Abstract
High-fat diets rapidly cause weight gain and glucose intolerance. We sought to determine whether these changes could be mitigated with prior exercise training. Male C57BL/6J mice were exercise-trained by treadmill running (1 h/day, 5 days/wk) for 4 wk. Twenty-four hours after the final bout of exercise, mice were provided with a high-fat diet (HFD; 60% kcal from lard) for 4 days, with no further exercise. In mice fed the HFD prior to exercise training, the results were blunted weight gain, reduced fat mass, and a slight attenuation in glucose intolerance that was mirrored by greater insulin-induced Akt phosphorylation in skeletal muscle compared with sedentary mice fed the HFD. When ad libitum-fed sedentary mice were compared with sedentary high-fat fed mice that were calorie restricted (−30%) to match the weight gain of the previously trained high-fat fed mice, the same attenuated impairments in glucose tolerance were found. Blunted weight gain was associated with a greater capacity to increase energy expenditure in trained compared with sedentary mice when challenged with a HFD. Although mitochondrial enzymes in white adipose tissue and UCP-1 protein content in brown adipose tissue were increased in previously exercised compared with sedentary mice fed a HFD, ex vivo mitochondrial respiration was not increased in either tissue. Our data suggest that prior exercise training attenuates high-fat diet-induced weight gain and glucose intolerance and is associated with a greater ability to increase energy expenditure in response to a high-fat diet.
Keywords: exercise training, high-fat diet, adipose tissue, glucose tolerance
obesity and the associated metabolic syndrome are due, in large part, to overnutrition and lack of physical activity (14). Exercise training has successfully been used to both treat (21, 30) and prevent (11) high-fat diet-induced obesity and the related impairments in glucose homeostasis. In addition to these noted effects on glucose metabolism, exercise is also known to possess potent anti-inflammatory properties (as reviewed in Ref. 14). In recent studies from our laboratory, we have examined how prior exercise training (4) or habitual physical activity (20) modifies the response to an acute inflammatory challenge. In these experiments, in the unstimulated condition, we saw little to no differences between sedentary and trained/active animals. However, a marked protective effect of training/physical activity was uncovered when mice were challenged with the β-3 adrenergic agonist CL 316,243 (4) or LPS (20). Although these findings highlight a relatively unappreciated aspect of regularly performed exercise, it has yet to be determined whether prior exercise training would also protect against metabolic insults, such as short-term high-fat diet-induced weight gain and glucose intolerance. Of interest, Morris et al. (18) recently reported that weight gain and markers of hepatic steatosis following a 3-day high-fat diet were attenuated in rats bred for high, compared with low, intrinsic aerobic capacity. Given these findings, and our prior work using inflammatory agents, we wanted to test the hypothesis that prior exercise training would blunt the effects of a short-term high-fat diet on weight gain and perturbations in glucose homeostasis. To test this supposition, mice were exercise-trained for 4 wk and then, in the absence of any additional exercise, were challenged with a high-fat diet for 4 days and changes in weight gain, adipose tissue accretion, glucose tolerance, skeletal muscle insulin signaling and whole body energy expenditure were determined.
METHODS
Materials
Rodent high-fat diet (HFD, 60% kcal from lard; D12492) and sucrose matched low-fat control diet (LFD, 10% kcal from lard; D12450J) were obtained from Research Diets (New Brunswick, NJ; Table 1). Phenylmethylsulfonylfluoride and protease inhibitor cocktail were obtained from Sigma (Mississauga, ON, Canada). NP40 lysis buffer, random primers, SuperScript II reverse transcriptase, dNTPs, DNase, and TaqMan gene expression assays (UCP-1, Mm01244861; GAPDH, 4352932E) were from Life Technologies (Burlington, ON, Canada). Reagents for SDS-PAGE, nitrocellulose membrane, and molecular weight marker were from Bio-Rad (Mississauga, ON, Canada). Antibodies against phosphorylated Thr-308 Akt (pAktThr-308; no. 4056) and total Akt (tAkt; no. 4685) were from Cell Signaling (Danvers, MA). Antibodies against pyruvate dehydrogenase subunit E1α (PDH E1α; no. MSP03), citrate synthase (CS; no. ab129095), Complex III subunit CORE 1 (CORE1; no. MS303), cytochrome oxidase subunit IV (COXIV; no. ab16056), GAPDH (no. ab8245), and α-tubulin (no. ab7291) were from Abcam (Cambridge, MA). Horseradish peroxidase-linked secondary antibodies were from Jackson ImmunoResearch (West Grove, PA). Enhanced chemiluminescent reagents were from Perkin Elmer (Woodbridge, ON, Canada). Substrates for respirometry experiments were from Sigma. All other general laboratory chemicals were from BioShop (Burlington, ON, Canada).
Table 1.
Diet composition
| % Weight |
% kcal |
|||
|---|---|---|---|---|
| LFD | HFD | LFD | HFD | |
| Protein | 19.2 | 26.2 | 20 | 20 |
| Carbohydrate | 67.3 | 26.3 | 70 | 20 |
| Fat | 4.3 | 34.9 | 10 | 60 |
| Weight, g | kcal | |||
|---|---|---|---|---|
| Casein | 200 | 200 | 800 | 800 |
| l-cystine | 3 | 3 | 12 | 12 |
| Corn starch | 506.2 | 0 | 2024.8 | 0 |
| Maltodextrin | 125 | 125 | 500 | 500 |
| Sucrose | 68.8 | 68.8 | 275.2 | 275.2 |
| Cellulose | 50 | 50 | 0 | 0 |
| Soybean oil | 25 | 25 | 225 | 225 |
| Lard | 20 | 245 | 180 | 2205 |
Both diets were obtained from Research Diets (New Brunswick, NJ).
LFD, low-fat diet (cat. no. D12450J), HFD high-fat diet (cat. no. D12492).
Animals
Mice (C57BL/6J, male, 8 wk of age, 156 total; Jackson Laboratories, Bar Harbor, ME) were individually housed in a temperature-controlled room (22°C) with a normal 12:12-h light-dark cycle (lights on at 0800). Upon arrival, animals were given ad libitum access to a LFD and water. All protocols followed the Canadian Council on Animal Care guidelines and were approved by the Animal Care Committee at the University of Guelph.
Training and feeding protocol.
Mice were acclimated to treadmill running during two short, low-intensity sessions (10 min, 15 m/min, 5% incline). Forty-eight hours after the last acclimation session, mice were randomly assigned to the training (TR) or sedentary (SED) group. Training consisted of treadmill running for 1 h/day, 5 days/wk for 4 wk (trained during the light cycle, between 0900 and 1100). Treadmill speed was increased weekly: 20, 22, 23, and 25 m/min. The incline was also increased weekly: 10%, 15%, and 20% (remaining at 20% for week 4). This training protocol has previously been shown by our laboratory to induce the expected increases in markers of mitochondrial content in skeletal muscle (4). During this training period, both SED and TR mice were provided with a LFD ad libitum. Twenty-four hours after the final exercise bout, mice either remained on the LFD or were provided HFD ad libitum (60% kcal from lard) for an additional 4 days. An additional group of SED mice were provided with the HFD but were given 30% fewer kilocalories per day (∼2.4 g of food) than the average daily food intake of the SED HFD ad libitum group of mice. This was done in an attempt to limit weight gain to the same amount as that seen in the trained ad libitum HFD group. Body weight and food consumption were recorded daily throughout the high-fat feeding.
Glucose Tolerance Test
An intraperitoneal glucose tolerance test (ipGTT) was performed following a 4-h fast on the third day of high-fat feeding. Blood glucose was assessed using a glucometer (Freestyle lite, Abbott Laboratories, Saint-Laurent, QC, Canada) through tail vein sampling at 0, 10, 20, 30, 45, 60, 90, and 120 min after an intraperitoneal injection of glucose (2 g/kg ip body wt).
Metabolic Caging
Mice were placed in a Comprehensive Lab Animal Monitoring System (CLAMS; Columbus Instruments, Columbus, OH) at the start of the third day of high-fat feeding. The first 24 h in the CLAMS caging was used as an acclimation period, and data collected over the subsequent 24 h were used for the determination of energy expenditure and physical activity levels. Animals were maintained on the same light-dark cycle and were provided water and food ad libitum. The light cycle data were averaged or summed per animal from 0900 to 2100, and the dark cycle data were averaged or summed from 2100 to 0900 of the second day only. The modified Weir equation (31) was used to calculate total energy expenditure (TEE): (3.9 × V̇o2) + (1.1 × V̇co2).
TEE was expressed as absolute energy expenditure over 24 h. Activity was estimated by summing the number of beam breaks in the x- and z-planes. Z beam breaks measure rearing activity, while x-ambulatory measures mouse ambulation in the caging. X-total is calculated as the difference between all beam breaks in the x-plane and x-ambulatory measures.
Tissue Sample Collection
After 4 days on a HFD (and 5 days after the final bout of exercise), mice were anesthetized (pentobarbital sodium, 60 mg/kg ip injection) for tissue collection at 0900 and in the fed state. The right epididymal white adipose tissue (eWAT) and inguinal subcutaneous adipose tissue (scAT) fat pads, quadriceps (rectus femoris, vastus lateralis, vastus medialis, and vastus intermedius), soleus, extensor digitorum longus, and plantaris muscles were removed, weighed, and flash frozen in liquid nitrogen. The mouse was then injected with a weight-adjusted bolus of insulin (1 U/kg body wt ip injection), and the contralateral fat pads and muscles, interscapular brown adipose tissue (BAT), and liver were removed after 15 min of insulin stimulation, weighed, and flash frozen in liquid nitrogen. As the liver could not be removed prior to the insulin injection in the previous experiments, an additional group of mice were anesthetized (pentobarbital sodium, 60 mg/kg ip injection) and injected with saline; the liver was removed 15 min later to compare with insulin-stimulated liver samples to nonstimulated control mice. All mice were euthanized by exsanguination. Insulin-stimulated tissues were only used for measuring phosphorylated Akt.
Western Blot Analysis
Adipose tissue, liver, and quadriceps muscle were homogenized (FastPrep-24, MP Biomedicals, Santa Ana, CA) in NP40 Lysis Buffer (3, 30, and 25 volumes, respectively) supplemented with phenylmethylsulfonylfluoride and protease inhibitor cocktail. Protein concentration was determined by the bicinchoninic acid method (24). An equal amount of protein was loaded and separated on SDS-PAGE gels. Protein was transferred to nitrocellulose membrane by wet transfer (100 V, 60 min). Membranes were blocked for 1 h in 7.5% BSA dissolved in Tris-buffered saline containing 0.1% Tween-20 (TBS-T). Membranes were incubated with appropriate primary antibody (diluted in TBS-T containing 7.5% BSA) over night at 4°C (anti-pAktThr-308, anti-tAkt, anti-PDH E1α, anti-CS, anti-CORE1, anti-COXIV, anti-UCP-1, anti-GAPDH, and anti-α tubulin all diluted to 1:1,000). Following a 1-h incubation with horseradish peroxidase-linked secondary antibody, bands were visualized using enhanced chemiluminescence reagent and quantified by densitometry (Alpha Innotech Fluorchem HD2, ThermoFisher Scientific, Ottawa, ON, Canada). Equal loading was confirmed both by Ponceau S staining and either GAPDH or α-tubulin housekeeping proteins.
Real-Time Quantitative PCR
Total RNA was extracted from scAT and eWAT using a combined QiaZol and RNeasy tissue kit protocol (Qiagen, Toronto, ON, Canada) followed by DNase treatment, according to the manufacturer's instructions (4). Quantity and purity were determined spectrophotometrically (NanoDrop2000, ThermoFisher Scientific). Total RNA (0.5 μg) was used to synthesize complementary DNA using SuperScript II Reverse Transcriptase, random primers, and dNTPs, and then it was diluted five-fold with ultrapure water after synthesis. Gene expression was determined in duplicate by real-time PCR (AB 7500; Applied Biosystems, Foster City, CA) using 5 μl of cDNA template and gene expression assays for UCP-1, and GAPDH (housekeeping gene). The difference in gene expression in the HFD condition was determined relative to its respective LFD condition using the 2−ΔΔCt method (16). GAPDH did not change with either diet or exercise.
Mitochondrial Respiration
Adipose tissue respiration was determined as previously described, with minor modifications (3, 13). Briefly, interscapular BAT or eWAT was excised from sedentary or previously trained high-fat fed mice, immediately placed in BIOPS buffer, and minced with scissors. Tissue was blotted and 2 mg (BAT) or 20 mg (eWAT) was used to determine rates of oxygen consumption by high-resolution respirometry (Oroboros Oxygraph-2k, Innsbruck, Austria). Respiration experiments were performed at 37°C in hyperoxygenated MiR05 buffer. Digitonin (10 μM) was used to permeabilize the membrane. For BAT, the sequential respiration protocol consisted of determining respiration in the presence of 10 mM pyruvate and 2 mM malate, maximal complex I, and complex II-supported respiration in the presence of 10 mM glutamate and 10 mM succinate, leak respiration in the presence of 7.5 μM oligomycin, and UCP-inhibited respiration in the presence of 4 mM GDP. For eWAT, the sequential respiration protocol consisted of determining respiration in the presence of 10 mM pyruvate + 2 mM malate, state III respiration in the presence 5 mM ADP (complex I), followed by the sequential addition of 10 mM glutamate (to maximize complex I) and 10 mM succinate (complex I + complex II) to determine maximal complex I and complex II-supported respiration. The subsequent titration of dinitrophenol yielded maximal respiration in both tissues and cytochrome c-stimulated respiration <10% in all experiments.
Skeletal Muscle and Liver Lipid Analysis
Frozen quadriceps muscle and liver were homogenized in 10 volumes of Tris·HCl buffer (pH 8.0) and used for lipid analysis. Total lipids were extracted (7), and diacyl- and triacylglycerols and ceramides from each sample were separated using thin-layer chromatography (12) and HPLC (34). Isolated ceramide, diacyl- and triacylglycerol samples were spiked with tridecanoic acid (13:0; internal standard) and were methylated (17); the fatty acid composition of each was then analyzed by gas chromatography, as previously described (2). Briefly, the fatty acid methyl esters were separated on an UFM-RTX WAX analytical column (Thermo Electron, Milan, Italy) using a gas chromatograph (Trace GC Ultra, Thermo Electron) fitted with a fast flame ionization detector, a split-splitless injector, and a Triplus AS autosampler. Fatty acids were identified by retention time compared with known standards (Supelco 37 component FAME mix, Supelco, Bellefonte, PA), and the absolute amount of each individual fatty acid was calculated using tridecanoic acid. Preliminary analyses indicated no detectable endogenous 13:0 in the samples analyzed (data not shown).
Statistical Analyses
Data were analyzed using a two-tailed unpaired Student's t-test, a two-way ANOVA or a three-way ANOVA with a Fisher least significant difference post hoc test where appropriate, using the Sigma Plot software package. A value of P < 0.05 was considered significant. All data are reported as means ± SE.
RESULTS
Characterization of the Exercise-Trained Model
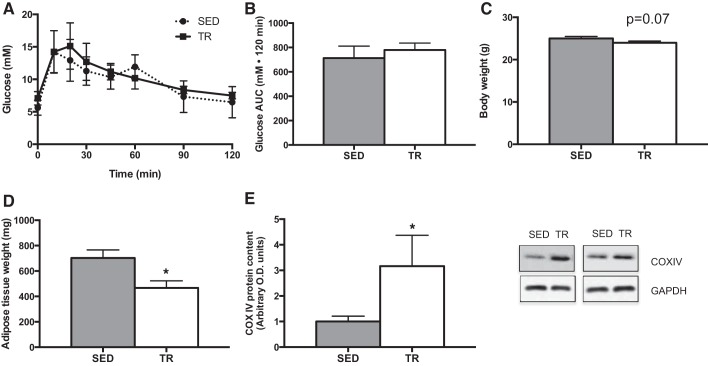
Before starting the low- or high-fat diets, the effects of the exercise training intervention were characterized. As shown in Fig. 1, A and B, there were no differences in glucose tolerance between sedentary and trained mice. There was a trend (P = 0.074) for exercise-trained mice to weigh less than sedentary animals (Fig. 1C), while the sum weight of the inguinal and epididymal adipose tissue depots was less in exercise-trained compared with sedentary mice (Fig. 1D). Consistent with what we have published previously with this training model (4, 9), COXIV protein content in quadriceps muscle was increased (P = 0.02) with exercise training (Fig. 1E).
Fig. 1.
Characterization of the effects of a 4-wk exercise training protocol. Intraperitoneal glucose tolerance test [A; sedentary (SED), ●; training (TR), ■], total area under the curve (AUC) (B; gray bar denotes SED, while white bar denotes TR), absolute terminal body weight (C), adipose tissue (combined epididymal and inguinal subcutaneous) weight (D), and quadriceps muscle cytochrome oxidase subunit IV (COX IV) content (E) following 4 wk of exercise training with increasing intensity in SED (gray bar) and TR (white bar) mice. Data are presented as means ± SE; n = 5. *Significantly different from SED, P < 0.05.
Prior Exercise Training Attenuates High-Fat Diet-Induced Weight Gain
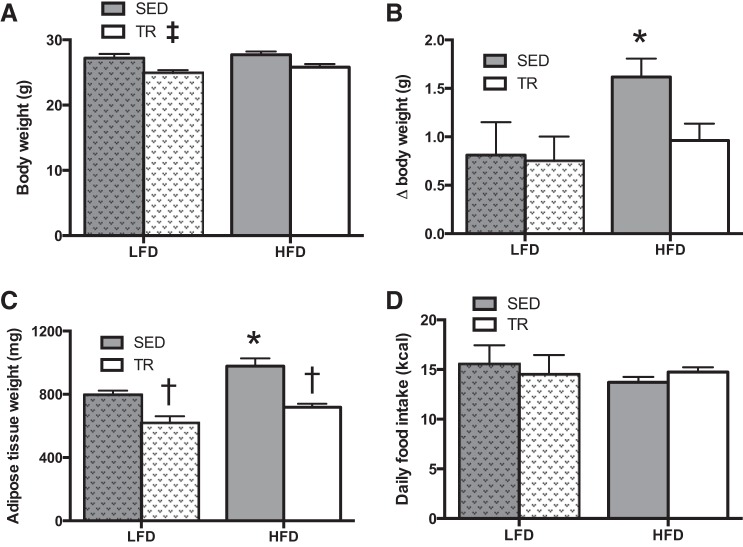
Next, we provided sedentary or previously exercise-trained mice on a LFD or HFD for 4 days. TR mice weighed less than SED mice (P = 0.013; Fig. 2A), and the degree of weight gain caused by the HFD was blunted with prior exercise training relative to the corresponding sedentary mice (P = 0.03; Fig. 2B). Additionally, white adipose tissue (sum of the epididymal and inguinal subcutaneous) fat pad weight was reduced in TR mice fed either the LFD or the HFD (Fig. 2C; P < 0.0001). Relative to body weight, the sum weight of the plantaris, extensor digitorum longus, and soleus muscles was greater (P < 0.0001) in previously TR compared with SED mice fed the HFD (Table 2). Differences in body and tissue weights were not explained by alterations in food intake (Fig. 2D). It should be noted that food intake data were obtained during the final 24 h in the CLAMS unit. Because of the design of the system, it is not possible to accurately determine the relative contribution of spilled/spoiled food in the food intake calculations. As such, these data should be viewed cautiously, as there could be an overestimation of LFD intake due to the consistency of the powdered diets.
Fig. 2.
Prior training protects against high-fat diet-induced weight gain. Absolute terminal body weight (A), weight gain (B), and adipose tissue (combined epididymal and inguinal subcutaneous) weight (C) following 4 days of high-fat or low-fat diet feeding in sedentary (gray bars) or trained (white bars) mice. D: absolute food intake in sedentary (gray bars) or trained (white bars) mice. Hatched bars indicate LFD to easily distinguish the two feeding treatments. Data are presented as means ± SE (A–C: n = 9; D: n = 17). *Significant effect of the HFD, P < 0.05. †Significant effect of exercise training, P < 0.05. ‡Main effect of training, P < 0.05.
Table 2.
Tissue mass relative to body weight in high fat diet fed mice
| Treatment |
|||||
|---|---|---|---|---|---|
| LFD |
HFD |
||||
| Relative Mass, mg/g body wt | SED | TR | SED | TR | P Value |
| Adipose tissue | 30.33 ± 1.14 | 28.29 ± 2.11 | 34.97 ± 1.58 | 28.29 ± 1.08 | *P = 0.038 †P < 0.001 |
| Skeletal muscle | 2.75 ± 0.04 | 2.63 ± 0.09 | 2.48 ± 0.08 | 2.95 ± 0.03 | ‡P = 0.015 #P = 0.023 §P < 0.001 |
Data are presented as means ± SE (n = 16–22, except muscle n = 3–4). White adipose tissue is composed of epididymal and inguinal subcutaneous, skeletal muscle was represented by soleus, extensor digitorum longus, and plantaris, as these muscles can be removed tendon to tendon to ensure complete removal.
SED, sedentary; TR, training.
Main effect of diet,
main effect of exercise,
interaction, #SED LFD vs. SED HFD,
SED HFD vs. TRD HFD, all P < 0.05.
Total food intake was also determined in sedentary and previously exercise-trained mice fed the HFD over the 4 days of the diet intervention. These measures were completed in the animal's home cage and were consistent with the data obtained in the CLAMS caging, which demonstrated that differences in weight gain on the HFD are not secondary to changes in food intake (cumulative 4-day intake: 59.0 ± 1.5 kcal trained, 57.8 ± 3.1 kcal, P = 0.74; n = 8 per group).
Prior Exercise Training Slightly Attenuates High-Fat Diet-Induced Glucose Intolerance
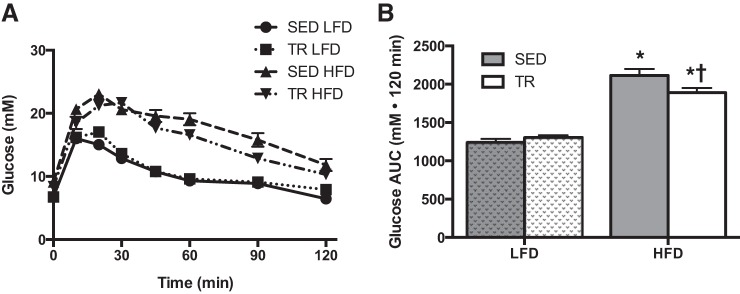
To examine the physiological importance of the blunted weight gain in previously trained mice, mice were challenged with an ipGTT on the third day of high-fat feeding. In both the SED and the TR condition, high-fat feeding induced glucose intolerance relative to the low-fat diet-fed animals (Fig. 3, A and B; P = 0.03). However, within the high-fat-fed groups, the impairment with high-fat feeding was slightly blunted (P = 0.016) with prior training compared with sedentary mice.
Fig. 3.
Prior training modestly blunts high-fat diet-induced glucose intolerance. Intraperitoneal glucose tolerance test (A; SED LFD, ●; TR LFD, ■; SED HFD, ▲, TR HFD, ▼) and total area under the curve (B: gray bars denote SED, while white bars denote TR) after 3 days of high-fat feeding. Stippled bars indicate LFD to easily distinguish the two feeding treatments. Data are presented as means ± SE (A and B: n = 9). *Significant effect of the high fat diet, P < 0.05. †Significant effect of exercise training, P < 0.05.
Limiting Weight Gain on a High-Fat Diet Mimics the Protective Effects of Prior Exercise Training on the Development of Glucose Intolerance
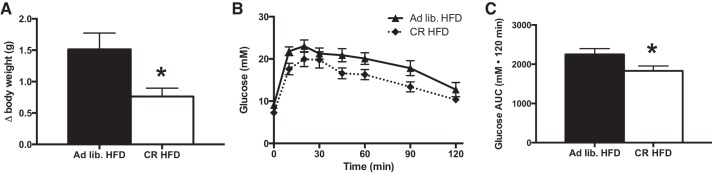
As prior exercise training protected against HFD-induced weight gain, it is difficult to determine whether the modest improvements with prior training on attenuating glucose intolerance were the result of training per se or perhaps secondary to the effects of reduced weight gain. In an attempt to answer this question, we completed an additional experiment in which the food intake of sedentary mice on a HFD was restricted to match the weight gain of previously trained mice. As shown in Fig. 4, restricting HFD consumption by ∼30% of the ad libitum intake led to an attenuation in weight gain to a similar extent as that observed in previously trained mice (Fig. 4A). Mice whose food intake was restricted displayed a similar blunting of HFD-induced glucose intolerance as that observed in the previously trained mice on the HFD (Fig. 4, B and C).
Fig. 4.
Attenuated weight gain by calorie restriction provides similar benefits as prior exercise training on the development of glucose intolerance following high-fat feeding. Change in body weight (A) and glucose tolerance [B; ad libitum HFD (▲), calorie restricted (CR) HFD (⧫)], and total glucose AUC (C) after 3 days of high-fat feeding (black bars denote ad libitum feeding while white bars = CR). Data are presented as means ± SE; n = 8. *Significantly different from ad libitum fed, P < 0.05.
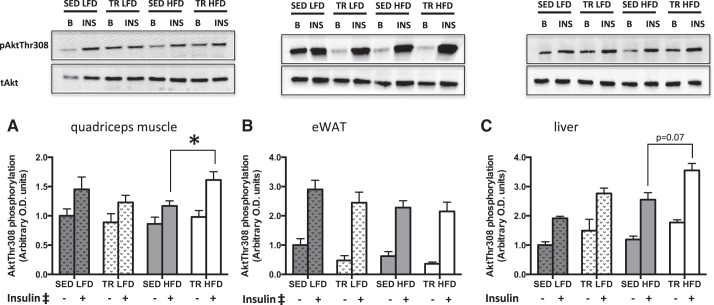
Akt Phosphorylation Is Greater in Skeletal Muscle From Previously Exercise-Trained Mice
We next sought to determine whether the slight blunting of glucose intolerance with prior exercise training in mice fed the HFD was associated with differences in insulin signaling. There was a main effect of insulin on threonine 308 Akt phosphorylation in both quadriceps muscle (P < 0.001) and eWAT (P < 0.001). In quadriceps, there was an interaction between diet and training, such that Akt phosphorylation in trained mice on the HFD was higher than that of sedentary mice on the HFD, (Fig. 5A; P = 0.041). Compared with HFD-fed sedentary mice, there was a trend toward increases in Akt phosphorylation in the liver from TR HFD (Fig. 5C; P = 0.07). Although higher in all conditions compared with basal, a main effect of insulin could not be properly interpreted due to the interaction between diet and insulin (P = 0.024) and the interaction between insulin and training (P = 0.041) in the liver.
Fig. 5.
Insulin signaling is enhanced in skeletal muscle from previously trained mice fed a high-fat diet. Basal (gray bar) and insulin-stimulated (white bar) phosphorylation of Akt (∼60 kDa) at Thr-308 in quadriceps muscle (A), epididymal white adipose tissue (B), and liver tissue (C) of low-fat (LFD) or high-fat (HFD) fed sedentary (SED) or trained (TR) mice. Stippled bars indicate LFD to easily distinguish the two feeding treatments. Data are presented as means ± SE; n = 9. *Significantly different from SED HFD, P < 0.05. ‡Main effect of insulin, P < 0.05.
Given the differences in AKT phosphorylation within the HFD condition only, we examined lipid content in liver and quadriceps muscle of SED and TR HFD mice to determine whether reactive lipids could be associated with the differences in Akt phosphorylation. As shown in Tables 3 and 4, there were no significant differences in the content of diacylglycerol or ceramides in liver and skeletal muscle from previously trained compared with sedentary mice fed a HFD. Both of these lipid moieties have been associated with insulin resistance (28, 35). Additionally, triacylglycerol content was not different in liver or skeletal muscle (Tables 3 and 4).
Table 3.
Quadriceps muscle triacylglycerol and diacylglycerol profiles
| Treatment |
|||
|---|---|---|---|
| Fatty Acid, μmol/g muscle | SED HFD | TR HFD | P Value |
| Total TG SFA | 3.41 ± 0.31 | 2.73 ± 0.28 | 0.15 |
| Total TG MUFA | 3.45 ± 0.37 | 2.89 ± 0.35 | 0.32 |
| Total TG PUFA | 0.60 ± 0.06 | 0.50 ± 0.03 | 0.25 |
| Total TG n3 | n.d. | n.d. | |
| Total TG n6 | 0.59 ± 0.06 | 0.50 ± 0.03 | 0.25 |
| Total triacylglycerol | 7.47 ± 0.73 | 6.12 ± 0.64 | 0.23 |
| Total DG MUFA* | 0.33 ± 0.12 | 0.25 ± 0.03 | 0.44 |
| Total ceramides | 1.10 ± 0.22 | 0.71 ± 0.13 | 0.16 |
Data are presented as means ± SE; n = 5–7.
No other species of DG was detected.
TG, triacylglycerols, SFA, saturated fatty acids, MUFA, monounsaturated acids, PUFA, polyunsaturated acids, DG, 1,2 diacylglycerols; n.d., not detected.
Table 4.
Liver triacylglycerol and diacylglycerol profiles
| Treatment |
|||
|---|---|---|---|
| Fatty Acid, μmol/g liver | SED HFD | TR HFD | P Value |
| Total TG SFA | 3.39 ± 0.44 | 3.37 ± 0.31 | 0.97 |
| Total TG MUFA | 8.06 ± 1.05 | 7.78 ± 0.76 | 0.83 |
| Total TG PUFA | 4.14 ± 0.53 | 3.94 ± 0.41 | 0.77 |
| Total TG n3 | n.d. | n.d. | |
| Total TG n6 | 4.14 ± 0.53 | 3.94 ± 0.41 | 0.77 |
| Total triacylglycerol | 15.59 ± 2.02 | 15.09 ± 1.45 | 0.84 |
| Total 1,2 DG SFA | 0.072 ± 0.031 | 0.012 ± 0.008 | 0.12 |
| Total 1,2 DG MUFA | 0.36 ± 0.15 | 0.28 ± 0.56 | 0.63 |
| Total 1,2 DG PUFA | 0.17 ± 0.064 | 0.31 ± 0.094 | 0.25 |
| Total 1,2 DG n3 | n.d. | n.d. | |
| Total 1,2 DG n6 | 0.17 ± 0.064 | 0.31 ± 0.064 | 0.25 |
| Total 1,2 diacylglycerol | 0.61 ± 0.23 | 0.60 ± 0.061 | 0.98 |
| Total 1,3 DG SFA | 1.59 ± 0.66 | 1.67 ± 0.60 | 0.93 |
| Total 1,3 DG MUFA | 3.99 ± 1.50 | 4.07 ± 1.38 | 0.97 |
| Total 1,3 DG PUFA | 1.88 ± 0.82 | 1.87 ± 0.72 | 0.99 |
| Total 1,3 DG n3 | n.d. | n.d. | |
| Total 1,3 DG n6 | 1.88 ± 0.82 | 1.86 ± 0.72 | 0.99 |
| Total 1,3 diacylglycerol | 7.45 ± 2.97 | 7.61 ± 2.69 | 0.97 |
| Total ceramides | 0.44 ± 0.09 | 0.53 ± 0.09 | 0.49 |
Data are presented as means ± SE; n = 5–7. No other species of DG was detected.
SFA, saturated fatty acids.
Prior Exercise Training Results in a Larger Shift in Energy Expenditure with High-Fat Diet Feeding
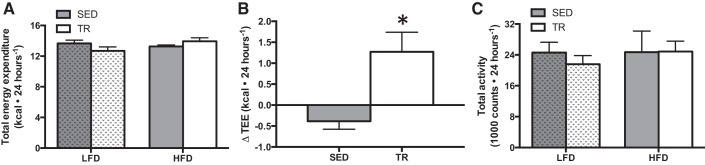
Despite consuming the same amount of food, previously trained mice gained less weight on the HFD than their sedentary counterparts. This suggested that there could be differences in energy expenditure. To examine this possibility, whole body oxygen consumption was measured in metabolic caging. Using the modified Weir equation (31), we calculated energy expenditure using V̇o2 and V̇co2. On an absolute level, there was a trend (P = 0.066) toward an interaction between training and diet in total energy expenditure (TEE), such that the TR HFD-fed mice expended more energy than the SED HFD-fed mice (Fig. 6A). When we calculated the difference in TEE between low-fat and high-fat conditions in each group, the change in TEE was much greater in previously trained compared to sedentary mice (Fig. 6B; P = 0.007). There were no differences in the respiratory exchange ratio between sedentary and previously trained mice fed the high-fat diet (0.818 ± 0.008 sedentary, 0.835 ± 0.003, trained). The changes in energy expenditure were independent of total activity (Fig. 6C).
Fig. 6.
The change in total energy expenditure is higher in trained compared with sedentary mice given a high-fat diet. Indirect calorimetric measures of total energy expenditure (TEE; A), change in TEE upon high-fat feeding (B) and total activity (C) on the fourth day of feeding in sedentary and previously trained mice (gray bars denote SED, while white bars denote TR). Stippled bars indicate LFD to easily distinguish the two feeding treatments. Data are presented as means ± SE; n = 7. *Significantly different from SED, P < 0.05.
Attenuated Weight Gain Is Associated with Increases in Select Mitochondrial Proteins, but not Respiration in Brown and White Adipose Tissue
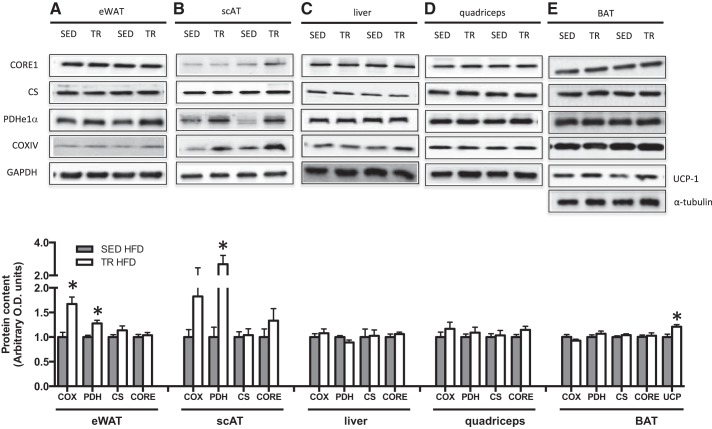
In the absence of differences in cage activity, previously trained mice had both attenuated weight gain and a trend toward increased TEE upon ingestion of a high-fat diet compared with SED mice. We next sought to explore a potential mechanism that could be mediating increases in energy expenditure independent of activity; thus, we measured markers of mitochondrial content in eWAT, scAT, quadriceps muscle, liver, and interscapular BAT. As differences in weight gain and energy expenditure were only evident in the presence of a high-fat diet, we focused our analysis on samples from these mice. Although controversial (32), it has been suggested that exercise training can increase both browning of WAT (32, 33) and activation and/or uncoupling of BAT (6, 23). These adaptations could lead to increased thermogenesis and as a result, perhaps, increased oxygen consumption. Five days after the final bout of exercise, PDH E1α protein was increased in eWAT and scAT of previously trained mice (P = 0.002 and P = 0.001 respectively; Fig. 7), while COX IV was increased in eWAT only. In samples from mice fed a LFD, there were no differences in COXIV or PDH E1α in either eWAT or scAT (data not shown). CORE1 and citrate synthase (CS) were not different between groups in both tissue depots. Markers of mitochondrial content in muscle, liver, and BAT (Fig. 7) were similar between previously trained and sedentary mice. This is not surprising, as it has previously been shown in muscle that citrate synthase activity returns to baseline levels after 7 days of detraining (19), and we collected our tissue 5 days after the final bout of exercise. There was no difference in UCP-1 mRNA expression in eWAT or scAT between trained and sedentary mice (Table 5). However, in BAT, UCP-1 protein content was increased (P = 0.008; Fig. 7) with previous training.
Fig. 7.
The content of mitochondrial proteins, is increased in eWAT, scAT, and BAT from previously trained HFD-fed mice. Protein content and representative Western blots of various markers of mitochondrial content in epididymal white adipose tissue (eWAT; A), subcutaneous inguinal white adipose tissue (scAT; B), liver (C), quadriceps tissue (D), and interscapular brown adipose tissue (BAT; E) in high-fat fed sedentary (gray bars, SED) and trained (white bars, TR) mice. Data are presented as mean ± SE; n = 7–15. *Significantly different from SED, P < 0.05. The approximate apparent molecular weights for the detected proteins are as follows: CORE1, 50 kDa; PDH E1α, 43 kDa; COXIV, 15 kDa; UCP-1, 33 kDa; GAPDH, 36 kDa, tubulin, 50 kDa.
Table 5.
mRNA expression of UCP-1 in epididymal and inguinal subcutaneous white adipose tissue
| Treatment |
||||
|---|---|---|---|---|
| SED LFD | TR LFD | SED HFD | TR HFD | |
| eWAT | 1.58 ± 0.49 | 1.39 ± 0.56 | 1.10 ± 0.22 | 0.84 ± 0.13 |
| scAT | 1.35 ± 0.38 | 1.13 ± 0.26 | 1.78 ± 0.68 | 1.12 ± 0.41 |
Data are presented as means ± SE; n = 8–9. mRNA expression is expressed relative to respective low-fat diet controls. GAPDH was used as the housekeeping gene.
UCP-1, uncoupling protein-1; eWAT, epididymal white adipose tissue; scAT, inguinal subcutaneous white adipose tissue.
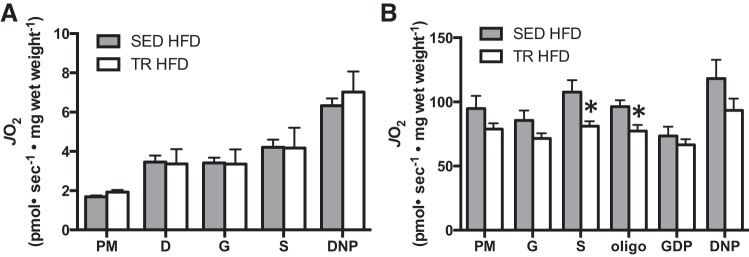
Measures of mitochondrial protein content can give insight into the maximal respiratory capacity of a given tissue (15). To gain insight into the functionality of the observed protein changes in white and brown adipose tissue, we used respirometry to measure mitochondrial function. We chose to use eWAT for these experiments, as increases in markers of mitochondrial content were more consistent in this tissue compared with scAT. Despite increases in mitochondrial markers (eWAT) and UCP-1 (BAT), these changes were not reflected in increased oxygen consumption in either tissue (Fig. 8, A and B), with some indices in BAT demonstrating decreased oxygen consumption.
Fig. 8.
Mitochondrial respiration in adipose tissue is not increased in previously trained mice fed a high-fat diet. Mitochondrial respiration measured by high-resolution respirometry in epididymal white adipose tissue (A) and interscapular brown adipose tissue (B). Gray bars denote SED HFD, while white bars denote TR HFD. P, pyruvate; M, malate; D, ADP; G, glutamate; S, succinate; oligo, oligomycin; DNP, dinitrophenol; JO2, rate of oxygen consumption. Data are presented as means ± SE; n = 7. *Significantly different from SED, P < 0.05.
DISCUSSION
The current study provides evidence that prior exercise training attenuates short-term HFD-induced weight gain, and this was associated with a greater change in TEE relative to SED mice when challenged with a HFD. Interestingly, despite an almost complete protection against HFD-induced weight gain, prior training only offered a modest protection against HFD-induced glucose intolerance, which was reflected in slightly greater levels of Akt phosphorylation.
An important novel aspect of this study is that mice were not exercising during the period of high-fat feeding and, thus, the prevention of weight gain cannot be explained by increases in energy expenditure during training sessions. Our findings are consistent with recent work demonstrating that rats bred for high instrinsic aerobic capacity compared with low intrinsic aerobic capacity are protected against short-term HFD-induced increases in markers of hepatic steatosis (18).
To help determine whether the slight beneficial effects of prior exercise training on glucose intolerance were related to an attenuation of HFD-induced weight gain, we completed an additional set of experiments in which we limited the food intake of sedentary mice to match the HFD-induced weight gain of that seen in previously trained mice. In this instance, we found that an attenuation of weight gain via caloric restriction led to the same protective effect of prior exercise training on HFD-induced glucose intolerance. Although the mechanisms of action leading to this reduction in weight gain are likely different in these two models, we interpret these findings as suggesting that the effects of prior training on glucose tolerance may be tied, in part, to the prevention of weight gain. It should be noted that glucose tolerance was assessed using a weight-adjusted, bolus injection of glucose. Because the sedentary mice were somewhat heavier than the previously trained mice, this results in the sedentary animals being challenged, in absolute terms, with more glucose. This could, in part, explain the differences in glucose tolerance. However, as we do not see differences in the clearance of blood glucose between LFD-fed sedentary or previously exercise-trained mice, nor in the absolute peak glucose within a diet treatment, we reason that this difference in glucose bolus does not have an appreciable effect.
As prior exercise attenuated HFD-induced body weight gain, despite similar food intake, we reasoned that whole body energy expenditure could be altered in previously trained mice. In fact, when the difference in TEE between mice fed a LFD and HFD was compared, this change was significantly greater in mice that had been previously trained. These results suggest, at least at the time point measured, that prior exercise training alters the ability of the organism to respond to nutrient challenges. While the increase in TEE in previously trained mice fed a HFD compared with LFD was subtle, this could be one potential mechanism explaining the protection against HFD-induced weight gain.
It is becoming increasingly well accepted that adipose tissue plays a key role in regulating whole body fuel metabolism (26, 27, 32). Several studies have reported increases in the thermogenic capacity of classical white adipose tissue depots with exercise training (1, 33). In fact, the transplantation of adipose tissue from trained mice into mice fed a high-fat diet increases whole body energy expenditure (27), although this was not associated with an appreciable degree of weight loss. Given this, we were interested in determining whether markers of mitochondrial content were increased along with the changes in energy expenditure. Although PDH E1α was increased in both eWAT and scAT from previously trained mice and COX IV was increased in eWAT, this occurred in the absence of an increase in UCP-1 expression, a marker of the thermogenic gene program (5). When mitochondrial oxygen consumption was measured, we found no difference in any measure between sedentary and previously trained HFD-fed mice, arguing against eWAT oxidative capacity being responsible for the observed changes in whole body metabolism.
Brown adipose tissue is thought to play a significant role in whole body oxygen consumption. For example, it has been estimated in humans that only 50 g of BAT (∼0.1% of body mass) could account for up to 20% of daily energy expenditure (22). This suggests that perhaps even modest increases in UCP-1 (∼20% seen in this study) could have implications on whole body energy expenditure and might be a potential mechanism to explain, at least a portion of the attenuation in weight gain that we observed in previously trained mice. Surprisingly though and in agreement with several previous studies (8, 29, 32), we did not observe an increase in BAT mitochondrial function when measured ex vivo, and in some measures, we even saw a decrease in oxygen consumption in BAT from trained mice. This, coupled with the fact that interscapular BAT mass was somewhat reduced (data not shown) suggests that changes in BAT likely do not explain the differences in whole body metabolism seen in the current investigation. With this being said, it should be noted that studying tissue function ex vivo cannot perfectly mimic the hormonal and metabolic milieu of the in vivo environment and, thus, we cannot rule out a potential role of in vivo BAT activation.
It has previously been shown that lean body mass is strongly correlated with energy expenditure (reviewed in Ref. 25). Additionally, it has been reported in humans that exercise itself has no effect on resting metabolic rate; however, the increase in lean body mass associated with regular exercise results in a modest change in energy expenditure (∼8–12%; Ref. 10). In the current study, the total and relative amount of epididymal and inguinal adipose tissue was reduced in previously trained compared with sedentary mice, and the combined plantaris, extensor digitorum longus, and soleus muscle weight was greater in trained compared with sedentary mice on the HFD. These findings, while admittedly only a crude proxy of body composition, would suggest that lean body mass is greater in the trained mice and could be contributing to changes in total energy expenditure under conditions of the HFD challenge.
Perspectives and Significance
In summary, we have provided novel data demonstrating that prior exercise training attenuates short-term high-fat diet-induced increases in weight gain and modestly protects against HFD-induced glucose intolerance. The difference in TEE between low- and high-fat-fed mice was greater in the mice that had been previously trained, and this could potentially account for some of the protective effects of prior exercise. Our findings highlight the powerful and surprisingly long-lasting effects of exercise training in protecting against a metabolic challenge.
GRANTS
This work was funded by the Canadian Institutes of Health Research (to D. C. Wright, Grant MOP 130525) and The Natural Sciences and Engineering Research Council of Canada (to P. J. LeBlanc, Grant 327015 and to G. P. Holloway, Grant 03656), and infrastructure was purchased with the assistance of the Canadian Foundation for Innovation/Ontario Research Fund (to D. C. Wright, Grant 218463 and to G. P. Holloway, Grant 25136). D. C. Wright is a Tier II Canada Research Chair in Lipids Metabolism and Health. L. A. Snook was supported by a Canadian Graduate Scholarship from the Natural Science and Engineering Research Council of Canada. R. E. K. MacPherson was supported by an Alzheimer's Society postdoctoral fellowship. C. Monaco was supported by a postgraduate scholarship from the Natural Science and Engineering Research Council of Canada. S. Frendo-Cumbo was supported by a postgraduate scholarship from the Natural Science and Engineering Research Council of Canada.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.A.S., P.J.L., G.P.H., and D.C.W. conception and design of research; L.A.S., R.E.K.M., C.M.F.M., S.F.-C., L.C., W.T.P., Z.G.A., S.L.B., and P.J.L. performed experiments; L.A.S., R.E.K.M., C.M.F.M., S.F.-C., L.C., W.T.P., Z.G.A., S.L.B., P.J.L., and D.C.W. analyzed data; L.A.S., R.E.K.M., S.F.-C., L.C., W.T.P., Z.G.A., S.L.B., G.P.H., and D.C.W. interpreted results of experiments; L.A.S. prepared figures; L.A.S., G.P.H., and D.C.W. drafted manuscript; L.A.S., C.M.F.M., S.F.-C., L.C., W.T.P., Z.G.A., S.L.B., P.J.L., G.P.H., and D.C.W. edited and revised manuscript; L.A.S., R.E.K.M., C.M.F.M., S.F.-C., L.C., W.T.P., Z.G.A., S.L.B., P.J.L., G.P.H., and D.C.W. approved final version of manuscript.
REFERENCES
- 1.Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Hojlund K, Gygi SP, Spiegelman BM. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481: 463–468, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradley NS, Heigenhauser GJ, Roy BD, Staples EM, Inglis JG, LeBlanc PJ, Peters SJ. The acute effects of differential dietary fatty acids on human skeletal muscle pyruvate dehydrogenase activity. J Appl Physiol (1985) 104: 1–9, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Canto C, Garcia-Roves PM. High-resolution respirometry for mitochondrial characterization of ex vivo mouse tissues. Curr Prot Mouse Biol 5: 135–153, 2015. [DOI] [PubMed] [Google Scholar]
- 4.Castellani L, Root-Mccaig J, Frendo-Cumbo S, Beaudoin MS, Wright DC. Exercise training protects against an acute inflammatory insult in mouse epididymal adipose tissue. J Appl Physiol (1985) 116: 1272–1280, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen P, Levy JD, Zhang Y, Frontini A, Kolodin DP, Svensson KJ, Lo JC, Zeng X, Ye L, Khandekar MJ, Wu J, Gunawardana SC, Banks AS, Camporez JP, Jurczak MJ, Kajimura S, Piston DW, Mathis D, Cinti S, Shulman GI, Seale P, Spiegelman BM. Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell 156: 304–316, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dinas PC, Nikaki A, Jamurtas AZ, Prassopoulos V, Efthymiadou R, Koutedakis Y, Georgoulias P, Flouris AD. Association between habitual physical activity and brown adipose tissue activity in individuals undergoing PET-CT scan. Clin Endocrinol (Oxf) 82: 147–154, 2015. [DOI] [PubMed] [Google Scholar]
- 7.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226: 497–509, 1957. [PubMed] [Google Scholar]
- 8.Gohil K, Henderson S, Terblanche SE, Brooks GA, Packer L. Effects of training and exhaustive exercise on the mitochondrial oxidative capacity of brown adipose tissue. Biosci Rep 4: 987–993, 1984. [DOI] [PubMed] [Google Scholar]
- 9.Herbst EA, Roussakis C, Matravadia S, Holloway GP. Chronic treadmill running does not enhance mitochondrial oxidative capacity in the cortex or striatum. Metabolism 64: 1419–1425, 2015. [DOI] [PubMed] [Google Scholar]
- 10.Horton TJ, Geissler CA. Effect of habitual exercise on daily energy expenditure and metabolic rate during standardized activity. Am J Clin Nutr 59: 13–19, 1994. [DOI] [PubMed] [Google Scholar]
- 11.Jordy AB, Kraakman MJ, Gardner T, Estevez E, Kammoun HL, Weir JM, Kiens B, Meikle PJ, Febbraio MA, Henstridge DC. Analysis of the liver lipidome reveals insights into the protective effect of exercise on high-fat diet-induced hepatosteatosis in mice. Am J Physiol Endocrinol Metab 308: E778–E791, 2015. [DOI] [PubMed] [Google Scholar]
- 12.Kishimoto K, Urade R, Ogawa T, Moriyama T. Nondestructive quantification of neutral lipids by thin-layer chromatography and laser-fluorescent scanning: suitable methods for “lipidome” analysis. Biochem Biophys Res Commun 281: 657–662, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Kraunsoe R, Boushel R, Hansen CN, Schjerling P, Qvortrup K, Stockel M, Mikines KJ, Dela F. Mitochondrial respiration in subcutaneous and visceral adipose tissue from patients with morbid obesity. J Physiol 588: 2023–2032, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lancaster GI, Febbraio MA. The immunomodulating role of exercise in metabolic disease. Trends Immunol 35: 262–269, 2014. [DOI] [PubMed] [Google Scholar]
- 15.Larsen S, Nielsen J, Hansen CN, Nielsen LB, Wibrand F, Stride N, Schroder HD, Boushel R, Helge JW, Dela F, Hey-Mogensen M. Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J Physiol 590: 3349–3360, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25: 402–408, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Mahadevappa VG, Holub BJ. Quantitative loss of individual eicosapentaenoyl-relative to arachidonoyl-containing phospholipids in thrombin-stimulated human platelets. J Lipid Res 28: 1275–1280, 1987. [PubMed] [Google Scholar]
- 18.Morris EM, Jackman MR, Johnson GC, Liu TW, Lopez JL, Kearney ML, Fletcher JA, Meers GM, Koch LG, Britton SL, Rector RS, Ibdah JA, MacLean PS, Thyfault JP. Intrinsic aerobic capacity impacts susceptibility to acute high-fat diet-induced hepatic steatosis. Am J Physiol Endocrinol Metab 307: E355–E364, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neufer PD, Shinebarger MH, Dohm GL. Effect of training and detraining on skeletal muscle glucose transporter (GLUT4) content in rats. Can J Physiol Pharmacol 70: 1286–1290, 1992. [DOI] [PubMed] [Google Scholar]
- 20.Peppler WT, Anderson ZG, Sutton CD, Rector RS, Wright DC. Voluntary wheel running attenuates lipopolysaccharide-induced liver inflammation in mice. Am J Physiol Regul Integr Comp Physiol 310: R934–R942, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Picklo MJ, Thyfault JP. Vitamin E and vitamin C do not reduce insulin sensitivity but inhibit mitochondrial protein expression in exercising obese rats. Appl Physiol Nutr Metab 40: 343–352, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rothwell NJ, Stock MJ. Luxuskonsumption, diet-induced thermogenesis and brown fat: the case in favour. Clin Sci 64: 19–23, 1983. [DOI] [PubMed] [Google Scholar]
- 23.Slocum N, Durrant JR, Bailey D, Yoon L, Jordan H, Barton J, Brown RH, Clifton L, Milliken T, Harrington W, Kimbrough C, Faber CA, Cariello N, Elangbam CS. Responses of brown adipose tissue to diet-induced obesity, exercise, dietary restriction and ephedrine treatment. Exp Toxicol Pathol 65: 549–557, 2013. [DOI] [PubMed] [Google Scholar]
- 24.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem 150: 76–85, 1985. [DOI] [PubMed] [Google Scholar]
- 25.Speakman JR, Selman C. Physical activity and resting metabolic rate. Proc Nutr Soc 62: 621–634, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Stanford KI, Middelbeek RJ, Townsend KL, An D, Nygaard EB, Hitchcox KM, Markan KR, Nakano K, Hirshman MF, Tseng YH, Goodyear LJ. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest 123: 215–223, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stanford KI, Middelbeek RJ, Townsend KL, Lee MY, Takahashi H, So K, Hitchcox KM, Markan KR, Hellbach K, Hirshman MF, Tseng YH, Goodyear LJ. A novel role for subcutaneous adipose tissue in exercise-induced improvements in glucose homeostasis. Diabetes 64: 2002–2014, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stratford S, Hoehn KL, Liu F, Summers SA. Regulation of insulin action by ceramide: dual mechanisms linking ceramide accumulation to the inhibition of Akt/protein kinase B. J Biol Chem 279: 36,608–36,615, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Terblanche SE, Gohil K, Packer L, Henderson S, Brooks GA. The effects of endurance training and exhaustive exercise on mitochondrial enzymes in tissues of the rat (Rattus norvegicus). Comp Biochem Physiol A Mol Integr Physiol 128: 889–896, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Vieira VJ, Valentine RJ, Wilund KR, Antao N, Baynard T, Woods JA. Effects of exercise and low-fat diet on adipose tissue inflammation and metabolic complications in obese mice. Am J Physiol Endocrinol Metab 296: E1164–E1171, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 109: 1–9, 1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu MV, Bikopoulos G, Hung S, Ceddia RB. Thermogenic capacity is antagonistically regulated in classical brown and white subcutaneous fat depots by high fat diet and endurance training in rats: impact on whole-body energy expenditure. J Biol Chem 289: 34,129–34,140, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu X, Ying Z, Cai M, Xu Z, Li Y, Jiang SY, Tzan K, Wang A, Parthasarathy S, He G, Rajagopalan S, Sun Q. Exercise ameliorates high-fat diet-induced metabolic and vascular dysfunction, and increases adipocyte progenitor cell population in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 300: R1115–R1125, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yano M, Kishida E, Muneyuki Y, Masuzawa Y. Quantitative analysis of ceramide molecular species by high performance liquid chromatography. J Lipid Res 39: 2091–2098, 1998. [PubMed] [Google Scholar]
- 35.Yu C, Chen Y, Cline GW, Zhang D, Zong H, Wang Y, Bergeron R, Kim JK, Cushman SW, Cooney GJ, Atcheson B, White MF, Kraegen EW, Shulman GI. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem 277: 50,230–50,236, 2002. [DOI] [PubMed] [Google Scholar]