Abstract
Leg thermotherapy (TT) application reduces blood pressure (BP) and increases both limb blood flow and circulating levels of anti-inflammatory mediators in healthy, young humans and animals. The purpose of the present study was to determine the impact of TT application using a water-circulating garment on leg and systemic hemodynamics and on the concentrations of circulating cytokines and vasoactive mediators in patients with symptomatic peripheral artery disease (PAD). Sixteen patients with PAD and intermittent claudication (age: 63 ± 9 yr) completed three experimental sessions in a randomized order: TT, control intervention, and one exercise testing session. The garment was perfused with 48°C water for 90 min in the TT session and with 33°C water in the control intervention. A subset of 10 patients also underwent a protocol for the measurement of blood flow in the popliteal artery during 90 min of TT using phase-contrast MRI. Compared with the control intervention, TT promoted a significant reduction in systolic (∼11 mmHg) and diastolic (∼6 mmHg) BP (P < 0.05) that persisted for nearly 2 h after the end of the treatment. The serum concentration of endothelin-1 (ET-1) was significantly lower 30 min after exposure to TT (Control: 2.3 ± 0.1 vs. TT: 1.9 ± 0.09 pg/ml, P = 0.026). In addition, TT induced a marked increase in peak blood flow velocity (∼68%), average velocity (∼76%), and average blood flow (∼102%) in the popliteal artery (P < 0.01). These findings indicate that TT is a practical and effective strategy to reduce BP and circulating ET-1 concentration and enhance leg blood flow in patients with PAD.
Keywords: intermittent claudication, thermotherapy, blood flow
peripheral artery disease (PAD) is a degenerative condition that affects one in ten 70-year olds and one in six people older than 80 years (19). The most common clinical presentation of PAD is intermittent claudication (IC), defined as pain in the leg caused by insufficient blood flow during walking. It is estimated that up to 40 million people worldwide suffer from IC (19). Current treatment for symptomatic PAD is based largely on two major goals: first, to reduce cardiovascular morbidity and mortality, and second, to alleviate the symptoms of exercise-induced muscle ischemia (6). The first is justified by the fact that patients with PAD have nearly doubled cardiovascular mortality rates compared with individuals without PAD (57). The latter stems from the notion that ischemic leg pain is the primary reason for the deterioration in the quality of life and functional impairment in these patients (43). Individuals with PAD have severely impaired leg function and lose their ability to walk and perform simple functional tasks at faster rates than individuals without the disease (45, 46). Few noninvasive, nonpharmacological, and widely accessible therapies that fulfill both treatment goals exist to treat PAD. Supervised exercise training in hospital settings is the primary and most effective treatment option for PAD (59). Unfortunately, the need for frequent visits to the few available clinical facilities over an extended time period and the presence of leg pain during the exercise bouts make this option challenging or not amenable for the vast majority of patients (55).
One emerging simple approach that has the potential to overcome these barriers is the use of thermotherapy (TT). Mounting evidence indicates that systemic or local mild heat stress evokes a number of beneficial physiological responses in healthy young individuals. First, as blood is diverted from the core to the periphery (i.e., skin) for effective core temperature regulation during hyperthermia, blood flow and wall shear-stress in major conduit arteries in the arms and legs are greatly increased (14). For instance, blood flow through the superficial femoral artery increases by nearly five-fold during mild whole body heat stress in healthy young subjects (12, 53). Second, the reduction in systemic vascular resistance during heat stress has been shown to induce a significant hypotensive effect in healthy individuals (12, 20, 22, 27, 31). Third, recent animal studies revealed that whole body heat stress elicits a temporary increase in the concentrations of anti-inflammatory cytokines and chemokines (e.g., IL-6 and IL-10) (7, 8, 62). Taken together, these hemodynamic and cytokine responses to TT could have potential therapeutic effects for patients with PAD, who are known to have severe peripheral vascular endothelial dysfunction (38), hypertension (34), and markedly increased systemic inflammation (9). Nonetheless, most of the aforementioned observations on the salutary effects of TT have been made in healthy, young humans and animals, and it is unclear whether similar effects occur in elderly patients with severe atherosclerosis and multiple comorbidities.
The purpose of the current study was to determine the effect of a single 90-min TT session on leg and systemic hemodynamics and on the concentrations of circulating cytokines and vasoactive mediators in patients with PAD and stable IC. A tube-lined, water-circulating garment that covered the legs, thighs, and buttocks was used for TT application. This strategy was chosen because, as opposed to other forms of TT, such as sauna or balneotherapy, water-circulating garments are portable and amenable for home-based application. On the basis of the available reports in healthy individuals, we hypothesized that TT would increase leg blood flow and the concentrations of anti-inflammatory cytokines and reduce blood pressure in patients with PAD.
METHODS
Subjects.
Sixteen patients with PAD, defined as an ankle-brachial index (ABI) below 0.9 in at least one leg and a history of stable IC, were recruited to participate in the study. All participants had significant aortoiliac or femoropopliteal disease, as established by noninvasive vascular imaging. Exclusion criteria included heart failure, chronic obstructive pulmonary disease, peripheral neuropathy, exercise-limiting comorbidities (e.g., angina and arthritis), and critical limb ischemia. As the protocol involved magnetic resonance imaging (MRI), patients with cardiovascular implants and devices (37) or other contraindications to MRI were not allowed to participate in the MRI visit. The study was approved by the Indiana University School of Medicine Institutional Review Board (no. 1409063619). All subjects provided written informed consent before participation.
Experimental design and general procedures.
Participants were asked to complete three experimental sessions, at least 72 h apart: 1) TT, 2) control, and 3) exercise testing. The order of the sessions was randomized. Individuals with no contraindications to MRI were asked to complete a fourth experimental session for the measurement of leg blood flow during exposure to TT. On all four sessions, participants were asked to have a light breakfast prior to the laboratory visits, to maintain their usual medication regimen, and to abstain from caffeine and alcohol for 12 h and from intense exercise for 24 h. Active smokers were asked to refrain from smoking for at least 4 h prior to the experiments. All experimental sessions were completed in the morning in a temperature-controlled room (22–24°C).
Thermotherapy and control interventions.
Participants received an ingestible core body temperature sensor (HQ, Palmetto, FL) and were asked to ingest it the night before the experiments (∼7–9 h before the experimental sessions) (63). Upon arriving at the laboratory, the subjects had their anthropometric characteristics (body weight and height) recorded. Four thermocouples (MLT422; ADInstruments, Colorado Springs, CO) were taped to the calf and thigh for measurement of mean leg skin temperature. Participants were asked to put on custom water-circulating trousers on top of underwear (Med-Eng, Ottawa, ON, Canada). To minimize evaporative heat loss during the interventions, participants wore polyvinyl chloride pants and had their legs covered with a thermal foil blanket. An intravenous catheter was placed in an antecubital vein for blood sampling. After instrumentation, participants were asked to rest in a semi-recumbent position for 30 min, after which the baseline blood sample was taken. The water-circulating garment was then connected to the heating circulator. In the TT trial, water at 48°C was perfused through the water-circulating garment with the goal to increase leg skin temperature to 39–40°C (27), while water at 33°C was used in the control intervention. After 90 min, the water circulator was turned off and the patients remained in the same position for another 2 h. Blood samples were taken 30 and 120 min after the completion of the interventions. Leg skin temperature and HR were recorded at 40 Hz using a data acquisition system (Powerlab and LabChart, ADInstruments, Colorado Springs, CO), and the last 2 min of every 5-min bins were averaged for the entire protocol. Systolic and diastolic blood pressures (BP) were measured every 5 min using an automated device (Tango+, Suntech Medical, Morrisville, NC), and mean arterial pressure (MAP) was calculated as diastolic pressure plus one-third pulse pressure (i.e., the difference between systolic and diastolic pressures). The area under the curve (AUC) over the entire experimental period was calculated for each blood pressure parameter.
Six-minute walk test.
The purpose of this session was to determine the severity of the walking impairment, as assessed by the six-minute walk test (44). After 30 min of quiet rest in the semi-recumbent position, participants were asked to perform two 6-min bouts of walking in a corridor, separated by 10 min of rest between each bout. Participants received standardized instructions by the investigators following the guidelines from the American Thoracic Society (33) and were asked to achieve the greatest distance possible. Blood pressure, core temperature, HR, and perceived pain in the leg (10-point Borg scale) were measured before and immediately after each test.
Phase-contrast MRI.
The purpose of this protocol was to determine the magnitude and temporal profile of changes in blood flow through the popliteal artery in the most symptomatic leg during 90 min of TT application using phase-contrast MRI. MRI scanning was performed on a Siemens 3 Tesla (T) Magnetom Prisma scanner (Siemens AG Healthcare Sector, Erlangen, Germany). Upon arriving at the imaging facility, patients were asked to put on the water-circulating garment and the polyvinyl chloride pants and to lie supine on the MRI scanner table. A transmit/receive knee coil was placed around the knee of the most symptomatic leg, and patients were instructed to rest quietly for 30 min. The water-circulating garment was then connected to the heating circulator, and water at 48°C was perfused through the garment for 90 min. Imaging was performed every 5 min throughout the entire protocol. For quantitative flow measurements, a two-dimensional gradient-echo technique was employed using the following acquisition parameters: TR 36.4 ms, TE 3.59 ms, flip angle 20°, FOV 200 mm, matrix size 256 × 256, and reconstructed voxel dimensions of 0.8 × 0.8 × 10.0 mm. A single slice was prescribed perpendicular to a straight section of the popliteal artery. Fifty dynamic phases were acquired to obtain flow waveforms over the cardiac cycle with a maximum range of flow velocity encoding of 100 cm/s. A quantitative flow analysis package (Argus, Flow Module, Siemens) was used to analyze phase-contrast flow data. A region of interest covering the entire visible cross section of the artery was manually drawn to calculate blood flow parameters (25).
Measurement of circulating inflammatory and vasoactive factors.
Venous blood samples were collected in serum-separating tubes (BD Vacutainer SST plus), allowed to clot for 30 min at room temperature, centrifuged for 15 min at 1,200 rpm, aliquoted, and stored at −80°C until analysis. The concentrations of endothelin-1 (ET-1), soluble vascular cell adhesion molecule-1 (sVCAM-1), and soluble intercellular adhesion molecule 1 (sICAM-1) were determined using ELISA kits (R&D Systems, Minneapolis, MN), according to manufacturer's instructions. Quantification of total nitric oxide (NOx) was performed using a commercial kit based on the Griess reaction (total nitric oxide parameter assay kit; R&D Systems). A custom Milliplex assay kit (EMD Millipore, Billerica, MA) was used to determine the concentrations of several angiogenic and inflammatory mediators, including IL-6, IL-8, IL-10, interleukin-1 receptor antagonist (IL-1Ra), IL1β, TNF-α, soluble tumor necrosis factor receptor 1 (sTNFR1), soluble tumor necrosis factor receptor 2 (sTNFR2), chemokine (C-C motif) ligand 2 (CCL2), and vascular endothelial growth factor (VEGF). The assay was performed following the manufacturer's protocol using the Bio-Plex 200 System (Bio-Rad, Hercules, CA) at Indiana University's Bio-Plex Core Facility.
Statistical analysis.
All statistical analyses were conducted using SAS (version 9.4; SAS Institute, Cary, NC) with results expressed as means ± SE. A mixed-effect (with fixed treatment and time effects, random individual effect, and repeated measurements under compound symmetric correlation structure) ANOVA model was used to compare the physiological responses and the concentrations of circulating inflammatory factors and vasoactive mediators between trials. If a significant main fixed effect was found, pairwise differences were identified using the Bonferroni post hoc procedure. The AUC for blood pressure was compared between trials using a paired Student's t-test. Percent changes from baseline for average blood flow velocity, peak velocity, and average flow in the popliteal artery were calculated for each subject. A one-way repeated-measures (with fixed time effect and random individual effect) ANOVA model was used to identify significant changes in these parameters from baseline, and post hoc analysis (Bonferroni) was performed when appropriate. For all analyses, P < 0.05 was considered statistically significant.
RESULTS
Patient demographic and clinical characteristics are listed in Table 1. Six patients were current smokers, and the remainder of patients had a history of smoking. All but two patients were taking at least one antihypertensive medication, and 12 patients were taking statins and antiplatelet agents. Physiological responses to the 6-min walk tests are shown on Table 2. All participants experienced mild-to-severe pain in at least one leg during the test, as revealed by the ratings of perceived leg pain (Table 2). There was no difference in the walk distance completed in the first and second tests (first test: 325 ± 32 m vs. second test: 327 ± 36 m, P = 0.69). The average distance achieved in the 6-min walk test (326 ± 34 m) matches previously reported values for this patient population (23, 47).
Table 1.
Clinical and demographic characteristics
| Characteristic | Value |
|---|---|
| No. | 16 |
| Age, yr | 65 ± 1 |
| Gender (male/female) | 14/2 |
| Body weight, kg | 80.9 ± 0.1 |
| BMI, kg/m2 | 26.6 ± 0.3 |
| ABI | 0.66 ± 0.06 |
| Race, % Caucasian | 78 |
| Current smoker, % | 37 |
| Diabetes, % | 19 |
| Medication, % | |
| Insulin | 19 |
| Antiplatelet Agents | 75 |
| Statins | 75 |
| Cilastazol | 19 |
| Antihypertensive agents | |
| β-blockers | 50 |
| Diuretics | 25 |
| Calcium channel blockers | 31 |
| ACE inhibitor | 56 |
| ANG II receptor antagonist | 6 |
Values shown are means ± SE or percentage. BMI, body mass index; ABI, ankle-brachial index.
Table 2.
Physiological responses to the 6-min walk tests
| Test 1 |
Test 2 |
|||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| Systolic blood pressure, mmHg | 140.0 ± 5.5 | 155.5 ± 6.0 | 137.4 ± 3.8 | 151.5 ± 5.7 |
| Diastolic blood pressure, mmHg | 75.4 ± 3.4 | 82.0 ± 4.0 | 80.4 ± 2.9 | 74.6 ± 3.2 |
| Perceived leg pain rating | 0.3 ± 0.1 | 4.8 ± 0.8 | 1.2 ± 0.4 | 5.4 ± 0.6 |
| Heart rate, bpm | 66 ± 4 | 95 ± 3.9 | 67 ± 3.7 | 98 ± 5.1 |
| Core temperature, °C | 36.9 ± 0.1 | 36.8 ± 0.1 | 36.9 ± 0.1 | 36.9 ± 0.09 |
Values shown are means ± SE.
Leg skin and body core temperature.
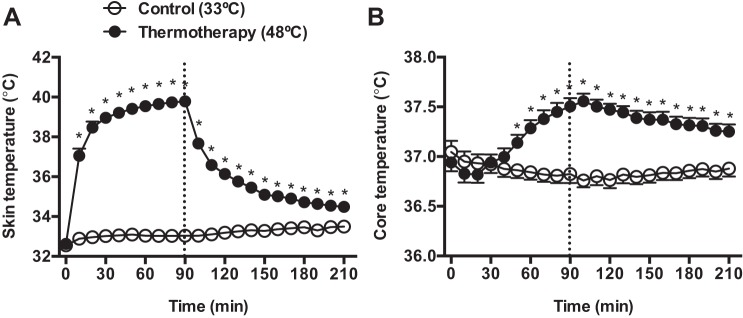
All patients completed the TT and control sessions, and no adverse reactions to the interventions were reported. The effects of TT on average leg skin temperature and body core temperature are shown on Fig. 1. Application of TT induced a gradual increase in leg skin temperature from 32.9 ± 0.3°C at baseline to 40 ± 0.7°C at 90 min. Body core temperature began to increase relative to the control trial after 50 min of TT application, peaked 10 min after the end of the intervention period (Control: 36.7 ± 0.07°C vs. TT: 37.5 ± 0.09°C, P < 0.01), and remained elevated until the end of the experiment (Fig. 1).
Fig. 1.
Leg skin temperature (A) and core body temperature (B) during and after exposure to 90 min of thermotherapy (TT) (●) and the control condition (○). The dashed line indicates the end of the intervention period. *Significantly different from the control treatment, P < 0.05.
Blood pressure and heart rate.
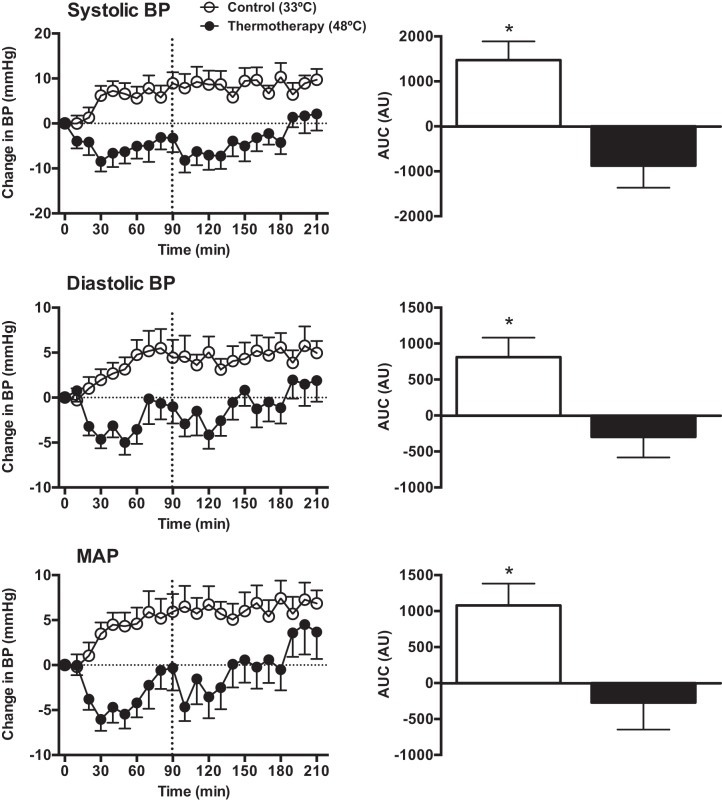
There were no differences between trials at baseline for systolic BP (Control: 133 ± 3 vs. TT: 132 ± 3 mmHg, P > 0.05), diastolic BP (Control: 76 ± 2 vs. TT: 74 ± 2 mmHg, P > 0.05), or MAP (Control: 95 ± 2 vs. TT: 94 ± 2 mmHg, P > 0.05). The effect of TT and the control intervention on changes in BP from baseline and the corresponding AUCs are shown in Fig. 2. Compared with the control treatment, application of TT led to a significant reduction in BP that persisted during most of the postintervention period. The mean reduction in systolic BP was 11 mmHg and diastolic BP was 6 mmHg (P < 0.01). Heart rate increased during exposure to TT from 64 ± 2 bpm at baseline to 76 ± 2 bpm at 90 min, while no changes were observed in the control trial (baseline: 70 ± 4 vs. 90 min: 67 ± 3 bpm).
Fig. 2.
Left: changes from baseline in systolic, diastolic and mean arterial pressure during and after TT (●) and the control intervention (○). Right: area under the curve, expressed as arbitrary units, for the entire intervention period. *Significantly different from TT treatment, P < 0.05.
Circulating inflammatory, angiogenic, and vasoactive mediators.
Exposure to TT did not affect the serum concentrations of selected cytokines, vascular adhesion molecules, angiogenic factors, and total nitric oxide (Table 3). At baseline, the serum concentration of vasoconstrictor ET-1 was similar between trials (Control: 1.8 ± 0.1 vs. TT: 1.7 ± 0.1 pg/ml, P = 0.82). However, TT led to a significant reduction in the concentration of the ET-1 compared with the control treatment 30 min after the intervention (P = 0.026, Fig. 3). A similar trend was observed 120 min after the end of treatment, but this change did not reach statistical significance (P = 0.09).
Table 3.
Serum concentrations of angiogenic, inflammatory and vasoactive mediators at baseline and 30 and 120 following exposure to TT and the control intervention
| Control (33°C) |
Thermotherapy (48°C) |
|||||
|---|---|---|---|---|---|---|
| Baseline | 30 min | 120 min | Baseline | 30 min | 120 min | |
| VEGF, pg/ml | 640.8 ± 249.3 | 695.4 ± 260.2 | 672.0 ± 272.2 | 700.7 ± 295.1 | 571.9 ± 195.0 | 617.1 ± 247.3 |
| MCP-1, pg/ml | 534.5 ± 60.0 | 533.6 ± 49.7 | 519.6 ± 54.5 | 541.9 ± 53.3 | 501.0 ± 44.2 | 502.5 ± 47.5 |
| IL1-Rα, pg/ml | 71.9 ± 38.0 | 86.8 ± 44.9 | 82.0 ± 41.9 | 94.7 ± 49.1 | 68.9 ± 28.5 | 77.9 ± 37.3 |
| IL-6, pg/ml | 15.6 ± 9.6 | 14.3 ± 7.7 | 15.5 ± 8.7 | 12.8 ± 7.7 | 9.2 ± 4.7 | 13.1 ± 7.9 |
| IL-10, pg/ml | 24.9 ± 13.3 | 27.9 ± 15.0 | 30.0 ± 14.5 | 23.7 ± 12.0 | 15.3 ± 3.8 | 24.2 ± 9.4 |
| TNF-α, pg/ml | 20.1 ± 9.5 | 22.9 ± 11.0 | 19.7 ± 9.2 | 18.6 ± 8.1 | 15.5 ± 4.9 | 17.6 ± 7.4 |
| IL-1β, pg/ml | 43.8 ± 33.4 | 46.8 ± 34.4 | 46.6 ± 37.8 | 35.0 ± 29.2 | 26.3 ± 20.2 | 30.4 ± 25.5 |
| IL-8, pg/ml | 13.8 ± 3.9 | 14.9 ± 3.9 | 13.4 ± 3.6 | 14.0 ± 3.8 | 13.9 ± 3.3 | 13.5 ± 3.7 |
| sTNFRI, pg/ml | 1649.1 ± 261.4 | 1624.5 ± 256.4 | 1625.6 ± 247.9 | 1662.5 ± 253.9 | 1668.3 ± 257.4 | 1657.7 ± 267.0 |
| sTNFRII, pg/ml | 9961.3 ± 1673.6 | 9928.7 ± 1652.7 | 9842.8 ± 1705.1 | 10089.3 ± 1630.6 | 9674.1 ± 1465.1 | 9623.4 ± 1527.2 |
| sVCAM-1, pg/ml | 37.3 ± 2.6 | 37.8 ± 3.0 | 38.4 ± 3.0 | 37.9 ± 2.8 | 37.8 ± 3.0 | 37.2 ± 3.0 |
| sICAM-1, pg/ml | 14.4 ± 1.5 | 14.2 ± 1.2 | 14.5 ± 1.5 | 14.5 ± 1.4 | 14.4 ± 1.4 | 14.3 ± 1.5 |
| NOx, pg/ml | 10.5 ± 1.1 | 10.0 ± 1.0 | 9.4 ± 1.0 | 10.6 ± 1.2 | 10.3 ± 1.1 | 9.5 ± 0.9 |
Values shown are means ± SE. See text for abbreviations.
Fig. 3.
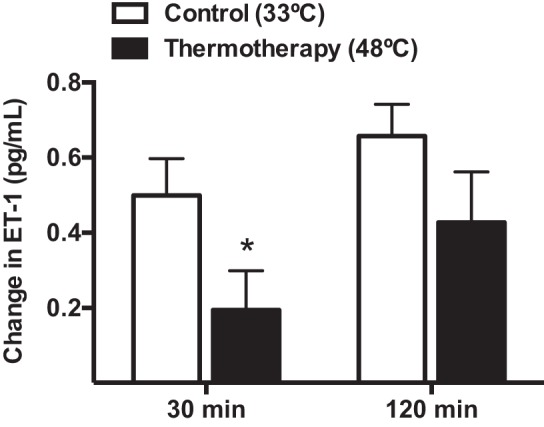
Changes in serum concentration of endothelin-1 (ET-1) from baseline at 30 and 120 min following exposure to TT (solid bars) or the control treatment (open bars). *Significantly different from the control treatment, P < 0.05.
Blood flow in the popliteal artery.
Six patients did not participate in the blood flow measurement session. Baseline values for average velocity, peak velocity, and flow rate in the popliteal artery were 6.6 ± 1.0 cm/s, 16.9 ± 2.4 cm/s, and 0.05 ± 0.01 l/min, respectively. Fig. 4 depicts the percent changes from baseline for these parameters during exposure to 90 min of TT. Blood flow began to increase above baseline ∼25 min after the onset of the treatment and reached peak levels within 80 min (Fig. 4).
Fig. 4.
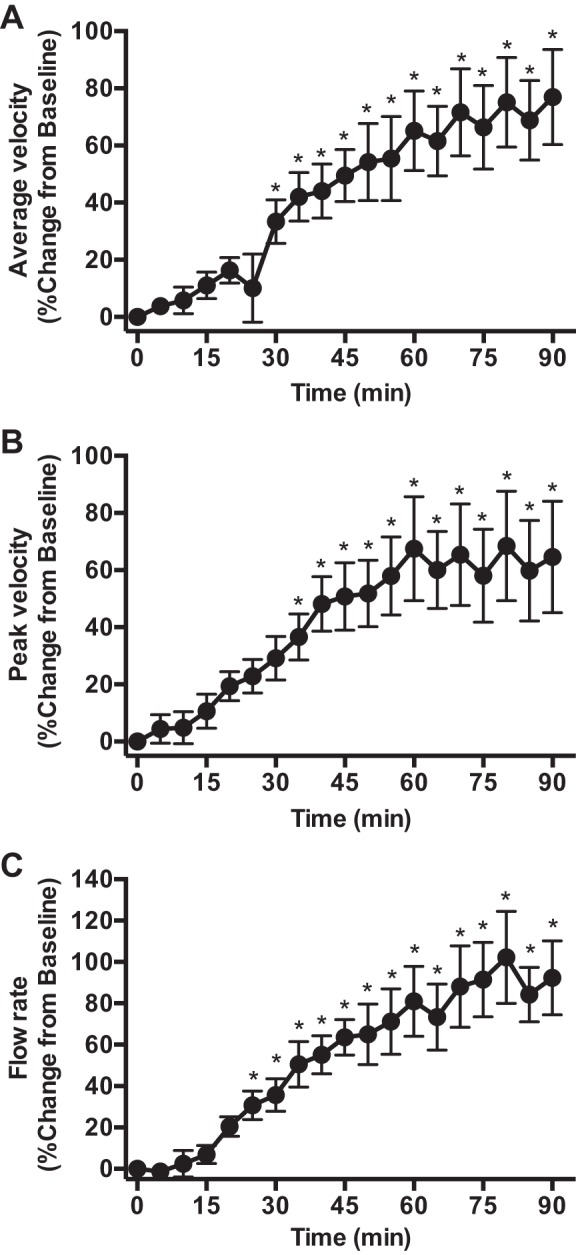
Percent change in average blood flow velocity (A), peak velocity (B) and average flow rate (C) in the popliteal artery during 90 min of TT treatment. *Significantly different from baseline, P < 0.05.
DISCUSSION
The overall goal of this study was to determine the effect of an acute session of TT on systemic and leg hemodynamics and on the levels of circulating inflammatory and vasoactive mediators in patients with symptomatic PAD. One novel feature and strength of our design is that TT was applied using a water-circulating garment system that is amenable for home-based treatment. The main findings were that a single 90-min session of TT in patients with PAD: 1) markedly reduced BP not only during but also for nearly 2 h after the treatment; 2) promoted a ∼60% reduction in the serum concentration of the vasoconstrictor and proinflammatory peptide endothelin-1 30 min after the therapy compared with the control intervention; and 3) elicited an increase (∼100%) in blood flow through the popliteal artery.
Effects of TT on blood pressure.
Hypertension is a major risk factor for PAD and a significant contributor to cardiovascular morbidity and mortality associated with this condition (13). It is estimated that more than half of patients with PAD are hypertensive (34). Patients with PAD also have an augmented BP response to walking (48), which has been shown to be an independent predictor of all-cause mortality and adverse cerebrovascular and cardiac events (18). Not surprisingly, antihypertensive therapy is one of the pillars of medical management of PAD, and there is growing evidence that intensive blood pressure lowering can result in a marked reduction in cardiovascular events in this patient population (6). Here, we report that exposure to 90 min of TT induced a robust reduction in BP compared with the control intervention, with average net reductions in systolic BP, diastolic BP, and MAP during the intervention of 11, 6, and 8 mmHg, respectively. Of note, this effect was sustained during the first 2 h after the therapy, with systolic BP, diastolic BP, and MAP being on average 12, 6, and 7 mmHg lower than in the control condition. The magnitude of this acute hypotensive effect is relevant and comparable to that achieved with well-established antihypertensive therapies in this patient population. For example, we recently reported that ingestion of two doses of 50 mg (30 min apart) of the vasodilator sildenafil induced an average reduction of brachial systolic BP of ∼15 mmHg in patients with PAD (56). Cucato et al. (15) showed that a 60-min session of intermittent walking in patients with PAD reduced systolic BP, diastolic BP, and MAP by 13, 5, and 7 mmHg, respectively (15). Although it is unclear whether the observed acute hypotensive response to TT would be sustained after regular treatment, evidence derived from exercise studies suggests that the response to a single session is predictive of long-term reductions in BP (26, 39). Indeed, recent studies in prehypertensive patients revealed that the magnitude of BP reduction following a single bout of exercise correlates strongly with chronic BP reduction in response to an endurance training program (26, 39). In addition, daily treatment with sauna therapy for as little as 2 wk has been shown to reduce resting BP levels in patients with cardiovascular risk factors (28, 42). Further studies are needed to determine the long-term impact of repeated leg TT on BP in patients with PAD.
Effects of TT on leg blood flow.
Leg heating to a level sufficient to induce an increase in core body temperature of ∼0.5–1°C has been shown to enhance blood flow through the common and superficial femoral arteries by three- to five-fold in healthy young individuals (11, 12, 53). This heat-induced hyperemia is the result of increases in blood flow not only to the skin but also to skeletal muscle, as elegantly shown by Heinonen et al. (27) and several others (11, 12, 53). In the present study, we observed that blood flow through the popliteal artery increased by approximately twofold, attaining peak levels at ∼80 min after the onset of the treatment (Fig. 4). On the basis of the aforementioned changes in femoral blood flow in healthy young individuals (approximately three- to five-fold) exposed to a similar thermal challenge (11, 12, 53), it is evident that the hyperemic response to TT is impaired in patients with PAD. This is expected given that vasodilator function of conduit and resistance vessels in the leg is known to be severely compromised in these patients (38). Nonetheless, it is conceivable that repeated increases in blood flow and the consequent increases in wall shear stress during treatment with TT can enhance peripheral vascular reactivity in patients with PAD. This notion is supported by several observations in healthy young subjects, as well as in different patient populations. Seminal studies by Green and colleagues (24, 49) revealed that repeated forearm heating for 8 wk induces a blood flow-dependent increase in conduit artery and skin vasodilatory function in healthy subjects. Similarly, treatment with far-infrared dry sauna has been shown to enhance flow-mediated dilation of the brachial artery in patients with chronic heart failure (32, 52) and patients with atherogenic risk factors (28). Combined, this compelling evidence indicates that TT may be a practical and effective strategy to improve peripheral vascular endothelial function, even in the presence of multiple comorbidities and risk factors for atherosclerosis.
Effects of TT on circulating ET-1 concentration.
Increases in limb blood flow during thermal stress reflect the combined vasodilation of the cutaneous and skeletal muscle circulations (14). The robust vasodilation of the cutaneous vasculature in response to heat stress is mediated, among others, by neural components and local production of vasodilators, such as nitric oxide (29). In skeletal muscle, the mechanistic basis of heat-induced vasodilation is less defined, but emerging evidence indicates that ATP released by erythrocytes play an important role (30). The observation of a reduction in circulating levels of ET-1 following TT (Fig. 3) is important, as this molecule is the most abundant vasoconstrictor produced by the vascular endothelium (60). This peptide exerts its effects through the activation of endothelin-A (ETA) receptors, located on vascular smooth muscle cells, as well as endothelin-B (ETB) receptors, present primarily on endothelial cells. There is strong evidence supporting a tonic vasoconstrictor influence of ET-1 on the peripheral vasculature (60), including the cutaneous circulation (36). Indeed, pharmacological blockade of ET-1 receptor A increases limb (58, 61) and skin blood flow (36) and results in marked reductions in BP in healthy subjects (58). The circulating concentration of ET-1 is known to be elevated in patients with atherosclerosis (35), including those with PAD (17, 41), and pharmacological blockade of ET-1 receptors has been consistently associated with improvements in blood flow and vasodilation in patients with atherogenic risk factors (10, 51) and coronary and peripheral artery disease (3, 4). On the basis of this extensive evidence, it is fair to speculate that the observed reduction in ET-1 concentration can explain, at least in part, the increases in leg blood flow and the consequent reduction in BP following exposure to TT in the present study (Figs. 2 and 4).
In addition to its potent vasoconstrictor effects, ET-1 is proinflammatory and mitogenic, and is strongly linked to endothelial dysfunction and the progression of atherosclerosis (5, 54). The levels of ET-1 are known to be an independent predictor of all-cause and cardiovascular mortality in patients with severe PAD (50). Mangiafico et al. (40) showed that the changes in the concentration of this factor could be a marker of clinical improvement following therapies such as prostaglandin (PG) E1 in PAD patients (40). Recently, De Haro et al. (16) documented that treatment with dual ET-receptor antagonist bosentan improved claudication distance and vascular function in patients with PAD (16). Whether similar effects can be achieved nonpharmacologically, by repeated exposures to TT, remains to be determined.
Clinical implications.
The prevalence of PAD is expected to rise drastically in the coming decades, and a pressing need remains for the development of a therapeutic strategy that is widely accessible and economically viable to treat patients with this condition. Exercise training is undoubtedly the most efficacious approach, but it is well known that only a very minor fraction of patients with symptomatic PAD participates in exercise rehabilitation (2, 55). Studies that have investigated the barriers behind the lack of adherence of these patients to physical activity programs have consistently shown that leg pain during exercise and environmental barriers, such as the lack of walkable areas, are among the top reasons (1, 21). Thermotherapy is an attractive tool in this regard because it is painless and amenable for home-based application. We chose to apply the therapy using a water-circulating garment coupled with a water pump because, as opposed to more traditional forms of TT, such as sauna, this set-up is portable and, therefore, patients do not need to travel to a clinical facility frequently to receive the treatment. In addition, TT can be used in patients with severe disease and limited locomotion for whom exercise therapy is not an option.
Limitations.
One limitation of the present study was that serum concentrations of inflammatory, angiogenic, and vasoactive factors were not corrected for the potential change in plasma volume induced by sweating during the TT protocol. Although we did not measure body mass after the interventions, it was evident that the heating protocol induced water loss and a potential consequent reduction in plasma volume. This is important, as the observed reduction in serum ET-1 concentration after TT would likely be larger when accounting for this response. A second limitation is that blood samples were taken from the arm, which was not directly heated during the TT protocol. It is conceivable that the responses of circulating factors to TT application would be different if the measurements were made in samples of venous blood draining from the heated legs. Lastly, it is important to point out that we focused solely on the recovery period after the intervention (30 and 120 min after TT), and it is unclear whether the responses of blood parameters measured at these time points are similar to the changes induced during the intervention. Additional studies are needed to determine the temporal profile of changes in the concentrations of serum cytokines and vasoactive mediators in response to TT in patients with PAD.
An additional limitation of this study is that popliteal blood flow, as determined using phase-contrast MRI, was not measured under a normothermic, control condition, in which thermoneutral water is perfused through the garment for 90 min. However, prior studies in young healthy individuals reported that circulating water at 33–34°C through a tube-lined suit covering the legs does not alter bulk leg blood flow and calf blood flow (27, 31). Also, popliteal blood flow was not measured in the recovery period following the TT intervention in the present study. Since tissue blood flow responses to heat stress are largely determined by local temperature-sensitive mechanisms (12), it is anticipated that leg blood flow would fall gradually toward baseline levels after the therapy, as observed for leg skin temperature (Fig. 1, left).
It is important to note that the fact that the participants wore the water-circulating garment and polyvinyl chloride pants during the postinterventional period following TT likely slowed the recovery kinetics of some physiological variables, including leg skin and core body temperature, leg blood flow, and blood pressure. Although the heated bath circulator was turned off after 90 min of TT, the water contained inside the tube-lined garment was still warm in the early recovery period. If the subjects had promptly removed the garment after 90 min of TT and exposed the legs to room temperature, it is possible that the physiological responses during recovery from TT would be different than observed in the present study. Future studies should determine the optimal treatment duration and recovery strategy to maximize the beneficial responses to this novel therapeutic approach.
Perspectives and Significance
Overall, the findings from the present study indicate that TT has the potential to fulfill the two most important goals of treatment for PAD: first, TT can likely impact cardiovascular risk by promoting reductions in BP and the circulating concentration of the potent vasoconstrictor and proinflammatory peptide ET-1; second, TT can enhance leg blood flow and, therefore, potentially alleviate the symptoms of walking-induced leg ischemia. These novel findings set the stage for future studies to investigate the impact of repeated treatment with TT on vascular function and functional capacity in patients with PAD.
GRANTS
Funding for this project was provided by the Indiana Clinical and Translational Science Institute (Project Development Teams Pilot Grant UL1TR001108) and the American Heart Association (Grant 16SDH-27600003).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.N., A.M.K., C.L., and B.T.R. performed experiments; D.N., A.M.K., C.L., and B.T.R. analyzed data; D.N., A.M.K., B.J.W., R.L.M., and B.T.R. interpreted results of experiments; D.N., C.L., B.J.W., R.L.M., and B.T.R. edited and revised manuscript; D.N., A.M.K., C.L., B.J.W., R.L.M., and B.T.R. approved final version of manuscript; B.J.W., R.L.M., and B.T.R. conception and design of research; B.T.R. prepared figures; B.T.R. drafted manuscript.
REFERENCES
- 1.Barbosa JP, Farah BQ, Chehuen M, Cucato GG, Farias Junior JC, Wolosker N, Forjaz CL, Gardner AW, Ritti-Dias RM. Barriers to physical activity in patients with intermittent claudication. Int J Behav Med 22: 70–76, 2015. [DOI] [PubMed] [Google Scholar]
- 2.Bartelink ML, Stoffers HE, Biesheuvel CJ, Hoes AW. Walking exercise in patients with intermittent claudication. Experience in routine clinical practice. Br J Gen Pract 54: 196–200, 2004. [PMC free article] [PubMed] [Google Scholar]
- 3.Bohm F, Ahlborg G, Johansson BL, Hansson LO, Pernow J. Combined endothelin receptor blockade evokes enhanced vasodilatation in patients with atherosclerosis. Arterioscler Thromb Vasc Biol 22: 674–679, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Bohm F, Beltran E, Pernow J. Endothelin receptor blockade improves endothelial function in atherosclerotic patients on angiotensin converting enzyme inhibition. J Int Med 257: 263–271, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Bohm F, Pernow J. The importance of endothelin-1 for vascular dysfunction in cardiovascular disease. Cardiovasc Res 76: 8–18, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Bonaca MP, Creager MA. Pharmacological treatment and current management of peripheral artery disease. Circ Res 116: 1579–1598, 2015. [DOI] [PubMed] [Google Scholar]
- 7.Bouchama A, Ollivier V, Roberts G, Al Mohanna F, de Prost D, Eldali A, Saussereau E, El-Sayed R, Chollet-Martin S. Experimental heatstroke in baboon: analysis of the systemic inflammatory response. Shock 24: 332–335, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Bouchama A, Roberts G, Al Mohanna F, El-Sayed R, Lach B, Chollet-Martin S, Ollivier V, Al Baradei R, Loualich A, Nakeeb S, Eldali A, de Prost D. Inflammatory, hemostatic, and clinical changes in a baboon experimental model for heatstroke. J Appl Physiol 98: 697–705, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Brevetti G, Giugliano G, Brevetti L, Hiatt WR. Inflammation in peripheral artery disease. Circulation 122: 1862–1875, 2010. [DOI] [PubMed] [Google Scholar]
- 10.Cardillo C, Kilcoyne CM, Cannon RO 3rd, Panza JA. Increased activity of endogenous endothelin in patients with hypercholesterolemia. J Am Coll Cardiol 36: 1483–1488, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Chiesa ST, Trangmar SJ, Gonzalez-Alonso J. Temperature and blood flow distribution in the human leg during passive heat stress. J Appl Physiol 120: 1047–1058, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiesa ST, Trangmar SJ, Kalsi KK, Rakobowchuk M, Banker DS, Lotlikar MD, Ali L, Gonzalez-Alonso J. Local temperature-sensitive mechanisms are important mediators of limb tissue hyperemia in the heat-stressed human at rest and during small muscle mass exercise. Am J Physiol Heart Circ Physiol 309: H369–H380, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clement DL, De Buyzere ML, Duprez DA. Hypertension in peripheral arterial disease. Curr Pharmaceut Design 10: 3615–3620, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Crandall CG, Wilson TE. Human cardiovascular responses to passive heat stress. Compr Physiol 5: 17–43, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cucato GG, Chehuen Mda R, Ritti-Dias RM, Carvalho CR, Wolosker N, Saxton JM, Forjaz CL. Post-walking exercise hypotension in patients with intermittent clau dication. Med Sci Sports Exerc 47: 460–467, 2015. [DOI] [PubMed] [Google Scholar]
- 16.De Haro J, Bleda S, Varela C, Esparza L, Acin F, Bosentan Population-Based Randomized Trial for C., and Endothelial Function Assessment on Endothelin Antagonist Therapy in Patients With Intermittent Claudication I . Effect of Bosentan on claudication distance and endothelium-dependent vasodilation in hispanic patients with peripheral arterial disease. Am J Cardiol 117: 295–301, 2016. [DOI] [PubMed] [Google Scholar]
- 17.de Haro Miralles J, Gonzalez AF, Varela Casariego C, Garcia FA. Onset of peripheral arterial disease: role of endothelin in endothelial dysfunction. Interact Cardiovasc Thorac Surg 10: 760–765, 2010. [DOI] [PubMed] [Google Scholar]
- 18.de Liefde II, Hoeks SE, van Gestel YR, Bax JJ, Klein J, van Domburg RT, Poldermans D. Usefulness of hypertensive blood pressure response during a single-stage exercise test to predict long-term outcome in patients with peripheral arterial disease. Am J Cardiol 102: 921–926, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, Norman PE, Sampson UK, Williams LJ, Mensah GA, Criqui MH. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet 382: 1329–1340, 2013. [DOI] [PubMed] [Google Scholar]
- 20.Gagnon D, Schlader ZJ, Crandall CG. Sympathetic activity during passive heat stress in healthy aged humans. J Physiol 593: 2225–2235, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galea MN, Bray SR, Ginis KA. Barriers and facilitators for walking in individuals with intermittent claudication. J Aging Phys Act 16: 69–83; quiz 84, 2008. [DOI] [PubMed] [Google Scholar]
- 22.Ganio MS, Brothers RM, Shibata S, Hastings JL, Crandall CG. Effect of passive heat stress on arterial stiffness. Exp Physiol 96: 919–926, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gardner AW, Parker DE, Montgomery PS, Blevins SM. Step-monitored home exercise improves ambulation, vascular function, and inflammation in symptomatic patients with peripheral artery disease: a randomized controlled trial. J Am Heart Assoc 3: e001107, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Green DJ, Carter HH, Fitzsimons MG, Cable NT, Thijssen DH, Naylor LH. Obligatory role of hyperaemia and shear stress in microvascular adaptation to repeated heating in humans. J Physiol 588: 1571–1577, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grozinger G, Pohmann R, Schick F, Grosse U, Syha R, Brechtel K, Rittig K, Martirosian P. Perfusion measurements of the calf in patients with peripheral arterial occlusive disease before and after percutaneous transluminal angioplasty using MR arterial spin labeling. J Magn Reson Imaging 40: 980–987, 2014. [DOI] [PubMed] [Google Scholar]
- 26.Hecksteden A, Grutters T, Meyer T. Association between postexercise hypotension and long-term training-induced blood pressure reduction: a pilot study. Clin J Sport Med 23: 58–63, 2013. [DOI] [PubMed] [Google Scholar]
- 27.Heinonen I, Brothers RM, Kemppainen J, Knuuti J, Kalliokoski KK, Crandall CG. Local heating, but not indirect whole body heating, increases human skeletal muscle blood flow. J Appl Physiol 111: 818–824, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imamura M, Biro S, Kihara T, Yoshifuku S, Takasaki K, Otsuji Y, Minagoe S, Toyama Y, Tei C. Repeated thermal therapy improves impaired vascular endothelial function in patients with coronary risk factors. J Am Coll Cardiol 38: 1083–1088, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Johnson JM, Minson CT, Kellogg DL Jr. Cutaneous vasodilator and vasoconstrictor mechanisms in temperature regulation. Compr Physiol 4: 33–89, 2014. [DOI] [PubMed] [Google Scholar]
- 30.Kalsi KK, Gonzalez-Alonso J. Temperature-dependent release of ATP from human erythrocytes: mechanism for the control of local tissue perfusion. Exp Physiol 97: 419–432, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keller DM, Sander M, Stallknecht B, Crandall CG. α-Adrenergic vasoconstrictor responsiveness is preserved in the heated human leg. J Physiol 588: 3799–3808, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kihara T, Biro S, Imamura M, Yoshifuku S, Takasaki K, Ikeda Y, Otuji Y, Minagoe S, Toyama Y, Tei C. Repeated sauna treatment improves vascular endothelial and cardiac function in patients with chronic heart failure. J Am Coll Cardiol 39: 754–759, 2002. [DOI] [PubMed] [Google Scholar]
- 33.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 166: 111–117, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Lane DA, Lip GY. Treatment of hypertension in peripheral arterial disease. Cochrane Database Syst Rev 12: CD003075, 2013. [DOI] [PubMed] [Google Scholar]
- 35.Lerman A, Edwards BS, Hallett JW, Heublein DM, Sandberg SM, Burnett JC Jr. Circulating and tissue endothelin immunoreactivity in advanced atherosclerosis. N Engl J Med 325: 997–1001, 1991. [DOI] [PubMed] [Google Scholar]
- 36.Leslie SJ, Rahman MQ, Denvir MA, Newby DE, Webb DJ. Endothelins and their inhibition in the human skin microcirculation: ET[1–31], a new vasoconstrictor peptide. Br J Clin Pharmacol 57: 720–725, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levine GN, Gomes AS, Arai AE, Bluemke DA, Flamm SD, Kanal E, Manning WJ, Martin ET, Smith JM, Wilke N, Shellock FS, American Heart Association Committee on Diagnostic and Interventional Cardiac C., American Heart Association Council on Clinical Catheterization, American Heart Association Council on Cardiovascular Radiology, and Intervention . Safety of magnetic resonance imaging in patients with cardiovascular devices: an American Heart Association scientific statement from the Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology, and the Council on Cardiovascular Radiology and Intervention: endorsed by the American College of Cardiology Foundation, the North American Society for Cardiac Imaging, and the Society for Cardiovascular Magnetic Resonance. Circulation 116: 2878–2891, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Liao JK, Bettmann MA, Sandor T, Tucker JI, Coleman SM, Creager MA. Differential impairment of vasodilator responsiveness of peripheral resistance and conduit vessels in humans with atherosclerosis. Circ Res 68: 1027–1034, 1991. [DOI] [PubMed] [Google Scholar]
- 39.Liu S, Goodman J, Nolan R, Lacombe S, Thomas SG. Blood pressure responses to acute and chronic exercise are related in prehypertension. Med Sci Sports Exerc 44: 1644–1652, 2012. [DOI] [PubMed] [Google Scholar]
- 40.Mangiafico RA, Malatino LS, Santonocito M, Messina R, Attina T, Dell'Arte S, Sarnataro F. Effects of a 4-week treatment with prostaglandin E1 on plasma endothelin-1 release in patients with intermittent claudication. Int J Clin Pharmacol Ther 37: 347–351, 1999. [PubMed] [Google Scholar]
- 41.Mangiafico RA, Malatino LS, Santonocito M, Sarnataro F, Dell'Arte S, Messina R, Santangelo B. Plasma endothelin-1 levels in patients with peripheral arterial occlusive disease at different Fontaine's stages. Panminerva Med 41: 22–26, 1999. [PubMed] [Google Scholar]
- 42.Masuda A, Miyata M, Kihara T, Minagoe S, Tei C. Repeated sauna therapy reduces urinary 8-epi-prostaglandin F(2α). Jpn Heart J 45: 297–303, 2004. [DOI] [PubMed] [Google Scholar]
- 43.McDermott MM. Lower extremity manifestations of peripheral artery disease: the pathophysiologic and functional implications of leg ischemia. Circ Res 116: 1540–1550, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McDermott MM, Guralnik JM, Criqui MH, Liu K, Kibbe MR, Ferrucci L. Six-minute walk is a better outcome measure than treadmill walking tests in therapeutic trials of patients with peripheral artery disease. Circulation 130: 61–68, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McDermott MM, Hoff F, Ferrucci L, Pearce WH, Guralnik JM, Tian L, Liu K, Schneider JR, Sharma L, Tan J, Criqui MH. Lower extremity ischemia, calf skeletal muscle characteristics, and functional impairment in peripheral arterial disease. J Am Geriatr Soc 55: 400–406, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McDermott MM, Liu K, Greenland P, Guralnik JM, Criqui MH, Chan C, Pearce WH, Schneider JR, Ferrucci L, Celic L, Taylor LM, Vonesh E, Martin GJ, Clark E. Functional decline in peripheral arterial disease: associations with the ankle brachial index and leg symptoms. JAMA 292: 453–461, 2004. [DOI] [PubMed] [Google Scholar]
- 47.McDermott MM, Liu K, Guralnik JM, Criqui MH, Spring B, Tian L, Domanchuk K, Ferrucci L, Lloyd-Jones D, Kibbe M, Tao H, Zhao L, Liao Y, Rejeski WJ. Home-based walking exercise intervention in peripheral artery disease: a randomized clinical trial. JAMA 310: 57–65, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muller MD, Reed AB, Leuenberger UA, Sinoway LI. Physiology in medicine: peripheral arterial disease. J Appl Physiol 115: 1219–1226, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Naylor LH, Carter H, FitzSimons MG, Cable NT, Thijssen DH, Green DJ. Repeated increases in blood flow, independent of exercise, enhance conduit artery vasodilator function in humans. Am J Physiol Heart Circ Physiol 300: H664–H669, 2011. [DOI] [PubMed] [Google Scholar]
- 50.Newton DJ, Khan F, McLaren M, Kennedy G, Belch JJ. Endothelin-1 levels predict 3-year survival in patients who have amputation for critical leg ischaemia. Br J Surg 92: 1377–1381, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Nohria A, Garrett L, Johnson W, Kinlay S, Ganz P, Creager MA. Endothelin-1 and vascular tone in subjects with atherogenic risk factors. Hypertension 42: 43–48, 2003. [DOI] [PubMed] [Google Scholar]
- 52.Ohori T, Nozawa T, Ihori H, Shida T, Sobajima M, Matsuki A, Yasumura S, Inoue H. Effect of repeated sauna treatment on exercise tolerance and endothelial function in patients with chronic heart failure. Am J Cardiol 109: 100–104, 2012. [DOI] [PubMed] [Google Scholar]
- 53.Pearson J, Low DA, Stohr E, Kalsi K, Ali L, Barker H, Gonzalez-Alonso J. Hemodynamic responses to heat stress in the resting and exercising human leg: insight into the effect of temperature on skeletal muscle blood flow. Am J Physiol Regul Integr Comp Physiol 300: R663–R673, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pernow J, Shemyakin A, Bohm F. New perspectives on endothelin-1 in atherosclerosis and diabetes mellitus. Life Sci 91: 507–516, 2012. [DOI] [PubMed] [Google Scholar]
- 55.Regensteiner JG. Exercise rehabilitation for the patient with intermittent claudication: a highly effective yet underutilized treatment. Curr Drug Targets Cardiovasc Haematol Disord 4: 233–239, 2004. [DOI] [PubMed] [Google Scholar]
- 56.Roseguini BT, Hirai DM, Alencar MC, Ramos RP, Silva BM, Wolosker N, Neder JA, Nery LE. Sildenafil improves skeletal muscle oxygenation during exercise in men with intermittent claudication. Am J Physiol Regul Integr Comp Physiol 307: R396–R404, 2014. [DOI] [PubMed] [Google Scholar]
- 57.Smith GD, Shipley MJ, Rose G. Intermittent claudication, heart disease risk factors, and mortality. The Whitehall Study Circulation 82: 1925–1931, 1990. [DOI] [PubMed] [Google Scholar]
- 58.Spratt JC, Goddard J, Patel N, Strachan FE, Rankin AJ, Webb DJ. Systemic ETA receptor antagonism with BQ-123 blocks ET-1 induced forearm vasoconstriction and decreases peripheral vascular resistance in healthy men. Br J Pharmacol 134: 648–654, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stewart KJ, Hiatt WR, Regensteiner JG, Hirsch AT. Exercise training for claudication. N Engl J Med 347: 1941–1951, 2002. [DOI] [PubMed] [Google Scholar]
- 60.Thorin E, Webb DJ. Endothelium-derived endothelin-1. Pflügers Arch 459: 951–958, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Verhaar MC, Strachan FE, Newby DE, Cruden NL, Koomans HA, Rabelink TJ, Webb DJ. Endothelin-A receptor antagonist-mediated vasodilatation is attenuated by inhibition of nitric oxide synthesis and by endothelin-B receptor blockade. Circulation 97: 752–756, 1998. [DOI] [PubMed] [Google Scholar]
- 62.Welc SS, Clanton TL, Dineen SM, Leon LR. Heat stroke activates a stress-induced cytokine response in skeletal muscle. J Appl Physiol 115: 1126–1137, 2013. [DOI] [PubMed] [Google Scholar]
- 63.Wilkinson DM, Carter JM, Richmond VL, Blacker SD, Rayson MP. The effect of cool water ingestion on gastrointestinal pill temperature. Med Sci Sports Exerc 40: 523–528, 2008. [DOI] [PubMed] [Google Scholar]