one-half of MS-exposed animals were classified as TA and one-half of NH-exposed animals were classified as TA (n = 10), with the remaining animals classified as NA (n = 10). Previous literature has documented a complex relationship between ELE and the emergence of aggressive behaviors. MS in Wistar rats has been reported to lead to increased offensive play-fighting behavior during the juvenile period, along with increased aggression toward a novel conspecific in adulthood (98, 99). In contrast, MS exposure in the Long-Evans rats does not lead to significant differences in juvenile aggressive behavior (104).
While it is well accepted that early-life environment has a strong influence on cardiovascular health and disease in adulthood (24), there has been a relative lack of preclinical studies addressing this issue. Loria et al. (52–55) utilized a very different paradigm in WKY rats to demonstrate that in their model MS precipitates adverse cardiovascular changes in adulthood (see below). In Wistar rats MS does not appear to alter resting HR levels but does lead to cardiac remodeling (95). Using a cross-fostering approach McCarty and colleagues (57, 58, 62) have demonstrated that cross-fostering pups from hypertensive strains with normotensive dams significantly lowered blood pressure in adulthood, indicating an important role of maternal care. The novelty of our findings is that in the stress-sensitive WKY rats our MS paradigm elicited adaptive behavioral and cardiovascular changes that persisted throughout adulthood.
Extensive studies in humans have documented differences in coping strategies, aggression, impulsivity, and hostility as correlating with differences in cardiovascular risk factors (22, 23, 28, 29, 44). Most of the preclinical data on the impact of aggression on cardiovascular function have been collected in the Groningen highly aggressive rats (21). Work in this model has implicated a correlation between high levels of aggression and aberrations in cardiac rhythm (12, 83). However, these rats are from the tail of the distribution that exhibits the highest levels of aggression with >55% offense (21). In contrast, TA rats in the present study exhibited offense scores of 22%, which corresponds to low-moderate levels of aggression in the general rat population (see Fig. 1 in Ref. 21). Our results suggest that low-moderate levels of aggression (termed TA in our study) correlate with elevations in resting blood pressure levels, which were accompanied by vascular remodeling and vascular sensitivity to PE, along with an increase in the cardiac norepinephrine content. On the other hand, very high levels of aggression in rats correlate with heart rhythm disturbances, heighten susceptibility to arrhythmias, and are characterized by cardiac vagal imbalance (12, 83).
Previous work in a highly heterogeneous rat population demonstrated that approximately a third of all animals are highly aggressive showing offensive behaviors >55% of the time, a third are intermediate aggressive with 15–55% of time of offense, and a third are low aggressive rats with <15% of offense (21). Observations in laboratory strains indicate that nearly 70% of these rats exhibit low levels of aggression (i.e., ∼4.5% offense of total time) with the rest of the animals showing intermediate aggression (offense score of 27.5% of the time) (21, 42, 68). Results of the present study are consistent with these reports, because the rats that we classified as TA spent 22% of their time in offensive displays, while the NA animals showed low aggression with 3% of offense.
Our previously published data (73) as well as the present HR and HRV findings suggest that MS elicits protective behavioral and cardiovascular adaptations in WKY rats. However, it is important to keep in mind that the details of the early-life stress are critically important and can have a strong impact on behavioral and physiological programming. Recent studies by Loria et al. (52–55) have utilized a different paradigm of ELE differences, where they separated pups from one-half of the litter daily for 180 min from P2-14. Pups from the other half of the litter remained with the dam and were not handled, and all of the pups were weaned on P28 (52–55). Using this model Loria et al. reported sensitization of the sympathetic nervous system (52) and enhanced sensitivity to angiotensin II-mediated vasoconstriction (54, 55) in MS-exposed rats. In contrast, in our studies we separated entire litters for either 15 or 180 min. from P1–P14, and weaned the pups at P21. It may be that separating the entire litter buffers the stress of the absent dam. Such separations may also closer mimic experiences in the wild when the dam leaves the nest to hunt for food. Likewise, the additional week spent with the dam, as a consequence of weaning on P28 (by Loria et al.) rather than on P21 (in the current study), may differentially program the cardiovascular system and contribute to the physiological differences. Previous studies have demonstrated significant behavioral differences, such as baseline levels of forced swim test immobility, in WKY rats from different commercial suppliers (6). This is due to breeding strategies and whether the rat line is maintained as an inbred strain. In the current study we purchased rats from the Charles River colony in Kingston, NY, which has demonstrated a stable behavioral phenotype over the years (6), while Loria et al. (52–55) obtained their animals from an in-house breeding colony. Given that significant genomic differences (∼33%) have been reported in WKY rats obtained from different commercial suppliers (79, 103), this genetic heterogeneity may also contribute to the divergent physiological effects of early-life MS.
Studies in clinical populations have demonstrated the importance of baseline cardiovascular parameters as prognostic factors for future morbidity and mortality. For example, for each 1 beat/min increase in resting HR there is a significant increase in the risk of heart failure and left ventricular dysfunction (70). Similarly, large clinical trials have documented that reductions in baseline HRV (including SDNN) significantly increase the risk of congestive heart failure, cardiac events, and death from cardiac causes (40, 96). The present observations that MS-exposed rats display decreased resting HR and increased SDNN indicates that ELE can program adaptive cardiovascular changes throughout adulthood. Similary, clinical studies have established a strong and direct relationship between resting SBP and DBP and overall mortality rates (15, 49). These data indicate that even a subtle, but persistent, difference in blood pressure can have a major impact on morbidity and mortality (15). Thus the increases in MAP, SBP, and DBP of 6–7 mmHg associated with TA throughout life may impair cardiovascular health.
Apart from baseline hemodynamic parameters, structural and functional characteristics of vasculature are also important to stratify the risk and progression of cardiovascular disease (32). TA rats showed arterial thickening in thoracic aorta evident by higher wall-to-lumen ratio suggestive of vascular remodeling. Similar results have been observed in clinical settings, where individuals with antagonistic and aggressive traits have greater increases in arterial thickening and increased intima-media thickness (5, 67, 90). Interestingly, depression and anxiety do not correlate with arterial thickness (67), which is consistent with our observations.
In addition to structural alterations, in TA rats we also detected increased sensitivity to PE as evidenced by the leftward shift of its dose-response curve and a decrease of the logEC50 in the induction of aortic contractions. This observation suggests increased sensitization of α1-adrenergic receptors to PE rather than increased receptor density, because the maximal response to PE did not differ between groups. This difference in sensitivity to PE may also be accompanied by other vascular alterations, such as impairment in the endothelium-dependent vasodilation as is common in hypertension (75). Although we did not evaluate vascular contractility in endothelium-denuded specimens, it is unlikely that TA rats manifest alterations in endothelial function (at least in thoracic aortae) since we did not detect a difference in acetylcholine-induced relaxation compared with NA rats (data not shown).
TA-related differences in receptor sensitivity may also be accompanied by increased sympathetic tone to specific peripheral organs. We did not quantify norepinephrine content within the aorta or the arterioles; however, we detected increased norepinephrine levels in the hearts, but not in plasma, of anesthetized TA rats. Extensive evidence indicates that sympathetic nerve activity is typically engaged in distinct patterns depending on the stimulus or environmental challenge (39, 61). Our findings of increased norepinephrine content in the heart, but not plasma, are consistent with this notion and suggest an increase in the sympathetic drive to the heart in TA rats, which may lead to increased cardiac contractility.
Potential mechanisms.
Extensive literature documents molecular and structural alterations in the hippocampus following differences in ELE. For example, MS alters hippocampal neurogenesis (47), cell proliferation (34), dendritic length (60), dendritic spine density (60), and mossy fiber density within the hippocampal subfields (35). These changes are accompanied by a host of molecular alterations: genetic and epigenetic changes, including differences in the expression of glucocorticoid receptors (46, 59), glutamate receptor subunits (71), histone deacetylases (88), and histone methyltransferases (88). These alterations are accompanied by increased histone methylation (89) and hypermethylation of the glucocorticoid receptor gene (43). In our preliminary studies we have detected a large increase in whole genome DNA methylation in the hippocampi of MS-exposed WKY rats, a change that was not detected in other brain regions (unpublished observations). Extensive evidence implicates hippocampus in behavioral regulation, including behavioral domains that were strongly altered by MS exposure in our model (73). In addition to behavior, the hippocampus has also been implicated in cardiovascular regulation. For example, hippocampal projections to the prefrontal cortex, amygdala, and subregions of the septum are thought to mediate cardiovascular responses to emotional stimuli (2, 7, 25, 33, 36, 101, 102). Hippocampal projection neurons synapse onto medial prefrontal cortical (mPFC) neurons that connect to the nucleus of the solitary tract (NTS) to regulate the baroreflex (9, 78, 91, 92, 102). Single unit recordings in humans show that a significant proportion (∼25–33%) of hippocampal neurons exhibit time-locked activity to the cardiac cycle and many respond to changes in HR (30). Hippocampal activation leads to decreases in MAP and HR, effects that are attenuated by atropine pretreatment indicating involvement of cardiac parasympathetic efferents (77). Moreover, such effects are prevented by mPFC lesions (78), suggesting that the hippocampus-mPFC-NTS circuit regulates sympathetic and parasympathetic nerve activity in the control of MAP and HR. It is feasible that the hippocampus-mPFC-NTS circuitry is altered via MS-induced hippocampal DNA hypermethylation to induce life-long decreases in HR and increases in HRV. Our future studies will test this hypothesis.
In a previous study we tested selectively bred high (bHR) and low (bLR) responder rats on the resident-intruder test, which showed that bHR resident rats spend ∼30% of their time in aggressive behavior, while bLR residents do so for <10% of the time (38). These percentages of aggressive behaviors in the bHR and bLR rats are similar to those of the TA and NA rats, respectively, in the current study (compare Fig. 1 in the present study with Fig. 1 in Ref. 38). Our data demonstrated that in response to intruder exposure the aggressive bHR rats manifested heightened activation (quantified with c-fos mRNA expression) of the hippocampus and diminished activation of the lateral septum (13), a region that has been strongly implicated in cardiovascular regulation. Classical studies that documented depressor responses following electrical stimulation within the lateral septum in diverse species (17). Its activation inhibits the paraventricular nucleus of the hypothalamus (PVN) (80, 85), which sends dense projections to the sympathetic preganglionic neurons in the spinal cord (16, 37). Thus diminished activity of the lateral septum associated with an aggressive phenotype may lead to disinhibition of the PVN and the subsequent increase in resting blood pressure.
Limitations.
It is possible that prior manipulations, including the behavioral test battery between P60 and P70 may have impacted our cardiovascular and behavioral results. However, it is unlikely that these significantly confounded our interpretation of the data, because baseline cardiovascular parameters, i.e., resting HR and MAP were stable over a long period of time, with resting HR showing appropriate age-related decline over the time course of the study. Similarly, our observed circulating corticosterone levels of ∼70 ng/ml are in line with basal circulating corticosterone levels of rats of a similar age (81). Likewise, it is unlikely that prior behavioral manipulations impacted the emergence of TA, because our earlier work and that by others have indicated that aggressive behavior is an inborn feature of rodent temperament and emotionality, which correlates with distinct endocrine, behavioral, cardiac, and neuronal responses (12, 13, 26, 38, 84). Nonetheless, we cannot definitvely rule out the effects of lifelong stress exposure in these animals. Therefore, it will be important to repeat the current study in animals that did not experience maternal separations, were not exposed to the behavioral test battery, and were single housed for a shorter period of time.
Perspectives and Significance
The present study highlights the importance of ELE as well as TA in modulating cardiovascular parameters in WKY rats. ELE impacted HR parameters, where MS-exposed WKY rats showed improved baseline cardiovascular function. Our previous data also indicate that in addition to these protective cardiovascular alterations, MS diminishes depressive- and anxiety-like behaviors along with an increase in social interaction the WKY rats, while eliciting the opposite changes (i.e., increased depressive- and anxiety-like behavior and decreased social interaction) in the stress-resilient Wistar rats (73). The protective cardiovascular and behavioral effects of early-life stress in WKY rats may be due to the adaptive responses triggered by MS, which programs the neonates to better adapt to stressful situations in adulthood. Such a predictive adaptive response is likely mediated by epigenetic mechanisms and has been proposed to be especially prominent in stress susceptible individuals (31, 82, 97). Future studies aimed at epigenetic effects in WKY rats with differences in ELE will be required to address this notion. On the other hand, TA seems to be an inborn trait that is not impacted by ELE in WKY rats and that correlates with specific vascular and blood pressure alterations. It is possible that these effects are mediated by the differential sympathetic activation of the nerves innervating the heart and vasculature. Future work will be required to evaluate the exact mechanisms by which ELE and/or TA shape cardiovascular function. Uncovering such mechanisms may contribute to the development of personalized therapies for cardiovascular disorders.
GRANTS
The study was funded by National Institutes of Health Grants R00-MH-081927 (to I. A. Kerman) UL1-TR-001417 (National Center for Advancing Translational Science), and P30-NS-47466 (Neuroscience Behavioral Assessment Core: University of Alabama at Birmingham). S. Rana was supported by the American Heart Association Predoctoral Fellowship 13PRE16940050. HPLC determinations were performed by the VBI/VKC Neurochemistry Core Laboratory at Vanderbilt University, which is supported by the Vanderbilt Kennedy Center for Research on Human Development and the Vanderbilt Brain Institute.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
S.R., P.C.P., E.K., J.M.W., H.M.S., C.R.W., S.M.C., and I.A.K. conception and design of research; S.R., P.C.P., E.K., S.A.S., and C.R.W. performed experiments; S.R., P.C.P., E.K., S.A.S., C.P.L., H.M.S., C.R.W., S.M.C., and I.A.K. analyzed data; S.R., P.C.P., E.K., S.A.S., C.P.L., J.M.W., H.M.S., C.R.W., S.M.C., and I.A.K. interpreted results of experiments; S.R. and I.A.K. prepared figures; S.R. and I.A.K. drafted manuscript; S.R., C.P.L., J.M.W., H.M.S., C.R.W., S.M.C., and I.A.K. edited and revised manuscript; S.R., P.C.P., E.K., S.A.S., C.P.L., J.M.W., H.M.S., C.R.W., S.M.C., and I.A.K. approved final version of manuscript.
REFERENCES
- 1.Anonymous. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 93: 1043–1065, 1996. [PubMed] [Google Scholar]
- 2.Arszovszki A, Borhegyi Z, Klausberger T. Three axonal projection routes of individual pyramidal cells in the ventral CA1 hippocampus. Front Neuroanat 8: 53, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med 66: 802–813, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Bertinieri G, di Rienzo M, Cavallazzi A, Ferrari AU, Pedotti A, Mancia G. A new approach to analysis of the arterial baroreflex. J Hypertens Suppl 3: S79–81, 1985. [PubMed] [Google Scholar]
- 5.Bleil ME, McCaffery JM, Muldoon MF, Sutton-Tyrrell K, Manuck SB. Anger-related personality traits and carotid artery atherosclerosis in untreated hypertensive men. Psychosom Med 66: 633–639, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Browne CA, van Nest DS, Lucki I. Antidepressant-like effects of buprenorphine in rats are strain dependent. Behav Brain Res 278: 385–392, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burns SM, Wyss JM. The involvement of the anterior cingulate cortex in blood pressure control. Brain Res 340: 71–77, 1985. [DOI] [PubMed] [Google Scholar]
- 8.Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci USA 95: 5335–5340, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canteras NS, Swanson LW. Projections of the ventral subiculum to the amygdala, septum, and hypothalamus: a PHAL anterograde tract-tracing study in the rat. J Comp Neurol 324: 180–194, 1992. [DOI] [PubMed] [Google Scholar]
- 10.Caramaschi D, de Boer SF, Koolhaas JM. Differential role of the 5-HT1A receptor in aggressive and non-aggressive mice: an across-strain comparison. Physiol Behav 90: 590–601, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Caramaschi D, de Boer SF, Koolhaas JM. Is hyper-aggressiveness associated with physiological hypoarousal? A comparative study on mouse lines selected for high and low aggressiveness. Physiol Behav 95: 591–598, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Carnevali L, Trombini M, Porta A, Montano N, de Boer SF, Sgoifo A. Vagal withdrawal and susceptibility to cardiac arrhythmias in rats with high trait aggressiveness. PLoS One 8: e68316, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clinton SM, Kerman IA, Orr HR, Bedrosian TA, Abraham AD, Simpson DN, Watson SJ, Akil H. Pattern of forebrain activation in high novelty-seeking rats following aggressive encounter. Brain Res 1422: 20–31, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clinton SM, Watson SJ, Akil H. High novelty-seeking rats are resilient to negative physiological effects of the early life stress. Stress 17: 97–107, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collins R, Peto R, MacMahon S, Hebert P, Fiebach NH, Eberlein KA, Godwin J, Qizilbash N, Taylor JO, Hennekens CH. Blood pressure, stroke, and coronary heart disease. Part 2, Short-term reductions in blood pressure: overview of randomised drug trials in their epidemiological context. Lancet 335: 827–838, 1990. [DOI] [PubMed] [Google Scholar]
- 16.Coote JH, Yang Z, Pyner S, Deering J. Control of sympathetic outflows by the hypothalamic paraventricular nucleus. Clin Exp Pharmacol Physiol 25: 461–463, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Covian MR. Studies on the neurovegetative and behavioral functions of the brain septal area. Prog Brain Res 27: 189–217, 1967. [DOI] [PubMed] [Google Scholar]
- 18.Daniels WM, Pietersen CY, Carstens ME, Stein DJ. Maternal separation in rats leads to anxiety-like behavior and a blunted ACTH response and altered neurotransmitter levels in response to a subsequent stressor. Metab Brain Dis 19: 3–14, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Daskalakis NP, Claessens SE, Laboyrie JJ, Enthoven L, Oitzl MS, Champagne DL, de Kloet ER. The newborn rat's stress system readily habituates to repeated and prolonged maternal separation, while continuing to respond to stressors in context dependent fashion. Horm Behav 60: 165–176, 2011. [DOI] [PubMed] [Google Scholar]
- 20.Daskalakis NP, Diamantopoulou A, Claessens SE, Remmers E, Tjalve M, Oitzl MS, Champagne DL, de Kloet ER. Early experience of a novel-environment in isolation primes a fearful phenotype characterized by persistent amygdala activation. Psychoneuroendocrinology 39: 39–57, 2014. [DOI] [PubMed] [Google Scholar]
- 21.de Boer SF, van der Vegt BJ, Koolhaas JM. Individual variation in aggression of feral rodent strains: a standard for the genetics of aggression and violence? Behav Genet 33: 485–501, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Denollet J. Type D personality. A potential risk factor refined. J Psychosom Res 49: 255–266, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Denollet J, Sys SU, Stroobant N, Rombouts H, Gillebert TC, Brutsaert DL. Personality as independent predictor of long-term mortality in patients with coronary heart disease. Lancet 347: 417–421, 1996. [DOI] [PubMed] [Google Scholar]
- 24.Elford J, Shaper AG, Whincup P. Early life experience and cardiovascular disease–ecological studies. J Epidemiol Community Health 46: 1–8, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fisk GD, Wyss JM. Descending projections of infralimbic cortex that mediate stimulation-evoked changes in arterial pressure. Brain Res 859: 83–95, 2000. [DOI] [PubMed] [Google Scholar]
- 26.Flagel SB, Waselus M, Clinton SM, Watson SJ, Akil H. Antecedents and consequences of drug abuse in rats selectively bred for high and low response to novelty. Neuropharmacology 76: 425–436, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Francis DD, Diorio J, Plotsky PM, Meaney MJ. Environmental enrichment reverses the effects of maternal separation on stress reactivity. J Neurosci 22: 7840–7843, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friedman M, Rosenman RH. Association of specific overt behavior pattern with blood and cardiovascular findings; blood cholesterol level, blood clotting time, incidence of arcus senilis, and clinical coronary artery disease. J Am Med Assoc 169: 1286–1296, 1959. [DOI] [PubMed] [Google Scholar]
- 29.Friedman M, Rosenman RH. Type A Behavior Pattern: its association with coronary heart disease. Ann Clin Res 3: 300–312, 1971. [PubMed] [Google Scholar]
- 30.Frysinger RC, Harper RM. Cardiac and respiratory correlations with unit discharge in human amygdala and hippocampus. Electroencephalogr Clin Neurophysiol 72: 463–470, 1989. [DOI] [PubMed] [Google Scholar]
- 31.Gluckman PD, Hanson MA, Beedle AS. Early life events and their consequences for later disease: a life history and evolutionary perspective. Am J Hum Biol 19: 1–19, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Harazny JM, Ritt M, Baleanu D, Ott C, Heckmann J, Schlaich MP, Michelson G, Schmieder RE. Increased wall:lumen ratio of retinal arterioles in male patients with a history of a cerebrovascular event. Hypertension 50: 623–629, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Herman JP, Mueller NK. Role of the ventral subiculum in stress integration. Behav Brain Res 174: 215–224, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Hulshof HJ, Novati A, Sgoifo A, Luiten PG, den Boer JA, Meerlo P. Maternal separation decreases adult hippocampal cell proliferation and impairs cognitive performance but has little effect on stress sensitivity and anxiety in adult Wistar rats. Behav Brain Res 216: 552–560, 2011. [DOI] [PubMed] [Google Scholar]
- 35.Huot RL, Plotsky PM, Lenox RH, McNamara RK. Neonatal maternal separation reduces hippocampal mossy fiber density in adult Long Evans rats. Brain Res 950: 52–63, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Jordan D. Autonomic changes in affective behavior. In: Central Regulation of Autonomic Functions, edited by Loewy AD, Spyer KM. New York: Oxford Univ. Press, 1990, p. 349–366. [Google Scholar]
- 37.Kerman IA, Akil H, Watson SJ. Rostral elements of sympatho-motor circuitry: a virally-mediated transsynaptic tracing study. J Neurosci 26: 3423–3433, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kerman IA, Clinton SM, Bedrosian TA, Abraham AD, Rosenthal DT, Akil H, Watson SJ. High novelty-seeking predicts aggression and gene expression differences within defined serotonergic cell groups. Brain Res 1419: 34–45, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kerman IA, McAllen RM, Yates BJ. Patterning of sympathetic nerve activity in response to vestibular stimulation. Brain Res Bull 53: 11–16, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Kleiger RE, Miller JP, Bigger JT Jr, Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol 59: 256–262, 1987. [DOI] [PubMed] [Google Scholar]
- 41.Koolhaas JM, Coppens CM, de Boer SF, Buwalda B, Meerlo P, Timmermans PJ. The resident-intruder paradigm: a standardized test for aggression, violence and social stress. J Vis Exp: e4367, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koolhaas JM, Schuurman T, Wiepkema PR. The organization of intraspecific agonistic behaviour in the rat. Prog Neurobiol 15: 247–268, 1980. [DOI] [PubMed] [Google Scholar]
- 43.Kundakovic M, Lim S, Gudsnuk K, Champagne FA. Sex-specific and strain-dependent effects of early life adversity on behavioral and epigenetic outcomes. Front Psychiatry 4: 78, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kupper N, Denollet J. Type D personality as a prognostic factor in heart disease: assessment and mediating mechanisms. J Pers Assess 89: 265–276, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Kupper N, Denollet J, de Geus EJ, Boomsma DI, Willemsen G. Heritability of type-D personality. Psychosom Med 69: 675–681, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Plotsky PM. Long-term adaptations in glucocorticoid receptor and mineralocorticoid receptor mRNA and negative feedback on the hypothalamo-pituitary-adrenal axis following neonatal maternal separation. Biol Psychiatry 55: 367–375, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Lajud N, Roque A, Cajero M, Gutierrez-Ospina G, Torner L. Periodic maternal separation decreases hippocampal neurogenesis without affecting basal corticosterone during the stress hyporesponsive period, but alters HPA axis and coping behavior in adulthood. Psychoneuroendocrinology 37: 410–420, 2012. [DOI] [PubMed] [Google Scholar]
- 48.Lehmann J, Feldon J. Long-term biobehavioral effects of maternal separation in the rat: consistent or confusing? Rev Neurosci 11: 383–408, 2000. [DOI] [PubMed] [Google Scholar]
- 49.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 360: 1903–1913, 2002. [DOI] [PubMed] [Google Scholar]
- 50.Lindenfors P, Tullberg BS. Evolutionary aspects of aggression the importance of sexual selection. Adv Genet 75: 7–22, 2011. [DOI] [PubMed] [Google Scholar]
- 51.Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science 277: 1659–1662, 1997. [DOI] [PubMed] [Google Scholar]
- 52.Loria AS, Brands MW, Pollock DM, Pollock JS. Early life stress sensitizes the renal and systemic sympathetic system in rats. Am J Physiol Renal Physiol 305: F390–F395, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Loria AS, D'Angelo G, Pollock DM, Pollock JS. Early life stress downregulates endothelin receptor expression and enhances acute stress-mediated blood pressure responses in adult rats. Am J Physiol Regul Integr Comp Physiol 299: R185–R191, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Loria AS, Kang KT, Pollock DM, Pollock JS. Early life stress enhances angiotensin II-mediated vasoconstriction by reduced endothelial nitric oxide buffering capacity. Hypertension 58: 619–626, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Loria AS, Pollock DM, Pollock JS. Early life stress sensitizes rats to angiotensin II-induced hypertension and vascular inflammation in adult life. Hypertension 55: 494–499, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Malkesman O, Maayan R, Weizman A, Weller A. Aggressive behavior and HPA axis hormones after social isolation in adult rats of two different genetic animal models for depression. Behav Brain Res 175: 408–414, 2006. [DOI] [PubMed] [Google Scholar]
- 57.McCarty R, Cierpial MA, Murphy CA, Lee JH, Fields-Okotcha C. Maternal involvement in the development of cardiovascular phenotype. Experientia 48: 315–322, 1992. [DOI] [PubMed] [Google Scholar]
- 58.McCarty R, Lee JH. Maternal influences on adult blood pressure of SHRs: a single pup cross-fostering study. Physiol Behav 59: 71–75, 1996. [DOI] [PubMed] [Google Scholar]
- 59.Meaney MJ, Diorio J, Francis D, Widdowson J, LaPlante P, Caldji C, Sharma S, Seckl JR, Plotsky PM. Early environmental regulation of forebrain glucocorticoid receptor gene expression: implications for adrenocortical responses to stress. Dev Neurosci 18: 49–72, 1996. [DOI] [PubMed] [Google Scholar]
- 60.Monroy E, Hernandez-Torres E, Flores G. Maternal separation disrupts dendritic morphology of neurons in prefrontal cortex, hippocampus, and nucleus accumbens in male rat offspring. J Chem Neuroanat 40: 93–101, 2010. [DOI] [PubMed] [Google Scholar]
- 61.Morrison SF. Differential control of sympathetic outflow. Am J Physiol Regul Integr Comp Physiol 281: R683–R698, 2001. [DOI] [PubMed] [Google Scholar]
- 62.Murphy CA, Fields-Okotcha C, McCarty R. Shared maternal influences in the development of high blood pressure in the spontaneously hypertensive (SHR) and Dahl salt-sensitive (SS/Jr) rat strains. Behav Neural Biol 57: 144–148, 1992. [DOI] [PubMed] [Google Scholar]
- 63.Musselman DL, Evans DL, Nemeroff CB. The relationship of depression to cardiovascular disease: epidemiology, biology, and treatment. Arch Gen Psychiatry 55: 580–592, 1998. [DOI] [PubMed] [Google Scholar]
- 64.Nam H, Clinton SM, Jackson NL, Kerman IA. Learned helplessness and social avoidance in the Wistar-Kyoto rat. Front Behav Neurosci 8: 109, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Natarajan D, Caramaschi D. Animal violence demystified. Front Behav Neurosci 4: 9, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nelson RJ, Trainor BC. Neural mechanisms of aggression. Nat Rev Neurosci 8: 536–546, 2007. [DOI] [PubMed] [Google Scholar]
- 67.Ohira T, Diez Roux AV, Polak JF, Homma S, Iso H, Wasserman BA. Associations of anger, anxiety, and depressive symptoms with carotid arterial wall thickness: the multi-ethnic study of atherosclerosis. Psychosom Med 74: 517–525, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Olivier B, Mos J, van Oorschot R, Hen R. Serotonin receptors and animal models of aggressive behavior. Pharmacopsychiatry 28, Suppl 2: 80–90, 1995. [DOI] [PubMed] [Google Scholar]
- 69.Olivier B, Young LJ. Animal models of aggression. In: Neuropsychopharmacology–5th Generation of Progress, edited by Davis KL, Charney D, Coyle JT, Nemeroff C. Philadelphia, PA: Lippincott, Williams, & Wilkins, 2002. [Google Scholar]
- 70.Opdahl A, Ambale Venkatesh B, Fernandes VR, Wu CO, Nasir K, Choi EY, Almeida AL, Rosen B, Carvalho B, Edvardsen T, Bluemke DA, Lima JA. Resting heart rate as predictor for left ventricular dysfunction and heart failure: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 63: 1182–1189, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pickering C, Gustafsson L, Cebere A, Nylander I, Liljequist S. Repeated maternal separation of male Wistar rats alters glutamate receptor expression in the hippocampus but not the prefrontal cortex. Brain Res 1099: 101–108, 2006. [DOI] [PubMed] [Google Scholar]
- 72.Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res Mol Brain Res 18: 195–200, 1993. [DOI] [PubMed] [Google Scholar]
- 73.Rana S, Pugh PC, Jackson N, Clinton SM, Kerman IA. Inborn stress reactivity shapes adult behavioral consequences of early-life maternal separation stress. Neurosci Lett 584: 146–150, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rensel MA, Wilcoxen TE, Schoech SJ. The influence of nest attendance and provisioning on nestling stress physiology in the Florida scrub-jay. Horm Behav 57: 162–168, 2010. [DOI] [PubMed] [Google Scholar]
- 75.Rossoni LV, Salaices M, Marin J, Vassallo DV, Alonso MJ. Alterations in phenylephrine-induced contractions and the vascular expression of Na+,K+-ATPase in ouabain-induced hypertension. Br J Pharmacol 135: 771–781, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation 99: 2192–2217, 1999. [DOI] [PubMed] [Google Scholar]
- 77.Ruit KG, Neafsey EJ. Cardiovascular and respiratory responses to electrical and chemical stimulation of the hippocampus in anesthetized and awake rats. Brain Res 457: 310–321, 1988. [DOI] [PubMed] [Google Scholar]
- 78.Ruit KG, Neafsey EJ. Hippocampal input to a “visceral motor” corticobulbar pathway: an anatomical and electrophysiological study in the rat. Exp Brain Res 82: 606–616, 1990. [DOI] [PubMed] [Google Scholar]
- 79.Sagvolden T, Dasbanerjee T, Zhang-James Y, Middleton F, Faraone S. Behavioral and genetic evidence for a novel animal model of attention-deficit/hyperactivity disorder predominantly inattentive subtype. Behav Brain Funct 4: 56, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Saphier D, Feldman S. Effects of septal and hippocampal stimuli on paraventricular nucleus neurons. Neuroscience 20: 749–755, 1987. [DOI] [PubMed] [Google Scholar]
- 81.Sapolsky RM, Krey LC, McEwen BS. The adrenocortical stress-response in the aged male rat: impairment of recovery from stress. Exp Gerontol 18: 55–64, 1983. [DOI] [PubMed] [Google Scholar]
- 82.Schmidt MV. Animal models for depression and the mismatch hypothesis of disease. Psychoneuroendocrinology 36: 330–338, 2011. [DOI] [PubMed] [Google Scholar]
- 83.Sgoifo A, Costoli T, Meerlo P, Buwalda B, Pico-Alfonso MA, De Boer S, Musso E, Koolhaas J. Individual differences in cardiovascular response to social challenge. Neurosci Biobehav Rev 29: 59–66, 2005. [DOI] [PubMed] [Google Scholar]
- 84.Sgoifo A, De Boer SF, Buwalda B, Korte-Bouws G, Tuma J, Bohus B, Zaagsma J, Koolhaas JM. Vulnerability to arrhythmias during social stress in rats with different sympathovagal balance. Am J Physiol Heart Circ Physiol 275: H460–H466, 1998. [DOI] [PubMed] [Google Scholar]
- 85.Silverman AJ, Hoffman DL, Zimmerman EA. The descending afferent connections of the paraventricular nucleus of the hypothalamus (PVN). Brain Res Bull 6: 47–61, 1981. [DOI] [PubMed] [Google Scholar]
- 86.Smith TW, Glazer K, Ruiz JM, Gallo LC. Hostility, anger, aggressiveness, and coronary heart disease: an interpersonal perspective on personality, emotion, and health. J Pers 72: 1217–1270, 2004. [DOI] [PubMed] [Google Scholar]
- 87.Suomi SJ. Early stress and adult emotional reactivity in rhesus monkeys. Ciba Found Symp 156: 171–183; discussion 183–178, 1991. [DOI] [PubMed] [Google Scholar]
- 88.Suri D, Bhattacharya A, Vaidya VA. Early stress evokes temporally distinct consequences on the hippocampal transcriptome, anxiety and cognitive behaviour. Int J Neuropsychopharmacol 17: 289–301, 2014. [DOI] [PubMed] [Google Scholar]
- 89.Suri D, Veenit V, Sarkar A, Thiagarajan D, Kumar A, Nestler EJ, Galande S, Vaidya VA. Early stress evokes age-dependent biphasic changes in hippocampal neurogenesis, BDNF expression, and cognition. Biol Psychiatry 73: 658–666, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sutin AR, Scuteri A, Lakatta EG, Tarasov KV, Ferrucci L, Costa PT Jr, Schlessinger D, Uda M, Terracciano A. Trait antagonism and the progression of arterial thickening: women with antagonistic traits have similar carotid arterial thickness as men. Hypertension 56: 617–622, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Swanson LW. A direct projection from Ammon's horn to prefrontal cortex in the rat. Brain Res 217: 150–154, 1981. [DOI] [PubMed] [Google Scholar]
- 92.Swanson LW, Cowan WM. An autoradiographic study of the organization of the efferent connections of the hippocampal formation in the rat. J Comp Neurol 172: 49–84, 1977. [DOI] [PubMed] [Google Scholar]
- 93.Talge NM, Neal C, Glover V, Early Stress TR; Early Stress, Translational Research and Prevention Science Network: Fetal and Neonatal Experience on Child and Adolescent Mental Health. Antenatal maternal stress and long-term effects on child neurodevelopment: how and why ? J Child Psychol Psychiatry 48: 245–261, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Thompson AL. Developmental origins of obesity: early feeding environments, infant growth, and the intestinal microbiome. Am J Hum Biol 24: 350–360, 2012. [DOI] [PubMed] [Google Scholar]
- 95.Trombini M, Hulshof HJ, Graiani G, Carnevali L, Meerlo P, Quaini F, Sgoifo A. Early maternal separation has mild effects on cardiac autonomic balance and heart structure in adult male rats. Stress 15: 457–470, 2012. [DOI] [PubMed] [Google Scholar]
- 96.Tsuji H, Larson MG, Venditti FJ Jr, Manders ES, Evans JC, Feldman CL, Levy D. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation 94: 2850–2855, 1996. [DOI] [PubMed] [Google Scholar]
- 97.van der Doelen RH, Kozicz T, Homberg JR. Adaptive fitness; early life adversity improves adult stress coping in heterozygous serotonin transporter knockout rats. Mol Psychiatry 18: 1244–1245, 2013. [DOI] [PubMed] [Google Scholar]
- 98.Veenema AH. Early life stress, the development of aggression and neuroendocrine and neurobiological correlates: what can we learn from animal models? Front Neuroendocrinol 30: 497–518, 2009. [DOI] [PubMed] [Google Scholar]
- 99.Veenema AH, Blume A, Niederle D, Buwalda B, Neumann ID. Effects of early life stress on adult male aggression and hypothalamic vasopressin and serotonin. Eur J Neurosci 24: 1711–1720, 2006. [DOI] [PubMed] [Google Scholar]
- 100.Veenema AH, Meijer OC, de Kloet ER, Koolhaas JM. Genetic selection for coping style predicts stressor susceptibility. J Neuroendocrinol 15: 256–267, 2003. [DOI] [PubMed] [Google Scholar]
- 101.Verberne AJ, Lam W, Owens NC, Sartor D. Supramedullary modulation of sympathetic vasomotor function. Clin Exp Pharmacol Physiol 24: 748–754, 1997. [DOI] [PubMed] [Google Scholar]
- 102.Verberne AJ, Owens NC. Cortical modulation of the cardiovascular system. Prog Neurobiol 54: 149–168, 1998. [DOI] [PubMed] [Google Scholar]
- 103.Zhang-James Y, Middleton FA, Faraone SV. Genetic architecture of Wistar-Kyoto rat and spontaneously hypertensive rat substrains from different sources. Physiol Genomics 45: 528–538, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zimmerberg B, Sageser KA. Comparison of two rodent models of maternal separation on juvenile social behavior. Front Psychiatry 2: 39, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]