Abstract
Obesity leads to altered autonomic reflexes that reduce stability of mean arterial pressure (MAP). Sympathoinhibitory reflexes such as baroreflexes are impaired, but reflexes that raise MAP appear to be augmented. In obese Zucker rats (OZR) sciatic nerve stimulation evokes larger increases in MAP by unknown mechanisms. We sought to determine the autonomic underpinnings of this enhanced somatic pressor reflex and whether other sympathoexcitatory reflexes are augmented. We also determined whether their final common pathway, glutamatergic activation of the rostral ventrolateral medulla (RVLM), was enhanced in male OZR compared with lean Zucker rats (LZR). Sciatic nerve stimulation or activation of the nasopharyngeal reflex evoked larger rises in splanchnic sympathetic nerve activity (SNA) (79% and 45% larger in OZR, respectively; P < 0.05) and MAP in urethane-anesthetized, ventilated, paralyzed adult OZR compared with LZR. After elimination of baroreflex feedback by pharmacological prevention of changes in MAP and heart rate, these two sympathoexcitatory reflexes were still exaggerated in OZR (167% and 69% larger, respectively, P < 0.05). In adult OZR microinjections of glutamate, AMPA, or NMDA into the RVLM produced larger rises in SNA (∼61% larger in OZR, P < 0.05 for each drug) and MAP, but stimulation of axonal fibers in the upper thoracic spinal cord yielded equivalent responses in OZR and LZR. In juvenile OZR and LZR, sympathoexcitatory reflexes and physiological responses to RVLM activation were comparable. These data suggest that the ability of glutamate to activate the RVLM becomes enhanced in adult OZR and may contribute to the development of exaggerated sympathoexcitatory responses independent of impaired baroreflexes.
Keywords: baroreflex, somatic pressor reflex, sciatic nerve, nasopharyngeal reflex, vagal, parasympathetic
obesity fosters the development of aberrant autonomic regulation of arterial pressure (AP), leading to hypertension and impaired short-term control of AP by baroreflexes (5, 16, 44, 52). Both of these attributes engender end-organ damage and a poor long-term prognosis (29, 45). Given the ever-increasing prevalence of obesity (43), a better understanding of the underpinnings for deleterious consequences of this condition is essential for optimizing treatments to improve life expectancy. Animal models of obesity that bear resemblance to the human condition are invaluable for investigating the physiological consequences of obesity (15). Obese Zucker rats (OZR), which lack functional leptin receptors, overeat and exhibit exaggerated weight gain compared with lean Zucker rats (LZR) with functional leptin receptors (22, 39). After the onset of obesity, adult OZR develop altered autonomic regulation of cardiovascular function with hypertension that is driven by elevated sympathetic nerve activity (SNA) (49, 52), as seen in obese humans (16). The rostral ventrolateral medulla (RVLM), the brain stem site that drives the SNA to cardiovascular targets, supports the enhanced sympathetic vasomotor tone to promote elevated AP in hypertensive OZR (21, 38). In addition, short-term regulation of AP by baroreflexes are impaired in adult OZR due to changes within the brain stem that reduce autonomic compensations to acute changes in AP (17, 22). Both parasympathetic control of HR and sympathetic control of the heart and vasculature by baroreflexes are diminished in adult OZR (5, 52), analogous to baroreflex deficits observed in obese humans (16). Likewise, activation of other sympathoinhibitory reflexes initiated by activation of vagal afferents to the brain stem, such as the Bezold-Jarisch reflex, also yields smaller reductions in SNA in adult OZR (20).
In contrast to impaired sympathoinhibitory reflexes, stimulation of the sciatic nerve evokes larger rises in AP and HR in OZR with a whole body adrenergic pressor reactivity that is comparable to LZR (47), suggesting an augmented sympathoexcitatory reflex in OZR. Our first aim was to examine the autonomic underpinnings of the exaggerated somatic pressor reflex and determine whether other acute sympathoexcitatory reflexes such as the nasopharyngeal reflex are similarly affected. Our second aim was to examine potential mechanisms underlying the augmented sympathetically mediated pressor responses in OZR. Inhibition of baroreceptor inputs or their brain stem site of termination in the nucleus tractus solitarius (NTS) enhances the somatic pressor reflex in Sprague-Dawley rats (24), so we determined whether augmented responses to sciatic nerve stimulation in OZR persisted in the absence of baroreceptor feedback. The somatic pressor reflex and nasopharyngeal reflex both require glutamatergic activation of the RVLM to increase SNA and MAP (26, 32). Therefore, we also sought to determine whether physiological responses to microinjections of glutamatergic agonists into the RVLM are augmented in OZR and whether changes in targets downstream of the RVLM could contribute. Lastly, we determined whether the development of enhanced sympathoexcitatory responses coincided with the onset of hypertension, impaired baroreflexes, and changes within the RVLM in OZR. Together, these experiments provide the first examination of when and how augmented sympathoexcitatory responses develop in OZR.
MATERIALS AND METHODS
Animals.
All experiments used male LZR (Lepr fa/Lepr+ and Lepr+/Lepr+) and OZR (Leprfa/Leprfa) that were purchased from Harlan. Rats were housed in centralized animal care facilities kept at consistent humidity (60 ± 5%), temperature (24 ± 1°C), and light cycle (0600–1800) and were given free access to tap water and standard rat chow (Teklad 8640, Purina 5GL3, or LabDiet Prolab RMH 1800). Lean and obese rats were housed separately, with rats housed 2–4 in a cage. The studies were performed on age-matched juvenile (7 wk old) and adult (13–17 wk old) rats. Experiments were performed in accordance with the National Institutes of Health's “Guide for Care and Use of Laboratory Animals” and the American Physiological Society's “Guiding Principles in the Care and Use of Vertebrate Animals in Research and Training.” Animal protocols were approved by Institutional Animal Care and Use Committees at University of North Texas Health Science Center and Medical College of Georgia.
Surgical preparations.
Rats were initially anesthetized with 5% isoflurane in 100% O2. Prior to tracheotomy, 2.5–3.5% isoflurane was administered through a nose cone. Adequate anesthesia level was confirmed by the absence of leg flexion in response to a firm toe pinch. Catheters were implanted into a femoral artery and vein to record AP and inject drugs, respectively. Mean AP (MAP) and heart rate (HR) were computed from the arterial pulse. The trachea was cannulated toward the lungs to allow for artificial ventilation (model no. 683, Harvard Apparatus). The LZR and OZR were age-matched and initially ventilated at ∼1 ml/100 g of LZR body weight with 2.0–2.4% isoflurane in 100% O2. Respiratory frequency was adjusted to maintain end-tidal CO2 at 3.8–4.2% (CapStar-100, CWE), and tidal volume was slightly increased for OZR to match frequency and end-tidal CO2 between LZR and OZR (21). For some rats, a second tube (PE-205) was inserted rostrally into the trachea and pushed to the choana to allow for stimulation of the nasopharyngeal reflex, as previously described (33, 55). Each rat was placed in a stereotaxic apparatus (David Kopf Instruments) with the bite bar set at −11 mm. To record SNA that targets the vasculature, the left greater splanchnic sympathetic nerve was isolated retroperitoneally, placed on two silver wires (Teflon-coated and bared 250 μm at tips; A-M Systems), and covered with Kwik-Sil (World Precision Instruments). The splanchnic sympathetic nerve was chosen because evoked increases in vascular resistance are greatest in the mesenteric vascular bed (compared to renal and hindquarter regions) with acute infusion of phenylephrine (49) or glutamatergic activation of the RVLM (65). For some rats, the right sciatic nerve was exposed, placed over the bared tips of two Teflon-coated sliver wires, and coated with Kwik-Sil. The wires were connected to an isolated stimulator (A330; World Precision Instruments) to elicit the somatic pressor reflex (47, 55). Incisions for implantation of catheters and silver wires around nerves were closed with wound clips. For experiments with microinjections into the brain stem, a partial occipital craniotomy was performed to expose the dorsal surface of the brain stem caudal to the cerebellum, as previously described (21, 55). For experiments with stimulation of the spinal cord, a dorsal midline incision was made to expose the spinal column. The dorsal processes of lower thoracic vertebrae were clamped to slightly elevate the rat and provide tension to minimize ventilatory movements and to allow for insertion of the stimulating electrode between two vertebrae. After excision of the disc and overlying dura mater, a bipolar stimulating electrode was placed in the dorsolateral funiculus between the first and second thoracic vertebrae. The spinal region was coated in mineral oil to prevent desiccation.
After surgical procedures were completed, isoflurane anesthesia was replaced by urethane (1.5 g/kg of LZR body weight in 1.5 g/5 ml, 50 μl/min iv, in age-matched OZR and LZR) to facilitate observation of reflexes (20, 21, 52). Once anesthetized with urethane, rats were allowed to recover for 30–45 min. After confirmation of an adequate level of anesthesia (<10 mmHg change in MAP to firm toe pinch), the neuromuscular blocker, pancuronium, was administered (1 mg/kg iv; supplemented hourly at 1/3 the dose). Rectal temperature was maintained at 37°C (TC-1000; CWE) throughout the experimental protocols.
Activation of sympathoexcitatory reflexes.
The somatic pressor reflex was evoked by a series of brief stimulations to the sciatic nerve. Similar to previous studies (47, 61), stimulations were delivered as 1-ms square-wave pulse trains of 5 s at 20 Hz using 5 intensities (100, 200, 400, 600, and 800 μA) with 2 min between stimulations. The nasopharyngeal reflex (simulated diving reflex) was evoked once by gently pushing 2 ml water followed by 8 ml air through the rostrally projecting tracheal catheter, which drained out of the nose (33, 55). A subset of these rats was given methylatropine (2 mg/kg iv) to block the parasympathetic effects on HR and allow isolated evaluation of the sympathetically mediated tachycardia. Because OZR have blunted baroreflexes that could enhance evoked responses (24, 50, 54), the responses to sciatic nerve stimulation were also evaluated in the absence of concomitant changes in MAP and HR. In addition to methylatropine, these rats were pretreated with the α-adrenergic receptor antagonist phentolamine (10 mg/kg iv) and the β-adrenergic receptor antagonist propranolol (5 mg/g iv) to prevent sympathetically mediated changes in MAP and HR.
Activation of the rostral ventrolateral medulla.
Although these sympathoexcitatory reflexes are initiated by different afferent nerves and conveyed through distinct central pathways, the somatic pressor reflex and the nasopharyngeal reflex both increase SNA and MAP by glutamatergic activation of the rostral ventrolateral medulla (RVLM) (26, 33). Therefore, we examined the ability of glutamate in the RVLM to increase SNA and MAP in OZR and LZR. The RVLM was located by mapping the region for maximal pressor responses to microinjections of glutamate (1 nmol in 50 nl) through glass pipettes (50 mm tip), as previously described (21, 50, 55). With the pipette tip angled 20° rostrally, a grid of sites separated by 0.2 mm were tested (1.6–2.0 mm lateral to the midline, 1.4–1.9 mm rostral to calamus scriptorius, and 2.3–2.9 mm ventral to the dorsal surface of the brain stem). Variables were allowed to return to baseline between injections (separated by ≥2 min). The most effective sites were usually located within 5–7 microinjections, and the optimal coordinates for evoking rises in MAP were 1.6 mm rostral to calamus scriptorius, 1.7 mm lateral from the midline, and 2.7 mm below the dorsal surface of the brain stem. Glutamate was injected bilaterally, and the microinjection site yielding the maximal pressor response on each side was used to calculate the average rise in MAP, HR, and SNA for each rat. The optimal site was marked by microinjection of green latex microspheres (5%, Lumiphore). After completion of the experimental protocol, the rats were perfused transcardially with PBS (250 ml, pH 7.4) followed by 4% formaldehyde (500 ml, pH 7.4). The brains were removed and stored in fixative for 48 h and then sectioned using a Vibratome (50-μm sections, coronal plane). The sections were mounted onto glass slides, and coverslips were applied with Krystalon. The microinjection sites were visualized via epifluorescence (BX40, Olympus) and were verified to be in the region of the RVLM, as previously described (30, 51). With the functional verification of the injection sites guided by stereotaxic coordinates, none were outside the region of presympathetic neurons of the RVLM (51).
Because sympathoexcitatory reflexes mediated by glutamatergic activation of the RVLM utilize different subtypes of ionotropic glutamate receptors (26, 63), we also examined changes in SNA, HR, and MAP by selective activation of N-methyl-d-aspartate (NMDA) receptors by microinjection of NMDA (10 pmol in 100 nl) or of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors by microinjection of AMPA (5 pmol in 100 nl). The doses for all three agonists were selected for their ability to increase SNA and MAP within the range of the magnitude of the evoked reflex responses. The selected doses were submaximal to allow for examination of changes in potency of glutamatergic activation within the operating range of the drug.
Spinally evoked changes in SNA, HR, and AP.
To examine whether exaggerated rises in SNA to activation of the RVLM in OZR could be explained by changes downstream of the RVLM at the intermediolateral cell column or sympathetic ganglia, descending axons were directly stimulated from the dorsolateral funiculus of the upper thoracic spinal cord. A single-pulse stimulation (1 ms at 0.5 Hz, for 50, 100, and 300 μA) was used to evoke a brief rise in SNA that did not alter MAP or HR (1). Baseline SNA (full-wave rectified voltage) was recorded for 30 s, and 0.2 ms after the stimulation was delivered, the response was measured (50 s for 50 μA, 70 ms for 100 μA, and 100 ms for 300 μA). This method of analysis allowed for examination of the entire sympathetic burst, taking into account the large range of conduction velocities of presympathetic RVLM neurons (51). Trains of stimulation (5-s train of 1-ms pulses of 300 μA at 10, 20, 50, 75, and 100 Hz with 2-min between trains) were used to evoke sympathetically mediated rises in MAP and HR (1). Baseline values were recorded for 30 s, and the immediate, maximal increases in MAP and HR following each stimulus were measured.
Data analysis.
Amplifiers and filters from the Neurolog system (Digitimer) were used to quantify AP, MAP, and HR. The HR was triggered from the rising phase of the AP pulse (Spike trigger, Neurolog). The raw SNA was amplified and filtered (10-Hz to 3-kHz, 60-Hz notch filter, differential AC amplifier 1700, A-M Systems). Analog physiological variables were converted to digital signals [Micro 1401; Cambridge Electronic Design (CED)] and viewed online (Spike2 software, CED). To analyze percent changes in SNA, the filtered signal was full-wave rectified and integrated into 1-s bins. Baseline integrated SNA was recorded for 30–60 s before each stimulus and was set at 100% (see individual protocols for timing). The minimum SNA (0%) was determined after ganglionic blockade with mecamylamine (10 mg/kg; Sigma) at the end of each experiment. Voltage changes in SNA with spinal cord stimulation were calculated using full-wave-rectified values after subtraction of voltage due to noise seen after ganglionic blockade. Values are expressed as X change from baseline voltage (53). Values in LZR and OZR were compared by unpaired t-tests or two-way ANOVA with repeated measures followed by Tukey-Kramer post hoc tests when significant F values were observed. Significant differences were reported when P < 0.05.
RESULTS
In juvenile age-matched rats (7.3 ± 0.2 wk old), OZR had a significantly greater body weight compared with LZR, but MAP and HR were comparable (Table 1), as previously reported (38, 52). In adult age-matched rats (14.9 ± 0.3-wk-old LZR and 15.6 ± 0.3-wk-old OZR), OZR weighed significantly more than the LZR and had a higher baseline MAP (Table 1), as previously reported. (21, 38)
Table 1.
Baseline values for juvenile and adult obese Zucker rats and lean Zucker rats
| Group | n | Age, days | Weight, g | MAP, mmHg | HR, bpm |
|---|---|---|---|---|---|
| Juvenile | |||||
| LZR | 13 | 51 ± 1 | 202 ± 6 | 111 ± 3 | 444 ± 9 |
| OZR | 13 | 49 ± 1 | 272 ± 7* | 112 ± 3 | 442 ± 6 |
| Adult | |||||
| LZR | 56 | 106 ± 2 | 376 ± 6 | 112 ± 2 | 425 ± 4 |
| OZR | 47 | 111 ± 2 | 585 ± 8* | 123 ± 3* | 428 ± 4 |
Values are expressed as means ± SE.
P < 0.05 compared to lean Zucker rats (LZR) at that age. OZR, obese Zucker rats; MAP, mean arterial pressure; HR, heart rate.
Effects of sciatic nerve stimulation on SNA, MAP, and HR in adult Zucker rats.
Stimulation of the sciatic nerve evoked brief, intensity-dependent rises in SNA, MAP, and HR in both LZR and OZR (Figs. 1 and 2). The rises in SNA and MAP were significantly larger in OZR compared with LZR (Fig. 2, A and C). In contrast, the rises in HR were comparable in LZR and OZR (Fig. 2B), suggesting the larger pressor response is due to enhanced increases in sympathetic vasomotor tone in OZR.
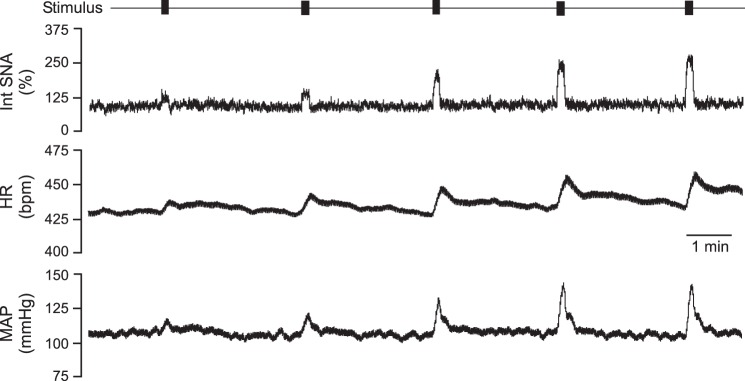
Fig. 1.
Representative tracings from an obese Zucker rat undergoing stimulation of the sciatic nerve. Bars across the top indicate each train of stimulation (1-ms pulses at 17 Hz for 5 s at 100, 200, 400, 600, and 800 μA), along with intensity-dependent, evoked rises in integrated sympathetic nerve activity (Int SNA), heart rate (HR), and mean arterial pressure (MAP).
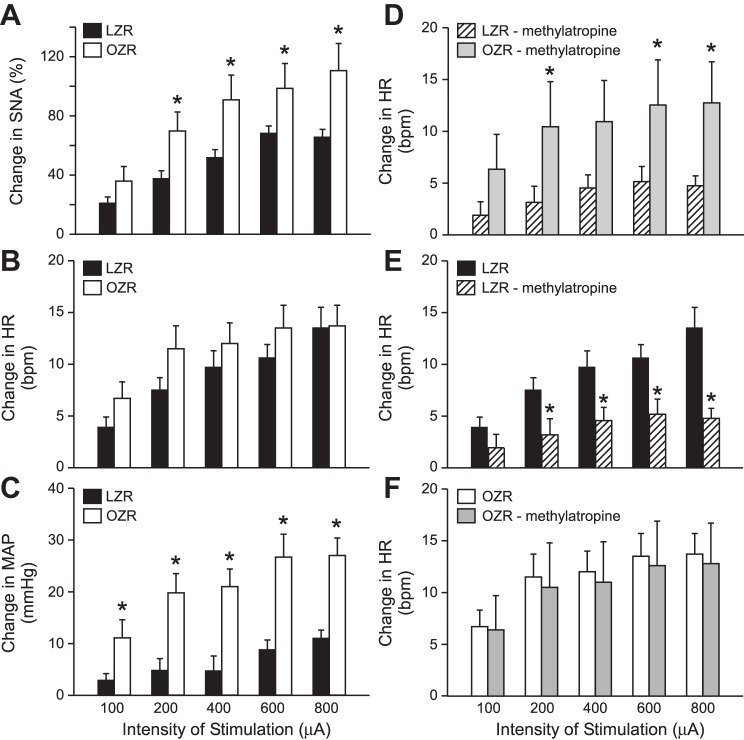
Fig. 2.
Summary data for stimulation of sciatic nerve in obese Zucker rats (OZR) and lean Zucker rats (LZR). Left: stimulation of the sciatic nerve evoked greater rises in sympathetic nerve activity (SNA; A) and MAP (C) in OZR (n = 13) compared with LZR (n = 12), with comparable rises in HR (B). Right: after treatment with methylatropine, stimulation of the sciatic nerve evoked greater rises in HR (D) in OZR (n = 5) compared with LZR (n = 5). Treatment with atropine reduced the evoked rises in HR in LZR (E), but had no effect on the evoked rises in HR in OZR (F). *P > 0.05 compared with untreated LZR at that stimulation intensity.
After elimination of parasympathetic influence on HR by pretreatment with methylatropine, stimulation of the sciatic nerve evoked exaggerated rises in HR in OZR compared with LZR (Fig. 2D), suggesting an enhanced sympathetically mediated rise in HR in OZR. This pretreatment reduced sciatic stimulation-induced rises in HR in LZR (Fig. 2E), illustrating the vagal withdrawal that normally contributes to the evoked rise in HR (64). The pretreatment did not alter the sciatic stimulation-mediated rises in HR in the OZR (Fig. 2F), suggesting minimal contribution of the parasympathetic nervous system to the evoked rise in HR in OZR.
Because differences in baroreflexes and the tonic influence of baroreceptor afferents under resting conditions in adult LZR and OZR could affect the magnitude of the sympathetic response to stimulation of the sciatic nerve (20, 21, 50), a subset of rats were also pretreated with phentolamine (to antagonize α-adrenergic receptors) and propranolol (to antagonize β-adrenergic receptors). These treatments eliminated sympathetically mediated vasoconstriction and sympathetic tone to the heart. The baseline MAP was reduced to 52 ± 6 mmHg in LZR and 60 ± 12 mmHg in OZR, which is below threshold for tonic activity of baroreceptor afferent nerves in Zucker rats (∼80 mmHg) (20). In addition, this pretreatment prevented sciatic nerve stimulation-mediated changes in MAP and HR to eliminate baroreceptor feedback (Fig. 3A). This pretreatment raised baseline SNA slightly more in LZR than OZR (48 ± 4% and 37 ± 4%, respectively, P < 0.05), similar to our previous observations in Zucker rats with nitroprusside-induced hypotension (52). Under these conditions, stimulation of the sciatic nerve evoked enhanced rises in SNA in adult OZR compared with LZR (Fig. 3B), suggesting the exaggerated sympathoexcitatory response occurs independent of impaired baroreflexes in the OZR. In a few rats (5/12), MAP drifted upward throughout the protocol (see Fig. 3A), perhaps due to release of vasopressin over time with repeated stimulation of the sciatic nerve (9).
Fig. 3.
Stimulation of sciatic nerve in OZR (n = 6) and LZR (n = 6) after antagonism of α- and β-adrenergic receptors. Left: After treatment, stimulation of the sciatic nerve evoked intensity-dependent rises in SNA but did not alter HR or MAP. Right: After treatment, stimulation of the sciatic nerve evoked enhanced rises in SNA in OZR compared with LZR. *P <0.05 compared with LZR at that stimulation intensity.
Effects of nasopharyngeal stimulation on SNA, HR, and MAP in adult Zucker rats.
In the LZR, passing water through the nasal cavity to simulate the diving or nasopharyngeal reflex evoked the expected rises in SNA and MAP along with bradycardia (Fig. 4, A and C–E) (33, 41). In the OZR, the rises in SNA and MAP were significantly larger compared with those observed in the LZR (Fig. 4, B, C, and E), and the bradycardia observed in the LZR was reversed to a tachycardia in the OZR (Fig. 4, B and D). Pretreatment with methylatropine did not significantly alter the rises in SNA and MAP in OZR or LZR compared with untreated rats (Fig. 4, C and E vs. F and H). As seen with sciatic nerve stimulation, pretreatment with methylatropine did not alter the nasopharyngeal reflex-induced tachycardia in OZR (Fig. 4, D and G), suggesting minimal vagal activation with the nasopharyngeal reflex in these rats. However, the bradycardia normally observed in the LZR was reversed to a tachycardia (Fig. 4G), uncovering the sympathetic component of the vagal-sympathetic coactivation that occurs with the diving reflex (41). In the absence of parasympathetic control of HR, activation of the nasopharyngeal reflex evoked a larger tachycardia in OZR compared with LZR (Fig. 4G). In addition, as seen with sciatic nerve stimulation, after pretreatment with phentolamine and propranolol to eliminate changes in HR and MAP, activation of the nasopharyngeal reflex produced enhanced rises in SNA in OZR (32 ± 4%) compared with LZR (19 ± 4%, P < 0.05).
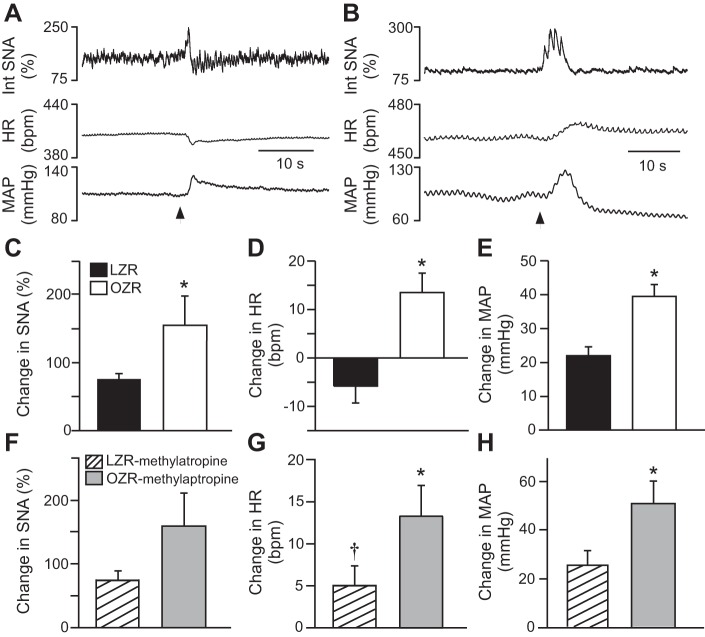
Fig. 4.
Nasopharyngeal reflex in OZR (n = 7) and LZR (n = 7). Activation of this reflex produced brief rises in SNA and MAP with a bradycardia in a lean Zucker rat (A), and rises in all three measures in an obese Zucker rat (B). The rises in SNA and MAP were larger in OZR than in LZR (C and E), and the HR responses were in opposite directions with a significant decrease in LZR and significant rises in the OZR. Pretreatment with methylatropine did not alter the rises in SNA (F) or MAP (H) in LZR (n = 5) or OZR (n = 5), but eliminated the bradycardia in the LZR (G). *P <0.05 compared with LZR.
Effects of activation of the RVLM on SNA, HR, and MAP in adult Zucker rats.
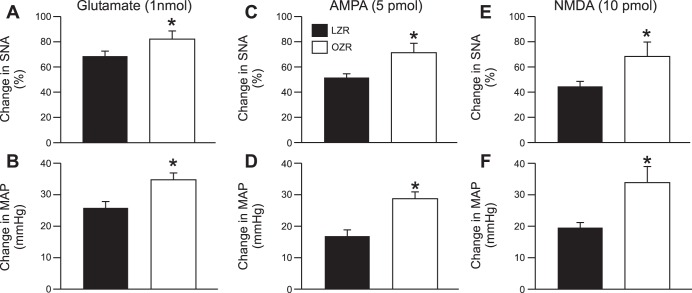
Microinjections of glutamate in the RVLM evoked enhanced rises in SNA and MAP in adult OZR compared with LZR (Fig. 5, A and B). Likewise, activation of AMPA or NMDA ionotropic glutamate receptors in the RVLM also produced exaggerated rises in SNA and MAP in adult OZR compared with LZR (Fig. 5, C–F). Changes in HR with microinjections were minimal in OZR and LZR and were not different for any of the agonists (data not shown).
Fig. 5.
Activation of glutamate receptors in the rostral ventrolateral medulla (RVLM). A and B: microinjections of glutamate into the RVLM evoked larger rises in SNA and MAP in the OZR (n = 18) compared with the LZR (n = 18). C and D: microinjections of AMPA into the RVLM evoked larger rises in SNA and MAP in the OZR (n = 9) compared with the LZR (n = 9). E and F: microinjections of NMDA into the RVLM evoked larger rises in SNA and MAP in the OZR (n = 9) compared with the LZR (n = 7). *P <0.05 compared with LZR.
Effects of spinal cord stimulation on SNA, HR, and MAP in adult Zucker rats.
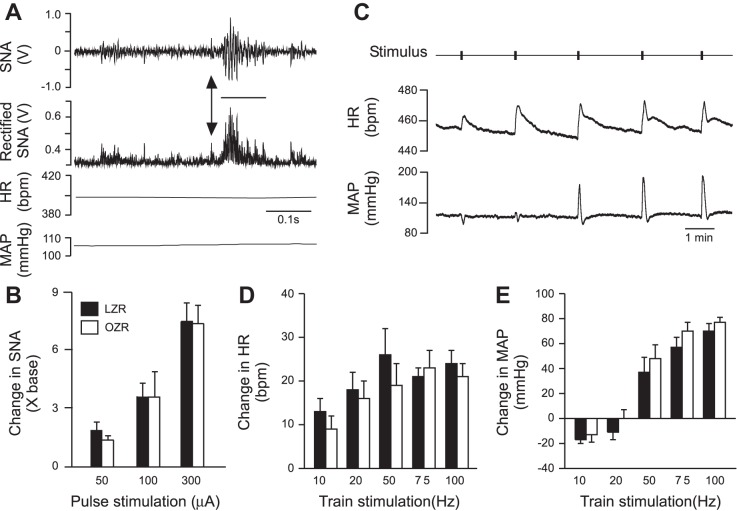
Single-pulse stimulation of an upper thoracic dorsolateral funiculus evoked a reliable burst of activity in the ipsilateral splanchnic nerve without altering HR or MAP (Fig. 6A). The evoked rises in SNA were intensity-dependent with no differences observed between OZR and LZR (Fig. 6B). Trains of stimulation-evoked rises in HR at all frequencies examined with no differences between OZR and LZR (Fig. 6, C and D). Trains of stimulation evoked small depressor responses or no change in MAP at the two lowest frequencies (10 and 20 Hz), and pressor responses at the three higher frequencies (50, 75, and 100 Hz) with no differences between LZR and OZR at any frequency examined (Fig. 6, B and E). The SNA was not quantified in the rats utilizing train stimulation due to stimulation artifact in some of the recordings.
Fig. 6.
Electrical activation of the dorsolateral funiculus of the upper thoracic spinal cord. A: typical example of pulse stimulation of the spinal cord of an obese Zucker rat using 300 mA, which caused a brief burst in SNA and no change in HR or MAP. B. Pulse stimulation of the spinal cord produced equivalent rises in SNA in OZR and LZR. C: typical example of train stimulation in a lean Zucker rat showing rises in HR and MAP. D: train stimulation evoked comparable rises in HR in LZR and OZR. E: train stimulation evoked reductions in MAP at lower frequencies and rises in MAP at higher frequencies that were all comparable in LZR and OZR.
Effects of sciatic nerve stimulation or activation of the RVLM on SNA, MAP, and HR in juvenile Zucker rats.
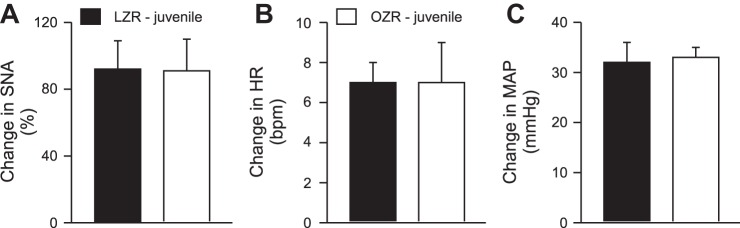
As observed in adult Zucker rats, stimulation of the sciatic nerve in juvenile Zucker rats evoked rises in SNA, HR, and MAP in all animals examined (Fig. 7). In contrast to the differences observed in the adult rats (Fig. 2), the evoked rises in SNA, HR, and MAP were not different in juvenile LZR compared with juvenile OZR (Fig. 7). Likewise, microinjections of glutamate in the RVLM evoked rises in SNA, HR, and MAP that were comparable in juvenile OZR and LZR (Fig. 8).
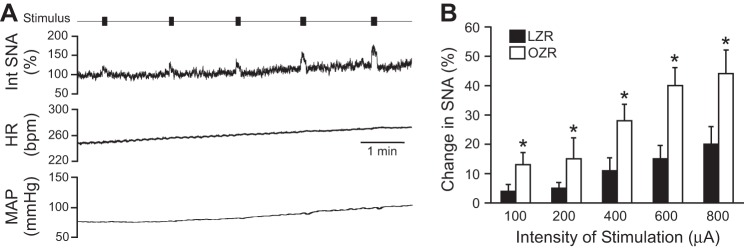
Fig. 7.
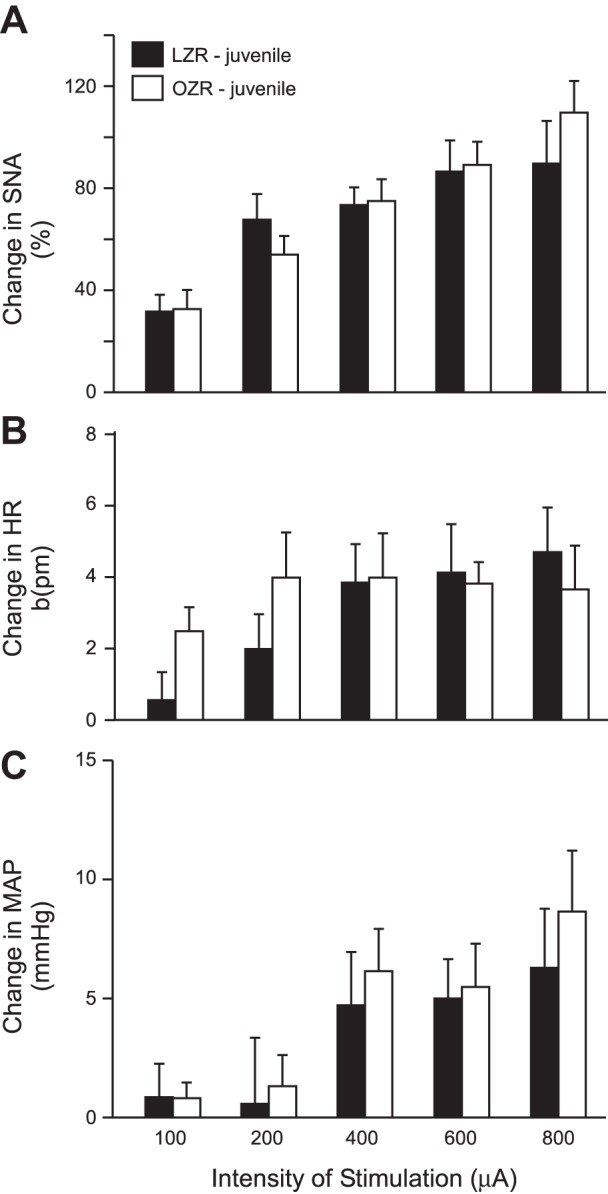
Stimulation of the sciatic nerve in juvenile LZR (n = 7) and OZR (n = 7) evoked rises in SNA (A), HR (B), and MAP (C) that were not different between groups. See baseline values in Table 1.
Fig. 8.
Activation of the RVLM by microinjections of glutamate in juvenile LZR (n = 6) and OZR (n = 6) evoked rises in SNA (A), HR (B), and MAP (C) that were not different between groups. See baseline values in Table 1.
DISCUSSION
Obesity and its accompanying metabolic syndrome are strongly associated with deleterious changes in the autonomic regulation of cardiovascular function. As seen in obese humans and animal models of obesity, adult OZR develop hypertension and impaired baroreflex-mediated control of SNA and HR (5, 16, 34, 44, 52, 60). Likewise, other stimuli that activate vagal afferents to the NTS, such as phenylbiguanide, also evoke smaller reductions in SNA in OZR (20). In contrast, stimulation of the sciatic nerve evokes enhanced rises in MAP and HR in OZR compared with LZR (47). Our novel findings are that rises in HR and MAP produced by stimulation of the sciatic nerve or nasopharyngeal reflex were accompanied by augmented activation of splanchnic SNA in adult male OZR. These larger rises in SNA persisted in the absence of changes in HR and MAP and tonic regulation by baroreceptor afferent nerves. Exaggerated reflex-mediated rises in HR were also observed in OZR after blockade of parasympathetic inputs to the heart. Glutamatergic activation of the RVLM, the site of convergence for these reflexes (26, 32, 61), yielded larger rises in SNA and MAP in OZR. However, activation of descending axons in the upper thoracic spinal cord evoked equivalent rises in SNA, HR, and MAP in adult OZR and LZR. In contrast to differences observed in the adult rats, activation of these reflexes or the RVLM produced comparable responses in juvenile OZR and LZR. These data suggest adult OZR develop exaggerated reflex-mediated rises in MAP and HR that are the result of augmented sympathoactivation to the heart and vasculature. Furthermore, enhanced sympathoexcitatory reflexes in adult OZR are not simply a consequence of impaired baroreflexes and likely involve enhanced activation of the RVLM by glutamate.
Stimulation of the sciatic nerve at intensities sufficient to activate nociceptive fibers evokes rises in SNA, HR, and MAP that are referred to as the somatic pressor reflex or the somatosympathetic reflex (26, 37, 61). In agreement with a previous report (47), stimulation of the sciatic nerve evoked larger rises in MAP in OZR. The present study also showed enhanced activation of splanchnic SNA, which raises MAP by increasing mesenteric vascular resistance (65). Activation of the nasopharyngeal reflex also elicited larger rises in splanchnic SNA and MAP in OZR compared with LZR. After treatment with methylatropine to block parasympathetic control of HR, the reflexes evoked larger rises in HR in OZR. These data suggest a generalized enhancement of reflexes that stimulate SNA to the heart and vasculature in adult OZR.
Although both reflexes elicit a robust sympathetic activation of the heart, they produce distinct parasympathetic effects upon HR. Stimulation of the sciatic nerve increases HR by a simultaneous activation of SNA and vagal withdrawal (64). Accordingly, in the present study, blockade of parasympathetic control of HR reduced the sciatic stimulation-induced tachycardia in adult LZR. However, in OZR, pretreatment with methylatropine did not alter the magnitude of the tachycardic response to activation of the somatosympathetic reflex. Impaired parasympathetic regulation of HR in OZR was also apparent with the nasopharyngeal reflex. In this case, stimulation of the anterior ethmoidal nerve evokes a simultaneous activation of both sympathetic and parasympathetic nerves to the heart (41, 48). As seen in adult LZR, this coactivation normally results in a bradycardia (41, 48), due to the dominance of vagal inputs upon HR. After blockade of parasympathetic inputs to the heart in LZR, the nasopharyngeal reflex evoked a tachycardia produced by unopposed sympathetic activation of HR. In contrast, activation of the nasopharyngeal reflex in OZR evoked a tachycardia that was not changed by pretreatment with methylatropine. These observations suggest an absence of significant reflexively mediated vagal responses in either direction with activation of sympathoexcitatory reflexes in the OZR, leaving sympathetic control of HR unchecked. Diminished parasympathetic control of HR has also been reported in obese dogs and humans (23, 66). Whether reduced parasympathetic responses in OZR occur by blunted changes in vagal efferent nerve activity or a reduced response of the heart to cholinergic inputs cannot be determined by the present study. However, regardless of the site of the deficit in OZR, the present study highlights the importance of considering parasympathetic contributions to reflex-mediated rises in HR.
Several observations strongly suggest that changes within the RVLM underlie the exaggerated rises in SNA evoked by the somatosympathetic and nasopharyngeal reflexes in adult OZR. These reflexes are initiated by different afferent nerves with distinct central pathways that converge at the RVLM (40, 69). Although correlative, in the OZR, the development of these enhanced sympathoexcitatory reflexes coincided with the emergence of heightened physiological responses to activation of the RVLM by glutamate, but comparable responses to stimulation of their axons in the thoracic spinal cord. In contrast to sympathoinhibitory reflexes, such as the baroreflex, that reduce SNA by GABAergic inhibition of the RVLM, activation of these sympathoexcitatory reflexes requires glutamatergic activation of the RVLM to raise SNA. Antagonism of ionotropic glutamate receptors in the RVLM minimizes or eliminates the rises in SNA and MAP evoked by the somatosympathetic and nasopharyngeal reflexes (4, 32, 61). Blockade of non-NMDA receptors, but not NMDA receptors in the RVLM, antagonizes the somatosympathetic reflex (26), but the subtype of glutamatergic receptor responsible for mediating the nasopharyngeal reflex is not known. Regardless, activation of the RVLM by NMDA or AMPA evoked larger rises in SNA and MAP in OZR, suggesting sympathoexcitatory responses that involve glutamatergic activation of the RVLM are likely to be enhanced in OZR.
A shift in the balance of tonic inhibitory and excitatory inputs to presympathetic RVLM neurons may underlie the enhanced ability of glutamate in the RVLM to increase SNA and MAP in adult OZR. Tonic GABAergic inhibition of the RVLM is reduced in adult OZR compared with the LZR (21), in conjunction with impaired baroreflex-mediated sympathoinhibition. The somatosympathetic reflex and nasopharyngeal reflex activate presympathetic RVLM neurons that are also inhibited by stimulation of baroreceptors, suggesting changes in baroreflex efficacy could affect the magnitude of sympathoexcitatory reflexes (32, 37). Reduced influence by baroreceptor afferents or the NTS may enhance the excitability of presympathetic RVLM neurons in OZR. However, in the absence of tonic influence by baroreceptors, the exaggerated sympathoexcitatory reflexes also persisted, suggesting baroreflexes may modulate the sympathoexcitatory reflexes but cannot fully explain the larger rises in SNA and MAP evoked by activation of the somatosympathetic and nasopharyngeal reflexes. Alternatively, the enhanced tonic activation of the RVLM by ANG II in OZR may augment activation by glutamate (21), because ANG II increases excitability of presympathetic RVLM neurons by reducing a resting potassium conductance (27). Further study is needed to clarify whether these changes foster enhanced glutamatergic activation in the RVLM and sympathoexcitatory reflexes in OZR.
Hypertension may contribute to the augmented rises in SNA evoked by glutamatergic activation of the RVLM and sympathoexcitatory reflexes, because these attributes are present in other rodent models of hypertension that do not exhibit metabolic syndrome. Spontaneously hypertensive rats (SHR) have reduced tonic GABAergic inhibition and elevated tonic angiotensinergic activation of the RVLM with augmented RVLM-driven SNA (31, 57), and SHR display exaggerated somatosympathetic reflexes independent of their impaired baroreflexes (58). Similarly, Sprague-Dawley rats exposed to chronic intermittent hypoxia develop elevated SNA and MAP with enhanced sympathoexcitatory reflexes and physiological responses to glutamate in the RVLM (55). The emergence of impaired baroreflexes in obese rats precedes the onset of hypertension (14, 52), but the time course for development of augmented glutamatergic activation of RVLM and sympathoexcitatory reflexes in relation to these other two deficits is not known. Whether or not hypertension causes enhanced sympathoexcitatory reflexes, elevated MAP likely exacerbates altered autonomic reflexes once it is established.
Obesity-induced changes in the levels and efficacy of circulating hormones are also likely to contribute to altered autonomic reflexes. However, examining the effects of these hormones in subjects of normal body weight or by short-term infusion may misrepresent their contributions. Furthermore, mechanisms underlying elevated basal SNA and MAP may not be the same as those that alter reflex-mediated changes in SNA and MAP. For example, corticosterone is elevated in OZR, and glucocorticoid receptor antagonism with mifepristone reduces MAP in OZR, whereas treatment with corticosterone raises MAP in LZR (8). However, 1 wk of dexamethasone treatment in Sprague-Dawley rats does not affect the somatosympathetic reflex (59). These data suggest that activation of glucocorticoid receptors promotes hypertension but not exaggerated sympathoexcitatory reflexes. However, whether activation of glucocorticoid receptors alters the somatosympathetic reflex in the obese rats is not known. Similarly, insulin levels are elevated in OZR, and insulin stimulates primarily SNA via an NMDA-dependent activation of the RVLM in rats of normal body weight (3, 47), but insulin has little effect upon MAP, in part, due to its vasodilatory properties (2, 5). The ability of insulin to raise SNA is also greatly impacted by other obesity-related attributes. For example, chronic activation of glucocorticoid receptors with dexamethasone prevents insulin-induced activation of lumbar SNA (59). In rats of normal body weight, acute infusion of insulin does not affect baroreceptor-mediated inhibition of lumbar SNA and actually enhances hypotension-induced rises in SNA (42). In contrast, 12 days of elevated insulin impairs baroreflex-mediated inhibition of splanchnic SNA, suggesting chronic hyperinsulinemia can diminish this sympathoinhibitory reflex in normotensive, euglycemic rats (6). Similarly, the impact of insulin upon sympathoexcitatory reflexes is not well understood. Augmented sympathoexcitatory reflexes are present in OZR with insulin resistance (47) and in humans with Type 2 diabetes (18), but the relative roles of elevated insulin and glucose are not known. These studies highlight the necessity of examining mechanisms that regulate basal states and reflexes independently, using long-term treatments to mimic chronic state changes and determining the impact of circulating factors upon reflexes within the milieu of metabolic syndrome.
Circulating leptin is elevated with obesity, and leptin raises SNA and MAP by activation of central melanocortin receptors (10). In normal-weight subjects, acute infusion of leptin can enhance, inhibit, or have no effect on baroreflexes depending on the site of administration and dose (7, 28). Furthermore, the impact of leptin upon sympathoexcitatory reflexes is not known. Because OZR do not have functional leptin receptors, leptin does not play a role in their development of hypertension or aberrant autonomic reflexes. The leptin receptor mutation itself cannot explain their deficits, because juvenile OZR have normal MAP, baroreflexes, and sympathoexcitatory reflexes (38, 52). Furthermore, the striking similarities between OZR and Sprague-Dawley rats and rabbits made obese by a high-fat diet (HFD) suggests their deficits arise by common mechanisms that do not require leptin. Both of these obese rat models develop elevated RVLM-driven SNA (21, 60) and hypertension that is dependent upon enhanced activation of central melanocortin receptors (11, 13). Both obesity models develop impaired baroreflex-mediated inhibition of SNA and HR by central mechanisms (17, 20, 34). In addition, other sympathoinhibitory reflexes that are initiated by vagal afferents to the NTS are also diminished in both obesity models. In rats made obese by a HFD, injection of cholecystokinin or expansion of blood volume evoke smaller reductions in SNA (19, 25), reminiscent of blunted phenylbiguanide-induced sympathoinhibition in OZR (20). In both models, the NTS is a key central site for the impairment of vagal sympathoinhibitory reflexes (17, 19). Thus, in obese subjects, leptin likely contributes to the rises in basal SNA and MAP, but in the absence of leptin, other obesity-related factors are capable of activating central melanocortin receptors to raise SNA and MAP (11). Furthermore, there is no clear evidence to support a role for leptin in producing the pattern of altered autonomic reflexes that develop with obesity.
In summary, adult OZR develop exaggerated sympathoexcitatory reflexes that impact SNA to the vasculature and heart to raise MAP and HR, akin to augmented sympathoexcitatory responses seen in humans with obesity and Type 2 diabetes (18, 23). These physiological effects are amplified by a concomitant dampening of parasympathetic control of HR (14, 23). Exaggerated sympathoexcitatory reflexes do not emerge in OZR until adulthood, coincident with the development of heightened responses to glutamate in the RVLM. Although adult OZR simultaneously develop impaired sympathoinhibitory reflexes, blunted baroreflexes are not necessary for the production of exaggerated sympathoexcitatory reflexes. These disparate deficits in autonomic regulation of cardiovascular function in OZR appear to occur by distinct mechanisms within different regions of the brain (17, Fig. 5), highlighting the complexity of the impact of metabolic syndrome upon neural control of cardiovascular function.
Perspectives and Significance
The stability of MAP is greatly affected by the short-term control of cardiovascular function by autonomic reflexes, including those that are tonically active, such as baroreflexes, and those that occur periodically, such as somatosympathetic reflexes. Heightened sympathoexcitatory reflexes can be present in the absence of impaired baroreflexes, and increased variability of MAP occurs with either condition (36, 56). These increased fluctuations in MAP, which can be gauged by the standard deviation of systolic blood pressure readings over the course of a day or a year, predicts detrimental cardiovascular outcomes independently of the mean blood pressure over that time period (45, 46, 68). Patients with successfully managed hypertension who have residual elevated MAP variability are still at increased risk for cardiovascular disease and stroke (46). Similar findings have been reported in animal studies. Increased MAP variability produced by chronic baroreceptor deafferentation in rats produces end-organ damage in the absence of hypertension (35, 62, 67). In SHR, the extent of end-organ damage to heart, kidneys, and aorta is more closely related to blood pressure variability than absolute MAP levels (36, 62), and organ protection with pharmacological antihypertensive treatments is strongly associated with a reduction in blood pressure variability (12, 29). With metabolic syndrome, even when hypertension is modest as seen in OZR, short-term regulation of MAP by autonomic reflexes is clearly disrupted. Furthering our understanding of causative attributes and underlying central mechanisms for changes in autonomic reflexes will provide a road map for treatments to improve cardiovascular health and reduce the premature morbidity and mortality associated with obesity.
GRANTS
This study was funded by grants from the Heart, Lung, and Blood Institute of the National Institutes of Health (R01HL086759 to AMS) and the American Heart Association (14GRNT18880005 to AMS).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.A.H. and A.M.S. conception and design of research; D.A.H. and A.M.S. performed experiments; D.A.H. and A.M.S. analyzed data; D.A.H. and A.M.S. interpreted results of experiments; D.A.H. and A.M.S. prepared figures; D.A.H. drafted manuscript; D.A.H. and A.M.S. edited and revised manuscript; D.A.H. and A.M.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors wish to thank Dr. Daniel Mandel and Dr. David W. Stepp for their valuable support and contributions for this project. Dr. Domitila Huber is currently an Assistant Professor in the Department of Physiological Sciences at the Federal University of Santa Catarina, Florianopolis, SC, Brazil.
REFERENCES
- 1.Adams JM, McCarthy JJ, Stocker SD. Excess dietary salt alters angiotensinergic regulation of neurons in the rostral ventrolateral medulla. Hypertension 52: 932–937, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson EA, Hoffman RP, Balon TW, Sinkey CA, Mark AL. Hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. J Clin Invest 87: 2246–2252, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bardgett ME, McCarthy JJ, Stocker SD. Glutamatergic receptor activation in the rostral ventrolateral medulla mediates the sympathoexcitatory response to hyperinsulinemia. Hypertension 55: 284–290, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bardgett ME, Sharpe AL, Toney GM. Activation of corticotropin-releasing factor receptors in the rostral ventrolateral medulla is required for glucose-induced sympathoexcitation. Am J Physiol Endocrinol Metab 307: E944–E953, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barringer DL, Buñag RD. Uneven blunting of chronotropic baroreflexes in obese Zucker rats. Am J Physiol Heart Circ Physiol 256: H417–H421, 1989. [DOI] [PubMed] [Google Scholar]
- 6.Buñag RD, Krizsan-Agbas D, Itoh H. Sympathetic activation by chronic insulin treatment in conscious rats. J Pharmacol Exp Ther 259: 131–138, 1991. [PubMed] [Google Scholar]
- 7.Ciriello J. Leptin in nucleus of the solitary tract alters the cardiovascular responses to aortic baroreceptor activation. Peptides 44: 1–7, 2013. [DOI] [PubMed] [Google Scholar]
- 8.Clapham JC, Turner NC. Effects of the glucocorticoid II receptor antagonist mifepristone on hypertension in the obese Zucker rat. J Pharmacol Exp Ther 282: 1503–1507, 1997. [PubMed] [Google Scholar]
- 9.Day TA, Sibbald JR. Noxious somatic stimuli excite neurosecretory vasopressin cells via A1 cell group. Am J Physiol Regul Integr Comp Physiol 258: R1516–R1520, 1990. [DOI] [PubMed] [Google Scholar]
- 10.do Carmo JM, da Silva AA, Dubinion J, Sessums PO, Ebaady SH, Wang Z, Hall JE. Control of metabolic and cardiovascular function by the leptin-brain melanocortin pathway. IUBMB Life 65: 692–698, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.do Carmo JM, da Silva AA, Rushing JS, Hall JE. Activation of the central melanocortin system contributes to the increased arterial pressure in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol 302: R561–R567, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Du WM, Miao CY, Liu JG, Shen FM, Yang XQ, Su DF. Effects of long-term treatment with ketanserin on blood pressure variability and end-organ damage in spontaneously hypertensive rats. J Cardiovasc Pharmacol 41: 233–239, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Dubinion JH, da Silva AA, Hall JE. Enhanced blood pressure and appetite responses to chronic central melanocortin-3/4 receptor blockade in dietary-induced obesity. J Hypertens 28: 1466–1470, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El-Wazir YM, Li SG, Smith R, Silox DL, Brown DR, Randall DC. Parasympathetic response to acute stress is attenuated in young Zucker obese rats. Auton Neurosci 143: 33–39, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fellmann L, Nascimento AR, Tibirica E, Bouquet P. Murine models for pharmacological studies of the metabolic syndrome. Pharmacol Ther 137: 331–340, 2013. [DOI] [PubMed] [Google Scholar]
- 16.Grassi G, Seravalle G, Dell'Oro R, Turri C, Bolla GB, Mancia G. Adrenergic and reflex abnormalities in obesity-related hypertension. Hypertension 36: 538–542, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Guimaraes PS, Huber DA, Campagnole-Santos MJ, Schreihofer AM. Development of attenuated baroreflexes in obese Zucker rats coincides with impaired activation of nucleus tractus solitarius. Am J Physiol Regul Integr Comp Physiol 306: R681–R692, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holwerda SW, Restaino RM, Manrique C, Lastra G, Fisher JP, Fadel PJ. Augmented pressor and sympathetic responses to skeletal muscle metaboreflex activation in type 2 diabetes patients. Am J Physiol Heart Circ Physiol 310: H300–H309, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.How JM, Wardak SA, Ameer SI, Davey RA, Sartor DM. Blunted sympathoinhibitory responses in obesity-related hypertension are due to aberrant central but not peripheral signalling mechanisms. J Physiol 592: 1705–1720, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huber DA, Schreihofer AM. Attenuated baroreflex control of sympathetic nerve activity in obese Zucker rats by central mechanisms. J Physiol 588: 1515–1525, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huber DA, Schreihofer AM. Altered regulation of the rostral ventrolateral medulla in hypertensive obese Zucker rats. Am J Physiol Heart Circ Physiol 301: H230–H240, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iida M, Murakami T, Ishida K, Mizumo A, Kuwajima M, Sharma K. Substitution at codon 269 (glutamine proline) of the leptin receptor (OB-R) cDNA is the only mutation found in the Zucker fatty (fa/fa) rat. Biochem Biophys Res Commun 224: 597–604, 1996. [DOI] [PubMed] [Google Scholar]
- 23.Indumathy J, Pal GK, Pal P, Ananthanarayanan PH, Parija SC, Balachander J, Dutta TK. Association of sympathovagal imbalance with obesity indices, and abnormal metabolic biomarkers and cardiovascular parameters. Obes Res Clin Pract 9: 55–66, 2015. [DOI] [PubMed] [Google Scholar]
- 24.Kawabe T, Kawabe K, Sapru HN. Cardiovascular responses to somatosensory stimulation and their modulation by baroreflex mechanisms. Clin Exp Hypertens 29: 403–418, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Khan SA, Sattar MZ, Abdullah NA, Rathore HA, Abdulla MH, Ahmad A, Johns EJ. Obesity depresses baroreflex control of renal sympathetic nerve activity and heart rate in Sprague-Dawley rats: role of the renal denervation. Acta Physiol 214: 390–401, 2015. [DOI] [PubMed] [Google Scholar]
- 26.Kiely JM, Gordon FJ. Non-NMDA receptors in the rostral ventrolateral medulla mediate somatosympathetic pressor responses. J Auton Nerv Syst 43: 231–239, 1993. [DOI] [PubMed] [Google Scholar]
- 27.Li YW, Guyenet PG. Angiotensin II decreases a resting K+ conductance in rat bulbospinal neurons of the C1 area. Circ Res 78: 274–282, 1996. [DOI] [PubMed] [Google Scholar]
- 28.Li B, Shi Z, Cassaglia PA, Brooks VL. Leptin acts in the forebrain to differentially influence baroreflex control of lumbar, renal, and splanchnic sympathetic nerve activity and heart rate. Hypertension 61: 812–19, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu ZA, Xie HH, Xu LP, Yin AF, Miao CY, Su DF. Restoration of arterial baroreflex function contributes to organ protection in spontaneously hypertensive rats treated with long-term hydrochlorothiazide mixture. Clin Exp Pharmacol Physiol 30: 49–54, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Mandel DA, Schreihofer AM. Glutamatergic inputs to the CVLM independent of the NTS promote tonic inhibition of sympathetic vasomotor tone in rats. Am J Physiol Heart Circ Physiol 295: H1772–H1779, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsuura T, Kumagai H, Kawai A, Onimaru H, Imai M, Oshima N, Sakata K, Saruta T. Rostral ventrolateral medulla neurons of neonatal Wistar-Kyoto and spontaneously hypertensive rats. Hypertension 40: 560–565, 2002. [DOI] [PubMed] [Google Scholar]
- 32.McCulloch PF, Panneton WM, Guyenet PG. The rostral ventrolateral medulla mediates the sympathoactivation produced by chemical stimulation of the rat nasal mucosa. J Physiol 516: 471–484, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCulloch PF, West NH. Cardiovascular responses to nasal water flow in rats are unaffected by chemoreceptor drive. Am J Physiol Regul Integr Comp Physiol 263: R1049–R1056, 1992. [DOI] [PubMed] [Google Scholar]
- 34.McCully BH, Brooks VL, Andresen MC. Diet-induced obesity severely impairs. Myelinated aortic baroreflex. Am J Physiol Heart Circ Physiol 302: H2083–H2091, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miao CY, Su DF. The importance of blood pressure variability in rat aortic and left ventricular hypertrophy produced by sinoaortic denervation. J Hypertens 20: 1865–1872, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Miao CY, Xie HH, Zhan LS, Su DF. Blood pressure variability is more important than blood pressure level in determination of end-organ damage in rats. J Hypertens 24: 1125–1135, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Morrison SF, Reis DJ. Reticulospinal vasomotor neurons in the RVL mediate the somatosympathetic reflex. Am J Physiol Regul Integr Comp Physiol 256: R1084–R1097, 1989. [DOI] [PubMed] [Google Scholar]
- 38.Osmond JM, Mintz JD, Dalton B, Stepp DW. Obesity increases blood pressure, cerebral vascular remodeling, and severity of stroke in the Zucker rat. Hypertension 53: 381–386, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Overton JM, Williams TD, Chambers JB, Rashotte ME. Cardiovascular and metabolic responses to fasting and thermoneutrality are conserved in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol 280: R1007–R1015, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Panneton WM, McCulloch PM, Sun W. Trigemino-autonomic connections in the muskrat: the neural substrate for the diving response. Brain Res 874: 48–65, 2000. [DOI] [PubMed] [Google Scholar]
- 41.Paton JF, Boscan P, Pickering AE, Nalivaiko E. The yin and yang of cardiac autonomic control: Vago-sympathetic interactions revisited. Brain Res Brain Res Rev 49: 555–565, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Pritcher MP, Freeman KL, Brooks VL. Insulin in the brain increases gain of baroreflex control of heart rate and lumbar sympathetic nerve activity. Hypertension 51: 512–520, 2008. [DOI] [PubMed] [Google Scholar]
- 43.Reuter S, Mrowka R. The metabolic syndrome: the future is now. Acta Physiol 214: 291–294, 2015. [DOI] [PubMed] [Google Scholar]
- 44.Rocchini AP, Mao HZ, Babu K, Marker P, Rocchini AJ. Clonidine prevents insulin resistance and hypertension in obese dogs. Hypertension 33: 548–533, 1999. [DOI] [PubMed] [Google Scholar]
- 45.Rothwell PM, Howard SC, Dolan E, O'Brien E, Dobson JE, Dahlöf B, Sever PS, Poulter NR. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet 375: 895–905, 2010. [DOI] [PubMed] [Google Scholar]
- 46.Rothwell PM. Does blood pressure variability modulate cardiovascular risk? Curr Hypertens Rep 13: 177–186, 2011. [DOI] [PubMed] [Google Scholar]
- 47.Ruggeri P, Brunori A, Cogo CE, Storace D, DiNardo F, Burattini R. Enhanced sympathetic reactivity associates with insulin resistance in the young Zucker rat. Am J Physiol Regul Integr Comp Physiol 291: R376–R382, 2006. [DOI] [PubMed] [Google Scholar]
- 48.Rybka EJ, McCulloch PF. The anterior ethmoidal nerve is necessary for the initiation of the nasopharyngeal response in the rat. Brain Res 1075: 122–132, 2006. [DOI] [PubMed] [Google Scholar]
- 49.Schreihofer AM, Hair CD, Stepp DW. Reduced plasma volume and mesenteric vascular reactivity in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol 288: R253–R261, 2005. [DOI] [PubMed] [Google Scholar]
- 50.Schreihofer AM, Ito S, Sved AF. Brain stem control of arterial pressure in chronic arterial baroreceptor-denervated rats. Am J Physiol Regul Integr Comp Physiol 289: R1746–R1755, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Schreihofer AM, Guyenet PG. Identification of C1 presympathetic neurons in the rat rostral ventrolateral medulla by juxtacellular labeling in vivo. J Comp Neurol 387: 524–536, 1997. [DOI] [PubMed] [Google Scholar]
- 52.Schreihofer AM, Mandel DA, Mobley SC, Stepp DW. Impairment of sympathetic baroreceptor reflexes in obese Zucker rats. Am J Physiol Heart Circ Physiol 293: H2543–H2549, 2007. [DOI] [PubMed] [Google Scholar]
- 53.Schreihofer AM, Stornetta RL, Guyenet PG. Regulation of sympathetic tone and arterial pressure by rostral ventrolateral medulla after depletion of C1 cells in rat. J Physiol 529: 221–236, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schreihofer AM, Sved AF. Use of sinoaortic denervation to study the role of baroreceptors in cardiovascular regulation. Am J Physiol Regul Integr Comp Physiol 266: R1705–R1710, 1994. [DOI] [PubMed] [Google Scholar]
- 55.Silva AQ, Schreihofer AM. Altered sympathetic reflexes and vascular reactivity in rats after exposure to chronic intermittent hypoxia. J Physiol 589: 1463–1476, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Simmonds SS, Lay J, Stocker SD. Dietary salt intake exaggerates sympathetic reflexes and increases blood pressure variability in normotensive rats. Hypertension 64: 583–589, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith JK, Barron KW. Cardiovascular effects of l-glutamate and tetrodotoxin microinjected into the rostral and caudal ventrolateral medulla in normotensive and spontaneously hypertensive rats. Brain Res 506: 1–8, 1990. [PubMed] [Google Scholar]
- 58.Smith SA, Williams MA, Leal AK, Mitchell JH, Garry MG. Exercise pressor reflex function is altered in spontaneously hypertensive rats. J Physiol 577: 1009–1020, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Steiner JL, Bardgett ME, Wolfgang L, Lang CH, Stocker SD. Glucocorticoids attenuate the central sympathoexcitatory actions of insulin. J Neurophysiol 112: 2597–2604, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stocker SD, Meador R, Adams JM. Neurons of the rostral ventrolateral medulla contribute to obesity-induced hypertension in rats. Hypertension 49: 640–646, 2007. [DOI] [PubMed] [Google Scholar]
- 61.Stornetta RL, Morrison SF, Ruggiero DA, Reis DJ. Neurons of rostral ventrolateral medulla mediate somatic pressor reflex. Am J Physiol Regul Integr Comp Physiol 256: R48–R462, 1989. [DOI] [PubMed] [Google Scholar]
- 62.Su DF, Miao CY. Blood pressure variability and organ damage. Clin Exp Pharmacol Physiol 28: 709–715, 2001. [DOI] [PubMed] [Google Scholar]
- 63.Sun MK, Reis DJ. NMDA receptor-mediated sympathetic chemoreflex excitation of RVL-spinal vasomotor neurones in rats. J Physiol 482: 53–68, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Terui N, Koizumi K. Responses of cardiac vagus and sympathetic nerves to excitation of somatic and visceral nerves. J Auton Nerv Syst 10: 73–91, 1984. [DOI] [PubMed] [Google Scholar]
- 65.Willette RN, Punnen-Grandy S, Krieger AJ, Sapru HN. Differential regulation of regional vascular resistance by the rostral and caudal ventrolateral medulla in the rat. J Auton Nerv Syst 18: 143–151, 1987. [DOI] [PubMed] [Google Scholar]
- 66.Van Vliet BN, Hall JE, Mizelle HL, Montani JP, Smith MJ Jr. Reduced parasympathetic control of heart rate in obese dogs. Am J Physiol Heart Circ Physiol 269: H629–H637, 1995. [DOI] [PubMed] [Google Scholar]
- 67.Van Vliet BN, Hu L, Scott T, Café L, Montani JP. Cardiac hypertrophy and telemetered blood pressure 6 wk after baroreceptor denervation in normotensive rats. Am J Physiol Regul Integr Comp Physiol 271: R1759–R1769, 1996. [DOI] [PubMed] [Google Scholar]
- 68.Vishram JK, Dahlöf B, Devereux RB, Ibsen H, Kjeldsen SE, Lindholm LH, Mancia G, Okin PM, Rothwell PM, Wachtell K, Olsen MH. Blood pressure variability predicts cardiovascular events independently of traditional cardiovascular risk factors and target organ damage: a LIFE substudy. J Hypertens 33: 2422–2430, 2015. [DOI] [PubMed] [Google Scholar]
- 69.Yu YG, Caous CA, Balan AC, Rae GA, Lindsey CJ. Cardiovascular responses to sciatic nerve stimulation are blocked by paratrigeminal nucleus lesion. Auton Neurosci 98: 70–74, 2002. [DOI] [PubMed] [Google Scholar]