Abstract
Renal ischemia-reperfusion (I/R) in male rats causes reductions in plasma testosterone, and infusion of testosterone 3 h postreperfusion is protective. We tested the hypotheses that acute high doses of testosterone promote renal injury after I/R, and that acute low-dose testosterone is protective by the following: 1) increasing renal IL-10 and reducing TNF-α; 2) its effects on nitric oxide; and 3) reducing intrarenal T-cell infiltration. Rats were subjected to renal I/R, followed by intravenous infusion of vehicle or testosterone (20, 50, or 100 μg/kg) 3 h postreperfusion. Low-dose testosterone (20 μg/kg) reduced plasma creatinine, increased nitrate/nitrite excretion, increased intrarenal IL-10, and reduced intrarenal TNF-α, whereas 50 μg/kg testosterone failed to reduce plasma creatinine, increased IL-10, but failed to reduce TNF-α. A higher dose of testosterone (100 mg/kg) not only failed to reduce plasma creatinine, but significantly increased both IL-10 and TNF-α compared with other groups. Low-dose nitro-l-arginine methyl ester (1 mg·kg−1·day−1), given 2 days before I/R, prevented low-dose testosterone (20 μg/kg) from protecting against I/R injury, and was associated with lack of increase in intrarenal IL-10. Intrarenal CD4+ and CD8+ T cells were significantly increased with I/R, but were attenuated with low-dose testosterone, as were effector T helper 17 cells. The present studies suggest that acute, low-dose testosterone is protective against I/R AKI in males due to its effects on inflammation by reducing renal T-cell infiltration and by shifting the balance to favor anti-inflammatory cytokine production rather than proinflammatory cytokines.
Keywords: acute kidney injury, inflammation, males, androgens, effector T cells, Th17
the incidence of acute kidney injury (AKI) among hospitalized patients is increasing such that nearly 2–5% of patients in intensive care units are diagnosed with AKI, with mortality rates of ∼45% (1, 7). Gender differences in susceptibility to AKI are also a factor, since the incidence of AKI is higher in men than women (1, 7). Animal studies also show that males are more susceptible to renal ischemia-reperfusion (I/R) than are females (15, 22, 26). Although numerous studies have been performed, the mechanisms by which males are more susceptible to I/R AKI are still unclear.
Studies by Park and colleagues to address the role of androgens were done in female mice given testosterone injections for 14 days that increased plasma testosterone to 75–100 pg/ml before renal I/R (18), a level that would be similar to that of untreated males. They found that plasma creatinine (PCr) levels were significantly increased with renal I/R in these females, implicating androgens as a mediator of AKI in this model.
Based on these findings, we performed previous studies to determine whether the timing of testosterone could impact the response to renal I/R in male rats. Our laboratory found that endogenous plasma testosterone falls to almost undetectable levels in both I/R and sham males within 3 h after reperfusion (24). By 24 h after reperfusion, testosterone levels were ∼60% of presurgery levels in shams (390 ng/dl), but remained very low (20 ng/dl) in rats subjected to I/R (24). In addition, our laboratory found that a single acute dose of testosterone (20 μg/kg body wt iv) 3 h postreperfusion reduced PCr, proteinuria, and kidney injury molecule-1, and improved medullary blood flow compared with rats subjected to I/R alone (24).
In our laboratory's previous studies, we did not evaluate potential mechanisms responsible for testosterone-mediated protection against I/R AKI. The mechanisms thought to play a role in mediating I/R AKI are numerous, but include the roles played by oxidative stress (28) with attenuation of nitric oxide (NO) production (5), and increase in inflammatory cytokines mediated, at least in part, by an increase in immune cell infiltration (4, 8–10).
In the present studies then, our goals were twofold. First, we tested the hypothesis that high doses of testosterone administered 3 h postreperfusion after renal ischemia would not be protective against I/R injury. We anticipated that this study would shed light on why in some studies such as ours testosterone is protective and in others testosterone promotes further renal injury after I/R.
The second goal was to determine potential mechanisms by which low-dose testosterone is protective against renal I/R. Based on studies that NO, inflammation, and immune cell infiltration play roles in mediating renal I/R, we tested the following hypotheses: 1) that low-dose testosterone in part protected against renal I/R injury by increasing renal tissue interleukin (IL)-10 and anti-inflammatory cytokine, and reducing renal TNF-α and proinflammatory cytokine; 2) that NG-nitro-l-arginine methyl ester (l-NAME) blockade before I/R would attenuate the protective effect of low-dose testosterone, suggesting that low-dose testosterone may work, in part, by improving NO production; and 3) that low-dose testosterone reduces intrarenal CD4+ and CD8+ T-cell infiltration, thus also causing a reduction in renal inflammation. We also determined whether effector T helper 17 (Th17) cells were present in renal tissue and were reduced by testosterone infusion postreperfusion.
MATERIALS AND METHODS
Animals.
Male Sprague-Dawley rats, aged 8–10 wk (290–300 g), were obtained from the vendor (Harlan Sprague-Dawley, Indianapolis, IN) and allowed to equilibrate in a temperature-controlled environment with 12:12-h light-dark cycle for at least 1 wk. Rats were allowed ad libitum access to rat chow (Teklad 1% NaCl) and tap water. All procedures were approved by the Animal Care and Use Committee of the University of Mississippi Medical Center and followed the Guidelines for the Care and Use of Laboratory Animals, 2011 edition. Rats were used at 9–14 wk of age.
I/R AKI surgical procedure.
I/R was performed as our laboratory previously described (24). Briefly, rats were anesthetized using isoflurane, and a midline abdominal incision was made. Renal vessels on both kidneys were exposed and clamped for 30 min. Sham rats had midline abdominal incision and the kidneys exposed. Throughout the surgical procedure, rat body temperature was maintained at 37 ± 0.5°C. During the period of renal ischemia, a catheter was placed in the right femoral vein for testosterone infusion. Three hours postreperfusion, rats received either vehicle (sham and I/R) or testosterone propionate (20, 50, or 100 μg/kg body wt in 0.75% ethanol in 2 ml saline iv) over 10 min. After 24 h recovery, rats were euthanized, blood was collected for PCr levels, and kidneys were collected for IL-10, TNF-α, and T-cell counts, as described below.
l-NAME pretreatment.
To determine whether the protective effect of testosterone was mediated via NO release, rats were treated with l-NAME (1 mg·kg−1·day−1) for 2 days before I/R. I/R was performed as described above in the presence or absence of acute testosterone infusion (20 μg/kg) 3 h after reperfusion, as described. Urine collections were taken through the next 24 h to evaluate excretion of urinary nitrate/nitrite (NOx). After 24 h, rats were euthanized, and blood was drawn for PCr, IL-10, and TNF-α.
PCr was measured using a commercial creatinine kit (Lab Assay, Wako Bio Products, catalog no. 290-65901) by Jaffe's method, according to the manufacturer's protocol, as our laboratory previously described (24).
Nitrate/nitrite excretion (NOx) was measured in urine from rats subjected to renal I/R that were placed in metabolism cages for the 24 h after reperfusion. Nitrate/nitrite was measured as our laboratory has previously described (11).
Renal tissue TNF-α and IL-10.
At 24 h post-I/R, kidneys were removed, minced, and homogenized by Polytron (PT 2100, Elkhart, IN) in ice cold RIPA buffer (1:7 ratio) containing protease inhibitor cocktail (Roche, Indianapolis, IN). Homogenate was then centrifuged at 4°C for 30 min and centrifuged (4°C, 20 min at 12,000 g). Pellet was discarded, and supernatant was saved. Renal protein concentrations in supernatant were measured by Lowry assay. Renal TNF-α was measured in renal homogenates using a commercially available kit (R&D Systems, Minneapolis, MN; RTA00), as our laboratory previous described (24). Data are presented as picograms of TNF-α per microgram of protein. Renal IL-10 was measured using a commercially available kit (Quantikine Rat IL-10 ELISA, R&D Systems, Minneapolis, MN), according to the manufacturer's directions, and data are expressed as picograms IL-10 per milligram protein.
Measurement of CD4+ and CD8+ T cells in kidney tissue.
At 24 h post-I/R, kidneys were collected and minced on ice with a razor blade, incubated in RPMI-1640 (Invitrogen, Grand Islands, NY) containing 125 U/ml collagenase IV (Invitrogen) and 200 μg/ml DNase I, (Sigma, St. Louis, MO) for 50 min at 37°C. Tissue digests were washed with RPMI-1640 and centrifuged at 1,600 rpm for 10 min. Pellets were suspended in RPMI-1640 and pushed through a 100-μm sieve (Fisher Scientific). The single-cell suspension was washed with RPMI-1640, centrifuged at 1,600 rpm for 10 min, and then layered over Lymphoprep (Accurate Chemical & Scientific, Westbury, NY). Lymphocytes were isolated according to the manufacturer's directions. Cells (6 × 106) were incubated with 100 μl of either mouse anti-rat-CD4, or -CD8a (BD Biosciences, San Jose, CA) on ice for 30 min. Cells were washed and stained with fluorescein isothiocyanate or phycoerythrin (PE) cyanine 5 (Southern Biotech, Birmingham, AL). To determine if I/R affected T regulatory (CD4+CD25+FOXP3+) or Th17 effector cells (CD4+CD25−RORγ+), another subset of cells was stained with mouse anti-rat CD4 and anti-rat CD25 (BD Biosciences) on ice for 30 min. Cells were washed and stained with fluorescein isothiocyanate or PE cyanine 5 on ice for 30 min, followed by another wash step. Cells were next permeabilized with Forkhead box P3 (FOXP3) permeabilization buffer per the manufacturer's directions (eBioscience, San Diego, CA) and incubated with RAR-related orphan receptor-γ (ROR-γ) conjugated to PE (R&D Systems) and FOXP3 conjugated to Alexa 647 (BD Biosciences). After staining, cells were washed and resuspended in 500 μl of FACS buffer (RPMI-1640 or for permeabilized cells Hanks' balanced salt solution + 1% fetal bovine serum, 0.5% 0.5 M EDTA, 0.1 mM sodium azide; pH 7.4) and analyzed by flow cytometry using a Beckman Coulter Gallios Flow Cytometer analyzer. Negative controls, for each rat, were performed using isotype controls matched to the host species of the primary antibody, and cells were treated as described above. The percentage of positively staining cells above the negative control was determined for each rat and averaged for each group [sham, sham + testosterone (20 μg/kg), I/R alone, and I/R + testosterone] were calculated.
Morphological studies: light microscopy.
Kidneys of rats were removed 24 h post I/R, placed in 10% buffered formalin, sectioned (5 μm), and stained with Masson's trichrome. Light microscopic examination of sections from four rat kidneys in each group (35 fields per slide) were examined for necrosis by a renal pathologist (JPG) who was blinded to the groups' identities. Data from kidneys in a group were averaged and are presented as average percent necrosis ± SE.
Analyses of degree of medullary tubular injury.
Fluorescence microscopy was performed on the same kidneys, according to the method of Regner and colleagues (21). For each kidney sample, five randomly chosen outer medullary fields were evaluated using Nikon Eclipse 5Si (Nikon Instruments, Melville, NY) epifluorescent microscope using a 540- to 580-nm excitation filter and a 593- to 668-nm emission filter. Parameter for image capture was constant for all images [focus (fast): 1,280 × 960; capture (quality): 1,280 × 960; mode: manual; exposure: 600 ms; gain: 3.4×, contrast: enhanced]. Following constant background thresholding, the percentage of the area containing fluorescent necrotic tubular epithelium or cast material was quantified using Nis-Elements image analysis software (version 3.03, Nikon Instruments). Data were expressed as percentage, and data for four rats in each group were averaged. Data are presented as average percentage ± SE for each group of rats. Representative photographs were taken (×800 total magnifications to the screen) with Digital Sight-DS2mBW Color Camera.
Statistical analysis.
Graphpad Prism 6 was used for statistical comparisons. Statistical comparisons were made by two-way ANOVA. A value of P < 0.05 was considered statistically significant. All data are expressed as means ± SE.
RESULTS
Body weights.
Body weights were similar for all groups in the dose-response study (sham: 326.1 ± 12.1 g; I/R + 0 testosterone: 310.1 ± 25.5 g; I/R + 20 μg/kg: 334.5 ± 12.4 g; I/R + 50 μg/kg: 329.0 ± 15.1 g; I/R + 100 μg/kg: 330.9 ± 14.0 g; P = nonsignificant for all comparisons).
Dose response to testosterone.
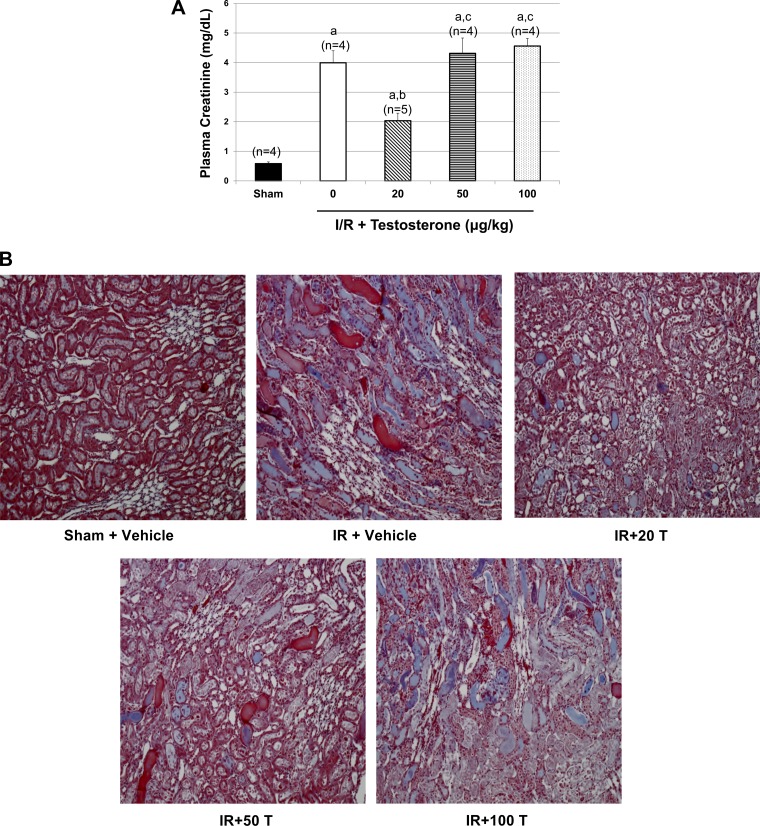
As shown in Fig. 1A, untreated rats subjected to renal I/R had significant increases in PCr compared with sham. As our laboratory showed previously (24), the dose of 20 μg testosterone reduced PCr in rats subjected to renal I/R. In contrast, 50 and 100 μg testosterone increased PCr to levels similar to I/R alone.
Fig. 1.
Effect of varying doses of testosterone on plasma creatinine, intrarenal IL-10, and TNF-α, 24 h following renal I/R. As described in materials and methods, rats were subjected to I/R, and testosterone was given via intravenous infusion at different doses (20, 50, or 100 μg/kg body wt) 3 h postreperfusion. A: plasma creatinine (PCr) levels were measured 24 h postreperfusion. B: representative morphology of outer medullary regions of the kidney 24 h post-I/R. Kidney sections were fixed in 10% buffered formalin and paraffin sectioned (5 μm). Sections were then stained with Masson's trichrome. Sections were examined using Nikon Eclipse 50i (Nikon Instruments, Melville, NY), magnification, ×100. T, testosterone. C: representative autofluorescence in outer medullary tubular epithelium, an indicator of necrosis, of the kidney 24 h post-I/R. Sections were examined using Nikon Eclipse 5Si (Nikon Instruments, Melville, NY) epifluorescent microscope equipped with Digital Sight-DS2mBW Color Camera, using a 540- to 580-nm excitation filter and a 593- to 668-nm emission filter. D: IL-10 was measured in kidney homogenates taken at 24 h postreperfusion. E: TNF-α was measured in kidney homogenates taken at 24 h postreperfusion. Values are means ± SE. Data were analyzed by two-way ANOVA. P < 0.05 vs. a|sham, bI/R, c20 μg/kg dose, and d50 μg/kg dose.
Figure 1B shows light microscopy of medullary region of representative kidneys from each group. As shown in Table 1, visible evaluation of necrosis in kidneys subjected to I/R showed that I/R alone significantly increased renal necrosis, and, while 20 μg/kg testosterone tended to reduce renal necrosis, it was not statistically significant. Doses of 50 and 100 μg testosterone were not different compared with I/R alone.
Table 1.
Necrosis score of renal tubules in kidneys of rats subjected to I/R ± testosterone infusion 3 h postreperfusion
| Group | n | Necrosis Score, % | SE |
|---|---|---|---|
| Sham | 4 | 0 | 0 |
| I/R | 4 | 66.25a | 5.46 |
| I/R + 20 T | 4 | 39.00a | 22.71 |
| I/R + 50 T | 4 | 62.50a | 21.15 |
| I/R + 100 T | 4 | 63.75a | 13.62 |
Necrosis score was determined by visual evaluation of at least 35 fields in n = 4 kidneys of each group. Data were averaged for each group. 20, 50, and 100 T, doses of testosterone propionate in μg/ml 0.9% NaCl given intravenously 3 h postreperfusion. Statistical analyses was done by two-way ANOVA. Significance defined as P < 0.05:
different compared with sham.
In our laboratory's previous studies (24), we showed that 20 μg/kg testosterone infused intravenously 3 h postreperfusion significantly reduced urinary protein excretion and kidney injury molecule-1 excretion and improved outer medullary blood flow measured 24 h postreperfusion. Based on these data, we evaluated outer medullary tubular epithelial necrosis using a fluorescent technique (21). Necrotic tubules exhibit marked autofluorescence compared with intact tubules. Figure 1C shows representative pictures of fluorescence in each group. As shown in Table 2, medullary autofluorescence was significantly increased after I/R and was reduced in I/R + 20 μg testosterone. The dose of 50 μg testosterone failed to attenuate the fluorescence as well, and the dose of 100 μg testosterone was not effective in attenuating autofluorescence compared with I/R alone.
Table 2.
Fluorescence of outer medullary necrotic tubular epithelium
| Group | n | Tubular Injury Score, % | SE |
|---|---|---|---|
| Sham | 4 | 1.683 | 0.475 |
| I/R | 4 | 19.685a | 3.339 |
| I/R + 20 T | 4 | 5.995a,b | 1.856 |
| I/R + 50 T | 4 | 10.632a,b,c | 2.617 |
| I/R + 100 T | 4 | 15.753a,c | 5.632 |
Autofluorescence analyses of trichrome staining in outer medullary region of kidneys was done in n = 4 rat kidneys of each group, as described in materials and methods. Data were averaged for each group. Statistical analyses was done by two-way ANOVA. Significance defined as P < 0.05:
different compared with sham;
different compared with I/R;
different compared with I/R + 20 T.
As shown in Fig. 1D, renal tissue levels of anti-inflammatory cytokine, IL-10, were increased by almost twofold compared with sham. Testosterone 20 μg and 50 μg doses increased intrarenal IL-10 by 60% compared with I/R alone. In I/R rats given 100 μg testosterone, renal IL-10 level was increased by 130% compared with I/R alone. As shown in Fig. 1E, renal tissue proinflammatory cytokine, TNF-α, was increased by ∼60% in I/R rats compared with sham controls. Testosterone 20 μg reduced renal TNF-α in I/R rats by 45% compared with I/R alone. In contrast, rats receiving 50 μg testosterone failed to reduce TNF-α compared with I/R alone, and 100 μg testosterone increased TNF-α by 25% compared with I/R alone.
NO synthase inhibition and I/R.
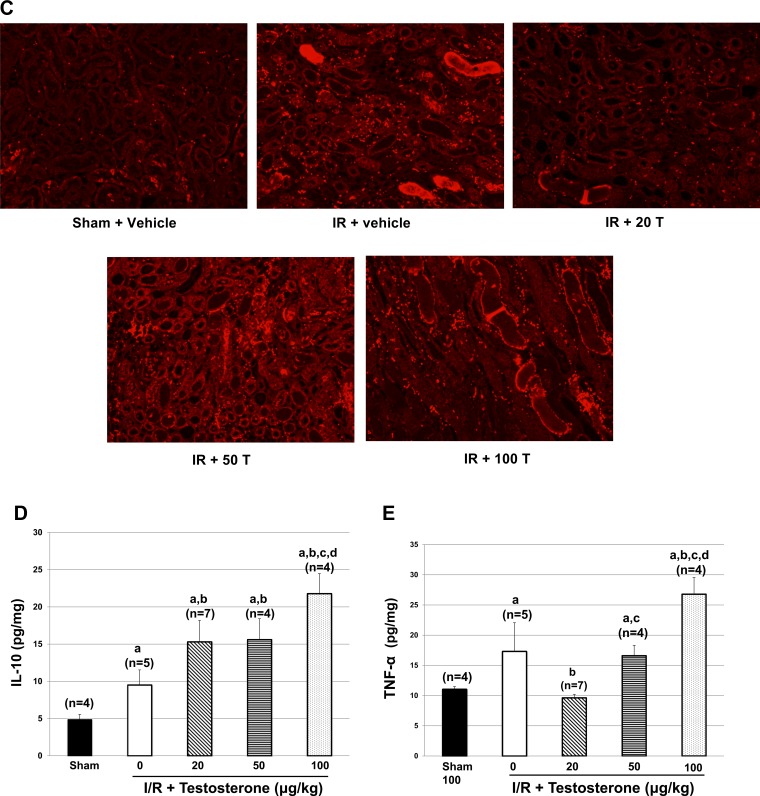
Rats subjected to I/R had ∼90% drop in NOx excretion compared with sham controls (see Fig. 2A). Rats that were pretreated with l-NAME for 2 days and subjected to I/R had a similar drop in NOx excretion and a similar increase in PCr as rats subjected to I/R alone (Fig. 2B). In rats subjected to I/R + 20 μg/kg testosterone without l-NAME pretreatment, NOx excretion was significantly increased, although not to sham levels, and PCr was significantly reduced compared with I/R alone or l-NAME-treated I/R rats. In rats given l-NAME for 2 days and then subjected to I/R and given 20 μg testosterone, testosterone was unable to either increase NOx or reduce PCr.
Fig. 2.
Pretreatment with l-NAME prevents testosterone (20 μg/kg) from improving plasma creatinine and prevents the increase in intrarenal IL-10 without affecting TNF-α. A: nitrate/nitrite excretion was measured in urine from rats exposed to I/R, as described in materials and methods. BW, body weight. B: plasma creatinine was measured at 24 h postreperfusion. C: IL-10 was measured in kidney homogenates taken at 24 h postreperfusion. D: TNF-α was measured in kidney homogenates taken at 24 h postreperfusion. Values are means ± SE. Data were analyzed by two-way ANOVA. P < 0.05 vs. a|sham, bI/R, cI/R + l-NAME, and dIR + 20 μg/kg testosterone alone.
Renal IL-10 levels were increased in I/R rats (Fig. 2C) and were unaffected by pretreatment with l-NAME. Testosterone 20 μg/kg further increased IL-10 compared with I/R alone or I/R + l-NAME. However, in I/R rats pretreated with l-NAME and given 20 μg/kg testosterone, IL-10 was reduced to levels even lower than in sham rats. As shown in Fig. 2D, TNF-α increased significantly with I/R alone, and pretreatment with l-NAME did not further augment the levels. In rats with I/R + 20 μg/kg testosterone, renal TNF-α was normalized to the levels found in sham rats. Pretreatment with l-NAME in rats subjected to I/R and given 20 μg/kg testosterone resulted in a further reduction in TNF-α levels that were even lower than sham.
Effect of testosterone on renal infiltration of T cells.
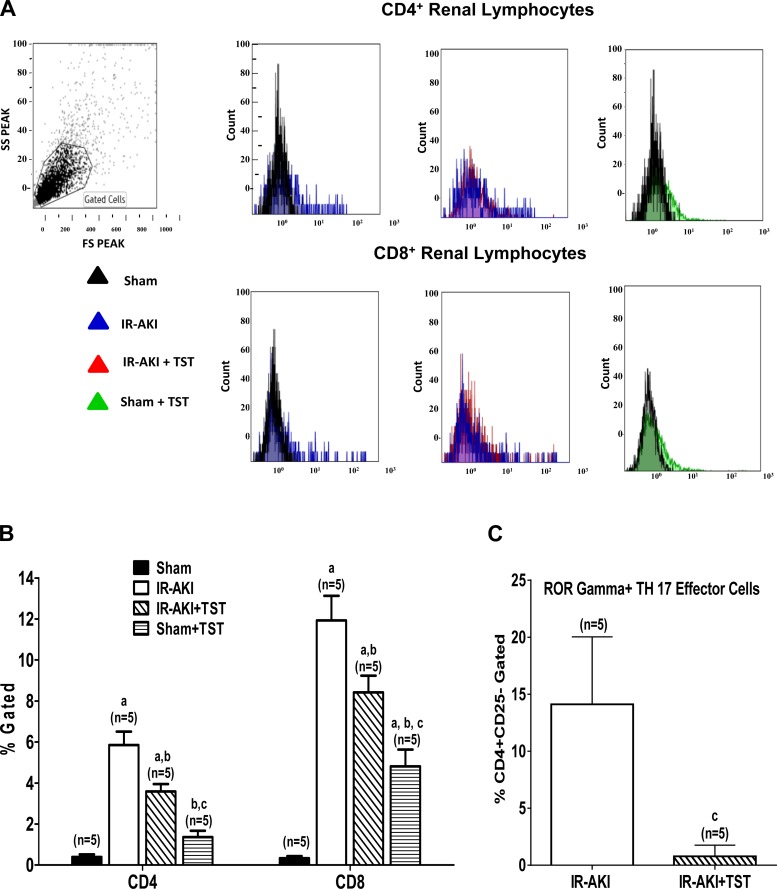
In this study, we evaluated the effect of low-dose (20 μg/kg) testosterone on renal T-cell infiltration. I/R significantly increased renal infiltration of both CD4+ and CD8+ T cells compared with sham rats (P < 0.001 and P < 0.001; Fig. 3, A and B). Treatment with 20 μg/kg testosterone in the I/R group significantly decreased infiltration of both CD4+ and CD8+ T cells compared with I/R alone (Fig. 3, A and B). Moreover, administration of testosterone significantly decreased renal Th17 effector cells (Fig. 3C). However, testosterone had no effect on renal T regulatory cells in rats subjected to I/R (data not shown).
Fig. 3.
Testosterone reduces the infiltration of CD4+ and CD8+ T cells into the kidneys of rats subjected to renal I/R. A: representative histogram overlays of CD4+ and CD8+ T cells between the groups of rats. TST, testosterone; SS, side scatter; FS, forward scatter. B: quantification of CD4+ and CD8+ T cells in kidneys of rats subjected to I/R with and without testosterone infusion given 3 h postreperfusion. C: Th17 effector cells were measured in I/R rats treated with and without testosterone. The percent positive ROR-γ cells gated in the CD4+CD25− renal cell population were measured. Values are means ± SE. Data were analyzed by ANOVA and are shown as T cells (%gated). aP < 0.005 vs. sham. P < 0.05 vs. bI/R alone, and cI/R + testosterone 20 μg/kg.
DISCUSSION
In the present study, the following novel observations were made: 1) while 20 μg/kg doses of testosterone attenuated PCr, reduced medullary tubular necrosis, as noted by fluorescence microscopy, and tended to reduce whole kidney necrosis (light microscopy) following renal I/R, higher doses (50 and 100 μg/kg) failed to protect; 2) one potential mechanism by which low-dose testosterone (20 μg/kg) protects against renal injury after I/R may be due to its effect to increase intrarenal IL-10 and reduce TNF-α, whereas higher doses of testosterone increase IL-10 but either fail to reduce (50 μg dose) or further increase (100 μg dose) TNF-α, compared with untreated renal I/R rats; 3) blockade of NO synthesis before I/R prevents testosterone from being able to reduce PCr and renal necrosis and is associated with attenuation of renal IL-10; 4) another potential mechanism for low-dose (20 μg/kg) testosterone-mediated protection against I/R is the significant reduction of infiltrating CD4+, Th17 cells, and CD8+ T cells in kidneys of male rats.
The present data support our laboratory's previous studies (24) that low doses of testosterone (20 μg/kg) attenuate the elevated PCr in male rats 24 h after renal I/R. However, higher doses of testosterone (50 and 100 μg/kg) were ineffective in reducing PCr. Based on our laboratory's previous studies that plasma testosterone is reduced by 90% 3 h post-renal reperfusion in untreated rats (24), our present data suggest that only small doses of testosterone replacement are protective against I/R injury, and even modest increases in doses of testosterone are detrimental following I/R injury. These new data may explain why Park and colleagues found that sustained injections of male-equivalent doses of testosterone into female mice worsened the injury following I/R (18).
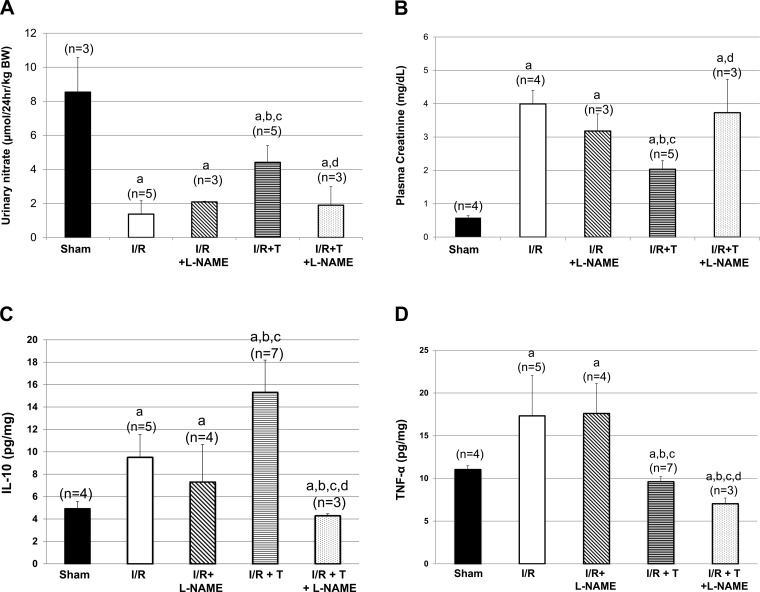
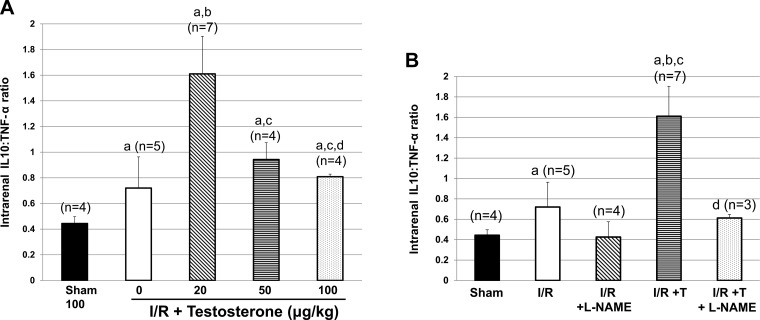
Our laboratory's previous studies characterized the renal consequences of testosterone infusion following renal I/R (24), but did not evaluate potential mechanisms by which testosterone attenuated PCr. In addition, the previous study only evaluated one dose of testosterone. In the present study, we found that testosterone infusion at all three doses (20, 50, and 100 μg/kg) increased the anti-inflammatory cytokine IL-10 in renal tissue of I/R rats. In contrast, only the 20 μg/kg testosterone dose significantly reduced renal tissue TNF-α compared with in untreated rats subjected to I/R, and the highest dose of testosterone (100 μg/kg) significantly increased the levels of TNF-α compared with in untreated I/R rats. As shown in Fig. 4A, the ratio of IL-10 to TNF-α is not significantly increased in rats subjected to renal I/R. In contrast, the ratio is significantly higher in rats given low-dose testosterone after I/R. High doses of testosterone failed to increase the IL-10-to-TNF-α ratios compared with rats subjected to I/R alone. These data suggest that low-dose testosterone infusion provides protection against renal I/R by promoting anti-inflammatory cytokine production and attenuating pro-inflammatory cytokine synthesis, thus shifting the balance to anti-inflammatory status.
Fig. 4.
IL-10-to-TNF-α ratios in rat subjected to I/R with and without testosterone in dose-response experiment and in the presence or absence of pretreatment with l-NAME. A: IL-10-to-TNF-α ratio is higher in I/R rats given 20 μg/kg testosterone, but reduced with higher doses. a|P < 0.05 vs. sham. b|P < 0.05 vs. I/R. c|P < 0.05 vs. 20 μg/kg dose. d|P < 0.05 vs. 50 μg/kg dose. B: pretreatment with l-NAME prevents the increase in IL-10-to-TNF-α ratios in rats given testosterone 3 h postreperfusion. Values are means ± SE. Data were analyzed by two-way ANOVA. aP < 0.05 vs. sham. bP < 0.05 vs. I/R. cP < 0.05 vs. I/R + l-NAME. dP < 0.05 vs. 20 μg/kg testosterone alone.
Our data also suggest that 20 μg/kg testosterone dose may be increasing NO synthesis in the hours after renal I/R, thus protecting against renal injury, as noted by the increase in PCr with testosterone infusion. l-NAME pretreatment attenuated the increase in IL-10 that occurred with testosterone alone after I/R, but also further reduced intrarenal TNF-α. Thus the IL-10-to-TNF-α ratios, shown in Fig. 4B, that were significantly increased with testosterone infusion alone were reduced with l-NAME in testosterone-treated rats to levels similar to I/R alone or I/R + l-NAME alone, causing a proinflammatory effect compared with I/R + testosterone.
Additional support for testosterone causing a reduction in inflammation following renal ischemia can be seen in our data showing that low-dose testosterone reduces the influx of inflammatory T cells, both CD4+ and CD8+ cells, into the kidney. Mechanisms that prevent or attenuate inflammatory T-cell movement into the kidney are protective after I/R (4, 8–10, 27, 29). Whether the infiltrated T cells were the source of the intrarenal TNF-α and IL-10 is not clear from our present studies. Similarly, Th17 cells secrete the inflammatory cytokine, IL-17, which has also been linked to increased protein levels of TNF-α and decreased IL-10. Low-dose testosterone has been demonstrated to decrease infiltrating CD4+ leukocytes and to reduce IL-17 in hepatic injury models (23). In our study, we demonstrate an important role for low-dose testosterone to significantly reduced total CD4+ T as well as intrarenal Th17, which could lead to less inflammation and renal oxidative stress. Furthermore, IL-17 plays a role in contributing to the development of renal I/R injury in mouse models (9), further supporting a relationship between these inflammatory cytokines and renal injury.
Although not tested in this study, it is possible that testosterone could also be causing renal vasodilation, especially in the renal medulla at a critical time following reperfusion. Testosterone is a vasodilator in numerous vascular beds (12, 17, 19), and treatment of animals with vasodilators has been shown to protect against renal I/R (3, 25). Indeed, our laboratory found in previous studies that testosterone causes a reduction in afferent resistance in kidneys using a renal microperfusion preparation (12). Muroya and colleagues (16) reported that medullary blood flow was reduced to 25% of baseline by 2–3 h postreperfusion after renal I/R in normal rats. Since this is the same time (3 h) that we are giving the acute dose of testosterone after reperfusion, it is possible that testosterone is increasing medullary perfusion and thus causing protection of the kidney in this way, as well as reducing inflammation. Muroya et al. (16) found that the reduction in PCr at 24 h following I/R was prevented in rats in which the medullary blood flow was improved to only 50% of baseline levels by an increase in 20-hydroxyeicosatetraenoic acid. Therefore, even just a small increase in medullary blood flow due to testosterone infusion could play an important role in reducing PCr at 24 h post-I/R. The mechanism by which testosterone causes acute vasodilation in other vascular beds is due to inhibition of calcium channels via the voltage-activated calcium channel and calcium-activated and voltage-sensitive potassium channels (5, 17, 20), rather than via NO release. However, our data that low-dose testosterone increases NOx and l-NAME attenuates this response and also prevents testosterone protection after I/R suggest a role for NO in renal protection by testosterone. Thus future studies will be necessary to determine whether testosterone improves medullary circulation and the mechanisms involved.
Perspectives
Whether androgen levels are reduced in men prior to development of AKI, thus allowing injury to occur, or whether testosterone levels are reduced once AKI is present, is unknown and should be studied in human populations. It has been well established that a reduction in circulating testosterone levels is associated with increased cardiovascular disease (2, 14) and increased mortality with coronary heart disease (13). If endogenous testosterone levels do decrease early in the development in AKI, and a low dose of testosterone could be found that protected men from worsening AKI, this could be a major breakthrough in the management of AKI in men. Future studies are necessary to determine whether indeed this is feasible.
GRANTS
This study was partly supported by grant funding from National Institutes of Health R01HL66072 (J. F. Reckelhoff), P01HL51971 (J. F. Reckelhoff), and R01HDo67541 (B. D. LaMarca) and from American Heart Association 14POST18640015 (M. Moulana).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.N.P., K.W., B.D.L., M.M., A.L.-R., A.S., and J.P.G. performed experiments; C.N.P., K.W., B.D.L., M.M., A.L.-R., A.S., J.P.G., and J.F.R. analyzed data; C.N.P., K.W., B.D.L., M.M., A.L.-R., A.S., L.A.J., J.P.G., and J.F.R. interpreted results of experiments; C.N.P., K.W., B.D.L., and J.F.R. prepared figures; C.N.P., B.D.L., M.M., L.A.J., and J.F.R. drafted manuscript; C.N.P., K.W., B.D.L., A.L.-R., A.S., L.A.J., J.P.G., and J.F.R. edited and revised manuscript; C.N.P., K.W., B.D.L., M.M., A.L.-R., A.S., L.A.J., J.P.G., and J.F.R. approved final version of manuscript; L.A.J. and J.F.R. conception and design of research.
REFERENCES
- 1.Centers for Disease Control and Prevention. Hospitalization discharge diagnoses for kidney disease–United States, 1980–2005. MMWR Morb Mortal Wkly Rep 57: 309–312, 2008. [PubMed] [Google Scholar]
- 2.Channer KS. Endogenous testosterone levels and cardiovascular disease in healthy men. Heart 97: 867–869, 2011. [DOI] [PubMed] [Google Scholar]
- 3.Cristol JP, Thiemermann C, Mitchell JA, Walder C, Vane JR. Support of renal blood flow after ischemic-reperfusion injury by endogenous formation of nitric oxide and of cyclo-oxygenase vasodilator metabolites. Br J Pharmacol 109: 188–194, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denecke C, Tullius SG. Innate and adaptive immune responses subsequent to ischemia-reperfusion injury in the kidney. Prog Urol 24, Suppl 1: S13–S19, 2014. [DOI] [PubMed] [Google Scholar]
- 5.Feitero J, Santos-Silva AJ, Verde I, Cairrao E. Testosterone and atrial natriuretic peptide share the same pathway to induce vasorelaxation of human umbilical artery. J Cardiovasc Pharmacol 63: 461–465, 2014. [DOI] [PubMed] [Google Scholar]
- 7.Hoste EA, Clermont G, Kersten A, Venkataraman R, Angus DC, De Bacquer D, Kellum JA. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care 10: R73, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kinsey GR, Sharma R, Okusa MD. Regulatory T cells in AKI. J Am Soc Nephrol 24: 1720–1726, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li L, Huang L, Vergis AL, Ye H, Bajwa A, Narayan V, Strieter RM, Rosin DL, Okusa MD. IL-17 produced by neutrophils regulates IFN-gamma-mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J Clin Invest 120: 331–342, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li L, Okusa MD. Macrophages, dendritic cells, and kidney ischemia-reperfusion injury. Semin Nephrol 30: 268–277, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopez-Ruiz AF, Iliescu R, Reckelhoff JF. Refractory blood pressure in female SHR to increased oxidative stress is not mediated by NO or by upregulation of renal antioxidant enzymes. Am J Physiol Regul Integr Comp Physiol 298: R266–R271, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu Y, Fu Y, Ge Y, Juncos LA, Reckelhoff JF, Liu R. The vasodilatory effect of testosterone on renal afferent arterioles. Gend Med 9: 103–111, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malkin CJ, Pugh PJ, Morris PD, Asif S, Jones TH, Channer KS. Low serum testosterone and increased mortality in men with coronary heart disease. Heart 96: 1821–1825, 2010. [DOI] [PubMed] [Google Scholar]
- 14.Morgentaler A, Miner MM, Caliber M, Guay AT, Khera M, Traish AM. Testosterone therapy and cardiovascular risk: advances and controversies. Mayo Clin Proc 90: 224–251, 2015. [DOI] [PubMed] [Google Scholar]
- 15.Muller V, Losonczy G, Heemann U, Vannay A, Fekete A, Reusz G, Tulassay T, Szabo AJ. Sexual dimorphism in renal ischemia-reperfusion injury in rats: possible role of endothelin. Kidney Int 62: 1364–1371, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Muroya Y, Fan F, Regner KR, Falck JR, Garrett MR, Juncos LA, Roman RJ. Deficiency in the Formation of 20-hydroxyeicosatetraenoic acid enhances renal ischemia-reperfusion injury. J Am Soc Nephrol 26: 2460–2469, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Navarro-Dorado J, Orensanz LM, Recio P, Bustamante S, Benedito S, Martinez AC, Garcia-Sacristan A, Prieto D, Hernandez M. Mechanisms involved in testosterone-induced vasodilatation in pig prostatic small arteries. Life Sci 83: 569–573, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Park KM, Kim JI, Ahn Y, Bonventre AJ, Bonventre JV. Testosterone is responsible for enhanced susceptibility of males to ischemic renal injury. J Biol Chem 279: 52282–52292, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Perusquia M, Espinoza J, Montano LM, Stallone JN. Regional differences in the vasorelaxing effects of testosterone and its 5-reduced metabolites in the canine vasculature. Vascul Pharmacol 56: 176–182, 2o12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramirez-Rosas MB, Cobos-Puc LE, Munoz-Islas E, Gonzalez-Hernandez A, Sanchez-Lopez A, Villalon CM, Maassenvandenbrink A, Centurion D. Pharmacological evidence that Ca2+ channels and, to a lesser extent, K+ channels mediate the relaxation of testosterone in the canine basilar artery. Steroids 76: 409–415, 2011. [DOI] [PubMed] [Google Scholar]
- 21.Regner KR, Zuk A, Van Why SK, Shames BD, Ryan RP, Falck JR, Manthati VL, McMullen ME, Ledbetter SR, Roman RJ. Protective effect of 20-HETE analogues in experimental renal ischemia reperfusion injury. Kidney Int 75: 511–517, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robert R, Ghazali DA, Favreau F, Mauco G, Hauet T, Goujon JM. Gender difference and sex hormone production in rodent renal ischemia reperfusion injury and repair. J Inflamm (Lond) 8: 14, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwinge D, Carambia A, Quaas A, Krech T, Wegscheid C, Tiegs G, Prinz I, Lohse AW, Herkel J, Schramm C. Testosterone suppresses hepatic inflammation by the downregulation of IL-17, CXCL-9, and CXCL-10 in a mouse model of experimental acute cholangitis. J Immunol 194: 2522–2530, 2015. [DOI] [PubMed] [Google Scholar]
- 24.Soljancic A, Ruiz AL, Chandrashekar K, Maranon R, Liu R, Reckelhoff JF, Juncos LA. Protective role of testosterone in ischemia-reperfusion-induced acute kidney injury. Am J Physiol Regul Integr Comp Physiol 304: R951–R958, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Srisawat U, Kongrat S, Muanprasat C, Chatsudthipong V. Losartan and sodium nitroprusside effectively protect against renal impairments after ischemia and reperfusion in rats. Biol Pharm Bull 38: 753–762, 2015. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka R, Tsutsui H, Ohkita M, Takaoka M, Yukimura T, Matsumura Y. Sex differences in ischemia/reperfusion-induced acute kidney injury are dependent on the renal sympathetic nervous system. Eur J Pharmacol 714: 397–404, 2013. [DOI] [PubMed] [Google Scholar]
- 27.Tsutahara K, Okumi M, Kakuta Y, Abe T, Yazawa K, Miyagawa S, Matsunami K, Otsuka H, Kaimori J, Takahara S, Nonomura N. The blocking of CXXR3 and CCR5 suppresses the infiltration of T lymphocytes in rat renal ischemia reperfusion. Nephrol Dial Transplant 27:3 799–3806, 2012. [DOI] [PubMed] [Google Scholar]
- 28.Wilcox CS, Liu R, Lai EY. Protective effect of tempol on acute kidney injury through PI3K/Akt/Nrf2 signaling pathway. Kidney Blood Press Res 41: 129–138, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xue C, Liu Y, Li C, Yang T, Xie L, Zhou P. Powerful protection against renal ischemia reperfusion injury by T cell-specific NF-κB inhibition. Transplantation 97: 391–396, 2014. [DOI] [PubMed] [Google Scholar]