Abstract
Purpose
The aim of this study was to investigate the impact of exercise-induced hypoxaemia (EIH) developed at sea-level on exercise responses at moderate acute altitude.
Methods
Twenty three subjects divided in three groups of individuals: highly trained with EIH (n = 7); highly trained without EIH (n = 8) and untrained participants (n = 8) performed two maximal incremental tests at sea-level and at 2,150 m. Haemoglobin O2 saturation (SpO2), heart rate, oxygen uptake (VO2) and several ventilatory parameters were measured continuously during the tests.
Results
EIH athletes had a drop in SpO2 from 99 ± 0.8% to 91 ± 1.2% from rest to maximal exercise at sea-level, while the other groups did not exhibit a similar decrease. EIH athletes had a greater decrease in VO2max at altitude compared to non-EIH and untrained groups (-22 ± 7.9%, -16 ± 5.3% and -13 ± 9.4%, respectively). At altitude, non-EIH athletes had a similar drop in SpO2 as EIH athletes (13 ± 0.8%) but greater than untrained participants (6 ± 1.0%). EIH athletes showed greater decrease in maximal heart rate than non-EIH athletes at altitude (8 ± 3.3 bpm and 5 ± 2.9 bpm, respectively).
Conclusion
EIH athletes demonstrated specific cardiorespiratory response to exercise at moderate altitude compared to non-EIH athletes with a higher decrease in VO2max certainly due to the lower ventilator and HRmax responses. Thus EIH phenomenon developed at sea-level negatively impact performance and cardiorespiratory responses at acute moderate altitude despite no potentiated O2 desaturation.
Introduction
In endurance sport, most training camps and competitions take place at altitude around 2000 m. At this altitude, aerobic performance and maximal oxygen uptake (VO2max) are reduced due to the decrease in partial pressure of inspired O2 (PIO2) [1]. However, altitude does not appear to affect every individual in an equal manner. Indeed it is now well documented that endurance-trained athletes demonstrate a larger decline in VO2max with increasing altitude compared to untrained subjects [2–4]. This particular sensitivity of endurance-trained athletes to moderate altitude has been related to an important decrease in arterial O2 saturation (SaO2) [2,3,5–7]. At sea level, 50% of healthy endurance-trained athletes develop exercise-induced hypoxaemia (EIH) [8]. “EIH athletes” show a difference between rest and maximal arterial O2 pressure (PaO2) values for at least 10 mmHg and/or a delta of SaO2 of at least 4% [9]. It is reported that EIH subsequently affects performance in response to high intensity exercise [10]. Physiopathology of EIH seems to be 1) a relative hypoventilation related to training adaptation and 2) gas exchange abnormality [11]. Each of these mechanisms appear to participate in EIH occurrence, with specific contribution depending on the training status and/or exercise mode and/or muscle mass involved [12]. However on average ~20% of the variation in PaO2 between individuals during exercise is due to the variation in hyperventilation response, with the remaining ~80% of the variance in PaO2 drop roughly divided evenly between ventilation-perfusion mismatch and diffusion limitation [13]. Because PIO2 is reduced with altitude, EIH exhibited at sea level by some endurance-trained athletes could be amplified at acute moderate altitude. Only a few studies have examined the consequences of EIH during a maximal exercise performed at altitude while the number of athletes involved in endurance mountaineering sports, who are prone to develop EIH, was greatly increased in the last decade. In fact, literature reports only four studies that have compared O2 desaturation and aerobic performance during maximal exercise at altitude between EIH athletes identified at sea level and non-EIH athletes [4,6,14,15]. If these studies have reported a greater decrease in VO2max in EIH athletes than in non-EIH athlete, they also have reported controversial results about SpO2 decrement and cardio-respiratory responses. Moreover none of these studies has examined the effect of a moderate altitude despite it is an usual altitude for training camps and competitions in endurance sports or mountaineering. The general aim of this study was to better understand the constraints applied on the cardiorespiratory system of EIH athletes at moderate altitude. More specifically, the purpose of this study was to evaluate 1) the level of O2 desaturation and 2) cardio-ventilatory responses of EIH athletes identified at sea level during a maximal exercise performed at acute moderate altitude.
Materials and Methods
Participants
Twenty-three healthy non-smoker males were recruited for this study. All the participants were sea-level residents. Fifteen participants were endurance-trained athletes involved in running or cycling activities (at least 10 hours per week for the past ten years). The remaining eight were sedentary or active in recreational sports, but had never been engaged in systematic endurance training. Before their inclusion in the protocol, each participant was informed about the procedures and the potential risks inherent to the experiments. They signed a written informed consent form. All procedures were approved by the ethics committee of the Consell General de l’Esport, Catalunya.
Design
Each participant was involved in two maximal incremental tests performed on a cycle ergometer (Kettler, Ense-Parsit, Germany). The exercises were separated by 7 days. The first test was performed at sea level (SL) and the second at 2150 meters in an altitude hut (ALT). After a 6-minutes warm-up at 30 Watts (W) for untrained participants and 60 W for trained participants, the power was increased every minute by 30 W until exhaustion. The test was considered to be maximal if at least three of the following criteria were presented: 1) an increase of VO2 of < 100 ml with the last increase in work rate, 2) achievement of age-predicted maximal heart rate (HR) [210-(0.65 age) ± 10%], 3) a respiratory exchange ratio (RER) above 1.1, and 4) the incapacity to maintain the pedalling frequency imposed (70 rpm minimum) despite maximum effort and verbal encouragement. During each test, cardiorespiratory parameters and O2 saturation were recorded. SL test allowed us to determine two groups of endurance-trained athletes: one group exhibiting EIH (n = 7) and a second group without EIH (n = 8). Mean characteristics of the three groups are presented in Table 1.
Table 1. Anthropometric and training data.
| EIH | Non-EIH | Untrained | |
|---|---|---|---|
| Age (years) | 40 ± 3.5 | 39 ± | 40 ± 1.6 |
| Body mass (kg) | 70 ± 7.2 * | 69 ± 7.3 * | 77 ± 5.9 |
| Height (cm) | 177 ± 5.2 | 175 ± 6.2 | 173 ± 3.0 |
| Body mass index (kg.m-2) | 22 ± 2.2 * | 22 ± 1.3 * | 25 ± 1.7 |
| Training (hours.week-1) | 14 ± 5.7 * | 11 ± 4.7 * | 2 ± 1.3 |
| Training (years) | 22 ± 5.7 | 21 ± 6.3 | / |
* Significantly different from untrained group (p < 0.05).
Measurements and EIH definition
Oxygen saturation level of haemoglobin was assessed by the peripheral capillary oxygen saturation (SpO2). SpO2 was measured continuously during tests using an ear-lobe pulse oximeter (Nonin, Minnesota, USA). PureSAT® technology used in Nonin Medical pulse oximeter guarantees an measurement accuracy of ± 2.1% compared to the gold standard which is CO-oximetry analysis of arterial blood samples. The ear was pre-warmed by a vasodilating capsaicin cream (Finalgon, Fher, Spain) to avoid poor perfusion during exercise. EIH may be considered to exist when SpO2 decreases of 4% between rest and maximal effort of the test at SL of at least the last 3 minutes [9]. At the same time, respiratory data were collected by a portable automatic breath-by-breath metabolic system (K4b2, Cosmed, Rome, Italy): VO2 (ml.min-1.kg-1), RER (VCO2/VO2), minute ventilation (VE, l.min-1), tidal volume (VT, l), breathing frequency (BF, breaths.min-1). K4b2 system was calibrated before each test according to the manufacturer’s specifications: using a 3-l syringe and a gas bottle of known O2 and CO2 concentrations (16 and 5%, respectively). Each subject was also equipped with a chest belt (Polar Electro, Kempele, Finland) to collect HR continuously (beats.min-1).
Statistical treatment
The results are expressed as means ± SD. Differences with no repeated measures among the three groups were analysed using one-way analysis of variance (ANOVA). Two-way ANOVA were used for repeated measures to analyse the main effect and interaction of altitude and group on measured parameters. Correlations between the variables were tested using Pearson’s product-moment correlation coefficient test. For all tests, the level of statistical significance was set at p < 0.05. Analyses were conducted using SigmaStat software (Ver 3.5).
Results
Characteristics at rest and in response to exercise at SL
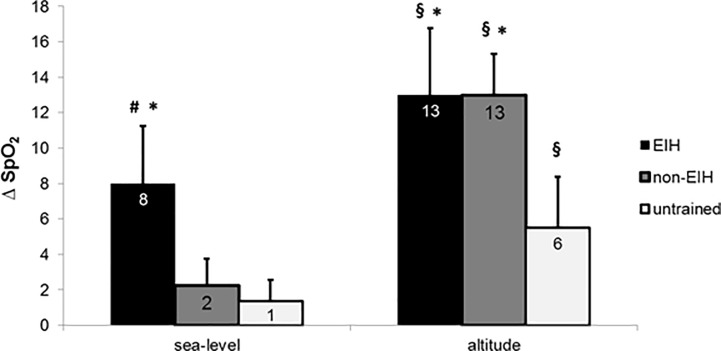
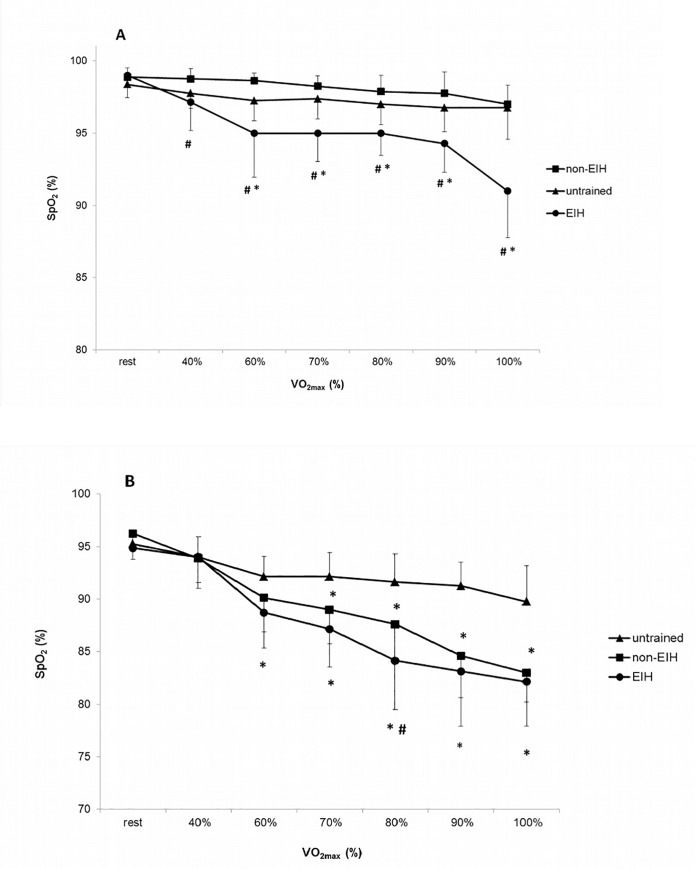
There was no difference in anthropometric data between EIH and non-EIH groups (Table 1). Untrained group had a higher body mass and body mass index compared to athletes (p < 0.05). Untrained participants presented significant differences in physiological data compared to endurance-trained groups (Table 1). At SL, there was no significant difference in SpO2 resting values between groups. SpO2 was normal (99 ± 0.8%) for all the participants at rest. Seven of the 15 endurance-trained athletes (~50%) exhibited EIH at SL. Delta of SpO2 (ΔSpO2, difference between rest and end of exercise values) at SL was significantly higher in EIH group than in non-EIH and untrained groups (Fig 1). Regarding SpO2 kinetics at SL in EIH group reported a greater SpO2 drop from the beginning until 60% of VO2max and the difference was reinforced from 90% to 100% of VO2max (Fig 2A). Performance and cardiorespiratory maximal parameters are presented in Table 2. Endurance-trained groups had a significant greater VO2max and Pmax than untrained group. EIH group exhibited a lower ventilatory equivalent for O2 (VE/VO2max) and CO2 (VE/VCO2max) at maximal exercise while no difference occurred in HRmax, BFmax, VTmax, VEmax and duration of test.
Fig 1. Delta of haemoglobin O2 saturation (ΔSpO2, difference between rest and maximal exercise values) at sea level and at 2150 meters for EIH, non-EIH and untrained participants.
§ Significantly different from sea level (p < 0.01); * Significantly different from untrained participants (p < 0.01); # Significantly different from non-EIH (p < 0.01).
Fig 2.
Kinetics of haemoglobin O2 saturation (SpO2) during rest and incremental maximal exercise at sea level (A) and altitude (B). * Significantly different from untrained (p < 0.05); # Significantly different from non-EIH (p < 0.05). Note that, at all intensities, SpO2 values at altitude are significantly different from sea level in the three groups (p < 0.05).
Table 2. Incremental maximal exercise parameters at sea level and at altitude.
| EIH | Non-EIH | Untrained | ||
|---|---|---|---|---|
| Effective | 7 | 8 | 8 | |
| VO2max(ml.min-1.kg-1) | SL | 62 ± 2.9 * | 59 ± 2.9 * | 41 ± 5.1 |
| ALT | 48 ± 5.4 *§ | 51 ± 4.1 *§ | 36 ± 7.9 § | |
| P-VO2max (Watt) | SL | 424 ± 53.2 * | 383 ± 47.4 * | 315 ± 39.3 |
| ALT | 373 ± 75.2 *§ | 341 ± 45.2 *§ | 285 ± 45.4 § | |
| HRmax (beats.min-1) | SL | 176 ± 8.5 | 173 ± 7.3 | 180 ± 8.3 |
| ALT | 168 ± 9.2 § | 168 ± 9.1 | 176 ± 8.0 | |
| VEmax (l.min-1) | SL | 149 ± 27.4 | 163 ± 27.8 * | 135 ± 22.1 |
| ALT | 136 ± 44.1 | 152 ± 23.9 | 136 ± 28.8 | |
| BFmax (breaths.min-1) | SL | 46 ± 10.5 | 54 ± 7.0 | 48 ± 8.5 |
| ALT | 40 ± 10.0 # | 49 ± 9.7 | 48 ± 8.6 | |
| VTmax (l) | SL | 3.3 ± 0.5 | 3.0 ± 0.5 | 2.8 ± 0.3 |
| ALT | 3.3 ± 0.5 | 3.2 ± 0.5 | 2.8 ± 0.4 | |
| VE/VO2max | SL | 35 ± 4.7 *# | 40 ± 3.9 | 43 ± 6.9 |
| ALT | 40 ± 7.2 * | 44 ± 7.5 | 49 ± 4.3 § | |
| VE/VCO2max | SL | 30 ± 3.8 # | 34 ± 2.5 | 34 ± 4.4 |
| ALT | 32 ± 5.1 *# | 36 ± 3.9 | 37 ± 2.8 |
VO2max: maximal oxygen uptake; P-VO2max: power achieved at VO2max; HRmax: heart rate at maximal exercise; VEmax: minute ventilation at maximal exercise; BFmax: breathing frequency at maximal exercise; VTmax: tidal volume at maximal exercise; VE/VO2max: ventilatory equivalent for O2 at maximal exercise; VE/VCO2max: ventilatory equivalent for CO2 at maximal exercise.
§ Significantly different from sea level (p < 0.05);
* Significantly different from untrained participants (p < 0.05);
# Significantly different from non-EIH athletes (p < 0.05).
Adaptations during exercise at ALT
At 2150 m, PIO2 is decreased and there was a significant effect of hypoxia on SpO2 resting values (99 ± 0.8% vs 95 ± 1.3% over all subjects) without difference among groups. SpO2 values at the end of exercise were significantly reduced in the three groups compared to SL condition, respectively: 82 ± 4.2% vs 91 ± 1.2% in EIH group; 83 ± 2.8% vs 97 ± 1.3% in non-EIH group; 90 ± 3.5% vs 97 ± 1.8% in untrained group (Fig 2). ΔSpO2 was significantly greater in EIH and non EIH groups compared with untrained group (Fig 1). Non-EIH group defined at SL have developed the same O2 desaturation than EIH group defined at SL. In all groups, there was an effect of ALT condition on ΔSpO2, reflecting O2 desaturation during exercise. Regarding SpO2 kinetic, most of SpO2 decrease occurred between 60 to 80% of VO2max in EIH group whereas it decrease between 80 to 100% of VO2max in non-EIH trained group (Fig 2B).
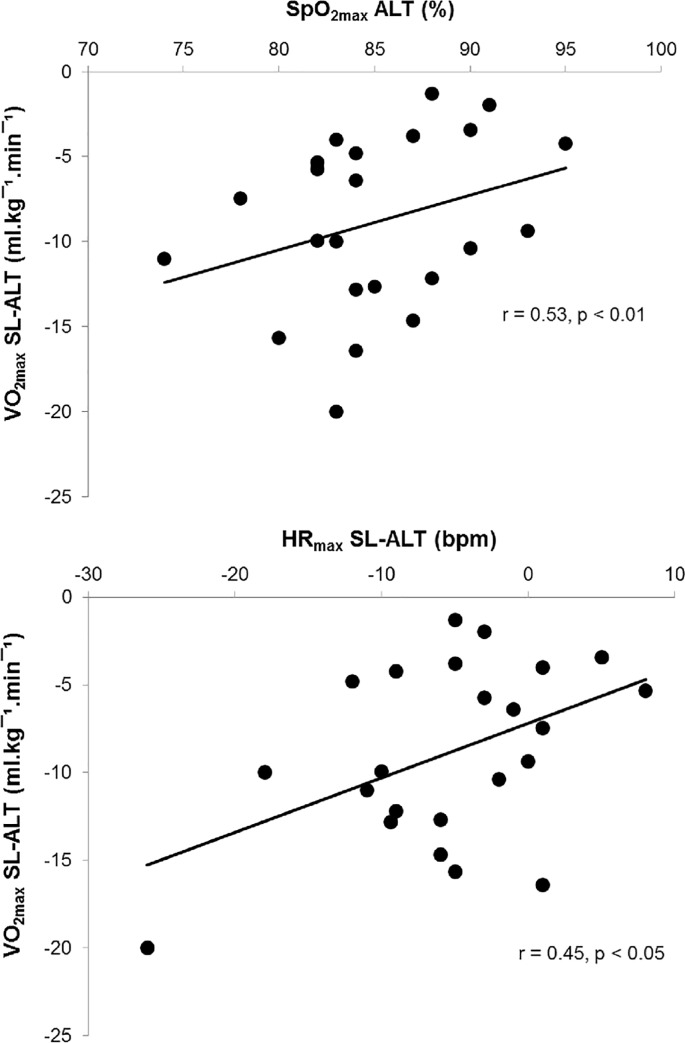
VO2max, power achieved at VO2max (P-VO2max) and duration of exercise at ALT were significantly lower than at SL in the three groups (Table 2). VO2max SL-ALT (difference between SL and ALT values) was significantly greater in EIH group compared to non-EIH and untrained groups in relative values (-22 ± 7.9%, -16 ± 5.3% and -13 ± 9.4%, respectively) and in absolute values. P-VO2max fall was around 10% in the three groups. Only EIH group exhibited a significant decrease of HRmax at ALT compared to SL condition (Table 2). When the data from all groups were pooled, there were significant relationship between VO2max SL-ALT and SpO2max at ALT (r = 0.53, p < 0.01) and also with HRmax SL-ALT (r = 0.45, p < 0.05; Fig 3). No significant effect of ALT has been observed for VEmax, BFmax and VTmax. BFmax and VE/VCO2max were lower in EIH group compared to non-EIH and untrained groups. VEmax tended to be lower in EIH group compared to non-EIH group (p = 0.09).
Fig 3. Correlations between decrease in VO2max (VO2max SL-ALT, difference between sea level and altitude values) with SpO2 values at the end of exercise at altitude (SpO2max ALT) and with decrease in HRmax (HRmax SL-ALT, difference between sea level and altitude values).
All participants were considered, n = 23.
Discussion
The main finding of this investigation is that non-EIH group had the same O2 desaturation level at ALT with EIH athletes identified at SL. However EIH athletes have a higher decline in VO2max, HRmax and an exercise ventilatory response possibly attenuated.
Methodological considerations
For ethical reasons this study used pulse oximetry, a non-invasive method to estimate SaO2 rather than blood arterial gases. Pulse oximetry method has been reported to deliver high precision, reproducibility and validity for O2 saturation above 75% when compared to O2 saturation measured from arterial blood gases at rest and exercise, at SL and at ALT [16,17]. Mollard et al. [17] have shown that the correlation between SaO2 and SpO2 was strong (r2 = 0.97, p < 0.001). In our study we are in agreement with the literature because SpO2 values were > 75%. Further we were very rigorous in the use of this method. Being aware of the limitations caused by the use of pulse oximetry, we used an EIH definition suitable at SpO2 measurement. Prefaut et al. [9] proposed that “EIH may be considered to exist when SaO2 decreases of 4% from baseline with 1 to 2% accounting for the accuracy of pulse oximeters and 1 to 2% accounting for the shift to the right of the oxyhemoglobin dissociation curve during exercise due to acidosis. EIH should persist for at least the last 3 steps of an incremental exercise test or the last 3 minutes of a steady state exercise test”.
O2 desaturation in EIH athletes at ALT
At ALT, the decline in PIO2 leads to a decline in PO2 down the cascade from the atmosphere, to the alveoli, to the arterial blood and finally into the capillary. However, impairment in PaO2 during exercise at ALT may not be universal for all subjects. In fact previous studies have shown a greater arterial O2 desaturation in athletes at maximal exercise in hypoxia [5,18]. Chapman [12] suggested that when PaO2 corresponds to the shoulder of the oxyhemoglobin dissociation curve (as for EIH) a small decline in PO2 leads to a relatively large decline in O2 saturation. As SpO2 during maximal exercise at SL in EIH group was on the shoulder of the oxyhemoglobin dissociation curve, we hypothesized that in response to a same exercise done at moderate altitude can result a significantly greater SpO2 fall in EIH group. Yet in our study, the same ΔSpO2 has been observed at ALT between EIH and non-EIH athletes. Previous studies at higher altitude (3000–4000m) have reported controversial results but seem indicate a higher decline in SpO2 for EIH athletes compared to non-EIH athletes [4,14,15]. Our results suggest that at ALT (~ 2000 m), reduce in PIO2 is not sufficient to induce a greater O2 desaturation in EIH athletes compared to non-EIH athletes. Our study is the first at moderate altitude, thus more studies are necessary to make a general trend.
Specific cardio-ventilatory responses of EIH athletes at ALT
At ALT, our results suggest an attenuate ventilatory response in EIH athletes but the small number of subjects in each group not allows us to clearly affirm this idea. In contrast, Gavin et al. [4] have clearly reported lower VEmax, VE/VO2max et VE/VCO2max at 4000 m in EIH athletes. It has been generally accepted that the ventilatory response to hypoxia is mainly mediated by the sensitivity of peripheral chemoreceptors [19]. Thus, the attenuated pulmonary ventilation at ALT in EIH group could be related to an alteration of the ventilatory response to hypoxia (HVR), as an index of peripheral chemosensitivity to hypoxia. Indeed, previous studies [20,21] have indicated that EIH athletes demonstrate a lower HVR suggesting that inadequate hyperventilatory response at ALT must be due to low chemoresponsiveness. However these studies have not measured ventilatory parameters during exercise in hypoxia. A potential low chemoresponsiveness in EIH group during exercise at ALT can be a surprising result because intermittent hypoxia exposure (as during training in EIH group) has been repeatedly shown to increase HVR [22–25]. This discrepancy can be explained by at least two reasons. Firstly, the increase in HVR seems to enhance exercise ventilation and improve SaO2 depending on the altitude where exercise is performed, since it was observed at high altitude (4500 m) [22] but not at moderate altitude (2500 m or below) [26,27]. An explanation can be the level of arterial oxygenation. In fact mean SpO2 values of our subjects during exercise at moderate ALT were around 80% whereas at 4500 m the values were around 60% [22]. As a consequence the stimulus to the peripheral oxygen sensing chemoreceptors was markedly lower in our subjects and could have contributed only slightly to the regulation of exercise ventilation. Secondly, in general, athletes are known to express low exercise ventilation and are reported to illustrate low chemoresponsiveness at SL [28]. The blunted chemosensitivity to HVR in the endurance athletes has been considered to be due to endurance training over long periods [29]. Thus exercise training and exposure to intermittent hypoxia might have opposite effects on HVR. At 4500 m, Katayama et al. [30] have observed that HVR increased significantly in control group but not in training group. These results would suggest that endurance training during intermittent exposure to hypoxia depresses the increment of chemosensitivity to hypoxia. Thus, the larger relative hypoventilation in EIH group during exercise in moderate ALT could be due to a larger effect of aerobic training on chemoreceptor desensitization.
Concerning cardiac response, Mollard et al.[7] have noted that HRmax decreased from 1000 m in trained subjects and from 2500 m in untrained subjects. Our results confirm the decrease in HRmax at acute moderate ALT in EIH athletes but not in non-EIH athletes. This greater decrease in HRmax at ALT in EIH athletes compared to non-EIH athletes has been observed in previous studies but not at moderate ALT [14,15]. Autonomic changes could be involved in the reduction in HRmax at ALT in EIH group. Indeed, prior studies have provided a gradual down regulation of β-adrenergic receptors [31] and an up-regulation of muscarinic receptors [32] over the time of exposure to ALT, as a possible mechanism of myocardial adaptation to hypoxia. Further it has been shown that chronic exercise, as chronic exposure to hypoxia, reduced the number of cardiac β receptors [33]. Perhaps the frequent repetition of long-duration exercises with training in subjects who exhibit an arterial O2 desaturation could induce a similar mechanism of myocardial adaptation and could explain the decrease in HRmax at ALT in EIH group.
Consequences of EIH on VO2max at moderate altitude
At SL, EIH is already known to negatively affect VO2max [8] and subsequently exercise performance [10]. In this study at 2150 m, EIH athletes demonstrated a higher decline in VO2max from SL than non-EIH athletes. From an investigation compiling the results of 11 studies examining the magnitude of VO2max decline with acute ALT exposure in endurance-trained athletes, VO2max declines by 7.7% for every 1000 m ascended above SL [1]. The mean decrease in VO2max in non-EIH athletes in this present study was 7.4% per 1000 m, which is in accordance with the literature. In EIH athletes, VO2max fall is higher (10% per 1000 m) exceeding the standards reported in the literature. Recently, Chapman et al. [12] suggested that the degree of arterial desaturation during maximal exercise at SL, and not baseline VO2max levels per se, is a primary limiting factor determining VO2max decline with exposure to acute moderate ALT. But as previously described, SpO2 fall at moderate ALT was equal in both EIH and non-EIH groups, bringing into question the contribution of other limiting factor(s) determining VO2max decline in EIH athletes. According to Fick equation, a fall in HRmax at ALT can have an effect on VO2max via a reduction of maximal cardiac output (Qmax). HRmax decrease under ALT was higher in EIH than in non-EIH athletes and even more compared to untrained participants. Thus greater VO2max decrement in EIH athletes could be explained by a larger reduction in O2 transport than non-EIH athletes. However in the present study, Qmax had not been measured; the effects of modification of HRmax on Qmax should be interpreted with caution. Further although EIH group shows a significant decrease in HRmax, this value is not significantly different between the two athletes groups at altitude.
This study has also shown a decrease of P-VO2max at altitude around 10% despite a VO2max decrease around 22% in EIH group. Thus it seems that the decrease of P-VO2max at altitude is not proportional with the VO2max decrease. As in our study, Peltonen and colleagues noted that hypoxia of 2 500 m reduced VO2max nearly twice as much as maximal power during a maximal exercise on cycle ergometer [34]. Several studies have shown that chronic [35] and acute [36,37] lack of oxygen supply to muscle, reduces the leftwards shift of the electromyogram power spectrum density during sustained static contractions of 60–80% of maximum voluntary contraction. This reduction in the power spectrum density corresponds to a preferential recruitment of fast motor units [38], which may constitute an adaptive process to limit the recruitment of slow motor units which are highly oxygen dependent [36]. The preferential recruitment of fast motor units could constitute an adaptive muscle response to a reduced oxygen supply and can explain the lesser decrease of P-VO2max compared to VO2max decrease in hypoxia. Further investigations are needed to confirm this hypothesis.
Conclusion
EIH athletes have shown a greater aerobic impairment than non-EIH athletes at altitude in spite of a same O2 desaturation level. Furthermore they also demonstrated a greater decrease in HRmax and potentially ventilatory adaptations at altitude. Thus, EIH athletes develop specific cardiorespiratory adaptations during exercise at acute moderate altitude maybe due to their frequent exposures to hypoxia. Based on these results, this study highlights the importance to discriminate EIH athletes in studies involving exercise adaptations in endurance athletes at altitude. Definitely further investigations into the mechanisms of EIH at moderate altitude are warranted.
Supporting Information
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
The authors give special thanks to the subjects for their enthusiastic participation and to Girona Faculty of Medicine and Masella ski resort for their hospitality.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Wehrlin JP, Hallén J. Linear decrease in VO2max and performance with increasing altitude in endurance athletes. Eur J Appl Physiol. 2006;96: 404–412. 10.1007/s00421-005-0081-9 [DOI] [PubMed] [Google Scholar]
- 2.Terrados N, Mizuno M, Andersen H. Reduction in Maximal Oxygen Uptake at Low Altitudes; Role of Training Status and Lung Function. Clin Physiol. 1985;5: 75–79. 10.1111/j.1475-097X.1985.tb00605.x [DOI] [PubMed] [Google Scholar]
- 3.Lawler J, Powers SK, Thompson D. Linear relationship between VO2max and VO2max decrement during exposure to acute hypoxia. J Appl Physiol Bethesda Md 1985. 1988;64: 1486–1492. [DOI] [PubMed] [Google Scholar]
- 4.Gavin TP, Derchak PA, Stager JM. Ventilation’s role in the decline in VO2max and SaO2 in acute hypoxic exercise. Med Sci Sports Exerc. 1998;30: 195–199. [DOI] [PubMed] [Google Scholar]
- 5.Gore CJ, Hahn AG, Scroop GC, Watson DB, Norton KI, Wood RJ, et al. Increased arterial desaturation in trained cyclists during maximal exercise at 580 m altitude. J Appl Physiol. 1996;80: 2204–2210. [DOI] [PubMed] [Google Scholar]
- 6.Chapman RF, Emery M, Stager JM. Degree of arterial desaturation in normoxia influences VO2max decline in mild hypoxia. Med Sci Sports Exerc. 1999;31: 658–663. [DOI] [PubMed] [Google Scholar]
- 7.Mollard P, Woorons X, Letournel M, Lamberto C, Favret F, Pichon A, et al. Determinants of maximal oxygen uptake in moderate acute hypoxia in endurance athletes. Eur J Appl Physiol. 2007;100: 663–673. 10.1007/s00421-007-0457-0 [DOI] [PubMed] [Google Scholar]
- 8.Dempsey JA, Wagner PD. Exercise-induced arterial hypoxemia. J Appl Physiol. 1999;87: 1997–2006. [DOI] [PubMed] [Google Scholar]
- 9.Prefaut C, Durand F, Mucci P, Caillaud C. Exercise-induced arterial hypoxaemia in athletes: a review. Sports Med Auckl NZ. 2000;30: 47–61. [DOI] [PubMed] [Google Scholar]
- 10.Grataloup O, Prieur F, Busso T, Castells J, Favier FB, Denis C, et al. Effect of hyperoxia on maximal O2 uptake in exercise-induced arterial hypoxaemic subjects. Eur J Appl Physiol. 2005;94: 641–645. 10.1007/s00421-005-1361-0 [DOI] [PubMed] [Google Scholar]
- 11.Durand F, Mucci P, Préfaut C. Evidence for an inadequate hyperventilation inducing arterial hypoxemia at submaximal exercise in all highly trained endurance athletes. Med Sci Sports Exerc. 2000;32: 926–932. [DOI] [PubMed] [Google Scholar]
- 12.Chapman RF. The individual response to training and competition at altitude. Br J Sports Med. 2013;47: i40–i44. 10.1136/bjsports-2013-092837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hopkins SR. Exercise induced arterial hypoxemia: the role of ventilation-perfusion inequality and pulmonary diffusion limitation. Adv Exp Med Biol. 2006;588: 17–30. [DOI] [PubMed] [Google Scholar]
- 14.Benoit H, Busso T, Castells J, Geyssant A, Denis C. Decrease in peak heart rate with acute hypoxia in relation to sea level VO(2max). Eur J Appl Physiol. 2003;90: 514–519. 10.1007/s00421-003-0899-y [DOI] [PubMed] [Google Scholar]
- 15.Grataloup O, Busso T, Castells J, Denis C, Benoit H. Evidence of decrease in peak heart rate in acute hypoxia: effect of exercise-induced arterial hypoxemia. Int J Sports Med. 2007;28: 181–185. 10.1055/s-2006-924216 [DOI] [PubMed] [Google Scholar]
- 16.Martin D, Powers S, Cicale M, Collop N, Huang D, Criswell D. Validity of pulse oximetry during exercise in elite endurance athletes. J Appl Physiol Bethesda Md 1985. 1992;72: 455–458. [DOI] [PubMed] [Google Scholar]
- 17.Mollard P, Bourdillon N, Letournel M, Herman H, Gibert S, Pichon A, et al. Validity of arterialized earlobe blood gases at rest and exercise in normoxia and hypoxia. Respir Physiol Neurobiol. 2010;172: 179–183. 10.1016/j.resp.2010.05.017 [DOI] [PubMed] [Google Scholar]
- 18.Martin D, O’Kroy J. Effects of acute hypoxia on the VO2 max of trained and untrained subjects. J Sports Sci. 1993;11: 37–42. 10.1080/02640419308729961 [DOI] [PubMed] [Google Scholar]
- 19.Dwinell MR, Janssen PL, Pizarro J, Bisgard GE. Effects of carotid body hypocapnia during ventilatory acclimatization to hypoxia. J Appl Physiol Bethesda Md 1985. 1997;82: 118–124. [DOI] [PubMed] [Google Scholar]
- 20.Derchak PA, Stager JM, Tanner DA, Chapman RF. Expiratory flow limitation confounds ventilatory response during exercise in athletes. Med Sci Sports Exerc. 2000;32: 1873–1879. [DOI] [PubMed] [Google Scholar]
- 21.Harms CA, Stager JM. Low chemoresponsiveness and inadequate hyperventilation contribute to exercise-induced hypoxemia. J Appl Physiol Bethesda Md 1985. 1995;79: 575–580. [DOI] [PubMed] [Google Scholar]
- 22.Katayama K, Sato Y, Morotome Y, Shima N, Ishida K, Mori S, et al. Intermittent hypoxia increases ventilation and Sa(O2) during hypoxic exercise and hypoxic chemosensitivity. J Appl Physiol Bethesda Md 1985. 2001;90: 1431–1440. [DOI] [PubMed] [Google Scholar]
- 23.Foster GE, McKenzie DC, Milsom WK, Sheel AW. Effects of two protocols of intermittent hypoxia on human ventilatory, cardiovascular and cerebral responses to hypoxia. J Physiol. 2005;567: 689–699. 10.1113/jphysiol.2005.091462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lusina S-JC, Kennedy PM, Inglis JT, McKenzie DC, Ayas NT, Sheel AW. Long-term intermittent hypoxia increases sympathetic activity and chemosensitivity during acute hypoxia in humans. J Physiol. 2006;575: 961–970. 10.1113/jphysiol.2006.114660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koehle MS, Sheel AW, Milsom WK, McKenzie DC. Two patterns of daily hypoxic exposure and their effects on measures of chemosensitivity in humans. J Appl Physiol Bethesda Md 1985. 2007;103: 1973–1978. 10.1152/japplphysiol.00545.2007 [DOI] [PubMed] [Google Scholar]
- 26.Faulhaber M, Gatterer H, Haider T, Patterson C, Burtscher M. Intermittent hypoxia does not affect endurance performance at moderate altitude in well-trained athletes. J Sports Sci. 2010;28: 513–519. 10.1080/02640410903581588 [DOI] [PubMed] [Google Scholar]
- 27.Katayama K, Sato K, Hotta N, Ishida K, Iwasaki K, Miyamura M. Intermittent hypoxia does not increase exercise ventilation at simulated moderate altitude. Int J Sports Med. 2007;28: 480–487. 10.1055/s-2006-955895 [DOI] [PubMed] [Google Scholar]
- 28.Scoggin CH, Doekel RD, Kryger MH, Zwillich CW, Weil JV. Familial aspects of decreased hypoxic drive in endurance athletes. J Appl Physiol. 1978;44: 464–468. [DOI] [PubMed] [Google Scholar]
- 29.Miyamura M, Ishida K. Adaptive changes in hypercapnic ventilatory response during training and detraining. Eur J Appl Physiol. 1990;60: 353–359. [DOI] [PubMed] [Google Scholar]
- 30.Katayama K, Sato Y, Ishida K, Mori S, Miyamura M. The effects of intermittent exposure to hypoxia during endurance exercise training on the ventilatory responses to hypoxia and hypercapnia in humans. Eur J Appl Physiol. 1998;78: 189–194. 10.1007/s004210050406 [DOI] [PubMed] [Google Scholar]
- 31.Kacimi R, Richalet JP, Corsin A, Abousahl I, Crozatier B. Hypoxia-induced downregulation of beta-adrenergic receptors in rat heart. J Appl Physiol Bethesda Md 1985. 1992;73: 1377–1382. [DOI] [PubMed] [Google Scholar]
- 32.Kacimi R, Richalet JP, Crozatier B. Hypoxia-induced differential modulation of adenosinergic and muscarinic receptors in rat heart. J Appl Physiol Bethesda Md 1985. 1993;75: 1123–1128. [DOI] [PubMed] [Google Scholar]
- 33.Werle EO, Strobel G, Weicker H. Decrease in rat cardiac beta 1- and beta 2-adrenoceptors by training and endurance exercise. Life Sci. 1990;46: 9–17. [DOI] [PubMed] [Google Scholar]
- 34.Peltonen JE, Tikkanen HO, Rusko HK. Cardiorespiratory responses to exercise in acute hypoxia, hyperoxia and normoxia. Eur J Appl Physiol. 2001;85: 82–88. [DOI] [PubMed] [Google Scholar]
- 35.Caquelard F, Burnet H, Tagliarini F, Cauchy E, Richalet JP, Jammes Y. Effects of prolonged hypobaric hypoxia on human skeletal muscle function and electromyographic events. Clin Sci Lond Engl 1979. 2000;98: 329–337. [PubMed] [Google Scholar]
- 36.Dousset E, Steinberg JG, Balon N, Jammes Y. Effects of acute hypoxemia on force and surface EMG during sustained handgrip. Muscle Nerve. 2001;24: 364–371. [DOI] [PubMed] [Google Scholar]
- 37.Bendahan D, Badier M, Jammes Y, Confort-Gouny S, Salvan AM, Guillot C, et al. Metabolic and myoelectrical effects of acute hypoxaemia during isometric contraction of forearm muscles in humans: a combined 31P-magnetic resonance spectroscopy-surface electromyogram (MRS-SEMG) study. Clin Sci Lond Engl 1979. 1998;94: 279–286. [DOI] [PubMed] [Google Scholar]
- 38.Badier M, Guillot C, Lagier-Tessonnier F, Jammes Y. EMG changes in respiratory and skeletal muscles during isometric contraction under normoxic, hypoxemic, or ischemic conditions. Muscle Nerve. 1994;17: 500–508. 10.1002/mus.880170506 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.