Abstract
Renal endothelin-1 (ET-1) and purinergic signaling systems regulate Na+ reabsorption in the renal medulla. A link between the renal ET-1 and purinergic systems was demonstrated in vitro, however, the in vivo interaction between these systems has not been defined. To test whether renal medullary activation of purinergic (P2) receptors promotes ET-dependent natriuresis, we determined the effect of increased medullary NaCl loading on Na+ excretion and inner medullary ET-1 mRNA expression in anesthetized adult male Sprague-Dawley rats in the presence and absence of purinergic receptor antagonism. Isosmotic saline (NaCl; 284 mosmol/kgH2O) was infused into the medullary interstitium (500 μl/h) during a 30-min baseline urine collection period, followed by isosmotic or hyperosmotic saline (1,800 mosmol/kgH2O) for two further 30-min urine collection periods. Na+ excretion was significantly increased during intramedullary infusion of hyperosmotic saline. Compared with isosmotic saline, hyperosmotic saline infused into the renal medulla caused significant increases in inner medullary ET-1 mRNA expression. Renal intramedullary infusion of the P2 receptor antagonist suramin inhibited the increase in Na+ excretion and inner medullary ET-1 mRNA expression induced by NaCl loading in the renal medulla. Activation of medullary P2Y2/4 receptors by infusion of UTP increased urinary Na+ excretion. Combined ETA and ETB receptor blockade abolished the natriuretic response to intramedullary infusion of UTP. These data demonstrate that activation of medullary P2 receptors promotes ET-dependent natriuresis in male rats, suggesting that the renal ET-1 and purinergic signaling systems interact to efficiently facilitate excretion of a NaCl load.
Keywords: endothelin-1, purinergic receptors, natriuresis, kidney, inner medulla
endothelin-1 (ET-1) is an autocrine inhibitor of Na+ and water reabsorption by the kidney and plays a central role for the regulation of Na+ homeostasis and blood pressure control. Within the renal medulla, ET-1 is released in response to a high-salt diet and inhibits tubular Na+ transport promoting natriuresis (14, 17). It appears that both ETA and ETB receptors are required for the full diuretic and natriuretic effects of ET-1 (7). However, the signaling mechanism by which NaCl loading to the renal medulla translates into an increase in ET-1 production and/or action is currently unknown.
Purinergic signaling has also emerged as another important system in the renal control of blood pressure and Na+ excretion (19, 31). In response to increased tubular flow, ATP is released from renal tubular cells inhibiting Na+ transport along the nephron (12, 19), mainly through P2Y2 receptor activation (18, 23). Both P2Y2 knockout mice and ETB-deficient rats develop salt-sensitive hypertension (23, 30), supporting the fundamental roles for these two systems in blood pressure regulation by controlling Na+ homeostasis. However, the interaction between renal ET-1 and purinergic signaling is relatively unexplored. Recently, it has been demonstrated that renal P2Y2 and P2X7 receptor blockade inhibits ET-1 production in inner medullary collecting duct cells (22). However, the interaction between the endothelin and the purinergic signaling systems within the renal medulla has yet to be explored in vivo. Taken together, we uniquely designed the current study to elucidate the potential interplay between ET-1 and purinergic signaling systems on renal Na+ excretory function in male rats.
We hypothesize that the activation of P2 receptors within the renal medulla promotes ET-dependent natriuresis. To test this hypothesis, we studied the effect of increased medullary NaCl loading on Na+ excretion and inner medullary ET-1 mRNA expression in adult male Sprague-Dawley rats in the presence or absence of P2 receptor antagonism. To determine the role of ET-1 system in response to increased NaCl loading to the renal medulla, we assessed the change in inner medullary ET-1 mRNA expression in response to infusion of hyperosmotic saline into the renal medulla. To assess whether inner medullary P2 receptors are involved in ET-dependent control of Na+ excretion, we evaluated the effect of P2 receptors blockade on natriuresis and ET-1 mRNA expression in inner medulla following increased medullary Na+ loading. Finally, to determine whether there is a cross talk between medullary ET-1 and purinergic signaling systems, we studied the effect of intramedullary infusion of the P2Y2/4 agonist UTP in the presence and absence of ETA and ETB receptor antagonists.
METHODS
General methods.
Male Sprague-Dawley rats (16–18 wk old, n = 5–7 per group; Harlan Laboratories, Indianapolis, IN) were used in the current study. Rats were housed in a temperature- and humidity-controlled room with a 12:12-h light-dark cycle, with free access to water. All protocols were in accordance with the Guide for the Care and Use of Laboratory Animals and were approved in advance by the University of Alabama at Birmingham Institutional Animal Care and Use Committee.
Acute intramedullary infusion.
Rats were anesthetized with the use of thiobutabarbitone (Inactin, hydrate 100 mg/kg ip; Sigma, St. Louis, MO) and surgically prepared for acute intramedullary infusion, as described in previous studies by our lab (6, 7). First, rats were placed on a heating surgical table to maintain body temperature at 37°C throughout the experiment, and a tracheotomy was performed to facilitate breathing. A catheter was inserted into the femoral vein for infusing 3% bovine serum albumin in phosphate-buffered saline at a rate of 1.2 ml/h to maintain euvolemia. Another catheter was inserted into the femoral artery, connected to a pressure transducer and a PowerLab data-acquisition system to monitor blood pressure over the course of the experiment. A midline incision was then performed and a catheter was placed in the left ureter to allow urine collection. The left renal artery was isolated and fitted with an ultrasonic perivascular flow probe (1PRB probe, Transonic Systems, Ithaca, NY) to measure the total renal blood flow continuously during each experiment. Finally, a stretched PE10 catheter was inserted into the left kidney as deep as the outer/inner medullary junction to allow infusion of isosmotic NaCl (0.9%) directly into the renal medulla (0.5 ml/h).
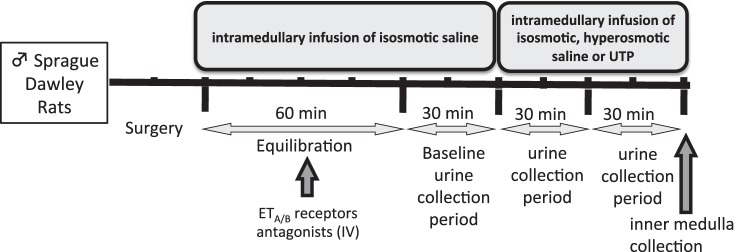
Following surgical preparation, a 60-min equilibration period and a 30-min baseline urine collection period were obtained during intramedullary infusion of isosmotic saline (284 mosmol/kgH2O, 0.9% NaCl, 154 mM NaCl). This was followed by isosmotic or hyperosmotic saline (1,800 mosmol/kgH2O, 5.7% NaCl, 976 mM NaCl) infusion for two further 30-min periods. The experimental timeline used in this intramedullary infusion experiment is illustrated in Fig. 1. It is important to highlight that we use the terms “isosmotic” and “hyperosmotic” relative to plasma osmolality.
Fig. 1.
Experimental timeline.
To determine the role of purinergic system in response to increasing intramedullary NaCl loading, we continuously infused the nonselective P2 receptor antagonist suramin (750 μg·kg−1·min−1, Sigma) into the renal medullary interstitium during the 30-min basal urine collection period. Suramin and hyperosmotic saline were then coinfused for two subsequent 30-min urine collection periods. To our knowledge, there are no available data on doses for intramedullary infusion of suramin. The suramin dose used in the current study originates from pilot studies conducted in our lab using a range of concentrations estimated based on prior intravenous studies (27).
To determine the role of ET-1 system in response to P2 receptor activation, rats received an intravenous bolus injection (0.5 ml/kg via femoral vein catheter) of vehicle or a combination of the selective ETA receptor antagonist ABT-627 (5 mg/kg) plus the selective ETB receptor antagonist A-192621 (10 mg/kg, Abbott Laboratories) 30 min before the end of the postsurgical equilibration period. These doses are known to maintain efficient blockade of both receptor subtypes for the duration of our experimental protocol as previously demonstrated (4, 7, 32). After the equilibration period and the 30-min baseline urine collection period during which isosmotic saline was intramedullary infused, the P2Y2/4 receptor agonists (200 pmol·kg−1·min−1 UTP, Sigma) were infused into the renal medullary interstitium for two subsequent 30-min urine collection periods.
Blood pressure and renal blood flow data were monitored using a PowerLab data-acquisition system (ADInstruments). Renal blood flow was normalized to left kidney weight. At the end of each experiment, the proper positioning of the catheter tip at the outer-inner medullary junction was confirmed by dissection of the kidney and inner medulla was harvested, snap-frozen in liquid nitrogen, and stored at −80°C until analysis.
Tissue analysis.
Renal inner medullary tissue was immediately harvested after the second 30-min urine collection period, and total RNA was isolated using Purelink Mini RNA extraction kit (Ambion) according to the manufacturer's instructions. Then, the isolated RNA was reverse transcribed using QuantiTect Reverse Transcription kit (Qiagen). Finally, the resulting cDNA was used to quantify ET-1 mRNA by Real Time-PCR (CFX96 Real-Time System, Biorad) using TaqMan primer gene expression assays with ET-1 (catalog no. Rn01775763_g1), P2Y2 receptor (catalog no. Rn02070661_s1), P2Y4 receptor (catalog no. Rn02133903_s1), P2Y6 receptor (catalog no. Rn02134326_s1), and GAPDH (catalog no. Rn01775763_g1) primers. We assessed the expression of these three P2Y receptors because UTP can directly activate both P2Y2 and P2Y4 and may be converted to UDP that is an agonist for P2Y6 receptors (11). ET-1 and P2Y receptor gene expression was quantified relative to GAPDH using 2−ΔΔCt method.
Urine analysis.
Urinary Na+ and K+ concentrations were measured with an atomic absorption spectrometer in the flame photometry mode (model 3100; Perkin Elmer, Rodgau, Germany). Osmolality of infused solutions and urine samples were determined by vapor pressure osmometer (VAPRO 5600, ELITechGroup, Logan, UT).
Statistics.
Data are presented as means ± SE. Statistical comparison of two experimental groups was performed by unpaired Student's t-test. When several groups were compared, we used one-way ANOVA followed by post hoc analysis with Bonferroni correction. A probability of P < 0.05 was considered significant.
RESULTS
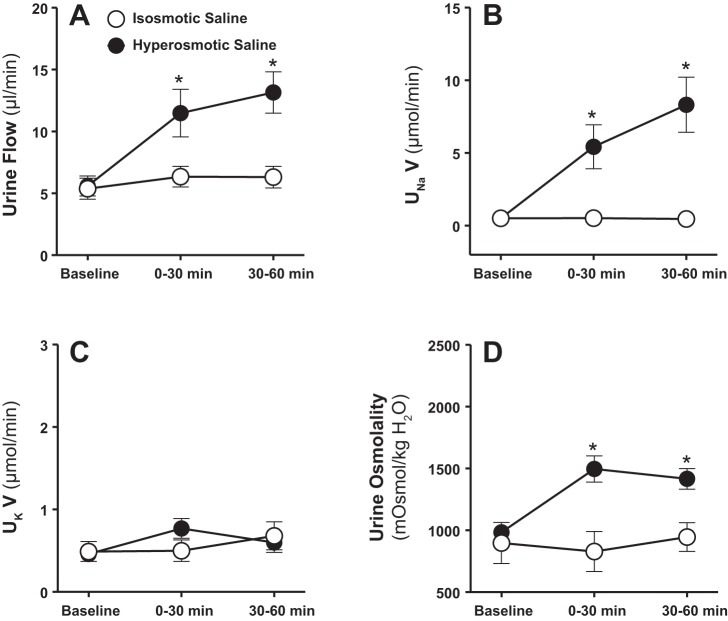
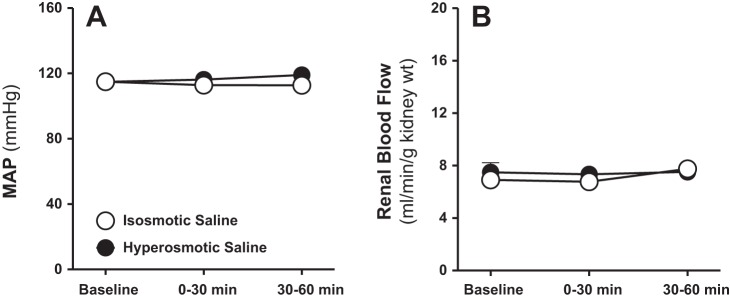
During infusion of isosmotic saline into the renal medulla, urine flow, electrolyte excretion, and urine osmolality remained unchanged (Fig. 2). Both urine flow and Na+ excretion increased markedly within the first 30 min of commencing intramedullary infusion of hyperosmotic saline (1,800 mosmol/kgH2O; Fig. 2, A and B). These diuretic and natriuretic responses demonstrated during the first 30 min of medullary NaCl loading continued further during the second 30-min time interval (Fig. 2, A and B). Increasing NaCl load into the renal medulla did not change urinary K+ excretion (Fig. 2C). Urine osmolality was significantly increased during the first as well as the second 30 min of intramedullary infusion of hyperosmotic saline (Fig. 2D). Mean arterial pressure (Fig. 3A) and total renal blood flow (Fig. 3B) remained unchanged during intramedullary infusions of isosmotic or hyperosmotic saline.
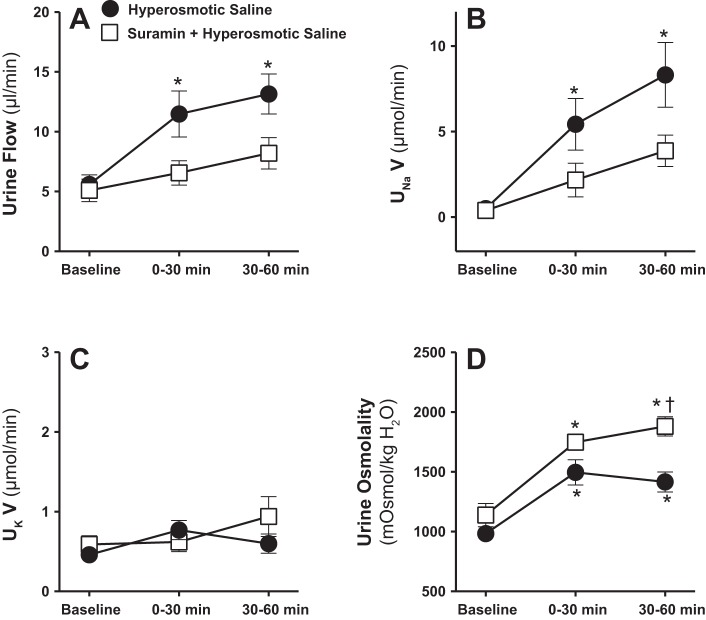
Fig. 2.
Effect of renal medullary interstitial infusions of isosmotic (284 mosmol/kgH2O) or hyperosmotic saline (1,800 mosmol/kgH2O) on urine flow (A), urinary excretion rate of Na+ (UNaV; B), K+ (UKV; C), and urine osmolality (D) in anesthetized male rats. Data are means ± SE of 5–7 samples in each group. Error bars for a few time points are not apparent due to very low SE. *P < 0.05 vs. corresponding isosmotic saline values.
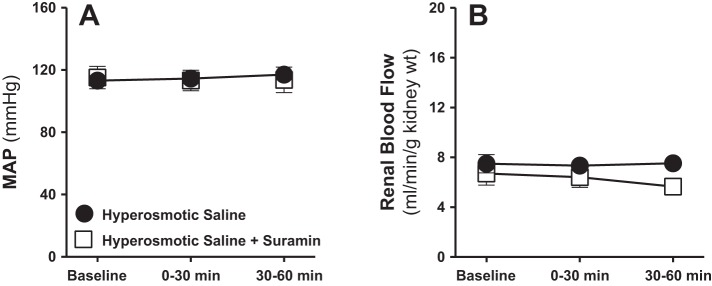
Fig. 3.
Effect of renal medullary interstitial infusions of isosmotic (284 mosmol/kgH2O) or hyperosmotic saline (1,800 mosmol/kgH2O) on mean arterial blood pressure (MAP; A) and total renal blood flow (B) in anesthetized male rats. Data are means ± SE of 5–7 samples in each group. Error bars for some time points are not apparent due to very low SE.
Increased medullary NaCl loading enhances ET-1 mRNA expression in the inner medulla.
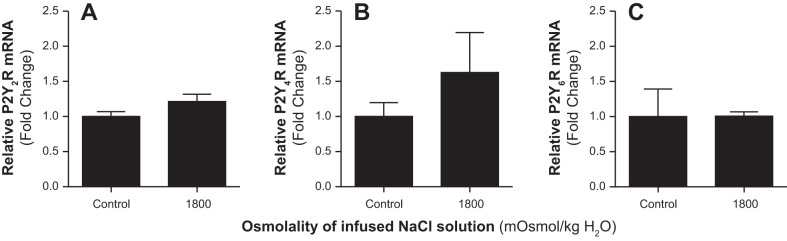
To determine whether the natriuretic effect of increasing medullary NaCl loading was associated with stimulation of the ET-1 system, we assessed the expression of ET-1 mRNA within the inner medulla in response to increased medullary NaCl loading. Infusion of hyperosmotic saline into the renal medulla for 60 min increased the expression of inner medullary ET-1 mRNA compared with corresponding values from rats infused with isosmotic saline (Fig. 4). Additionally, we assessed the expression of inner medullary P2Y receptors in response to NaCl loading. Intramedullary infusion of hyperosmotic saline for 60 min had no significant effect on the expression of P2Y2, P2Y4, or P2Y6 receptors within the inner medulla (Fig. 5).
Fig. 4.
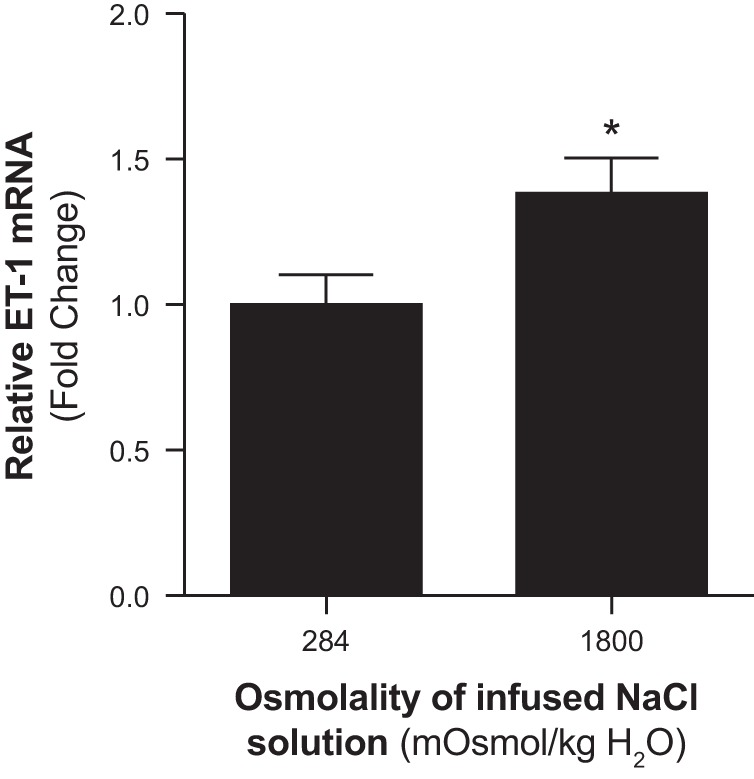
Effect of renal medullary interstitial infusions of isosmotic (284 mosmol/kgH2O) or hyperosmotic saline (1,800 mosmol/kgH2O) on the relative inner medullary mRNA expression of endothelin-1 (ET-1) in anesthetized male rats. Values represent fold change from corresponding isosmotic saline levels. Data are means ± SE of 5–7 samples in each group. *P < 0.05 vs. corresponding isosmotic saline values.
Fig. 5.
Effect of renal medullary interstitial infusions of hyperosmotic saline (1,800 mosmol/kgH2O) on relative inner medullary mRNA expression of purinergic receptors (P2Y2 R, P2Y4 R, and P2Y6 R) in anesthetized male rats. Values represent fold change from corresponding control (contralateral kidney) levels. Data are means ± SE of 4–5 samples in each group.
Blockade of P2 receptors attenuated natriuresis in response to increased medullary NaCl loading.
To determine the role of P2 receptor activation in mediating the natriuretic effect of increasing the medullary NaCl delivery, we tested the effect of intramedullary infusion of hyperosmotic saline with or without the nonselective antagonist of P2 receptors, suramin. Intramedullary infusion of suramin (750 μg·kg−1·min−1) significantly attenuated the diuretic as well as the natriuretic responses to increased medullary NaCl during the first as well as the second 30-min urine collection periods (Fig. 6, A and B). Increases in urine osmolality in response to intramedullary infusion of hyperosmotic saline were still evident in the presence of suramin, and even rising further during the second 30-min time interval (Fig. 6D). Urinary K+ excretion, mean arterial pressure, and total renal blood flow were not significantly altered by intramedullary infusion of hyperosmotic saline alone or when combined with the purinergic (P2) antagonist suramin (Figs. 6C and 7).
Fig. 6.
Effect of renal medullary interstitial infusions of hyperosmotic saline (1,800 mosmol/kgH2O) with or without the nonselective antagonist of purinergic (P2) receptors, suramin, on urine flow (A), UNaV (B), UKV (C), and urine osmolality (D) in anesthetized male rats. Data are means ± SE of 5–6 samples in each group. Error bars for some time points are not apparent due to very low SE. *P < 0.05 vs. corresponding baseline values. †P < 0.05 vs. corresponding hyperosmotic saline values.
Fig. 7.
Effect of renal medullary interstitial infusions of hyperosmotic saline (1,800 mosmol/kgH2O) with or without the nonselective antagonist of P2 receptors, suramin, on MAP (A) and total renal blood flow (B) in anesthetized male rats. Data are means ± SE of 5–6 samples in each group. Error bars for some time points are not apparent due to very low SE.
Blockade of P2 receptors attenuated the increase in ET-1 mRNA expression in response to increased medullary NaCl.
To evaluate the possibility that medullary P2 receptors stimulate the medullary ET-1 system following NaCl loading, we assessed the expression of ET-1 mRNA within the inner medulla after 60-min infusion of hyperosmotic saline alone or combined with suramin, the nonselective blocker of P2 receptors. Interestingly, the increase in inner medullary ET-1 mRNA expression in response to increased medullary NaCl loading was completely abolished by suramin (Fig. 8).
Fig. 8.
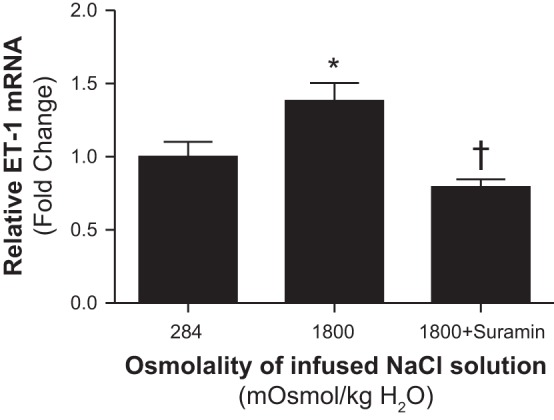
Effect of renal medullary interstitial infusions of isosmotic (284 mosmol/kgH2O), hyperosmotic saline (1,800 mosmol/kgH2O) with or without the nonselective antagonist of P2 receptors, suramin, on the relative inner medullary mRNA expression of ET-1 in anesthetized male and female rats. Values represent fold change from corresponding isosmotic saline levels. Data are means ± SE of 5–7 samples in each group. *P < 0.05 vs. corresponding isosmotic saline values. †P < 0.05 vs. corresponding hyperosmotic saline values.
Dual blockade of ETA and ETB receptors inhibited the natriuretic response to P2Y2/4 receptor activation.
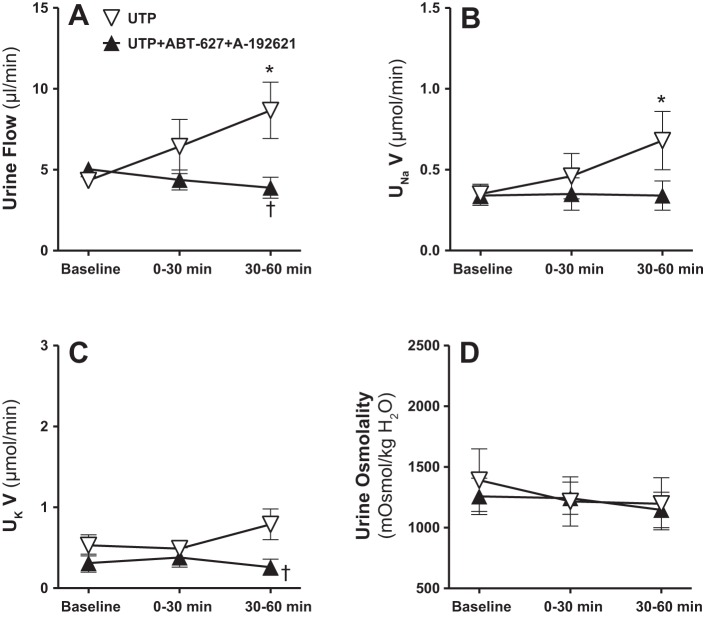
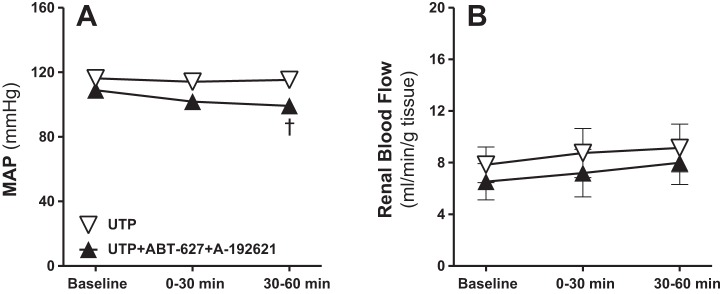
To determine a more direct interaction between the renal medullary ET-1 and purinergic systems, separate groups of male rats received intramedullary infusions of the P2Y2/4 receptor agonist UTP (200 pmol·kg−1·min−1) with or without combined ETA and ETB receptor blockade using the selective ETA antagonist (ABT-627) plus the selective ETB antagonist (A-192621). Intramedullary P2Y2/4 receptor activation with UTP enhanced the urine flow and Na+ excretion significantly during the second 30-min period, compared with baseline as demonstrated in Fig. 8, A and B. These diuretic and natriuretic responses to P2Y2/4 receptor activation were completely abolished by dual blockade of ETA and ETB receptors (Fig. 9, A and B). Urinary K+ excretion was unchanged by intramedullary P2Y2/4 activation, but was significantly lower during the second 30-min urine collection period in antagonist-infused rats compared with those given UTP alone (Fig. 9C). Urine osmolality was not significantly changed during these experiments (Fig. 9D). Mean arterial pressure remained stable during intramedullary infusion of UTP, and was slightly, but significantly, decreased in rats receiving intravenous ETA and ETB receptor blockers during the second 30-min time period, compared with the corresponding time point in the UTP group (Fig. 10A). However, no statistically significant differences were detected between the second 30-min time point during dual blockade of ET receptors and corresponding baseline values. Total renal blood flow was not significantly different between these two groups (Fig. 10B).
Fig. 9.
Effect of renal medullary interstitial infusions of the P2Y2/4 receptor agonist UTP with or without the selective ETA receptor blocker (ABT-627) plus the selective ETB receptor blocker (A-192621) on urine flow (A), UNaV (B), UKV (C), and urine osmolality (D) in anesthetized male rats. Data are means ± SE of 5–7 samples in each group. *P < 0.05 vs. corresponding baseline values. †P < 0.05 vs. corresponding UTP values.
Fig. 10.
Effect of renal medullary interstitial infusions of the P2Y2/4 receptor agonist UTP with or without the selective ETA receptor blocker (ABT-627) plus the selective ETB receptor blocker (A-192621) on MAP (A) and total renal blood flow (B) in anesthetized male rats. Data are means ± SE of 5–7 samples in each group. †P < 0.05 vs. corresponding UTP values.
DISCUSSION
The current study shows that 1) increased NaCl load to the renal medulla of male rats results in a diuretic and a natriuretic response associated with an increase in inner medullary ET-1 mRNA expression, 2) blockade of P2 receptors within the renal medulla inhibits the natriuresis and the increase in ET-1 mRNA expression following medullary NaCl loading, and 3) blockade of ET receptors inhibits the diuretic and natriuretic response to UTP infusion. Together, these findings suggest an interaction between the ET-1 and the purinergic signaling systems within the renal medulla to efficiently excrete increased NaCl load.
Several earlier studies suggest that extracellular osmolality enhances ET-1 production and release by freshly isolated or cultured renal tubular or collecting duct cells (9, 16, 22, 33). This in vitro evidence was supported by in vivo studies from our lab showing that infusion of hyperosmotic saline into the renal medulla resulted in diuresis, natriuresis, and increases in urinary ET-1 excretion (6). The diuretic response following intramedullary infusion of hyperosmotic saline was attenuated by dual blockade of ETA and ETB receptors (7), suggesting that infusion of hyperosmotic saline to the renal medulla results in ET-dependent diuresis (7). This is in line with accumulating evidence highlighting a central role for renal medullary derived ET-1 in the control of renal excretory function (6, 7, 14, 15, 17, 21, 26).
In the current study, we demonstrate that increasing the medullary NaCl load enhances water and Na+ excretion in male rats. This diuretic and natriuretic effect is demonstrated during the first 30 min of infusing hyperosmotic saline and extended during the second 30-min urine collection period. Our lab has provided evidence that exposing the renal medulla to hyperosmotic saline stimulates ET-1 release in male rats over this time frame (6). Importantly, our current study further supported this evidence by showing significant increases in ET-1 mRNA expression within the renal inner medullary tissues in response to intramedullary infusion of hyperosmotic saline for 60 min.
The signaling mechanism by which a high medullary NaCl load is translated into an increase in ET-1 production/release in vivo is currently unclear. Our findings suggest that the purinergic receptors might be involved. This idea is originally based on a recent study revealing that P2 receptors are required for flow-stimulated ET-1 release in inner medullary collecting duct cells (22). Our data showed that blockade of medullary P2 receptors by suramin inhibited the diuretic and natriuretic response to increased medullary NaCl loading. More importantly, suramin completely abolished the increase in inner medullary ET-1 mRNA expression following intramedullary infusion of hyperosmotic saline. These data provide strong in vivo evidence that activation of the medullary P2 receptors is necessary for the ET-1-dependent natriuretic response in male rats. As suramin is a nonselective blocker of P2, it is unclear which P2 receptors could be involved in the regulation of ET-1 production in response to medullary NaCl loading. Pandit and colleagues' (22) findings suggest that P2X7 and P2Y2 receptors play a central role. Accordingly, the potential contribution of different subtypes of medullary P2Y and P2X receptors to ET-1-dependent natriuresis remains unclear and further studies are needed to address this particular point.
Our results showed that P2Y2, P2Y4, and P2Y6 receptor mRNA is expressed in renal inner medullary tissues. Previous studies have shown that P2Y2 and P2Y4 receptors are expressed in the proximal convoluted tubule and outer medullary collecting ducts (2). Additionally, Cha and colleagues (8) found P2Y2 in the outer medullary collecting tubule. In the descending limb of Henle, P2Y2 mRNA was found, but P2Y4 was not expressed (2). Additionally, it has been shown that P2Y6 receptor mRNA is expressed in most segments of the rat nephron but basolateral expression of this protein is restricted to the proximal tubule (3). Our results showed that intramedullary NaCl loading did not significantly affect the expression of P2Y2, P2Y4, or P2Y6 receptors within the renal inner medulla.
It is important to comment on the effect of dual blockade of ETA and ETB receptors on the diuretic and natriuretic response to intramedullary infusion of UTP. As expected, intramedullary infusion of UTP for 60 min enhanced the urine flow and Na+ excretion. This is in line with the well-established role for the purinergic signaling system in regulating Na+ excretion (19, 24, 31). P2 receptors are expressed along the nephron, suppressing Na+ transport at several sites (19). Our studies also revealed an inhibitory effect of combined blockade of ETA and ETB receptors on the diuretic and natriuretic effect of UTP. We used the approach of blocking both receptor subtypes given evidence that both ETA and ETB receptors could participate in ET-1-dependent natriuresis even though the predominant action is via the ETB receptor (15, 29). Use of selective ETB receptor antagonists has the unfortunate consequence of increasing ETA receptor activity, which would make interpretation of such results problematic. Nonetheless, our findings further support the possibility of an interaction between the renal purinergic and ET-1 system within the renal medulla.
Collectively, our findings that pharmacologic blockade of P2 or both ETA and ETB receptors attenuated the diuretic and natriuretic response to intramedullary infusion of hyperosmotic saline or UTP, respectively, uncovers an important cross talk between the medullary purinergic and ET-1 signaling systems in regulating Na+ excretion in male rats. This is in line with recent findings demonstrating that blockade of P2Y2 and P2X7 receptors inhibits ET-1 production in inner medullary collecting duct cells (22). Furthermore, nonrenal studies demonstrated that purinergic and ET-1 signaling interact in sensory neurons (5), blood vessels (1, 13), as well as myocardium (10). Barr et al. (5) provided evidence that mechanical hypersensitivity caused by cutaneous ET-1 involves sensitization of P2X4 receptors. In porcine pancreatic arteries, P2Y14 receptor plays vasocontractile role that involves the release of ET-1 (1). Moreover, loss of mouse P2Y4 receptors on cardiac microvascular endothelial cells protects against myocardial infarction through ET-1 downregulation (10). Thus, there is fairly clear evidence to suggest that ET-1 and purinergic receptors are important in controlling renal tubular reabsorption of Na+ and water. The tubular segment contributing to the interaction between ET-1 and purinergic receptors in controlling Na+ excretion is not specifically known; however, the inner medullary collecting ducts are potential contributors based on recent data (22). Roles for proximal tubule and thick ascending limb are also postulated.
Finally, it is imperative to pinpoint that increasing the medullary NaCl load by intramedullary infusion of hyperosmotic saline is not used in the current study to specifically mimic the effect of high-salt diet, but rather, it is used as an established stimulus for ET-1 production. Changes in the osmolality of the renal medulla resulting from water loading (20), diuretics (28), dehydration (25), and drinking salty water (9) have been reported in the literature. Drinking 1% NaCl in water for 7 days has been reported to increase the medullary osmolality in rats (9); however, the effect of high-salt diet on medullary Na+ concentration is not yet totally clear.
It is important to note that our study used suramin as an antagonist for all P2 receptors and so a limitation of our study is that we cannot specifically assign a role for subtypes of P2 receptors, P2Y or P2X, or furthermore, subtypes of these two major classes of purinergic receptor. Additionally, we used UTP that can activate P2Y2 as well as P2Y4 receptors. UTP may also be converted to UDP that can activate P2Y6 receptors (11). Future studies using more specific P2 agonists and antagonists will help clarify the exact receptor subtypes involved in the interaction between ET-1 and purinergic signaling in the renal medulla.
In summary, our current findings suggest that P2 receptors activate the ET-1-dependent natriuresis in male rats (Fig. 11). Consistently, ET-1 appears to contribute to the diuretic and natriuretic response to intramedullary infusion of UTP. Together, these observations provide strong in vivo evidence for possible renal medullary interplay between ET-1 and purinergic systems in controlling Na+ and water homeostasis in male rats.
Fig. 11.

Hypothetical sequence of the interaction between the purinergic and ET-1 signaling.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant P01 HL095499 (to D. M. Pollock) and American Heart Association Postdoctoral Grant 15POST25090329 (to E. Y. Gohar).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Dr. Gohar is also affiliated with Department of Pharmacology and Toxicology, Faculty of Pharmacy, Alexandria University, Egypt.
AUTHOR CONTRIBUTIONS
E.Y.G., M.K., and D.M.P. conception and design of research; E.Y.G., J.S.S., and C.J. performed experiments; E.Y.G. analyzed data; E.Y.G., J.S.S., M.K., and D.M.P. interpreted results of experiments; E.Y.G. prepared figures; E.Y.G. drafted manuscript; E.Y.G., J.S.S., M.K., C.J., and D.M.P. edited and revised manuscript; E.Y.G., J.S.S., M.K., C.J., and D.M.P. approved final version of manuscript.
REFERENCES
- 1.Alsaqati M, Latif ML, Chan SL, Ralevic V. Novel vasocontractile role of the P2Y14 receptor: characterization of its signalling in porcine isolated pancreatic arteries. Br J Pharmacol 171: 701–713, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey MA, Imbert-Teboul M, Turner C, Marsy S, Srai K, Burnstock G, Unwin RJ. Axial distribution and characterization of basolateral P2Y receptors along the rat renal tubule. Kidney Int 58: 1893–1901, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Bailey MA, Imbert-Teboul M, Turner C, Srai SK, Burnstock G, Unwin RJ. Evidence for basolateral P2Y6 receptors along the rat proximal tubule: functional and molecular characterization. J Am Soc Nephrol 12: 1640–1647, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Ballew JR, Watts SW, Fink GD. Effects of salt intake and angiotensin II on vascular reactivity to endothelin-1. J Pharmacol Exp Ther 296: 345–350, 2001. [PubMed] [Google Scholar]
- 5.Barr TP, Hrnjic A, Khodorova A, Sprague JM, Strichartz GR. Sensitization of cutaneous neuronal purinergic receptors contributes to endothelin-1-induced mechanical hypersensitivity. Pain 155: 1091–1101, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boesen EI, Pollock DM. Acute increases of renal medullary osmolality stimulate endothelin release from the kidney. Am J Physiol Renal Physiol 292: F185–F191, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Boesen EI, Pollock DM. Cooperative role of ETA and ETB receptors in mediating the diuretic response to intramedullary hyperosmotic NaCl infusion. Am J Physiol Renal Physiol 299: F1424–F1432, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cha SH, Sekine T, Endou H. P2 purinoceptor localization along rat nephron and evidence suggesting existence of subtypes P2Y1 and P2Y2. Am J Physiol Renal Physiol 274: F1006–F1014, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Herrera M, Garvin JL. A high-salt diet stimulates thick ascending limb eNOS expression by raising medullary osmolality and increasing release of endothelin-1. Am J Physiol Renal Physiol 288: F58–F64, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Horckmans M, Esfahani H, Beauloye C, Clouet S, di Pietrantonio L, Robaye B, Balligand JL, Boeynaems JM, Dessy C, Communi D. Loss of mouse P2Y4 nucleotide receptor protects against myocardial infarction through endothelin-1 downregulation. J Immunol 194: 1874–1881, 2015. [DOI] [PubMed] [Google Scholar]
- 11.Jacobson KA, Costanzi S, Ohno M, Joshi BV, Besada P, Xu B, Tchilibon S. Molecular recognition at purine and pyrimidine nucleotide (P2) receptors. Curr Topics Med Chem 4: 805–819, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jensen ME, Odgaard E, Christensen MH, Praetorius HA, Leipziger J. Flow-induced [Ca2+]i increase depends on nucleotide release and subsequent purinergic signaling in the intact nephron. J Am Soc Nephrol 18: 2062–2070, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Joseph EK, Green PG, Bogen O, Alvarez P, Levine JD. Vascular endothelial cells mediate mechanical stimulation-induced enhancement of endothelin hyperalgesia via activation of P2X2/3 receptors on nociceptors. J Neurosci 33: 2849–2859, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kohan DE. The renal medullary endothelin system in control of sodium and water excretion and systemic blood pressure. Curr Opin Nephrol Hypertens 15: 34–40, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Kohan DE, Inscho EW, Wesson D, Pollock DM. Physiology of endothelin and the kidney. Compr Physiol 1: 883–919, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kohan DE, Padilla E. Osmolar regulation of endothelin-1 production by rat inner medullary collecting duct. J Clin Invest 91: 1235–1240, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kohan DE, Rossi NF, Inscho EW, Pollock DM. Regulation of blood pressure and salt homeostasis by endothelin. Physiol Rev 91: 1–77, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lehrmann H, Thomas J, Kim SJ, Jacobi C, Leipziger J. Luminal P2Y2 receptor-mediated inhibition of Na+ absorption in isolated perfused mouse CCD. J Am Soc Nephrol 13: 10–18, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Menzies RI, Unwin RJ, Bailey MA. Renal P2 receptors and hypertension. Acta Physiol (Oxf) 213: 232–241, 2015. [DOI] [PubMed] [Google Scholar]
- 20.Muller E, Neuhofer W, Burger-Kentischer A, Ohno A, Thurau K, Beck F. Effects of long-term changes in medullary osmolality on heat shock proteins HSp25, HSP60, HSP72 and HSP73 in the rat kidney. Pflügers Arch 435: 705–712, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Nakano D, Pollock JS, Pollock DM. Renal medullary ETB receptors produce diuresis and natriuresis via NOS1. Am J Physiol Renal Physiol 294: F1205–F1211, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pandit MM, Inscho EW, Zhang S, Seki T, Rohatgi R, Gusella L, Kishore B, Kohan DE. Flow regulation of endothelin-1 production in the inner medullary collecting duct. Am J Physiol Renal Physiol 308: F541–F552, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pochynyuk O, Bugaj V, Rieg T, Insel PA, Mironova E, Vallon V, Stockand JD. Paracrine regulation of the epithelial Na+ channel in the mammalian collecting duct by purinergic P2Y2 receptor tone. J Biol Chem 283: 36599–36607, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Praetorius HA, Leipziger J. Intrarenal purinergic signaling in the control of renal tubular transport. Annu Rev Physiol 72: 377–393, 2010. [DOI] [PubMed] [Google Scholar]
- 25.Saikia TC. Composition of the renal cortex and medulla of rats during water diuresis and antidiuresis. Q J Exp Physiol Cogn Med Sci 50: 146–157, 1965. [DOI] [PubMed] [Google Scholar]
- 26.Schneider MP, Ge Y, Pollock DM, Pollock JS, Kohan DE. Collecting duct-derived endothelin regulates arterial pressure and Na excretion via nitric oxide. Hypertension 51: 1605–1610, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schnermann J. Maintained tubuloglomerular feedback responses during acute inhibition of P2 purinergic receptors in mice. Am J Physiol Renal Physiol 300: F339–F344, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sone M, Ohno A, Albrecht GJ, Thurau K, Beck FX. Restoration of urine concentrating ability and accumulation of medullary osmolytes after chronic diuresis. Am J Physiol Renal Fluid Electrolyte Physiol 269: F480–F490, 1995. [DOI] [PubMed] [Google Scholar]
- 29.Stuart D, Rees S, Woodward SK, Koesters R, Strait KA, Kohan DE. Disruption of the endothelin A receptor in the nephron causes mild fluid volume expansion. BMC Nephrol 13: 166, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor TA, Gariepy CE, Pollock DM, Pollock JS. Gender differences in ET and NOS systems in ETB receptor-deficient rats: effect of a high salt diet. Hypertension 41: 657–662, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Vallon V, Rieg T. Regulation of renal NaCl and water transport by the ATP/UTP/P2Y2 receptor system. Am J Physiol Renal Physiol 301: F463–F475, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vassileva I, Mountain C, Pollock DM. Functional role of ETB receptors in the renal medulla. Hypertension 41: 1359–1363, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Yang T, Terada Y, Nonoguchi H, Ujiie K, Tomita K, Marumo F. Effect of hyperosmolality on production and mRNA expression of ET-1 in inner medullary collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 264: F684–F689, 1993. [DOI] [PubMed] [Google Scholar]