Abstract
The hypothalamus is responsible for the control of many of our physiological responses, including energy homeostasis. Of interest, there are a number of instances of sexual dimorphism documented with regard to metabolic processes. This review will discuss the necessity of utilizing both male and female models when studying the mechanisms underlying energy homeostasis, particularly those originating at the level of the hypothalamus. Because obesity often results in central neuroinflammation, we describe markers that could be used to study differences between male and female models, both the whole organism and also at the cellular level. Our laboratory has generated a wide array of immortalized hypothalamic cell models, originating from male and female rodents that we suggest could be beneficial for these types of studies. It is imperative that both sexes are considered before any recommendations for therapeutic interventions are considered.
Keywords: sexual dymorphism, cell biology, gene expression, saturated fatty acid, neuroinflammation, hypothalamus
obesity has become a global epidemic because of its rising prevalence worldwide and is associated with severe consequences, including diabetes mellitus, cardiovascular disease, metabolic syndrome, and cancer (15). Although debated, much of this increase is attributed to diet and nutrition, giving rise to diet-induced obesity (15). The complications of obesity are well-documented in males and less studied in females. Males become insulin resistant in diet-induced obese states, whereas premenopausal women remain more insulin sensitive (29). Because of the sexually dimorphic nature of many disease processes, granting agencies now call for the inclusion of males and females in studies (9). Considering the existence of a sexual dimorphism in the mechanisms and control of energy balance in males and females (16, 29), it is important to study both male and female humans and rodents in obesity-related studies.
Sexual Dimorphism in Peripheral Tissues
Males and females in an obese state deposit fat differently (23). Females tend to have more subcutaneous fat, whereas males have more visceral fat (16, 23). In addition to having more visceral fat, males have increased lipolysis of this specific type of fat (1). Adler et al. (1) have shown in rats that there are increased neuronal projections to the visceral adipose tissue in males and to the subcutaneous adipose tissue in females, thereby resulting in differential sex-dependent lipolysis. Estrogens play a role in this phenomena by increasing the sympathetic nerve activity to these fat depots (1).
Inflammatory cytokines and free fatty acids increase hepatic glucose production and hyperinsulinemia, essentially leading to insulin resistance (27). Lipolysis of visceral fat is a source of increased circulating levels of cytokines and free fatty acids (27). Considering the male-specific accumulation of visceral fats, the increased level of pro-inflammatory cytokines and fatty acids may explain why males become insulin resistant much quicker than females. In addition, leptin levels are higher in females compared to males independent of adipocyte mass (13). These differences may contribute to the sex-dependent susceptibility to comorbidities of diet-induced obesity seen in humans and rodent models (15, 16, 29).
Sexually Dimorphic Hypothalamic Control of Energy Balance
The primary control of energy balance occurs in the hypothalamus, where regions including the ventromedial area (VMH), paraventricular nucleus (PVN), and the arcuate nucleus (ARC) modulate feeding and energy expenditure (17, 34). In the ARC, peripheral signals, such as leptin, ghrelin, and insulin, activate or inhibit orexigenic neuropeptide Y/Agouti-related peptide (NPY/AgRP) and anorexigenic proopiomelanocortin (POMC) neurons to control energy homeostasis. Dysregulation of this system has been shown to lead to obesity and further metabolic perturbations (17). Evidence of sexual dimorphism in the hypothalamic regions involved in energy balance exist (3, 34). Deletion of estrogen receptor-α (ERα) from steroidogenic factor-1 neurons of the VMH led to abdominal obesity, whereas deletion of ERα in POMC neurons lead to hyperphagia specifically in female mice (23, 34). Furthermore, specific populations of POMC and 5-HT2cR coexpressing neurons in the ARC seem to modulate energy intake and insulin sensitivity in both males and females, while only contributing to improved physical activity and energy expenditure in males (3).
Diet-induced obesity due to consumption of a high-fat diet gives rise to dysregulation of energy balance by increasing circulating levels of fatty acids and pro-inflammatory cytokines (10, 20). Hyperlipidemia and inflammation induce insulin resistance and decreased glucose sensitivity in the periphery. More importantly, a high-fat diet in rodents has been shown to change the expression of hypothalamic feeding related neuropeptides (NPY and POMC) (10, 20). In fact, the hypothalamus plays a critical and primary role in mediating the inflammatory effects of diet-induced obesity as markers of hypothalamic neuroinflammation increase before changes in the periphery (19, 28). Neuroinflammatory cytokine expression is induced in neuronal cells as well as glial cells, giving rise to low-grade chronic inflammation in the hypothalamus (10). This low-grade chronic inflammation is suggested to lead to cellular insulin resistance (10), mediating the aberrant response of specific populations of hypothalamic neurons to the increased levels of insulin after a meal.
The hypothalamus of female rodents does not respond to a high-fat diet in the same way as male rodents. Upon a 20-wk high-fat diet in males, there is a dysregulation in neuropeptide levels and an induction of inflammatory cytokines (21). Morselli et al. (21) found that despite similar weight gain, a 20-week high-fat diet leads to the accumulation of higher amounts of palmitic acid in male hypothalamii. Palmitic acid is a 16-carbon saturated fatty acid, the most abundant fatty acid in the diet. This increase in palmitic acid was also accompanied by increased levels of metabolites of palmitate, including ceramides and sphingomyelins, in male, but not in female brains. In addition, induction of the inflammatory cytokines IL6, TNF-α, and IL-1β was only seen in male mice (21). Functionally, these hypothalamic changes were associated with decreased myocardial function that was also only seen in male mice. This sexually dimorphic response to a high-fat diet in male mice may mediate differences in the development of insulin resistance and comorbidities seen with diet-induced obesity.
Role of Hormones
The observation that premenopausal women are at a lower risk for obesity-related comorbidities and that postmenopause the occurrence of metabolic disorders increase significantly in women, point to the potential role of estrogens in the sexually dimorphic regulation of energy balance (4). Estrogen treatment has been shown to downregulate inflammatory markers in the hypothalamus and in adipose tissue (13). Activation of astrocytes by estrogen led to increased leptin sensitivity, ameliorating the resistance to leptin seen in obesity (13). In addition, as females have increased circulating levels of leptin, the anorexigenic effects of leptin in the arcuate nucleus of the hypothalamus may be more prominent in females (13). Progesterone and androgens have also been shown to mediate some of these effects (6, 23).
However, more important than levels of circulating estrogens in the protection against obesity-related complications may be the expression of the estrogen receptor ERα. In fact, Morselli et al. (21), in the above-mentioned study, illustrate the role of ERα in mediating the sex-dependent effects seen with a high-fat diet. Palmitate exposure in neurons and astrocytes led to a decrease in ERα in a peroxisome proliferator-activated receptor gamma, coactivator-1α (PCG-1α)-dependent manner, leading to reduced inflammation (21). This finding supported previous studies showing that ERα is required to induce estrogen-mediated protection against metabolic disorders and to reduce inflammatory cytokine production (13, 21, 30). Thus reduced levels of ERα in male hypothalamii as a result of high-fat diets may induce hypothalamic inflammation and the resulting insulin resistance seen in males, but not in females. This begs the question of whether it is only the hormonal milieu that is responsible for diet-induced changes in ERα expression or whether it is the intrinsic nature of male cells that lead to the sex-dependent effect.
Hypothalamic Cell Models
In vitro studies are necessary to study these potential intrinsic differences in male and female hypothalamic cells. Particularly, in terms of the hypothalamus, as it is structurally heterogeneous, it is difficult to study responses of individual cell populations in vivo (33). Furthermore, in vitro mechanistic studies are essential to study biochemical and signaling pathways of compounds (26), such as palmitate. Primary culture of hypothalamic neurons prove ineffective due to the difficulty of culturing, their limited lifespan, and their heterogeneous composition. The need for immortalized hypothalamic cell lines representing populations of individual neuronal subtypes is evident. The Belsham lab has generated over 250 neuronal cell models to date, both clonal and nonclonal in composition (33). Clonal cell lines consist of both embryonic and adult models. These were generated by immortalization with SV40 large T-antigen as described previously (33). Briefly, upon hypothalamic extraction and primary culture, cells were incubated with a vector containing SV40 T-antigen and a neomycin resistance gene. For the adult-derived cells, before incubation with the vector, cells were treated with ciliary neurotrophic factor (2), which induces neuronal proliferation as SV40 T-antigen will only incorporate into actively dividing cells. Immortalized cells were selected using geneticin and subcloned to create individual populations of genetically identical cells (2, 33). We have previously reported differences between the embryonic-derived versus the adult-derived male NPY-expressing cell lines (11). Thus we would expect that there may also be unique characteristics of the male versus female cell lines in terms of mechanisms for sensing hormones and nutrients (Fig. 1). Currently, we have a number of models expressing key feeding-related neuropeptides from both the male and female mouse and rat, both from embryonic- and adult-derived primary hypothalamic cultures.
Fig. 1.
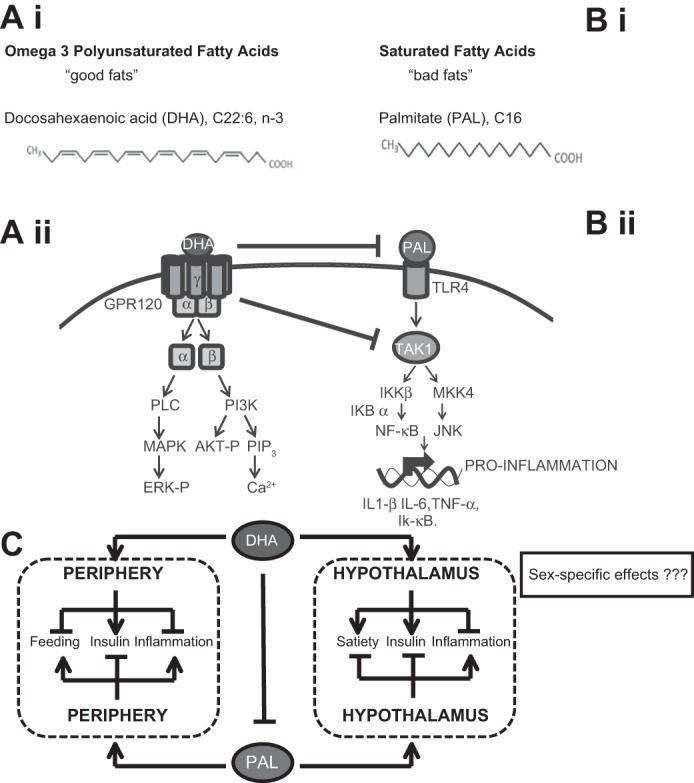
Potential effects of polyunsaturated versus saturated long chain fatty acids. Ai: structure of doxahexanoic acid (DHA). Aii: stimulation of the omega-3 fatty acid receptor GPR120 activates mitogen-activated protein kinase (MAPK) and PI3K. Bi: structure of palmitate (PAL). Bii: PAL activates Toll-like receptor 4 (TLR4) and initiates inhibitor of κB kinase (IKK) and mitogen-activated protein kinase kinase 4 (MKK4) activity. C: omega-3 fatty acid consumption promotes satiety, enhances insulin signaling, and reduces inflammation in the periphery and central nervous system. Palmitate impedes insulin signaling, promotes inflammation, and enhances feeding. Despite the clear connection between nutrients and neuronal function, little is currently known about how fatty acids affect hypothalamic neurons in a sex-specific manner.
Nonclonal or mixed population cell lines are all adult derived. These were created by extracting the hypothalamii of mice expressing enhanced green fluorescent protein (eGFP) under the control of a neuron-specific promoter, such as the POMC, NPY, or gonadotropin-releasing hormone (GnRH) promoter. Upon immortalization with SV40 T-antigen and selection, cells were sorted with fluorescence-activated cell sorting to obtain the entire immortalized population of POMC, NPY, or GnRH-expressing cells in the hypothalamus (22, 33). These cell lines have neuronal characteristics, including the expression of neuronal markers, neuronal morphology, and secretory properties. They have been shown to express specific neuropeptides and receptors and respond to hormonal stimulation, making them functional models to study mechanisms underlying feeding, reproduction, circadian rhythms, and metabolic disorders. More importantly, male and female models from embryonic and adult hypothalamii are available to study sex-specific differences in cells with respect to feeding and obesity (33).
Nutrient-Sensing Mechanisms in Hypothalamic Cell Models
Some of these cell lines have been used to delineate hormone- and nutrient-sensing mechanisms in specific cell types from the hypothalamus by our group and others. A number of studies have demonstrated leptin and insulin signaling mechanisms (5, 18, 22, 24, 31). Previous research has shown that these cell lines sense nutrients and are able to respond to glucose, amino acids, and palmitate (33). Glucose-sensing mechanisms have been reported in specific Agouti-related peptide (AgRP) and POMC models (5, 7), whereas the embryonic mouse hypothalamus cell line 46 (mHypoE-46) cell line has been shown to sense amino acid fluctuations (8). Palmitate is enriched in high-fat diets, can cross the blood-brain barrier, and become elevated up to concentrations of 300 μM in obese states (25). Consistent with what is seen with a high-fat diet, 100 μM or 50 μM of palmitate has been shown to induce expression of inflammatory cytokines IL-1β, TNF-α, and IK-κB in rat (rHypoE-7) and murine (mHypoE-46) embryonic male-derived NPY/AgRP cell lines (J. A. Chalmers and D. D. Belsham, unpublished observations). NPY and AgRP are orexigenic neuropeptides that increase feeding and are reduced with insulin and leptin signaling (17). Exposure to palmitate for 12 to 33 h leads to increased expression of NPY and AgRP in the mHypoE-46 line, despite being in a nutrient-rich state (J. A. Chalmers and D. D. Belsham, unpublished observations). Fick et al. (12) have also shown that exposure to palmitate for 21 h, but not for 1 or 9 h, directly increases NPY expression in the mHypoE-44 cell line. Discrepancies between changes in neuropeptide levels in cell lines compared with mouse models following palmitate or high-fat diet exposure may be attributed to length of exposure, composition of the high-fat diet, and characteristics of individual cell populations. The effect of palmitate in inducing inflammation and neuropeptide dysregulation is at least partially mediated by the inhibitor of κB kinase (IKK)-β/nuclear factor-κB (NF-κB) pathway. Upstream events include binding of palmitate to Toll-like receptor 4, thereby activating IKK-β, leading to the translocation of NF-κB into the nucleus and the transcriptional regulation of proinflammatory mediators. As previously stated, this event seems to be a crucial mediator of dysregulation of energy balance as hypothalamic inflammation occurs before changes in the inflammatory and insulin-sensing states of peripheral tissues and body weight (19, 28). In contrast, the polyunsaturated fatty acid doxahexanoic acid (DHA) normalizes the increase in inflammatory cytokines seen with palmitate treatment and decreases AgRP neuropeptide levels (32). These effects of DHA are thought to be mediated through G protein-coupled receptor GPR120, which signals through P13K and mitogen-activated protein kinase (MAPK) pathways, which in turn may inhibit the IKK-β/NF-κB pathway, leading to the anti-inflammatory effects of DHA (32).
Gondatrophin-releasing hormone (GnRH) neurons in the hypothalamus coordinate reproductive function (28). Tran et al. (28a) determined whether a GnRH neuronal cell model is able to sense free fatty acids. One hundred micrograms of palmitate or DHA induced GnRH mRNA expression at 2 h in the mHypoA-GnRH/GFP neurons, a female-derived cell line. As in the rHypoE-7 cell line, the effect of DHA was shown to be mediated through GPR120 and downstream PI3K or MAPK signaling by using small molecule inhibitors. However, the effect of palmitate on GnRH mRNA expression is independent of TLR4 and was thought to occur through metabolism to palmitoyl-coA synthesis and P13K signaling. Furthermore, DHA, in contrast to the rHypoE-7 cell line, was shown to induce inflammatory cytokines in the mHypoA-GnRH/GFP cell line (28). Whether these unique findings are due to the specific cell type or whether these are a result of the female origins of the cell line is unknown, but represents a potential sex-dependent difference in the mechanisms through which free fatty acids signal in hypothalamic neurons.
Sexual Dimorphism in Cell Models
To study sex differences in the response of feeding-related hypothalamic neurons to palmitate, the mHypoA-2/12 and mHypoA-59 cells, an adult, male and adult, female-derived NPY/AgRP cell line respectively, were used. Neuropeptide and inflammatory cytokine gene expression was determined at 4 and 16 h after 50 μM palmitate exposure. NPY expression decreased in both cell lines at 4 h, as would be expected following food intake. However, at 16 h, representing prolonged exposure to palmitate, female cells were resistant to palmitate-induced increase of AgRP expression seen in male cells (N. Loganathan and D. D. Belsham, unpublished data). This points to a dysregulation in the control of energy metabolism at the hypothalamic cellular level in males and not in females, a finding supporting the above-mentioned in vivo studies. Furthermore, the male-derived mHypoA-2/12 cells respond to palmitate with a greater increase in proinflammatory cytokines TNF-α and IL-1β at 16 h compared with the mHypoA-59 cell line (N. Loganathan and D. D. Belsham, unpublished data). The mechanisms behind this intrinsic sex-dependent cellular difference to palmitate exposure is currently being studied. Future experiments warrant investigation into the involvement of ERα in modulating this difference as suggested in vivo.
Clearly, there appears to be an intrinsic difference in male- and female-derived cell lines in how they respond to dietary fatty acids, suggesting the hormonal environment does not entirely explain why premenopausal women are protected against metabolic syndrome. Male embryos are generally larger than female embryos before the effect of sex hormones (6), suggesting hormone-independent mediators of weight gain and energy metabolism. In fact, Chen et al. (6) report that the number of X chromosomes regardless of type of gonad had effects on food intake, weight gain, and hyperinsulinemia in response to a high-fat diet. They suggest the role of the degree of X-chromosome inactivation on metabolic phenotype, particularly after menopause, with reduced levels of circulating estrogen (6, 14). Nevertheless, even without intrinsic differences in male- and female-derived cell lines, culture media containing hormones is often used during experiments, and unless this media is devoid of hormones, there may be differential responses depending on the sex of the cells (26).
Perspectives and Significance
Differences exist in male and female humans, rodents, and cell lines in nutrient sensing and the development of obesity and its associated comorbidities. We suggest that it is necessary to include both male and female humans, rodents, and cell lines when studying sexually dimorphic disease processes, especially those for which targeted therapies are being developed, as these differences should be considered before recommendations are prescribed.
GRANTS
This research was funded by the Natural Sciences and Engineering Research Council (NSERC), Canadian Institutes for Health Research (CIHR), and Canada Foundation for Innovation and Canada Research Chairs Program (DDB).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
N.L. performed experiments; N.L. and D.D.B. analyzed data; N.L. and D.D.B. interpreted results of experiments; N.L. drafted manuscript; N.L. and D.D.B. edited and revised manuscript; D.D.B. conception and design of research; D.D.B. prepared figures; D.D.B. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank all of the Belsham Lab members, past and present, who have contributed to the data presented in this review.
REFERENCES
- 1.Adler ES, Hollis JH, Clarke IJ, Grattan DR, Oldfield BJ. Neurochemical characterization and sexual dimorphism of projections from the brain to abdominal and subcutaneous white adipose tissue in the rat. J Neurosci 32: 15913–15921, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belsham DD, Fick LJ, Dalvi PS, Centeno ML, Chalmers JA, Lee PK, Wang Y, Drucker DJ, Koletar MM. Ciliary neurotrophic factor recruitment of glucagon-like peptide-1 mediates neurogenesis, allowing immortalization of adult murine hypothalamic neurons. FASEB J 23: 4256–4265, 2009. [DOI] [PubMed] [Google Scholar]
- 3.Burke LK, Doslikova B, D'Agostino G, Greenwald-Yarnell M, Georgescu T, Chianese R, Martinez de Morentin PB, Ogunnowo-Bada E, Cansell C, Valencia-Torres L, Garfield AS, Apergis-Schoute J, Lam DD, Speakman JR, Rubinstein M, Low MJ, Rochford JJ, Myers MG, Evans ML, Heisler LK. Sex difference in physical activity, energy expenditure and obesity driven by a subpopulation of hypothalamic POMC neurons. Mol Metab 5: 245–252, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab 88: 2404–2411, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Chalmers JA, Jang JJ, Belsham DD. Glucose sensing mechanisms in hypothalamic cell models: glucose inhibition of AgRP synthesis and secretion. Mol Cell Endocrinol 382: 262–270, 2014. [DOI] [PubMed] [Google Scholar]
- 6.Chen X, McClusky R, Chen J, Beaven SW, Tontonoz P, Arnold AP, Reue K. The number of x chromosomes causes sex differences in adiposity in mice. PLoS Genet 8: e1002709, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng H, Isoda F, Belsham DD, Mobbs CV. Inhibition of agouti-related peptide expression by glucose in a clonal hypothalamic neuronal cell line is mediated by glycolysis, not oxidative phosphorylation. Endocrinology 149: 703–710, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Cheung MK, Gulati P, O'Rahilly S, Yeo GSH. FTO expression is regulated by availability of essential amino acids. Int J Obes 37: 744–747, 2013. [DOI] [PubMed] [Google Scholar]
- 9.Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature 509: 282–283, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Souza CT, Araujo EP, Bordin S, Ashimine R, Zollner RL, Boschero AC, Saad MJA, Velloso LA. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology 146: 4192–4199, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Dhillon SS, Gingerich S, Virtanen C, Belsham DD. Gene array analysis of embryonic- versus adult-derived hypothalamic NPY-expressing cell lines. Mol Cell Endocrinol 358: 116–126, 2012. [DOI] [PubMed] [Google Scholar]
- 12.Fick LJ, Fick GH, Belsham DD. Palmitate alters the rhythmic expression of molecular clock genes and orexigenic neuropeptide Y mRNA levels within immortalized, hypothalamic neurons. Biochem Biophys Res Commun 413: 414–419, 2011. [DOI] [PubMed] [Google Scholar]
- 13.Fuente-Martin E, Garcia-Caceres C, Morselli E, Clegg DJ, Chowen JA, Finan B, Brinton RD, Tschöp MH. Estrogen, astrocytes and the neuroendocrine control of metabolism. Rev Endocr Metab Disord 14: 331–338, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Link JC, Chen X, Arnold AP, Reue K. Metabolic impact of sex chromosomes. Adipocyte 2: 74–79, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lutz TA, Woods SC. Overview of animal models of obesity. Curr Protoc Pharmacol. doi: 10.1002/0471141755.ph0561s58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Macotela Y, Boucher J, Tran TT, Kahn CR. Sex and depot differences in adipocyte insulin sensitivity and glucose metabolism. Diabetes 58: 803–812, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev 34: 309–338, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Metlakunta AS, Sahu M, Yasukawa H, Dhillon SS, Belsham DD, Yoshimura A, Sahu A. Neuronal suppressor of cytokine signaling-3 deficiency enhances hypothalamic leptin-dependent phosphatidylinositol 3-kinase signaling. Am J Physiol Regul Integr Comp Physiol 300: R1185–R1193, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Milanski M, Arruda AP, Coope A, Ignacio-Souza LM, Nunez CE, Roman EA, Romanatto T, Pascoal LB, Caricilli AM, Torsoni MA, Prada PO, Saad MJ, Velloso LA. Inhibition of hypothalamic inflammation reverses diet-induced insulin resistance in the liver. Diabetes 61: 1455–1462, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milanski M, Degasperi G, Coope A, Morari J, Denis R, Cintra DE, Tsukumo DML, Anhe G, Amaral ME, Takahashi HK, Curi R, Oliveira HC, Carvalheira JBC, Bordin S, Saad MJ, Velloso LA. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J Neurosci 29: 359–370, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morselli E, Fuente-Martin E, Finan B, Kim M, Frank A, Garcia-Caceres C, Navas CR, Gordillo R, Neinast M, Kalainayakan SP, Li DL, Gao Y, Yi CX, Hahner L, Palmer BF, Tschöp MH, Clegg DJ. Hypothalamic PGC-1α protects against high-fat diet exposure by regulating ERα. Cell Reports 9: 633–645, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nazarians-Armavil A, Chalmers JA, Lee CB, Ye W, Belsham DD. Cellular insulin resistance disrupts hypothalamic mHypoA-POMC/GFP neuronal signaling pathways. J Endocrinol 220: 13–24, 2013. [DOI] [PubMed] [Google Scholar]
- 23.Palmer BF, Clegg DJ. The sexual dimorphism of obesity. Mol Cell Endocrinol 402: 113–119, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piessevaux J, Lavens D, Montoye T, Wauman J, Catteeuw D, Vandekerckhove J, Belsham D, Peelman F, Tavernier J. Functional cross-modulation between SOCS proteins can stimulate cytokine signaling. J Biol Chem 281: 32953–32966, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Sabin MA, De Hora M, Holly JMP, Hunt LP, Ford AL, Williams SR, Baker JS, Retallick CJ, Crowne EC, Shield JPH. Fasting nonesterified fatty acid profiles in childhood and their relationship with adiposity, insulin sensitivity, and lipid levels. Pediatrics 120: e1426–e1433, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Shah K, McCormack CE, Bradbury NA. Do you know the sex of your cells? Am J Physiol Cell Physiol 306: C3–C18, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med 371: 1131–1141, 2014. [DOI] [PubMed] [Google Scholar]
- 28.Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, Zhao X, Sarruf DA, Izgur V, Maravilla KR, Nguyen HT, Fischer JD, Matsen ME, Wisse BE, Morton GJ, Horvath TL, Baskin DG, Tschöp MH, Schwartz MW. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest 122: 153–162, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28a.Tran DQ, Ramos EH, Belsham DD. Induction of Gnrh mRNA expression by the ω-3 polyunsaturated fatty acid docosahexaenoic acid and the saturated fatty acid palmitate in a GnRH-synthesizing neuronal cell model, mHypoA-GnRH/GFP. Mol Cell Endocrinol 426: 125–135, 2016. [DOI] [PubMed] [Google Scholar]
- 29.Varlamov O, Bethea CL, Roberts CT Jr. Sex-specific differences in lipid and glucose metabolism. Front Endocrinol (Lausanne) 5: 241, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vegeto E, Belcredito S, Etteri S, Ghisletti S, Brusadelli A, Meda C, Krust A, Dupont S, Ciana P, Chambon P, Maggi A. Estrogen receptor-alpha mediates the brain antiinflammatory activity of estradiol. Proc Natl Acad Sci USA 100: 9614–9619, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wauman J, De Smet AS, Catteeuw D, Belsham D, Tavernier J. Insulin receptor substrate 4 couples the leptin receptor to multiple signaling pathways. Mol Endocrinol 22: 965–977, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wellhauser L, Belsham DD. Activation of the omega-3 fatty acid receptor GPR120 mediates anti-inflammatory actions in immortalized hypothalamic neurons. J Neuroinflammation 11: 60, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wellhauser L, Gojska NM, Belsham DD. Delineating the regulation of energy homeostasis using hypothalamic cell models. Front Neuroendocrinol 36: 130–149, 2015. [DOI] [PubMed] [Google Scholar]
- 34.Xu Y, Nedungadi TP, Zhu L, Sobhani N, Irani BG, Davis KE, Zhang X, Zou F, Gent LM, Hahner LD, Khan SA, Elias CF, Elmquist JK, Clegg DJ. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab 14: 453–465, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]


