Abstract
Homeostasis stabilizes critical biological variables within appropriate limits via corrective regulatory effector responses that adequately counter disturbing effects. Identifying individual effects and responses, and distinguishing their individual influences on a regulated state, is challenging. Studying effector responses can reveal regulatory phenomena that depart from homeostasis into the realm of allostasis.
Keywords: homeostasis, allostasis, sign reversal, addiction, obesity, regulation, thermoregulation, nitrous oxide
This Discovery article focuses on a key concept emphasized in a recent review by Ramsay and Woods seeking to clarify the roles of homeostasis and allostasis in physiological regulation.1 We have argued1,2 that challenging an organism and simply measuring changes of a regulated variable to understand physiological regulation can promote erroneous conclusions. A critique of the use of core temperature to study regulation illustrates our rationale. Core temperature during a challenge is an integral of numerous influences on heat loss and heat production, e.g., their rates at baseline, the direct and indirect effects of the challenge, and importantly, the consequences of the biobehavioral regulatory effector responses recruited during the challenge. Because changes of core temperature per se convey limited information on thermoregulatory system engagement, defining a change of core temperature as a physiological response is misleading, and even worse is employing changes of core temperature to quantify a subject's degree of responsiveness to a challenge. Analogously, in addiction research, individuals exhibiting the greatest changes in a variable when initially administered a drug have long been described as having a high level of response to the drug, whereas individuals exhibiting minimal change in the variable are described as having “a low level of response.”3 When alcohol is administered to humans, for example, they exhibit wide variability in changes of diverse measured states ranging from behavioral to endocrine to physiological.3 Similarly, while most rats exhibit hypothermia during initial nitrous oxide inhalation, wide variability is observed: some rats exhibit marked and prolonged hypothermia whereas others exhibit little change of core temperature over several hours.4 But a simple analogy indicates why “a low level of response” should not be inferred from the relative stability of a regulated variable during a homeostatic challenge. Small mammals such as rats exhibit euthermia over a wide range of ambient temperature (e.g., 14° to 30°C), yet euthermia at 14°C derives not from being unresponsive to cold but rather from effective thermoeffector response activation including markedly elevated heat production.
Recent work underscores the limitations of traditional interpretations in drug research. When rats were administered nitrous oxide in a combined direct and indirect calorimeter to simultaneously measure core temperature and its thermal determinants, heat loss consistently increased in all subjects, yet some individuals were nonetheless resistant to hypothermia because they activated a heat-generating response sufficient to obviate the drug-induced heat loss effect. Thus, insensitive rats were actually more responsive and consequently better able to offset the drug effect than were sensitive rats that exhibited a large decrease of core temperature but were in fact relatively unresponsive at the level of initiating compensatory responses.4
We previously highlighted the importance of identifying the actual response made during an initial drug challenge to understand the consequences of repeated drug administrations.2 Briefly, highly responsive individuals, who rapidly activate a robust compensatory response to a drug's pharmacological effect during an initial administration, will appear relatively insensitive at the level of a feedback-controlled output (e.g., core temperature). Our data indicate that these individuals are poised to rapidly acquire chronic tolerance and subsequently progress to a form of hyper-tolerance indicative of a departure from homeostasis.1,5 Rats exhibiting little change of core temperature during an initial nitrous oxide challenge developed a persistent intra-administration hyperthermic sign-reversal over subsequent nitrous oxide challenges.5 While nitrous oxide continued to elevate heat loss in these rats similarly as during the initial exposure, the rats acquired heat-generating responses that overcompensated for the drug-induced heat loss (Fig. 1). The important point is that initially insensitive individuals who are highly responsive in terms of activating compensatory responses to a hypothermic drug challenge are also faster to develop a hyperthermic sign reversal with repeated use.5
Figure 1.
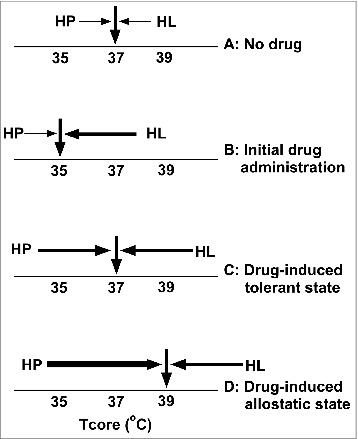
Simple thermoregulatory heuristic distilled from our research findings illustrating the importance of identifying actual regulatory responses for studying regulatory adaptation. (A) Prior to administration of the hypothermia-promoting drug nitrous oxide, core temperature (Tcore) rests at its characteristic normothermic balance point via the actions of an intricate regulatory system in which heat loss (HL) and heat production (HP) are balanced at that point (equal opposing arrows with minimal energetic cost). (B) Initial administration of ≥ 60% nitrous oxide to rats results in hypothermia by selectively promoting an increase in HL (a “primary drug effect”). (C) Following several nitrous oxide administrations, Tcore remains at or near its characteristic normothermic balance point during nitrous oxide inhalation. Note, however, that designating Tcore as a response and measuring only this outcome during nitrous oxide administration would suggest that the subject has become insensitive to nitrous oxide. However, the state of chronic tolerance does not reflect the development of insensitivity to nitrous oxide's effect to promote HL, but instead reflects thermoregulatory adaptations that confer the ability to effectively and promptly match the drug's largely intact HL effect with a centrally mediated countervailing HP regulatory response. (D) Additional nitrous oxide administrations result in a striking acquired thermoregulatory phenotype wherein the HP response during nitrous oxide inhalation substantially exceeds the drug's effect to promote HL, revealing a state of disordered (allostatic) regulation. This state is inconsistent with canonical homeostatic theory, and we propose that thermoregulatory allostasis reflects the interaction of a non-naturalistic stressor with a thermoregulatory system comprised of multiple relatively independent effector loops that are not subservient to an overarching Tcore set-point. [Numerous factors in addition to the drug's effect and centrally elicited thermoregulatory system responses contribute to the balance point of Tcore, e.g., environmental factors determining HL via conduction, convection, radiation and evaporation; the gradient between Tcore and ambient temperature; recruitment of extra-thermoregulatory stress responses that might alter thresholds and gains associated with individual thermoeffector loops.]
As detailed elsewhere,1 sign reversals are inconsistent with principles of homeostasis. Traditional interpretations of homeostasis argue that when critical bodily variables are perturbed (e.g., core temperature, blood glucose, blood pressure and volume), a centrally coordinated battery of corrective reflexes is elicited that has the net effect of obviating the perturbation. For example, as core temperature declines upon cold exposure, heat producing and heat conserving responses are recruited; if blood glucose increases during a carbohydrate-rich meal, glucose-lowering responses are activated. Several features of this description typically attributed to homeostasis are important to note.1 First, the responses are post-hoc, triggered by the already-occurring perturbation. Thus no role is proposed for anticipatory responses that would preempt impending perturbations. Second, the level of the variable (e.g., core temperature; blood glucose) will re-acquire its pre-perturbation state (sometimes considered a set point). Finally, the recruited set of responses is “wisely” coordinated by relevant brain circuits such that regulated variable is stabilized with optimal efficiency (see1). However, our recent investigation of the behavioral and autonomic effector responses observed during repeated nitrous oxide exposures reveals inefficient concurrent competition between thermoregulatory responses during the development of chronic tolerance and a subsequent hyperthermic sign reversal.6 These findings depart from the canonical homeostatic framework and are more compatible with an allostatic interpretation.
Although homeostasis has been the predominant explanation for biological regulation for almost a century, some fundamental tenets have been challenged in recent years.1 First, compelling evidence indicates a major role for preemptive responses that are made in anticipation of likely perturbations. Second, the set-point concept has numerous flaws such that the adoption of a balance-point perspective seems more appropriate.1 Finally, in contrast to the homeostatic model's premise of efficient central coordination of regulatory effector activity, considerable evidence suggests that key variables are regulated via multiple separate, and often independent, sensor-effector loops.1 Allostasis was initially proposed to account for specific shortcomings of homeostasis by incorporating preemptive responding and a variable set point, changes that are also incorporated in contemporary models of homeostasis.1 One goal of our recent review1 was to explicate the situations where allostasis could be used to explain phenomena that are beyond the scope of a contemporary homeostatic model (e.g., excessive responses that lead to sign reversals, concurrently competing responses, and excessively persistent responses). In situations where the homeostatic model is inconsistent with empirical observations, the allostatic framework appears to be the more popular alternative.
Allostasis has become the term du jour to describe what occurs when regulated variables are maintained inefficiently and/or at potentially dangerous levels in a health sense (i.e., allostatic load). Examples include elevated glucose in type-2 diabetes, obesity and drug addiction. These disorders have been considered to be allostatic conditions in which certain (often poorly understood) antecedent factors cause vital variables to acquire persistent equilibria at unhealthy levels. Returning to our previous example, rats given repeated nitrous oxide exposures develop tolerance to its initial hypothermic effect, but this can progress to a hyperthermic sign-reversal state due to excessive heat-producing responses. Notably, however, these excessive heat-producing responses occur in tandem with increased cool-seeking behavior6 revealing inefficient concurrent effector competition that fits well with an allostatic model of regulation.1
The significance of being highly responsive to challenges extends well beyond thermoregulatory allostasis. Piazza and colleagues7 have assessed numerous variables in rats when challenging them with a novel environment. Those exhibiting stronger initial responses such as greater stress hormone secretion (corticosterone) and a greater release of dopamine in brain reward circuits were more likely to self-administer drugs of abuse in the future. Thus, being hyper-responsive appears to predispose to drug use or abuse. Consistent with this, humans who appear to have a “low level of response” to alcohol's effects are most likely to abuse alcohol later in life.3 We hypothesize that initially insensitive subjects who appear least impacted by the ethanol are in fact most effective at mounting compensatory responses. Based on an allostatic model of drug addiction,1 we are currently investigating whether initially insensitive rats to nitrous oxide-induced hypothermia are also more likely to self-administer nitrous oxide. Collectively, these observations imply that quantitative differences in the way that individuals initially respond to challenges (e.g., during an initial drug administration or even during non-drug challenges) may be indicative of the propensity to develop an allostatic versus homeostatic phenotype.
Many of the ailments of modern life can be attributed to allostatic activity. These include drug abuse, obesity and perhaps depression as well. Our regulatory systems are increasingly being confronted with challenges that were not present in an evolutionary sense, such as by drugs of abuse and the ready availability of highly palatable and hedonically pleasing high-caloric foods. Analogously, as stressors have shifted from mainly physical to predominantly psychological over time, a stress-response system that persistently over-responds to challenges may predispose to depression. An important point is that it is becoming increasingly feasible to identify the actual responses that are made to challenges. Measuring these responses should make it possible to screen individuals for an allostatic phenotype and quantify and even mitigate their risk for developing disease.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Ramsay DS, Woods SC. Clarifying the roles of homeostasis and allostasis in physiological regulation. Psychol Rev 2014; 121:225-47; PMID:24730599; http://dx:doi.org/ 10.1037/a0035942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ramsay DS, Woods SC. Biological consequences of drug administration: implications for acute and chronic tolerance. Psychol Rev 1997; 104:170-93; PMID:9009884; http://dx:doi.org/ 10.1037/0033-295X.104.1.170 [DOI] [PubMed] [Google Scholar]
- 3. Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. Am J Psychiatry 1994; 151:184-9; PMID:8296886 [DOI] [PubMed] [Google Scholar]
- 4. Kaiyala KJ, Butt S, Ramsay DS. Direct evidence for systems-level modulation of initial drug (in)sensitivity in rats. Psychopharmacology (Berl) 2007; 191:243-51; PMID:17237917; http://dx:doi.org/ 10.1007/s00213-006-0657-z [DOI] [PubMed] [Google Scholar]
- 5. Kaiyala KJ, Chan B, Ramsay DS. Robust thermoregulatory overcompensation, rather than tolerance, develops with serial administrations of 70% nitrous oxide to rats. J Therm Biol 2012; 37:30-40; PMID:22247586; http://dx:doi.org/ 10.1016/j.jtherbio.2011.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ramsay DS, Woods SC, Kaiyala KJ. Repeated nitrous oxide exposure in rats causes a thermoregulatory sign-reversal with concurrent activation of opposing thermoregulatory effectors. Temperature 2014; 1:151-61; http://dx:doi.org/ 10.4161/23328940.2014.944809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Piazza PV, Le Moal M. Pathophysiological basis of vulnerability to drug abuse: role of an interaction between stress, glucocorticoids, and dopaminergic neurons. Annu Rev Pharmacol Toxicol 1996; 36:359-78; PMID: 8725394; http://dx:doi.org/ 10.1146/annurev.pa.36.040196.002043 [DOI] [PubMed] [Google Scholar]


