Abstract
Injectable hydrogels can be used to deliver drugs in situ over a sustained period of time. We hypothesized that sustained delivery of interleukin-10 (IL-10) following acute kidney injury (AKI) would mitigate the local and systemic proinflammatory cascade induced by AKI and reduce subsequent fibrosis. Wild-type C57BL/6 mice underwent ischemia-reperfusion AKI with avertin anesthesia. Three days later, mice were treated with either hyaluronic acid injectable hydrogel with or without IL-10, or IL-10 suspended in saline, injected under the capsule of the left kidney, or hydrogel with IL-10 injected subcutaneously. Untreated AKI served as controls. Serial in vivo optical imaging tracked the location and degradation of the hydrogel over time. Kidney function was assessed serially. Animals were killed 28 days following AKI and the following were evaluated: serum IL-6, lung inflammation, urine neutrophil gelatinase-associated lipocalin, and renal histology for fibroblast activity, collagen type III deposition and fibrosis via Picrosirius Red staining and second harmonic imaging. Our model shows persistent systemic inflammation, and renal inflammation and fibrosis 28 days following AKI. The hydrogels are biocompatible and reduced serum IL-6 and renal collagen type III 28 days following AKI even when delivered without IL-10. Treatment with IL-10 reduced renal and systemic inflammation, regardless of whether the IL-10 was delivered in a sustained manner via the injectable hydrogel under the left kidney capsule, as a bolus injection via saline under the left kidney capsule, or via the injectable hydrogel subcutaneously. Injectable hydrogels are suitable for local drug delivery following renal injury, are biocompatible, and help mitigate local and systemic inflammation.
Keywords: acute kidney injury, chronic kidney disease, hyaluronic acid, injectable hydrogels, interleukin-10
acute kidney injury (AKI) has been estimated to contribute to as many as 7 million deaths per year worldwide (22), and a single episode of AKI can predispose patients to the development of chronic kidney disease (CKD) (24). Epidemiologic data strongly suggest that AKI can result in CKD (5–8). Murine models of ischemia-reperfusion injury have shown an early proinflammatory response (10, 11). Murine ischemic AKI studies evaluating later time points following injury have shown an increase in inflammatory mediators, implicating prolonged inflammation as a likely culprit for AKI-to-CKD progression (9, 21, 26). Herein, we aim to develop a murine model of AKI-to-CKD transition following ischemic AKI, and attempt to abate the proinflammatory cascade leading to renal fibrosis by delivering interleukin-10 (IL-10). IL-10 is a potent anti-inflammatory cytokine that inhibits inflammatory pathways implicated in kidney injury. In AKI, studies have shown that IL-10 can reduce the recruitment of neutrophils and the production of proinflammatory cytokines (10). We hypothesize that by meliorating the early inflammation that results from AKI, we will reduce the subsequent progression to CKD.
Hydrogels are crosslinked polymer networks that can deliver encapsulated cargo such as cytokines or cells. Toward therapeutic delivery, injectable hydrogels are of particular interest because they allow for facile injection directly to the target site as well as retention at the site of injection with subsequent controlled release of encapsulated therapeutics (14). Herein, we have utilized shear-thinning injectable hydrogels composed of hyaluronic acid (HA) modified by the guest-host pair adamantane (Ad) and β-cyclodextrin (CD) to enable hydrogel assembly, shear-thinning behavior for injection, and controlled therapeutic delivery (Fig. 1). HA is a naturally occurring glycosaminoglycan found widely throughout the human body. As such, HA is considered biocompatible (4); hydrogels have not previously been studied in ischemic AKI for safety and efficacy. In this study, we tested the safety and efficacy of our injectable hydrogels in a murine model of ischemic AKI and used the hydrogels for local and sustained release of IL-10. We hypothesize that amelioration of the proinflammatory cascade that follows AKI will abate the progression of AKI-to-CKD.
Fig. 1.
Overview of material and study design. A: injectable hyaluronic acid (HA) hydrogel formed through guest-host interaction and self-assembly of adamantane (Ad; guest, blue) and β-cyclodextrin (CD; host, red) modified HA (black) in the presence of interleukin-10 (IL-10; green). When shear stress is applied to the hydrogel (i.e., the push of the syringe), the guest-host complexes break, and the hydrogel becomes a liquid to allow injection under the kidney capsule where the material reforms and subsequently degrades to release IL-10 in situ. B: summary of animal model, wherein acute kidney injury (AKI) is created by ischemia-reperfusion and examined at 28 days for renal and systemic outcomes. In treatment groups, therapy is introduced at day 3.
METHODS
Hydrogel synthesis.
Injectable guest-host HA hydrogels using Ad-HA and CD-HA were synthesized, formulated, and prepared with or without inclusion of murine IL-10 (5 μg/15 μl) as previously described (23). The therapeutic suspensions containing IL-10 also contained 0.1 wt% mouse serum albumin (Sigma, St. Louis, MO) to stabilize the murine IL-10. The final weight-percent of the injectable hydrogel was 3.5%.
Animals.
Eight- to 10-week-old wild-type C57BL/6 male mice were procured (JAX, Bar Harbor, ME). Animals were maintained for the study duration with food and water provided ad libitum. All protocols were submitted to and approved by the University of Colorado Institutional Animal Use & Care Committee (IACUC).
Surgical protocol.
Mice were anesthetized with intraperitoneal Avertin (Sigma-Aldrich, Milwaukee, WI) and underwent bilateral renal ischemia-reperfusion with clamp time of 22 min as previously described (3). Following induction of AKI, mice received 0.5 ml saline resuscitation subcutaneously daily for 5 days. For all surgical procedures, fascia and skin were closed in two layers. Animals who did not exhibit AKI on the postoperative day 1 blood urea nitrogen (BUN) and serum creatinine (Cr) were excluded from the study.
Hydrogel and IL-10 treatment.
On postoperative day 3, the mice received 15 μl of therapy based on cohort. Five cohorts with ischemic AKI were studied (n = 5): 1) HA hydrogel injected under the left kidney capsule (AKI + LK gel); 2) 5 μg murine IL-10 (Ebioscience) injected under the left kidney capsule (AKI + LK IL-10); 3) HA hydrogel containing 5 μg IL-10 injected under the left kidney capsule (AKI + LK gel/IL-10); 4) HA hydrogel containing 5 μg IL-10 injected subcutaneously (dorsum) (AKI + SQ gel/IL-10); 5) control (AKI without treatment). Injection of therapy under the left kidney capsule was achieved via retroperitoneal surgical approach as previously described (30). Overall mortality for the AKI and therapeutic intervention surgical procedures combined was 16%.
Blood and tissue collection.
On postoperative day 1, blood was collected via retro-orbital venipuncture and serum BUN and Cr were measured to confirm AKI. Following therapeutic intervention on postoperative day 3, serum was again collected on postoperative day 4. Twenty-eight days following the AKI procedure, mice were killed via pentobarbital sodium with cardiac puncture. Serum, urine, and tissue were collected for analysis at the time of death. Blood samples were centrifuged at 3,000 g for 10 min, serum was collected, and then was centrifuged again at 3,000 g for 2 min with final serum collected for analysis. Specimens showing gross hemolysis were discarded.
Detection of lung myeloperoxidase.
Lungs were homogenized using ice-cold hexadecyltrimethylammoniun bromide (HTAB) buffer [0.5% HTAB (Avantor Performance Materials, Deventer, Overijssel, Holland) and 50 mM KPO4 (Fisher Scientific, Fair Lawn, NJ); pH 6.0]. Lung homogenates were sonicated at 50% power for 10 s using an ultrasonic homogenizer (Branson 450 digital sonifier, Branson Ultrasonics, Danbury, CT). Homogenates were centrifuged at 14,000 g for 30 min at 4°C, and the supernatant was collected. Twenty microliters of supernatant were added to a 96-well plate (Fisher Scientific) followed by 200 μl of reaction buffer [16.7 mg O-dianisidine (Sigma, St. Louis, MO), 100 ml: 90% water and 10% 50 mM KPO4 buffer + 0.0005% H2O2 (Fisher Scientific)]. The optical density was immediately read at 450 nm and then again 30 s later (Benchmark microplate reader, Bio-Rad, Hercules, CA). Protein concentration was determined by a detergent-compatible protein assay (Bio-Rad).
Optical imaging.
The near-infrared marker Cy7.5 was covalently bound to the Ad-HA component of the hydrogel as previously described, through methacrylation of the HA (∼10% modification) and subsequent addition reaction with the peptide GCKKG-Cy7.5 (27). Treatment cohorts that received the injectable HA hydrogel (AKI + LK gel, AKI + LK gel/IL-10, and AKI + SQ gel/IL-10) underwent serial in vivo optical imaging to track and quantify hydrogel location over time. Animals were imaged using the Pearl Impulse (LI-COR, Lincoln, NE) before injection of the hydrogel, immediately postinjection, and then serially on days 1, 4, 8, 11, 15, 18, 22, and 25. Signal intensity measured in photons/pixel/second was analyzed (Pearl Impulse Software v2.0), normalized per animal, and averaged per cohort.
Assessment of kidney function and inflammation.
BUN (BioAssay Systems, Hayward, CA) and serum Cr (Point Scientific, Canton, MI) were analyzed per manufacturer instructions. Serum IL-6, IL-10, and urine neutrophil gelatinase-associated lipocalin (NGAL) were measured via ELISA per manufacturer instructions (R&D Systems, Minneapolis, MO).
Histological evaluation.
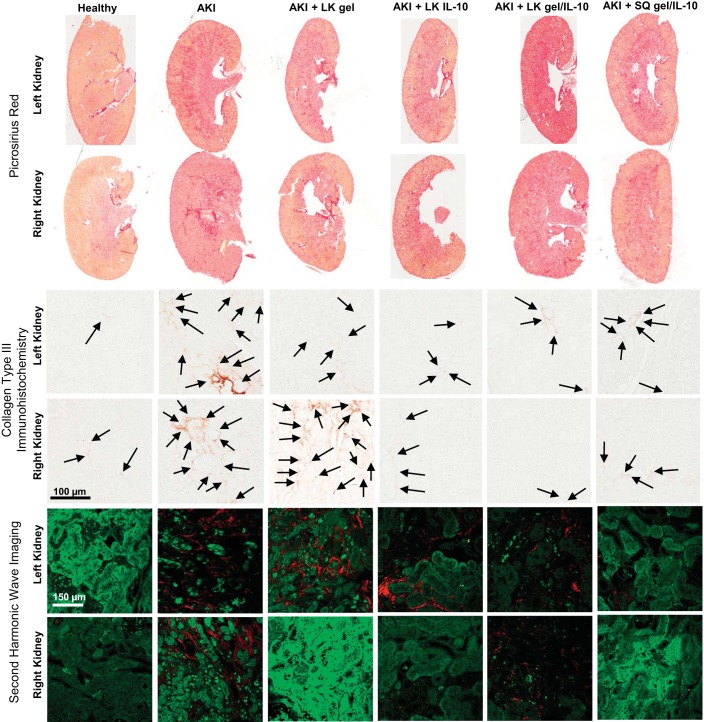
At the time of animal death, the kidneys, liver, spleen, and partial lung were collected. Half of each kidney as well as segments of the spleen, liver, and lung were flash-frozen while the other half of each kidney and the rest of the spleen, liver, and lungs were collected, formalin-fixed, and paraffin-embedded. Histological staining was performed on 4-μm sections. Picrosirius red staining was performed on kidney and cardiac tissue using standard histological staining procedures using Sirius red F3B (Sigma). Imaging software (Image J) was used to calculate the percent area of fibrosis of each sample. Kidney tissue analysis was limited to the renal cortex and excluded the medulla. Trichrome (American MasterTech, Lodi, CA) staining was performed on lung tissue per standard protocol, and samples were scored for fibrosis using the Modified Ashcroft Score (17). Immunohistochemistry for collagen type III (Col III) was performed following standard protocols using goat anti-type III collagen antibody (Southern Biotech, Birmingham, AL) (1:50) for 3 h at 4°C followed by rabbit anti-goat horseradish peroxidase (Dako, Carpinteria, CA) (1:200) for 45 min. Twenty cortical images at ×20 magnification were obtained per sample and imaging software (Aperio) was used to detect positive cells per high-powered field. Second harmonic imaging (Zeiss LSM780 spectral) to detect collagen deposition was performed on mounted slides with representative images obtained from the right and left kidneys of each cohort.
Protein detection via Western blotting for α-smooth muscle actin.
Kidneys were homogenized using 1× RIPA buffer (Cell Signaling Technology, Danvers, MA). Protein concentration was determined by a detergent-compatible protein assay (Bio-Rad). Twenty micrograms of total protein were loaded into each lane of a 10% Tris·HCl precast gel (Bio-Rad) for SDS-PAGE analysis and then transferred to polyvinylidene difluoride membranes (Millipore, Billerica, MA). The membranes were incubated with primary antibody (ab32575, Abcam, Cambridge, MA) and visualized using a horseradish peroxidase secondary antibody (#4050-05, SouthernBiotech) and SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher, Rockford, IL). Chemiluminescence was recorded with an All Pro Imaging 100 Plus film processor. Results were analyzed using ImageJ software. The blots were analyzed for vinculin (ab129002, Abcam) as a loading control.
Statistical analysis.
Data are reported as means ± SD. For optical imaging, ANOVA with α < 0.05 was used to determine significance. For outcome analysis, statistical significance between two groups was determined by unpaired t-test with α < 0.05, and ANOVA was performed to determine significance between multiple cohorts with Bonferroni correction, α < 0.05.
RESULTS
Model of AKI-to-CKD.
To determine whether ischemic AKI leads to fibrosis in our model of ischemic AKI, we subjected healthy wild-type C57BL/6 mice to 22 min of bilateral ischemia-reperfusion renal injury, monitored the mice for 28 days, collected serum on postoperative days 1 and 4, and collected lung and kidney tissue, urine, and serum at the time of death (28 days postischemic reperfusion). On days 1, 4, and 28 post-AKI, serum Cr and BUN were increased compared with healthy control (Fig. 2, A and B, respectively). Creatinine peaked 1 day following AKI induction and both serum Cr and BUN remained elevated 28 days following injury.
Fig. 2.
Model of AKI to chronic kidney disease (AKI-to-CKD). Twenty-eight days following AKI, serum creatinine (Cr), blood urea nitrogen (BUN), urine neutrophil gelatinase-associated lipocalin (NGAL), and serum IL-6 remain elevated (A, B, C, and D). There is no difference in serum IL-10 (E). Lung myeloperoxidase (MPO) is significantly increased (F). There is evidence of renal fibrosis via Pricrosirius Red staining (G) as well as immunohistochemistry staining for collagen type III (H), and in detection of α-smooth muscle actin (SMA) via Western blot (I, J); n = 5, *P < 0.05.
Twenty-eight days following AKI, urine NGAL (Fig. 2C), a urinary biomarker for tubular injury, and serum IL-6 (Fig. 2D), a proinflammatory cytokine, were elevated. There was no difference in serum IL-10 (Fig. 2E) between the AKI group and healthy control 28 days following AKI. Lung myeloperoxidase (MPO), a marker of lung inflammation, was significantly elevated in the AKI cohort (Fig. 2F). Quantitative analysis of Picrosirius Red (Fig. 2G) staining demonstrated increased renal fibrosis 28 days following AKI; representative images are shown in Fig. 3. Quantitative analysis of immunohistochemistry for Col III, a precursor for fibrosis, (Fig. 2H); representative images are shown in Fig. 3. Quantitative analysis of Western blot for α-smooth muscle actin (α-SMA), a marker of fibroblast activity (Fig. 2I), was also increased in the AKI cohort at 28 days. Qualitatively, collagen deposition as detected by second wave harmonic imaging (Fig. 3) was increased in AKI.
Fig. 3.
Representative images of left and right kidneys of healthy controls and each cohort 28 days following AKI. The top 2 rows show whole kidney sections stained via Picrosirius Red. The middle 2 rows show immunohistochemistry staining for collagen type III. The bottom 2 rows show cortical collagen deposition (red) via second harmonic imaging.
Evaluation of guest-host HA hydrogel delivery.
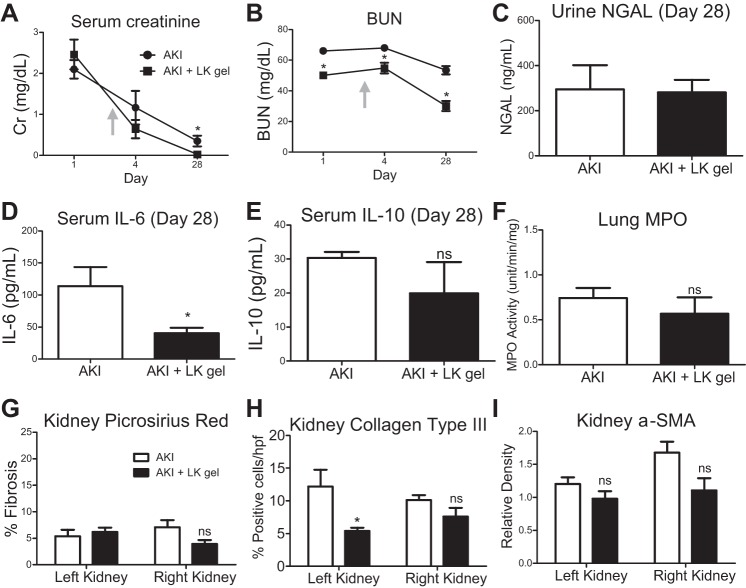
After the AKI-to-CKD transition model was developed, we sought to determine the safety and efficacy of local HA hydrogel for potential therapeutic intervention in ischemic AKI. In situ delivery of the HA hydrogel under the left kidney capsule allows for sustained and local delivery of therapeutic cargo, such as IL-10. We hypothesized that such sustained and local delivery of IL-10 following ischemic AKI would ameliorate the AKI-to-CKD transition by mitigating the proinflammatory cascade induced by ischemic injury. Before using the HA hydrogel to deliver IL-10 to treat AKI, we investigated the effect of delivering the HA hydrogel alone to the left kidney (LK gel) 3 days following AKI. In vivo optical imaging demonstrated clearance of the hydrogel injected under the kidney capsule within 15 days, with ∼40% remaining 4 days after injection (Fig. 4). Serum Cr was lower at 28 days vs. the untreated AKI control (Fig. 5A) and BUN was lower at 1, 4, and 28 days vs. untreated AKI control (Fig. 5B). Of note, the initial postoperative day 1 BUN—which was obtained 2 days before delivery of the HA hydrogel—of the LK gel cohort was statistically lower than the untreated AKI cohort. In our model of AKI, serum Cr correlates with severity of tubular injury, thus, because serum Cr at day 1 was similar between the two groups, these data suggest that injectable HA hydrogel alone may have a beneficial effect on kidney function recovery following ischemic AKI, and delivery of the hydrogel in situ to the kidney has no untoward effect on kidney function. Twenty-eight days after injury, urine NGAL was the same in the LK gel cohort vs. the untreated AKI controls (Fig. 5C); serum IL-6 was lower in the LK gel cohort compared with untreated AKI control (Fig. 5D), and both serum IL-10 and lung MPO were unchanged between the LK gel cohort vs. the untreated AKI controls (Fig. 5, E and F).
Fig. 4.

A: serial in vivo optical imaging of Cy7.5-labeled HA hydrogel 1, 4, 8, and 11 days after injection under the left kidney capsule 3 days after AKI surgery. B: normalized signal intensity demonstrating clearance of the HA hydrogel in vivo over time after injection under the left kidney capsule (LK gel) with or without IL-10, as well as subcutaneously with IL-10 (SQ +IL-10). The hydrogel degraded in vivo within 15 days in each group. The hydrogel injected subcutaneously demonstrated less degradation on day 4 compared with the hydrogel injected under the kidney capsule; n = 5, *P < 0.005.
Fig. 5.
AKI vs. AKI treated with hydrogel under the left kidney capsule (LK gel). Twenty-eight days following AKI, compared with the untreated AKI cohort, the group treated on day 3 (arrow) with hydrogel under the left kidney capsule (LK gel) demonstrated an improvement in serum Cr and BUN by day 28 (A, B); there was no change in urine NGAL (C); however, serum IL-6 was reduced in the treatment group (D). There was no difference in serum IL-10 (E). Lung MPO was not significantly reduced (F). There was no reduction in fibrosis in either kidney via Pricrosirius Red staining (G); however, there was a reduction in collagen type III formation in the left kidneys of the treatment group, but not the right kidneys (H). There was no significant change in α-SMA by Western blot (I); n = 5, *P < 0.05.
We next evaluated the histological effect of the HA hydrogel on renal tissue to evaluate for any evidence of renal inflammation resulting from delivery of the hydrogel under the capsule of the left kidney. Histological analysis separated the treated left kidneys from the untreated right kidneys to distinguish the direct effect of the injection, while also monitoring for any effect on the untreated kidneys. Both the treated left and untreated right kidneys were compared with the untreated AKI control kidneys. Picrosirius Red staining showed that fibrosis in the left and right kidneys of the LK gel cohort was similar to the fibrosis in the left and right kidneys of the untreated AKI control (Fig. 5G). Immunohistochemistry for Col III was reduced in the treated left kidneys but was unchanged in the untreated right kidneys compared with untreated AKI controls (Fig. 5H). There was no significant difference in α-SMA expression in either the left treated or right untreated kidneys in the LK gel cohort compared with untreated AKI controls at the 28-day time point (Fig. 5I). Second wave harmonic imaging comparing the LK gel and AKI cohorts demonstrated no qualitative difference in collagen deposition on the treated left kidney and an apparent decrease in fibrosis in the untreated right kidney compared with untreated AKI controls (Fig. 3). Taken in sum, these data demonstrate that delivery of HA hydrogel under the kidney capsule has no deleterious effect on renal outcomes following AKI, and indeed, may improve renal function, as well as reduce systemic biomarkers of inflammation.
Evaluation of local and systemic delivery of IL-10.
After the biocompatibility of the HA hydrogel and its suitability for local sustained delivery of therapeutics in the AKI model was demonstrated, we next studied the intervention of delivering IL-10 in three ways: 1) under the left kidney capsule suspended in saline (LK IL-10); 2) under the left kidney capsule suspended in HA hydrogel (LK gel/IL-10); and 3) subcutaneously suspended in HA hydrogel (SQ gel/IL-10). Histological evaluation of these treatment groups was separated into left kidney (treated) vs. right kidney (untreated). Any effect of treatment on the right kidney is presumed to be from either diffusion of therapy into the systemic circulation with subsequent delivery to the right kidney or altered systemic inflammation from treatment on the left kidney with subsequent “kidney cross-talk” via effects on levels of systemic cytokines.
Delivery of IL-10 in saline delivered under the left kidney capsule (LK IL-10) 3 days after AKI.
Five micrograms of IL-10 suspended in 15 μl saline were injected under the left kidney capsule 3 days after the AKI procedure. Without the HA hydrogel to provide sustained release of the IL-10, this injection was a bolus delivery of IL-10 to the kidney—delivered all at once to the surrounding tissue before clearing either via blood stream clearance, or secretion into the urine, or adsorption by surrounding tissue. Compared with the untreated AKI controls, this cohort exhibited no change in serum Cr or BUN at the 1- and 4-day time points, but did show a significant improvement in both markers of renal function at the 28-day time point (Fig. 6, A and B). There was no significant difference in urine NGAL between the treatment group compared with the untreated AKI control at the 28-day time point (Fig. 6C); however, there was a significant reduction in serum IL-6 in the cohort treated with IL-10 (Fig. 6D). There was no difference in the amount of serum IL-10 detected between the two groups at 28 days (Fig. 6E). Lung MPO was similarly unchanged between the treatment group and the untreated AKI control (Fig. 6F).
Fig. 6.
AKI vs. AKI treated with IL-10 under the left kidney capsule (LK IL-10). Twenty-eights days following AKI, compared with the untreated AKI cohort, the group treated on day 3 (arrow) with IL-10 suspended in saline under the left kidney capsule (LK IL-10) demonstrated an improvement in serum Cr and BUN by day 28 (A, B). There was no change in urine NGAL (C); however, serum IL-6 was reduced in the treatment group (D). There was no difference in serum IL-10 (E). There was no reduction in lung MPO (F). There was no reduction in fibrosis in either kidney via Pricrosirius Red staining (G); however, there was a reduction in collagen type III formation in both the left and right kidneys of the treatment group (H). There was a significant reduction in α-SMA detection in the right but not the left kidneys (I); n = 5, *P < 0.05.
Histologically, the delivery of IL-10 via saline to the left kidney did not significantly alter the amount of fibrosis detected via Pricrosirius Red staining in either the left or right kidneys (Fig. 6G); however, Col III deposition via immunohistochemistry was reduced in the both the left and right kidneys in the treatment group (Fig. 6H). And while there was no difference in α-SMA detected in the left kidney, there was a reduction noted in the right kidneys of the treatment group (Fig. 6I). These findings were supported qualitatively by the second harmonic wave imaging (Fig. 3) that showed improvement in the right but not the left kidney.
In summary, bolus injection of IL-10 under the kidney capsule improved marker of kidney function, reduced serum IL-6, and demonstrated a variable reduction in markers of fibrosis in both the treated left and untreated right kidneys.
Delivery of IL-10 via HA hydrogel under the left kidney capsule (LK gel/IL-10) 3 days after AKI.
In the second treatment group, we delivered the 5 μg IL-10 suspended in 15 μl HA hydrogel under the left kidney capsule 3 days after the AKI procedure (LK gel/IL-10). The HA hydrogel allows for sustained and local delivery of the IL-10 over time. In vivo optical imaging demonstrated that the HA hydrogel containing IL-10 cleared at the same rate as HA hydrogel without IL-10 (Fig. 4) when injected under the kidney capsule. We previously showed that HA hydrogel degradation corresponds with release of therapeutic cargo, including IL-10 (24, 25).
Compared with the untreated AKI cohort, the group treated with LK gel/IL-10 exhibited no statistically significant change in serial Cr or BUN measurements except for an improvement in BUN at the 28-day time point (Fig. 7, A and B). While there was likewise no change in urine NGAL (Fig. 7C) detected between the two groups at day 28, there was a significant reduction in serum IL-6 (Fig. 7D) in the group treated with LK gel/IL-10. The LK gel/IL-10 group also demonstrated a significant reduction in the amount of serum IL-10 detected on day 28 (Fig. 7E). There was no change in lung MPO detected between the treatment group compared with the untreated AKI control (Fig. 7F).
Fig. 7.
AKI vs. AKI treated with hydrogel containing IL-10 under the left kidney capsule (LK gel/IL-10). Twenty-eight days following AKI, compared with the untreated AKI cohort, the group treated on day 3 (arrow) with HA containing 5 μg of IL-10 under the left kidney capsule (LK gel/IL-10) demonstrated no improvement in serum Cr (A), although serum BUN on day 28 was reduced (B). There was no change in urine NGAL (C); however, serum IL-6 was reduced in the treatment group (D). Serum IL-10 was reduced in the treatment group (E). There was no change in lung MPO (F). There was an increase in fibrosis via Picrosirius Red staining in the left kidneys of the treatment groups, but no change in the right kidneys (G). There was a reduction in collagen type III formation in both the left and right kidneys of the treatment group (H). There was a reduction in α-SMA in the right but not the left kidneys of the treatment group (I); n = 5, *P < 0.05.
Histologically, the delivery of IL-10 via HA hydrogel to the left kidney increased the amount of fibrosis detected via Picrosirius Red in the left kidney; there was no change demonstrated in the untreated right kidneys (Fig. 7G). Immunohistochemistry for Col III, however, was reduced in the treatment group in both the left and right kidneys (Fig. 7H). α-SMA detection was unchanged in the left kidneys of the treatment group and was reduced in the right kidneys (Fig. 7I). Second wave harmonic imaging comparing the LK gel/IL-10 cohort with the untreated AKI showed no qualitative difference between the groups in either the left or right kidneys. These discordant findings indicate that delivery of IL-10 via degradable HA hydrogel to the left kidney has unequivocal results in the right and left kidney and seems to be less beneficial overall than delivery of either HA hydrogel or IL-10 alone.
Delivery of IL-10 via HA hydrogel subcutaneously (SQ gel/IL-10) 3 days following AKI.
Five micrograms of IL-10 suspended in 15 μl of HA hydrogel were injected subcutaneously on the dorsum of the mouse, over the area of the left kidney, 3 days after the initial AKI procedure. In vivo optical imaging demonstrated that subcutaneously administered HA hydrogel degraded at an attenuated rate during early time points relative to hydrogel injection under the kidney capsule (Fig. 4). Specifically, 4 days after injection only ∼20% of the hydrogel had cleared in the subcutaneous cohort compared with ∼60% clearance by that time of hydrogel injected under the kidney capsule. Serologically, compared with the untreated AKI cohort, this treatment group exhibited no significant improvement in serum Cr but there was a decrease in BUN at the 28-day time point (Fig. 8, A and B). Of note, the control group did have a significantly higher serum Cr on post-AKI day 1 compared with the untreated AKI control, while the treatment group had a higher initial BUN than the control AKI group. There was no change in urine NGAL detected between the two groups (Fig. 8C), while there was a decrease in both serum IL-6 and serum IL-10 in the treatment group (Fig. 8, D and E). There was no significant difference in lung MPO detection between the two groups (Fig. 8F).
Fig. 8.
AKI vs. AKI treated with hydrogel containing IL-10 subcutaneously (SQ gel/IL-10). Twenty-eight days following AKI, compared with the untreated AKI cohort, the group treated on day 3 (arrow) with HA containing 5 μg of IL-10 injected subcutaneously on the dorsum (SQ gel/IL-10) exhibited a higher post-AKI day 1 serum Cr, but there was no difference in Cr on days 4 or 28 (A). There was a similar increase in day 1 serum BUN in the treatment group with a reduction seen on day 28 (B). There was no change in urine NGAL (C); however, serum IL-6 was reduced in the treatment group (D). Serum IL-10 was reduced in the treatment group (E). Lung MPO was unchanged (F). There was no change in fibrosis via Picrosirius Red staining in either the left or right kidneys of the treatment group (G). There was a reduction in collagen type III formation in both the left and right kidneys of the treatment group (H). There was a reduction in α-SMA in the right but not the left kidneys of the treatment group (I); n = 5, *P < 0.05.
Histologically, there was no change in renal fibrosis detected via Picrosirius Red staining in either the left or right kidneys (Fig. 8G); however, immunohistochemistry staining for Col III was reduced in both the left and right kidneys of the treatment group (Fig. 8H) while α-SMA detection was reduced in the right and not the left kidneys (Fig. 8I). Second wave harmonic imaging demonstrated a qualitative improvement in the treatment group, more so in the right kidney than the left treated kidney (Fig. 3). In sum, subcutaneous delivery of HA hydrogel with IL-10 demonstrated similar systemic and renal effects as delivery under the left kidney capsule.
ANOVA comparison among the treatment groups.
There was no difference among the treatment groups in day 28 BUN, serum Cr, serum IL-10, or lung MPO. ANOVA analysis of serum IL-6 28 days following AKI did demonstrate that groups treated with IL-10 either in saline under the left kidney capsule (LK IL-10) or subcutaneously within gel (SQ gel/IL-10) had significantly lower levels of serum IL-6 compared with the group treated with gel and IL-10 under the left kidney capsule (LK gel/IL-10), P = 0.0043. ANOVA analysis of urine NGAL data demonstrated that the group treated with IL-10 in saline under the left kidney capsule (LK IL-10) had significantly lower urine NGAL at day 28 compared with the group treated with gel and IL-10 under the left kidney capsule (LK gel/IL-10), P = 0.0033.
In the left kidneys, ANOVA analysis of the Picrosirius Red staining demonstrated further reduction of fibrosis in the groups treated with gel alone under the kidney capsule (LK gel), as well as IL-10 delivered in saline under the kidney capsule (LK IL-10) or subcutaneously within gel (SQ gel/IL-10) compared with the group treated with gel and IL-10 under the left kidney capsule (LK gel/IL-10), P = 0.0079. There was no difference in Picrosirius Red staining results among the treatment groups in the right kidneys.
ANOVA analysis of Col III via immunohistochemistry demonstrated several differences between the treatment groups. Compared with the group treated with gel under the kidney capsule (LK gel), the three other treatment groups—IL-10 under the kidney capsule (LK IL-10), gel with IL-10 delivered under the kidney capsule (LK gel/IL-10), or subcutaneously (SQ gel/IL-10)—each demonstrated statistically significantly reduced Col III deposition in both the left and right kidneys. Even further reduction in Col III deposition was noted in the left kidneys of the group treated with gel and IL-10 subcutaneously (SQ gel-10) compared with the group treated with gel and IL-10 under the left kidney capsule (LK gel/IL-10). In the right kidneys, further reduction of Col III was noted in the group treated with Il-10 under the left kidney capsule (LK IL-10) compared with the group treated with gel containing IL-10 under the kidney capsule (LK gel/IL-10), P < 0.0001.
ANOVA comparison of the α-SMA detection among the treatment groups demonstrated no difference in the left kidneys. However, there was a significantly reduced amount of α-SMA detected in the right kidneys of groups treated with IL-10 either in saline under the left kidney capsule (LK IL-10) or IL-10 within gel delivered subcutaneously (SQ gel/IL-10) compared with the group that received gel alone under the left kidney capsule (LK gel), P = 0.046.
DISCUSSION
In our prior work, we demonstrated the immunomodulatory effect of local delivery of IL-10 via injectable HA hydrogels in a unilateral ureteral obstruction (UUO) murine model of chronic kidney disease (24). Although histological markers of inflammation were reduced in the treatment groups, a primary limitation of the UUO model was the inability to assess any alteration of renal function because the targeted kidney was permanently obstructed and nonfunctioning. Herein, we built on this methodology by developing a murine model of AKI-to-CKD transition suitable for examination of histological, functional, and systemic effects of potential AKI therapy through local or systemic delivery of IL-10 via injectable hydrogel depots. Furthermore, the bilateral ischemia-reperfusion model recapitulates the bilateral injury most common in clinical practice and allows for comparison of outcomes on both the left and right kidney following asymmetric delivery of therapeutics (hydrogel with or without IL-10 under the left kidney capsule).
AKI-to-CKD.
By following the ischemia-reperfusion model of AKI out to 28 days, we demonstrated the late-term sequels of renal fibrosis and persistent inflammation via a significant increase in serum IL-6, urine NGAL, lung MPO, and collagen deposition 28 days following AKI despite near-normalization of serum Cr. We previously demonstrated the importance of fluid resuscitation and outcomes of BUN and Cr following AKI which suggest this may relate to the relatively elevated BUN levels at the 28-day time point (3). Because we limit fluid resuscitation, our model of bilateral ischemia is severe (even though the ischemia time is somewhat shorter than other models) with a rise in serum Cr up to 2.0 and acute tubular necrosis scores of 4 to 5 at 24 h (23); furthermore, we specifically demonstrated that this model is similar to human AKI (9, 22, 23). These findings further support the growing appreciation that AKI and CKD are interconnected, rather than two distinct clinical entities, and exist along a continuum of the same disease process, although the pathophysiological timing and signaling that cause AKI to result in CKD remain unclear. Our findings also support the notion that serum Cr is an insensitive biomarker of disease and that biomarkers such as urine NGAL may be useful in predicting disease progression (13). Further studies that investigate disease pathophysiology along this temporal spectrum will improve our understanding of the pathogenesis of acute-to-chronic kidney disease progression and may elicit immune-modulating targets for therapeutic intervention. In light of the clinical burden of both AKI and CKD, such therapies would have a profound and positive impact on patient healthcare and quality of life.
Biocompatibility of injectable HA hydrogels.
HA is widely accepted as a biocompatible material that can be utilized for therapeutic intervention in a variety of disease models, including kidney disease (4, 24). Here, it has been demonstrated that delivery of the HA hydrogel to an injured kidney has no untoward effect on histological outcomes and may improve upon some outcomes such as renal fibrosis. Furthermore, we showed that renal function is not only protected, but may be improved with HA delivery. These results are in agreement with prior studies utilizing the UUO model, wherein histological evaluation demonstrated attenuation of macrophage infiltration, TGF-β expression, and fibrosis by hydrogel injection alone (24, 25). We hypothesized that the injectable hydrogels have a beneficial immune-modulating effect by quenching proinflammatory cytokines near the site of injury. While beyond the scope of this study, further studies are needed to specifically investigate this hypothesis and we propose excising the HA hydrogel several days following injection for ex vivo analysis of proinflammatory cytokines such as IL-6.
Sustained vs. bolus local delivery.
We previously showed that IL-10 delivered via saline suspension under the kidney capsule, such as what the AKI + LK IL-10 cohort received, clears the kidney at a faster rate than IL-10 delivered within a hydrogel (25). While no change in renal fibrosis was seen in the LK IL-10 cohort, there was still a beneficial effect on serum IL-6 as well as renal function and Col III deposition.
Subcutaneous delivery vs. delivery under the kidney capsule.
A notable finding from our study was that sustained delivery of IL-10 via injectable hydrogel was effective in improving outcomes even when delivered subcutaneously. Indeed, this delivery site likely provides inherent advantages as it enables facile clinical intervention through minimally invasive means without further perturbation of the injured renal tissue, and it also provides comparable treatment to both kidneys compared with unilateral treatment in the subcapsular injection. In vivo degradation rates are affected by the site of injection, and serial optical imaging of the subcutaneous delivery of HA demonstrated a slower degradation profile over the first several days following injection. Our prior work showed that release of IL-10 corresponds with hydrogel degradation rate (24); the effect of IL-10 delivery therefore likely varies between groups and may not be ruled out as a confounding factor in comparison of these therapeutic approaches.
Timing of therapeutic intervention.
The degradation profile and subsequent release of IL-10 also lead to discussion regarding the timing of therapeutic intervention. We chose to deliver our HA/IL-10 therapy 3 days after AKI as a clinically relevant model for future therapeutic intervention. While this is indeed relatively late in the sense that most proinflammatory markers have already peaked by this time, patients with AKI are often diagnosed several days into the disease process, making this model quite relevant to clinical interventions. Certainly, the positive effect with more mitigated early release seen in the subcutaneous cohort (AKI + SQ gel/IL-10) indicates that perhaps even later delivery of IL-10 might still be beneficial.
Asymmetric effect.
Another notable finding of this work was that despite therapeutic intervention to only one of the injured kidneys (the left), in several cohorts, there was asymmetrical improvement in histologic outcomes with more benefit seen in the untreated (right) kidney. For example, in the cohorts treated with IL-10 either suspended in saline or HA hydrogel and injected under the kidney capsule (AKI + LK IL-10, and AKI LK gel/IL-10), there was a decrease in α-SMA detected in the right untreated kidneys 28 days following AKI, but no such improvement was seen in the left treated kidneys. We hypothesize that these effects are a result of mitigation of the proinflammatory cascade on the left kidney by the injected hydrogel and/or IL-10, with a subsequent decrease in systemic release of proinflammatory cytokines. This “kidney cross-talk” results in subsequent improvement in the contralateral, untreated kidney. We previously showed that AKI mediates systemic inflammation and subsequent lung injury via an increase in serum IL-6 (2) and we demonstrated a decrease in serum IL-6 28 days after AKI in our treated cohorts compared with untreated AKI control. Furthermore, the untreated right kidney did not undergo the same surgical manipulation on day 3 required for subcapsular delivery to the left treated kidney. These findings further support the notion that subcapsular delivery might not be necessary to optimize therapeutic effect and that other delivery sites such as the subcutaneous delivery method may indeed be just as beneficial. Interestingly, this asymmetric effect was also seen in the group that received the HA hydrogel containing IL-10 injected subcutaneously (AKI + SQ gel/IL-10). Of note, the subcutaneous injection was made on the dorsum of the animal overlying the left kidney, so even barring any surgical manipulation of the left kidney, it seems that there was more of a beneficial effect on the contralateral kidney.
Future directions.
Perhaps the most interesting finding of our study was that local delivery of either IL-10 in saline or HA hydrogel alone under the kidney capsule improved renal fibrosis following AKI; however, delivery of both (LK gel/IL-10) actually increased renal fibrosis in the treated left kidney. While this antagonizing effect is not yet understood, we previously demonstrated that while delivery of either IL-10 alone or anti-TGB-β alone improved renal fibrosis in an animal model of CKD, delivery of both IL-10 and anti-TGF-β together similarly antagonized the fibrotic response (24). This elucidates the need for further understanding of the cell signaling pathways that lead to fibrosis, as well determination of the optimal dose and timing of therapeutic interventions. Until such time that these mechanistic pathways are better understood, it will be challenging to optimize interventions. One promising avenue is the delivery of mesenchymal stem cells (MSCs) to the site of injury. MSCs have been shown to have anti-inflammatory, anti-fibrotic paracrine effects that respond uniquely to their surrounding milieu. As such, MSCs hold therapeutic promise in the treatment of kidney injury, and are already undergoing clinical trials (18–21). One major obstacle in MSC therapy is lack of a viable delivery method, with most studies relying on systemic delivery that can result in distal organ delivery and embolism in either the lungs or spleen. As such, delivery of MSCs via injectable hydrogels would be a promising alternative mode of delivery allowing for in situ delivery of MSCs for optimal paracrine effect. Furthermore, encapsulation of MSCs in injectable hydrogels has been shown to increase MSC viability, retention, and therapeutic efficacy (1, 12, 13).
In sum, we demonstrated that injectable HA hydrogels can be utilized to deliver therapeutics to injured kidneys and improve both histological and functional outcomes of kidney disease, as well as reduce systemic inflammation several weeks following AKI. Further studies are needed to optimize both the cargo to be delivered as well as the timing of therapeutic intervention and location of in situ delivery. Injectable hydrogels hold great promise in translational therapy for mitigating renal disease along the spectrum of AKI-to-CKD transition.
GRANTS
We are grateful for support from the Research Institute at Children's Hospital Colorado (to D. E. Soranno) as well as a Predoctoral Fellowship from the American Heart Association (to C. B. Rodell). Second wave harmonic Imaging was performed in the University of Colorado Anschutz Medical Campus Advance Light Microscopy Core supported in part by National Institutes of Health (NIH)/NCATS Colorado CTSI Grant Number UL1 TR001082. Contents are the authors' sole responsibility and do not necessarily represent official NIH views.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
D.E.S., C.B.R., J.A.B., and S.F. conception and design of research; D.E.S., C.B.R., C.A., and A.A.-H. performed experiments; D.E.S. and J.D. analyzed data; D.E.S., J.A.B., and S.F. interpreted results of experiments; D.E.S. and C.B.R. prepared figures; D.E.S. drafted manuscript; D.E.S., C.B.R., J.D., and S.F. edited and revised manuscript; D.E.S., J.A.B., and S.F. approved final version of manuscript.
REFERENCES
- 1.Aguado BA, Mulyasasmita W, Su J, Lampe KJ, Heilshorn SC. Improving viability of stem cells during syringe needle flow through the design of hydrogel cell carriers. Tissue Eng Part A 18: 806–815, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahuja N, Andres-Hernando A, Altmann C, Bhargava R, Bacalja J, Webb RG, He Z, Edelstein CL, Faubel S. Circulating IL-6 mediates lung injury via CXCL1 production after acute kidney injury in mice. Am J Physiol Renal Physiol 303: F864–F872, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andres-Hernando A, Altmann C, Bhargava R, Okamura K, Bacalja J, Hunter B, Ahuja N, Soranno D, Faubel S. Prolonged acute kidney injury exacerbates lung inflammation at 7 days post-acute kidney injury. Physiol Rep 2: e12084, 1–12, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burdick JA, Prestwich GD. Hyaluronic acid hydrogels for biomedical applications. Adv Mater 23: H41–H56, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chawla LS. Acute kidney injury leading to chronic kidney disease and long-term outcomes of acute kidney injury: the best opportunity to mitigate acute kidney injury? Contrib Nephrol 174: 182–190, 2011. [DOI] [PubMed] [Google Scholar]
- 6.Chawla LS, Amdur RL, Amodeo S, Kimmel PL, Palant CE. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int 79: 1361–1369, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chawla LS, Eggers PW, Star RA, Kimmel PL. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med 371: 58–66, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chawla LS, Kimmel PL. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int 82: 516–524, 2012. [DOI] [PubMed] [Google Scholar]
- 9.Chuang ST, Kuo YH, Su MJ. Antifibrotic effects of KS370G, a caffeamide derivative, in renal ischemia-reperfusion injured mice and renal tubular epithelial cells. Sci Rep 4: 5814, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng J, Kohda Y, Chiao H, Wang Y, Hu X, Hewitt SM, Miyaji T, McLeroy P, Nibhanupudy B, Li S, Star RA. Interleukin-10 inhibits ischemic and cisplatin-induced acute renal injury. Kidney Int 60: 2118–2128, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Furuichi K, Kaneko S, Wada T. Chemokine/chemokine receptor-mediated inflammation regulates pathologic changes from acute kidney injury to chronic kidney disease. Clin Exp Nephrol 13: 9–14, 2009. [DOI] [PubMed] [Google Scholar]
- 12.Gaffey AC, Chen MH, Venkataraman CM, Trubelja A, Rodell CB, Dinh PV, Hung G, MacArthur JW, Soopan RV, Burdick JA, Atluri P. Injectable shear-thinning hydrogels used to deliver endothelial progenitor cells, enhance cell engraftment, and improve ischemic myocardium. J Thorac Cardiovasc Surg 150: 1268–1276, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao J, Liu R, Wu J, Liu Z, Li J, Zhou J, Hao T, Wang Y, Du Z, Duan C, Wang C. The use of chitosan based hydrogel for enhancing the therapeutic benefits of adipose-derived MSCs for acute kidney injury. Biomaterials 33: 3673–3681, 2012. [DOI] [PubMed] [Google Scholar]
- 14.Guvendiren M, Lu HD, Burdick JA. Shear-thinning hydrogels for biomedical applications. Soft Matter 8: 260–272, 2012. [Google Scholar]
- 15.Hubner RH, Gitter W, El Mokhtari NE, Mathiak M, Both M, Bolte H, Freitag-Wolf S, Bewig B. Standardized quantification of pulmonary fibrosis in histological samples. Biotechniques 44: 507–511, 2008. [DOI] [PubMed] [Google Scholar]
- 16.Humphreys BD. Introduction: stem cells and kidney regeneration. Semin Nephrol 34: 349–350, 2014. [DOI] [PubMed] [Google Scholar]
- 17.Humphreys BD. Kidney injury, stem cells and regeneration. Curr Opin Nephrol Hypertens 23: 25–31, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Humphreys BD. Kidney structures differentiated from stem cells. Nat Cell Biol 16: 19–21, 2014. [DOI] [PubMed] [Google Scholar]
- 19.Humphreys BD, Bonventre JV. Mesenchymal stem cells in acute kidney injury. Annu Rev Med 59: 311–325, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Lameire NH, Bagga A, Cruz D, De Maeseneer J, Endre Z, Kellum JA, Liu KD, Mehta RL, Pannu N, Van Biesen W, Vanholder R. Acute kidney injury: an increasing global concern. Lancet 382: 170–179, 2013. [DOI] [PubMed] [Google Scholar]
- 21.Liu G, Zhao L, Wang T, Zhang M, Pei H. Optimal therapeutic dose and time window of picroside II in cerebral ischemic injury. Neural Regen Res 9: 1437–1445, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehta RL, Cerda J, Burdmann EA, Tonelli M, Garcia-Garcia G, Jha V, Susantitaphong P, Rocco M, Vanholder R, Sever MS, Cruz D, Jaber B, Lameire NH, Lombardi R, Lewington A, Feehally J, Finkelstein F, Levin N, Pannu N, Thomas B, Aronoff-Spencer E, Remuzzi G. International Society of Nephrology's 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): a human rights case for nephrology. Lancet 385: 2616–2643, 2015. [DOI] [PubMed] [Google Scholar]
- 23.Rodell CB, MacArthur JW, Dorsey SM, Wade RJ, Wang LL, Woo YJ, Burdick JA. Shear-thinning supramolecular hydrogels with secondary autonomous covalent crosslinking to modulate viscoelastic properties in vivo. Adv Funct Mater 25: 636–644, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodell CB, Rai R, Faubel S, Burdick JA, Soranno DE. Local immunotherapy via delivery of interleukin-10 and transforming growth factor beta antagonist for treatment of chronic kidney disease. J Control Release 206: 131–139, 2015. [DOI] [PubMed] [Google Scholar]
- 25.Soranno DE, Lu HD, Weber HM, Rai R, Burdick JA. Immunotherapy with injectable hydrogels to treat obstructive nephropathy. J Biomed Mater Res A 102: 2173–2180, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang L, Chen H, Liu XH, Chen ZY, Weng XD, Qiu T, Liu L, Zhu HC. Ozone oxidative preconditioning inhibits renal fibrosis induced by ischemia and reperfusion injury in rats. Exp Ther Med 8: 1764–1768, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]