Abstract
Background
HIV-associated neurocognitive disorders (HAND) persist despite combination antiretroviral therapy (cART), supporting the need to better understand HIV neuropathogenesis. Magnetic resonance spectroscopy (MRS) of the brain has demonstrated abnormalities in HIV-infected individuals despite cART. We examined the associations between MRS metabolites and selected cerebrospinal fluid (CSF) biomarkers reflecting monocyte/macrophage activation and chemotaxis.
Methods
A multicenter cross-sectional study involving five sites in the United States was conducted. The following CSF biomarkers were measured: soluble CD14 (sCD14), monocyte chemotactic protein 1 (MCP-1), interferon inducible protein 10 (IP-10), and stromal cell derived growth factor 1 alpha (SDF-1α). The following MRS metabolites were measured from basal ganglia (BG), frontal white matter (FWM) and frontal gray matter (FGM): N-acetyl-aspartate (NAA), Myo-inositol (MI), Choline (Cho), and Creatine (Cr). CSF biomarkers were compared to absolute MRS metabolites as well as metabolite/Cr ratios using linear regression.
Results
83 HIV-infected individuals were included, 78% on cART and 37% with HAND. The most robust positive correlations were between MCP-1 and Cho in BG (R2 0.179, p<0.001) as well as MCP-1 and MI in FWM (R2 0.137, p=0.002). Higher Cr levels in FWM were associated with MCP-1 (R2 0. 075, p=0.01) and IP-10 (R2 0.106, p=0.003). Comparing biomarkers to MRS metabolite/Cr ratios impacted some relationships, e.g., higher sCD14 levels were associated with lower Cho/Cr ratios in FGM (R2 0.224, p<0.001), although higher MCP-1 levels remained associated with Cho/Cr in BG.
Conclusion
These findings provide evidence that monocyte activation and chemotaxis continue to contribute to HIV-associated brain abnormalities in cART-treated individuals.
Background
Over 30 million people are infected with Human Immunodeficiency Virus (HIV) worldwide and over 1 million of these people reside in the United States.(Fenton, 2007) While the incidence of HIV-associated dementia has declined with the use of combination antiretroviral therapy (cART), the milder forms of HIV-associated neurocognitive disorder (HAND) remain common with an overall frequency of approximately 50%.(Heaton et al, 2010) HAND is associated with decreased quality of life and increased risk of mortality.(Heaton et al, 1994; Tozzi et al, 2003; Vivithanaporn et al, 2010) Its persistence suggests that brain injury continues during cART treatment, at least in some individuals. Indeed, studies of cerebrospinal fluid (CSF) biomarkers and neuroimaging show persistent inflammation, neuronal injury, and disrupted connectivity in treated individuals.(Harezlak et al, 2011; Marcotte et al, 2013; Thomas et al, 2013) Further research is needed to better understand how the central nervous system is adversely affected during treated HIV disease.
Proton magnetic resonance spectroscopy (MRS) provides a sensitive and noninvasive in vivo method to detect metabolite changes in the brain.(Rosen and Lenkinski, 2007) MRS studies of HIV-infected individuals have shown higher levels of metabolites of membrane turnover (e.g., Choline or Cho) and glial activation (e.g., Myo-inositol or MI).(Chang et al, 2014; Harezlak et al, 2011) In addition, neuronal integrity (estimated by N-acetyl-aspartate or NAA) appears to be reduced in those with HAND.(Suwanwelaa et al, 2000; Yiannoutsos et al, 2004)
In parallel, multiple CSF biomarkers have been linked to HAND. These include markers of monocyte/macrophage activation such as soluble CD14 (sCD14) and chemokines such as monocyte chemotactic protein-1 (MCP-1), interferon inducible protein-10 (IP-10), and stromal cell derived growth factor-1α (SDF-1α).(Kamat et al, 2012; Kelder et al, 1998; Kolb et al, 1999) A small number of studies involving individuals with chronic HIV disease have identified associations between CSF biomarkers and MRS metabolites.(Chang et al, 2004; Cysique et al, 2013; Letendre et al, 2011; Vera et al, 2012) For example, higher concentrations of MCP-1 have been linked to higher MI and lower NAA/Cr in FWM. Findings from these studies have been somewhat restricted by small sample sizes or have yet to be independently confirmed. The purpose of this study was to investigate the associations of CSF biomarkers reflecting activation and chemotaxis of the monocyte/macrophage cell lineage with MRS metabolites in a robust sampling of HIV-infected individuals.
Methods
The CNS HIV Antiretroviral Effects Research (CHARTER) study is an observational cohort study of HIV-infected individuals in the United States. For this investigation, 83 HIV-infected participants without significant CNS confounding conditions were included from five CHARTER sites (Galveston, Baltimore, New York, Seattle, and San Diego). CNS confounding conditions included history of AIDS-defining opportunistic infection of the CNS, ongoing substance abuse disorders, and other conditions as defined in the 2007 nosology of HAND statement and used in previous studies.(Antinori et al, 2007; Heaton et al, 2010; Jernigan et al, 2011) The protocol was approved by the institutional review board at each location and written consent was obtained from subjects.
A cross-sectional analysis was performed of CSF and MRS data at baseline imaging assessments that occurred between May 2004 and July 2006.(Jernigan et al, 2011) CSF biomarkers were measured by immunoassay, either traditional plate-based (sCD14, R&D Systems, DC14) or bead suspension array-based (IP-10, MCP-1, SDF-1α, EMD Millipore) assays. HIV RNA levels in plasma and CSF were measured by RT-PCR (Amplicor v.1.5, Roche Diagnostics, lower limit of quantitation 50 copies/mL). MRS was performed using a standardized protocol.(Lin et al, 2011) Images were acquired with General Electric 1.5 Tesla scanners using point-resolved spectroscopy. Echo time (TE) was 35 milliseconds and relaxation time (TR) was 3000 milliseconds. Three regional voxels were acquired: frontal gray matter (FGM) at 20mm3 and 64 acquisitions, frontal white matter (FWM) at 20mm3 and 64 acquisitions, and basal ganglia (BG) at 15mm3 and 96 acquisitions (See Figure 1 for voxel locations). For image processing, MRS concentrations of N-acetylaspartate (NAA), choline (Cho), myo-inositol (MI), and creatine (Cr) were quantified using LCModel with water suppression.(Provencher, 2001) Water suppression allows for the examination of absolute metabolites, our primary association of interest. Only metabolite estimates for appropriately placed voxels with adequate spectra (standard deviation <21) were used, therefore, sample size varied by MRS region or metabolite.
Figure 1.
Representative Axial T1 sections with MRS voxels: Frontal Gray (FGM) & White (FWM) Matter; and Basal Ganglia (BG).
Linear regressions were used to investigate associations between biomarkers and MRS metabolites. Biomarker values were log-transformed prior to analyses to approximate normality necessary for parametric analyses (with the exception of IP-10, which had a normal distribution). Separate models were fitted for each biomarker-metabolite association. All models included scanner identifier (6 levels, one for each scanner at 5 sites, one site having two scanners) and the proportion of relevant tissue volume within each voxel (e.g., amount of gray matter in FGM) to control for measurement error and individual variability in brain volumes. Since Cr, a measure of energy metabolism, is often used as a reference metabolite in MRS studies to reduce variability, we also explored the same associations with metabolite ratios, using Cr as the denominator (for example, NAA/Cr).(Jansen et al, 2006) We chose to emphasize the associations with absolute measures because we found significant associations between Cr and HIV RNA in this sample, violating the assumption that Cr is a relatively constant measure unaffected by disease. Specifically, Cr in FWM and FGM was associated with HIV RNA levels in CSF and plasma. For example, in FWM, higher HIV RNA levels in CSF correlated with higher Cr (β=0.326, p=0.01). Further supporting this approach, Cr levels also significantly correlated with nadir CD4+ T-cell counts, an indicator of severity of past immune suppression and a known correlate of HAND.
P-values for multiple analyses for associations between all considered biomarkers and each MRS metabolite were adjusted for multiple comparisons with false discovery rate, which was chosen for its gain in power over other methods like Bonferroni.(Bejamini, 1995) Only those associations that yielded a p-value less than 0.20 were pursued further in multivariable models that adjusted for covariates. In the final multivariable models, only p-values less than 0.05 were considered as significant results. Age, hepatitis C virus (HCV) serostatus, nadir CD4+ T-cell count, plasma HIV RNA (<50 copies/ml, all of whom had CSF HIV RNA <50 copies/ml), and estimated duration of HIV were included as covariates, if their effect was significant (p<0.05). All models included scanner identifier and the proportion of relevant tissue volume within each voxel, as described above. Interactions between these covariates and biomarkers were analyzed and included in models when significant (p<0.05). Effect sizes of associations between biomarkers and MRS metabolites were measured by R2 values, which were estimated from multivariable models that accounted for the covariates described above. Metabolite/Cr ratios were analyzed using the same methods. To focus on the most clinically relevant subjects, we performed a series of subanalyses that sequentially eliminated participants who had three characteristics that might affect the findings: HCV seropositivity, detectable HIV RNA in plasma, and detectable HIV RNA in CSF (see Tables 2 and 3). All tests were two-sided and considered significant at the 0.05 level, unless stated otherwise. All analyses were performed using R 3.0.1.(R Core Team; R Foundation for Statistical computing, 2013)
Table 2.
Effects of CSF biomarkers on absolute MRS metabolites as measured by R-squared derived from multivariable models. All associations were positive. All analyses performed on the full sample (N=83, Column 2) were repeated on four clinically relevant subsets (Columns 3-6). Models that did not yield significant biomarker effect in the full sample analysis (N=83) are not shown.
| MRS metabolite CSF Biomarker | Effect of Biomarker: R2 (p-value) |
||||
|---|---|---|---|---|---|
| All (N=83) | HCV-negative (N=65) | Undetectable CSF (N=56) | Undetectable Plasma and CSF (N=42) | HCV-negative Undetectable Plasma and CSF (N=29) | |
| NAA FWM | |||||
| IP-10 | 0.045 (0.046) | 0.042 (0.074) | 0.054 (0.079) | 0.052 (0.115) | 0.078 (0.057) |
| Cr FWM | |||||
| IP-101 | 0.106 (0.003) | 0.113 (0.006) | 0.076 (0.044) | 0.107 (0.035) | 0.105 (0.094) |
| MCP-12 | 0.075 (0.01) | 0.069 (0.032) | 0.076 (0.046) | 0.074 (0.085) | 0.128 (0.062) |
| MI FWM | |||||
| IP-103 | 0.05 (0.036) | 0.056 (0.063) | 0.01 (0.446) | 0.021 (0.334) | 0.125 (0.068) |
| MCP-13,4 | 0.137 (0.002) | 0.151 (<0.001) | 0.031 (0.159) | 0.021 (0.313) | 0.096 (0.125) |
| Cho BG | |||||
| MCP-1 | 0.179 (<0.001) | 0.227 (<0.001) | 0.158 (0.005) | 0.195 (0.007) | 0.307 (0.003) |
Models control for scanner, imaging covariate, and significant covariates (p<0.05)
nadir CD4
undetectable plasma HIV (except for the last two columns in which by definition Plasma HIV was undetectable)
HCV (except for the second and last columns, in which by definition HCV was negative)
age. Cho = choline; Cr = creatine; CSF = cerebrospinal fluid; FWM = frontal white matter; HCV = hepatitis C virus; MI = myo-inositol; MRS = magnetic resonance spectroscopy; NAA = N-acetylaspartate.
Table 3.
Effects of CSF biomarkers on MRS metabolite/creatine ratios as measured by R-squared derived from significant multivariable models. Associations were negative (in bold) between NAA/Cr FWM and MCP-1; NAA/Cr FGM and sCD14; and Cho/Cr FGM and sCD14. The remaining associations were positive. All analyses performed on the full sample (N=83, Column 2) were repeated on four clinically relevant subsets (Columns 3-6). Models that did not yield significant biomarker effect in the full sample analysis (N=83) are not shown.
| MRS metabolite CSF Biomarker | Effect of Biomarker: R2 (p-value) |
||||
|---|---|---|---|---|---|
| All (N=83) | HCV-negative (N=65) | Undetectable CSF (N=56) | Undetectable Plasma and CSF (N=42) | HCV-negative Undetectable Plasma and CSF (N=29) | |
| NAA/Cr FWM | |||||
| MCP-1 | 0.102 (0.005) | 0.107 (0.012) | 0.04 (0.14) | 0.048 (0.169) | 0.089 (0.136) |
| SDF-1α1 | 0.056 (0.037) | 0.092 (0.018) | 0.057 (0.082) | 0.071 (0.093) | 0.081 (0.156) |
| NAA/Cr FGM | |||||
| sCD14 | 0.068 (0.019) | 0.047 (0.084) | 0.063 (0.057) | 0.048 (0.163) | 0.004 (0.736) |
| Cho/Cr FGM | |||||
| sCD142 | 0.224 (<0.001) | 0.176 (0.001) | 0.163 (0.001) | 0.14 (0.01) | 0.155 (0.027) |
| Cho/Cr BG | |||||
| MCP-12 | 0.055 (0.035) | 0.060 (0.042) | 0.066 (0.047) | 0.064 (0.083) | 0.08 (0.061) |
Models control for scanner, imaging covariate, and significant covariates (p<0.05)
undetectable plasma HIV (except for the last two columns in which by definition plasma HIV was undetectable)
estimated duration of HIV infection. Cho = choline; Cr = creatine; CSF = cerebrospinal fluid; FGM = frontal gray matter; FWM = frontal white matter; HCV = hepatitis C virus; MRS = magnetic resonance spectroscopy; NAA = N-acetylaspartate.
Results
Participants were mostly men (83%) on cART (78%) with a median age of 44 years (Table 1). Median current CD4+ T-cell count was 462/mm3, and median nadir CD4+ T-cell count was 147/mm3. 49% had plasma HIV RNA ≥50 copies/mL and 32% had CSF HIV RNA ≥50 copies/mL. 22% of subjects were HCV seropositive and 37% met criteria for HAND. The CSF collection occurred a mean of 7.3 days before the neuroimaging assessment (standard deviation 14.2 days). The median interval between these visits was 2 days (interquartile range 0-7 days).
Table 1.
Demographic and medical characteristics of participants (n=83)
| Variable | Statistic |
|---|---|
| Age (years)a | 45 (7.7) [27-62] |
| Ethnicity | |
| Caucasian | 52% |
| African American | 39% |
| Other | 9% |
| Sex (% Men) | 83% |
| Self-Reported Duration of HIV Disease (months)a | 133 (74) [7-285] |
| Antiretroviral Therapy Use (% Currently Taking) | 78% |
| AIDS (%) | 67% |
| Current CD4+ T-cell Count (cells/mm3)b | 462 [339, 602] |
| Nadir CD4+ T-cell Count (cells/mm3)b | 147 [24, 256] |
| Plasma HIV RNA (% <50 c/mL) | 51% |
| CSF HIV RNA (% <50 c/mL) | 68% |
| HAND (%) | 37% |
| ANI | 25% |
| MND/HAD | 12% |
| HCV seropositive | 22% |
mean (standard deviation) [range]
median [interquartile range]
AIDS = acquired immunodeficiency syndrome; ANI = asymptomatic neurocognitive impairment; CSF = cerebrospinal fluid; HAD = HIV-associated dementia; HCV = hepatitis C virus; MND = mild neurocognitive disorder; RNA = ribonucleic acid.
Associations between CSF biomarkers and absolute MRS metabolites
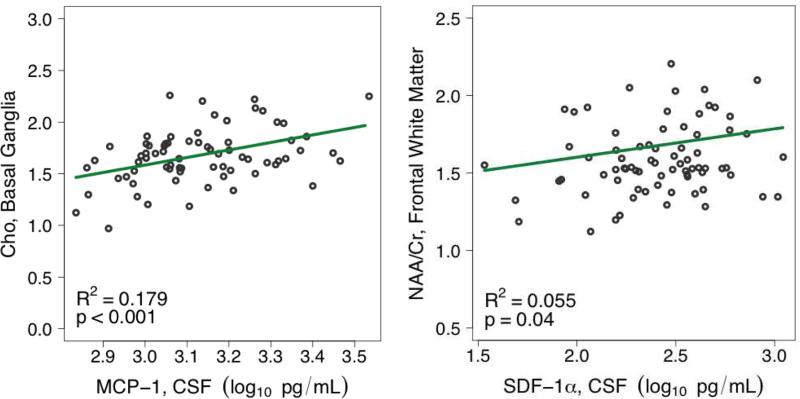
Depending on the individual model, the following covariates were found to be statistically significant and were incorporated: nadir CD4+, undetectable plasma HIV RNA, HCV serostatus, and age (see Table 2). Henceforth, higher levels of MCP-1 and IP-10 were each associated with higher levels of MI and Cr in FWM. A particularly strong association was found between higher MCP-1 and higher Cho in BG (R2 0.179, p<0.001) (figure 2) while a weaker association was found between higher IP-10 and higher NAA in FWM (R2 0.045, p=0.046). No statistically significant associations were present between sCD14 or SDF-1α and individual MRS metabolites after adjusting for scanner and imaging covariates.
Figure 2.
Associations between selected CSF biomarkers and cerebral metabolites. Slopes, R-squared, and p-values were derived from multivariable models controlling for scanner, relevant imaging covariate, and significant medical covariates.
Associations between CSF biomarkers and MRS metabolite/Cr ratios
Depending on the individual model, the following covariates were found to be statistically significant and were incorporated: undetectable plasma HIV RNA and estimated duration of HIV infection (see Table 3). Similar to the association found with absolute values, higher MCP-1 remained associated with higher Cho/Cr in BG (R2 0.055, p= 0.035). However, different associations were demonstrated in FWM and FGM. In FWM, lower NAA/Cr ratios correlated with higher MCP-1 (R2 0.102, p= 0.005) and lower SDF-1α (R2 0.056, p= 0.037) (figure 2). In FGM, higher sCD14 correlated with lower NAA/Cr (R2 0.068, p= 0.019) and lower Cho/Cr (R2 0.224, p<0.001).
Relationships in HCV seronegative subjects
Sixty-five subjects in the study were HCV seronegative. When limiting the analyses to these subjects, four direct relationships between CSF biomarkers and MRS absolute metabolites remained statistically significant (Table 2). Higher levels of IP-10 (R2 0.113, p=0.006) and MCP-1 (R2 0.069, p=0.032) were each associated with higher Cr in FWM. Associations appeared to strengthen between MCP-1 and both Cho in BG (R2 0.227, p<0.001) and MI in FWM (R2 0.151, p<0.001). When using MRS metabolite/Cr ratios, biomarker relationships remained similar in HCV seronegative subjects (Table 3), except for the correlation between sCD14 and NAA/Cr in FGM, which weakened below statistical significance.
Relationships in subjects with undetectable HIV RNA levels
To focus on the most clinically relevant subgroup, additional analyses were performed among the subgroups of subjects who had HIV RNA levels below 50 copies/mL in either CSF (n=56) or in plasma and CSF (n=42). In the subgroup with CSF HIV RNA below 50 copies/ml, three positive correlations remained statistically significant: IP-10 with Cr in FWM (R2 0.076, p=0.044), MCP-1 with Cr in FWM (R2 0.076, p=0.046) and MCP-1 with Cho in BG (R2 0.158, p=0.005). When examining metabolite/Cr ratios in this subgroup, only the positive correlation between MCP-1 and Cho/Cr in BG (R2 0.066, p=0.047) and the negative correlation between sCD14 and Cho/Cr in FGM (R2 0.163, p=0.001) remained statistically significant. In the subgroup with concurrent undetectable CSF and plasma HIV RNA, positive correlations remained between IP-10 and Cr in FWM (R2 0.107, p=0.035) as well as between MCP-1 and Cho in BG (R2 0.195, p=0.007). In this same group of subjects, the only biomarker-metabolite/Cr relationship that remained significant was a negative correlation between sCD14 and Cho/Cr in FGM (R2 0.14, p=0.01). In the analysis of the 29 participants who were HCV seronegative and had both plasma and CSF HIV RNA below 50 copies/mL, higher MCP-1 again correlated with higher Cho (R2 0.307, p=0.003) in BG. While the relationship between MCP-1 and Cho/Cr in BG weakened (R2 0.08, p=0.061), sCD14 again negatively correlated with Cho/Cr in FGM (R2 0.155, p=0.027).
Discussion
Given the relatively high prevalence of HAND and mounting evidence that brain inflammation and injury may persist despite cART, continued investigation is needed to better understand the effects of HIV on the central nervous system. MRS studies of HIV-infected individuals demonstrate abnormalities despite cART, but the mechanisms by which these abnormalities occur are not fully understood.(Harezlak et al, 2011) In our study of 83 HIV-infected adults without confounding CNS conditions, we identified several associations between CSF biomarkers and MRS metabolites in multivariable analysis. As with prior investigations, the associations were not similar across brain regions (FWM, FGM, and BG), suggesting that susceptibility to monocyte/macrophage-mediated injury may have regional variability.
We found that higher IP-10 and MCP-1 levels in CSF were associated with higher levels in FWM of Cr, an indicator of energy metabolism. These associations remained statistically significant even after eliminating potentially confounding conditions, such as HCV co-infection and detectable HIV replication. Associations with Cr are rarely reported in part because MRS studies in HIV infected individuals typically focus on metabolite/Cr ratios. Our study demonstrated that Cr correlated with HIV RNA levels in two of three regions examined, supporting our secondary analyses which excluded subjects with detectable HIV RNA. This correlation should be considered in future analyses involving Cr in the setting of HIV infection. Our analyses of metabolite/Cr ratios identified shifts in several associations between CSF biomarkers and cerebral metabolites, supporting the possibility that biomarkers such as IP-10 and MCP-1 may influence Cr itself (or vice versa). These findings should be kept in mind when interpreting associations between these two biomarkers and MRS findings that involve Cr ratios in the setting of HIV. In other disease conditions such as epilepsy, Cr levels are not necessarily constant throughout brain regions(Lundbom et al, 2001) and for this reason some authors suggest caution when interpreting MRS/Cr ratios.(Jansen et al, 2006)
The strongest association, in the analyses of absolute metabolites, was the correlation between MCP-1 with Cho in BG. This association was particularly robust in the most clinically relevant subgroup, i.e., HCV seronegative participants who had undetectable plasma and CSF HIV RNA (R2= 0.307). These findings extend prior evidence of the association between CSF MCP-1 levels and a composite score of glial markers that included Cho in BG.(Chang et al, 2004) Thus, monocyte chemotaxis as indicated by MCP-1 may have a significant role in membrane turnover and inflammation in the basal ganglia, which is known to be particularly susceptible to injury during HIV disease.(Yiannoutsos et al, 2004) It is not clear why this association increased among individuals with suppressed HIV RNA, but may be indicative of more robust cellular immunity and monocyte recruitment in such individuals.
There were two associations involving MCP-1 from this study that are concordant with findings from previously published studies. The correlation between MCP-1 and MI in FWM matches that of a study in the early cART era involving subjects three months after starting cART.(Chang et al, 2004) Our larger study provides further support for this association which links MCP-1 and glial activation during HIV disease. It should be noted that the presence of liver disease could confound this relationship based on existing literature. One study of HCV-infected subjects (HIV status not reported) showed increased MI/Cr in FWM compared to HCV-uninfected controls.(Forton et al, 2008) Conversely, multiple studies of cirrhotic individuals (not all of whom were HCV infected, and again HIV status was not reported) have shown that MI and MI/Cr is actually decreased in certain brain regions.(Bajaj et al, 2012; Long et al, 2009) In our study, the correlation between MCP-1 and MI in FWM appeared to strengthen after eliminating HCV seropositive subjects and to weaken in the subgroup of subjects who had undetectable plasma and CSF HIV RNA. These findings support the possibility that the association between MCP-1 and MI in FWM may be driven by HIV. It is possible that HIV-infected microglia, which are one of the main cellular reservoirs of HIV in the brain, are attracted via MCP-1 to this region and that MI (a glial marker) reflects their activity. The correlation between MCP-1 and NAA/Cr in FWM confirms that of a prior study, in which the association also weakened when limiting the analysis to subjects with undetectable plasma and CSF HIV RNA.(Letendre et al, 2011) Although small sample size reduced the power of our subgroup analyses, the effect sizes were also reduced, suggesting that ongoing HIV replication may mediate this relationship.
Two biomarker associations were significant only in analyses of metabolite/Cr ratios. Soluble CD14, a marker of monocyte activation that has been implicated in other studies of the HIV-infected brain,(Kamat et al, 2012) negatively correlated with NAA/Cr in FGM. While this association weakened in subgroup analyses, the relationship may reflect a detrimental impact of monocyte activation on neuronal integrity. Somewhat counterintuitively, sCD14 also inversely correlated with Cho/Cr in FGM. We would expect that increased monocyte activation would instead be associated with increased inflammation and membrane turnover. While this relationship appeared to weaken in the multivariable analyses, we acknowledge that this finding is somewhat contrary and we did not find a direct correlation between sCD14 and Cr that could have contributed to the finding. One possible explanation relates to CD14 being a cellular receptor for bacterial lipopolysaccharide (LPS), a byproduct of microbial translocation across an HIV-damaged gut wall (Brenchley et al, 2006). While higher sCD14 levels in blood have been linked to HAND, LPS has not been measured in CSF from HIV+ adults. Findings from an animal model support that LPS-associated alterations in the immune response in the CNS (reduced IP-10 expression and T-cell infiltration(Maingat et al, 2010)) could have neuroprotective properties, consistent with our findings between sCD14 and Cho/Cr.
Higher levels of SDF-1α (CXCL-12) correlated with higher NAA/Cr ratios, supporting a neuroprotective effect of this chemokine. SDF-1α is a ligand for CXCR4, one of the co-receptors for HIV cell entry(Nagasawa, 2014), and has pleiotropic effects with evidence of involvement in multiple aspects of immune surveillance and tissue homeostasis.(Karin, 2010) SDF-1α can also have antiviral effects, inhibiting HIV infection of peripheral blood mononuclear cells (PBMCs) in vitro.(Bleul et al, 1996) Our finding linking SDF-1α and NAA/Cr (a marker of neuronal integrity) in FWM may reflect some degree of HIV entry blockade, resulting in an overall benefit. While sCD14 and SDF-1α were not found to be associated with Cr in the analyses of direct association (Table 2), these findings should be noted in the context of the significant underlying associations between other biomarkers (including plasma and CSF HIV RNA levels) and Cr.
The fact that the relationships found in our study were not exactly the same from region to region is not surprising. Other MRS studies have demonstrated that metabolic abnormalities may differ between basal ganglia, gray matter, and white matter regions in HIV-infected individuals.(Harezlak et al, 2011) One possible explanation for this is uneven distribution of microglia in the brain. Microglia are target cells for HIV in the brain with population sizes that differ by orders of magnitude between white and grey matter in individuals from general medical populations.(Mittelbronn et al, 2001) Differences across regions in the population size of microglia and other cells that reflect immune activation could affect MRS findings.
Our study is limited in the scope of CSF biomarkers and MRS metabolites examined. Additional biomarkers found to be salient to HIV neuropathogenesis, such as neopterin or neurofilament light chain, were not examined in this study, and thus our analysis did not address all potentially important associations.(Burdo et al, 2013; Hagberg et al, 2010; Valcour et al, 2012) Similarly, we did not examine MRS metabolites such as glutamate/glutamine, which are markers of excitotoxicity.(Harezlak et al, 2011) Lastly, biomarkers in the CSF do not necessarily reflect biomarkers in the brain parenchyma and thus inferences based on CSF are indirect. Despite these limitations, the relatively large size of our study as well as subgroup analyses focusing on subjects with the clinically relevant subgroup of HCV seronegative subjects with suppressed plasma and CSF HIV RNA provide further insight into metabolic changes of the brain that are found in HIV-infected individuals during the cART era. Further investigations, including continued direct examination of HIV-infected brain specimens, are needed to better understand HIV neuropathogenesis.
Acknowledgements
This study was presented in part (abstract 487) at the Conference on Retroviruses and Opportunistic Infections (CROI) in Boston, Massachusetts, March 3-6, 2014.
This work was supported by awards from the National Institutes of Health for the CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) [N01 MH2205 and HHSN271201000036C] and P30 MH62512. The views expressed in this article are those of the authors and do not reflect the official policy or position of the United States Government. AMA is supported by NIH K23 MH095679 and SLL is supported by K24 MH097673.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–99. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajaj JS, Hylemon PB, Ridlon JM, Heuman DM, Daita K, White MB, Monteith P, Noble NA, Sikaroodi M, Gillevet PM. Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. Am J Physiol Gastrointest Liver Physiol. 2012;303:G675–85. doi: 10.1152/ajpgi.00152.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejamini YH, Y Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B (Methodological) 1995;57:289–300. [Google Scholar]
- Bleul CC, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer TA. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–33. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, Blazar BR, Rodriguez B, Teixeira-Johnson L, Landay A, Martin JN, Hecht FM, Picker LJ, Lederman MM, Deeks SG, Douek DC. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–71. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- Burdo TH, Weiffenbach A, Woods SP, Letendre S, Ellis RJ, Williams KC. Elevated sCD163 in plasma but not cerebrospinal fluid is a marker of neurocognitive impairment in HIV infection. AIDS. 2013;27:1387–95. doi: 10.1097/QAD.0b013e32836010bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Ernst T, St Hillaire C, Conant K. Antiretroviral treatment alters relationship between MCP-1 and neurometabolites in HIV patients. Antivir Ther. 2004;9:431–40. doi: 10.1177/135965350400900302. [DOI] [PubMed] [Google Scholar]
- Chang L, Jiang C, Cunningham E, Buchthal S, Douet V, Andres M, Ernst T. Effects of APOE epsilon4, age, and HIV on glial metabolites and cognitive deficits. Neurology. 2014 doi: 10.1212/WNL.0000000000000526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cysique LA, Moffat K, Moore DM, Lane TA, Davies NW, Carr A, Brew BJ, Rae C. HIV, vascular and aging injuries in the brain of clinically stable HIV-infected adults: a (1)H MRS study. PLoS One. 2013;8:e61738. doi: 10.1371/journal.pone.0061738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton KA. Changing epidemiology of HIV/AIDS in the United States: implications for enhancing and promoting HIV testing strategies. Clin Infect Dis. 2007;45(Suppl 4):S213–20. doi: 10.1086/522615. [DOI] [PubMed] [Google Scholar]
- Forton DM, Hamilton G, Allsop JM, Grover VP, Wesnes K, O'Sullivan C, Thomas HC, Taylor-Robinson SD. Cerebral immune activation in chronic hepatitis C infection: a magnetic resonance spectroscopy study. J Hepatol. 2008;49:316–22. doi: 10.1016/j.jhep.2008.03.022. [DOI] [PubMed] [Google Scholar]
- Hagberg L, Cinque P, Gisslen M, Brew BJ, Spudich S, Bestetti A, Price RW, Fuchs D. Cerebrospinal fluid neopterin: an informative biomarker of central nervous system immune activation in HIV-1 infection. AIDS Res Ther. 2010;7:15. doi: 10.1186/1742-6405-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harezlak J, Buchthal S, Taylor M, Schifitto G, Zhong J, Daar E, Alger J, Singer E, Campbell T, Yiannoutsos C, Cohen R, Navia B, Consortium HIVN Persistence of HIV-associated cognitive impairment, inflammation, and neuronal injury in era of highly active antiretroviral treatment. AIDS. 2011;25:625–33. doi: 10.1097/QAD.0b013e3283427da7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Jr., Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, Group C. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–96. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Velin RA, McCutchan JA, Gulevich SJ, Atkinson JH, Wallace MR, Godfrey HP, Kirson DA, Grant I. Neuropsychological impairment in human immunodeficiency virus-infection: implications for employment. HNRC Group. HIV Neurobehavioral Research Center. Psychosom Med. 1994;56:8–17. doi: 10.1097/00006842-199401000-00001. [DOI] [PubMed] [Google Scholar]
- Jansen JF, Backes WH, Nicolay K, Kooi ME. 1H MR spectroscopy of the brain: absolute quantification of metabolites. Radiology. 2006;240:318–32. doi: 10.1148/radiol.2402050314. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Taylor MJ, Theilmann RJ, Julaton MD, Notestine RJ, Wolfson T, Letendre SL, Ellis RJ, Heaton RK, Gamst AC, Franklin DR, Jr., Clifford DB, Collier AC, Gelman BB, Marra C, McArthur JC, McCutchan JA, Morgello S, Simpson DM, Grant I, Group C. Clinical factors related to brain structure in HIV: the CHARTER study. J Neurovirol. 2011;17:248–57. doi: 10.1007/s13365-011-0032-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamat A, Lyons JL, Misra V, Uno H, Morgello S, Singer EJ, Gabuzda D. Monocyte activation markers in cerebrospinal fluid associated with impaired neurocognitive testing in advanced HIV infection. J Acquir Immune Defic Syndr. 2012;60:234–43. doi: 10.1097/QAI.0b013e318256f3bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin N. The multiple faces of CXCL12 (SDF-1alpha) in the regulation of immunity during health and disease. J Leukoc Biol. 2010;88:463–73. doi: 10.1189/jlb.0909602. [DOI] [PubMed] [Google Scholar]
- Kelder W, McArthur JC, Nance-Sproson T, McClernon D, Griffin DE. Beta-chemokines MCP-1 and RANTES are selectively increased in cerebrospinal fluid of patients with human immunodeficiency virus-associated dementia. Ann Neurol. 1998;44:831–5. doi: 10.1002/ana.410440521. [DOI] [PubMed] [Google Scholar]
- Kolb SA, Sporer B, Lahrtz F, Koedel U, Pfister HW, Fontana A. Identification of a T cell chemotactic factor in the cerebrospinal fluid of HIV-1-infected individuals as interferon-gamma inducible protein 10. J Neuroimmunol. 1999;93:172–81. doi: 10.1016/s0165-5728(98)00223-9. [DOI] [PubMed] [Google Scholar]
- Letendre SL, Zheng JC, Kaul M, Yiannoutsos CT, Ellis RJ, Taylor MJ, Marquie-Beck J, Navia B, Consortium HIVN Chemokines in cerebrospinal fluid correlate with cerebral metabolite patterns in HIV-infected individuals. J Neurovirol. 2011;17:63–9. doi: 10.1007/s13365-010-0013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K, Taylor MJ, Heaton R, Franklin D, Jernigan T, Fennema-Notestine C, McCutchan A, Atkinson JH, Ellis RJ, McArthur J, Morgello S, Simpson D, Collier AC, Marra C, Gelman B, Clifford D, Grant I, group C. Effects of traumatic brain injury on cognitive functioning and cerebral metabolites in HIV-infected individuals. J Clin Exp Neuropsychol. 2011;33:326–34. doi: 10.1080/13803395.2010.518140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long LL, Li XR, Huang ZK, Jiang YM, Fu SX, Zheng W. Relationship between changes in brain MRI and (1)H-MRS, severity of chronic liver damage, and recovery after liver transplantation. Exp Biol Med (Maywood) 2009;234:1075–85. doi: 10.3181/0903-RM-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundbom N, Gaily E, Vuori K, Paetau R, Liukkonen E, Rajapakse JC, Valanne L, Hakkinen AM, Granstrom ML. Proton spectroscopic imaging shows abnormalities in glial and neuronal cell pools in frontal lobe epilepsy. Epilepsia. 2001;42:1507–14. doi: 10.1046/j.1528-1157.2001.15301.x. [DOI] [PubMed] [Google Scholar]
- Maingat F, Viappiani S, Zhu Y, Vivithanaporn P, Ellestad KK, Holden J, Silva C, Power C. Regulation of lentivirus neurovirulence by lipopolysaccharide conditioning: suppression of CXCL10 in the brain by IL-10. J Immunol. 2010;184:1566–74. doi: 10.4049/jimmunol.0902575. [DOI] [PubMed] [Google Scholar]
- Marcotte TD, Deutsch R, Michael BD, Franklin D, Cookson DR, Bharti AR, Grant I, Letendre SL, Group C. A concise panel of biomarkers identifies neurocognitive functioning changes in HIV-infected individuals. J Neuroimmune Pharmacol. 2013;8:1123–35. doi: 10.1007/s11481-013-9504-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelbronn M, Dietz K, Schluesener HJ, Meyermann R. Local distribution of microglia in the normal adult human central nervous system differs by up to one order of magnitude. Acta Neuropathol. 2001;101:249–55. doi: 10.1007/s004010000284. [DOI] [PubMed] [Google Scholar]
- Nagasawa T. CXC chemokine ligand 12 (CXCL12) and its receptor CXCR4. J Mol Med (Berl) 2014;92:433–9. doi: 10.1007/s00109-014-1123-8. [DOI] [PubMed] [Google Scholar]
- Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001;14:260–4. doi: 10.1002/nbm.698. [DOI] [PubMed] [Google Scholar]
- R Core Team. R Foundation for Statistical computing VA R: A language and environment for statistical computing. 2013.
- Rosen Y, Lenkinski RE. Recent advances in magnetic resonance neurospectroscopy. Neurotherapeutics. 2007;4:330–45. doi: 10.1016/j.nurt.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwanwelaa N, Phanuphak P, Phanthumchinda K, Suwanwela NC, Tantivatana J, Ruxrungtham K, Suttipan J, Wangsuphachart S, Hanvanich M. Magnetic resonance spectroscopy of the brain in neurologically asymptomatic HIV-infected patients. Magn Reson Imaging. 2000;18:859–65. doi: 10.1016/s0730-725x(00)00173-9. [DOI] [PubMed] [Google Scholar]
- Thomas JB, Brier MR, Snyder AZ, Vaida FF, Ances BM. Pathways to neurodegeneration: effects of HIV and aging on resting-state functional connectivity. Neurology. 2013;80:1186–93. doi: 10.1212/WNL.0b013e318288792b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozzi V, Balestra P, Galgani S, Murri R, Bellagamba R, Narciso P, Antinori A, Giulianelli M, Tosi G, Costa M, Sampaolesi A, Fantoni M, Noto P, Ippolito G, Wu AW. Neurocognitive performance and quality of life in patients with HIV infection. AIDS Res Hum Retroviruses. 2003;19:643–52. doi: 10.1089/088922203322280856. [DOI] [PubMed] [Google Scholar]
- Valcour V, Chalermchai T, Sailasuta N, Marovich M, Lerdlum S, Suttichom D, Suwanwela NC, Jagodzinski L, Michael N, Spudich S, van Griensven F, de Souza M, Kim J, Ananworanich J, Group RSS. Central nervous system viral invasion and inflammation during acute HIV infection. J Infect Dis. 2012;206:275–82. doi: 10.1093/infdis/jis326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera JH, Garvey LJ, Allsop JM, Kaye S, McClure MO, Back D, Taylor-Robinson SD, Winston A. Alterations in cerebrospinal fluid chemokines are associated with maraviroc exposure and in vivo metabolites measurable by magnetic resonance spectroscopy. HIV Clin Trials. 2012;13:222–7. doi: 10.1310/hct1304-222. [DOI] [PubMed] [Google Scholar]
- Vivithanaporn P, Heo G, Gamble J, Krentz HB, Hoke A, Gill MJ, Power C. Neurologic disease burden in treated HIV/AIDS predicts survival: a population-based study. Neurology. 2010;75:1150–8. doi: 10.1212/WNL.0b013e3181f4d5bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiannoutsos CT, Ernst T, Chang L, Lee PL, Richards T, Marra CM, Meyerhoff DJ, Jarvik JG, Kolson D, Schifitto G, Ellis RJ, Swindells S, Simpson DM, Miller EN, Gonzalez RG, Navia BA. Regional patterns of brain metabolites in AIDS dementia complex. Neuroimage. 2004;23:928–35. doi: 10.1016/j.neuroimage.2004.07.033. [DOI] [PubMed] [Google Scholar]