Abstract
The adverse effects of amphetamine- (AMPH) and methamphetamine- (METH) induced hyperthermia on vasculature, peripheral organs and peripheral immune system are discussed. Hyperthermia alone does not produce amphetamine-like neurotoxicity but AMPH and METH exposures that do not produce hyperthermia (≥40°C) are minimally neurotoxic. Hyperthermia likely enhances AMPH and METH neurotoxicity directly through disruption of protein function, ion channels and enhanced ROS production. Forebrain neurotoxicity can also be indirectly influenced through the effects of AMPH- and METH- induced hyperthermia on vasculature. The hyperthermia and the hypertension produced by high doses amphetamines are a primary cause of transient breakdowns in the blood-brain barrier (BBB) resulting in concomitant regional neurodegeneration and neuroinflammation in laboratory animals. This BBB breakdown can occur in the amygdala, thalamus, striatum, sensory and motor cortex and hippocampus. Under these conditions, repetitive seizures greatly enhance neurodegeneration in hippocampus, thalamus and amygdala. Even when the BBB is less disrupted, AMPH- or METH- induced hyperthermia effects on brain vasculature may play a role in neurotoxicity. In this case, striatal and cortical vascular function are adversely affected, and even greater ROS, immune and damage responses are seen in the meninges and cortical surface vasculature. Finally, muscle and liver damage and elevated cytokines in blood can result when amphetamines produce hyperthermia. Proteins, from damaged muscle may activate the peripheral immune system and exacerbate liver damage. Liver damage can further increase cytokine levels, immune system activation and increase ammonia levels. These effects could potentially enhance vascular damage and neurotoxicity.
Keywords: hyperthermia, neurotoxicity, amphetamine, methamphetamine, cerebral vasculature, meninges, blood-brain barrier, immune system
Abbreviations
- AMPH
amphetamine
- BBB
blood-brain barrier
- CBF
cerebral blood flow
- CSF
cerebrospinal fluid
- EIH
environmentally-induced hyperthermia
- LPS
lipopolysaccharides
- METH
methamphetamine
- MAV
meninges and associated vasculature
- ROS
reactive oxidative stress
Earlier History of Amphetamine- and Methamphetamine-Induced Hyperthermia and Neurotoxicity
Introductory remarks
The terms amphetamine (AMPH) and methamphetamine (METH) neurotoxicity are often used interchangeably in this review. This is because research from our laboratory has not been able to identify any appreciable difference in the neurotoxicity produced in rodent by AMPH compared to METH.1,2 Figure 1 shows the profiles of the temperature changes produced by either exposure to amphetamines or environmentally-induced hyperthermia (EIH) that we have observed in our laboratory. The interactive effects that hyperthermia has with respect to toxicity during either AMPH or METH exposures are complex. Table 1 summarizes the physiological and pathological effects that can be produced by 1) EIH, 2) AMPH or METH exposures producing significantly hyperthermic conditions or 3) AMPH or METH exposures when life-threatening hyperthermia occurs. Table 2 provides information on the role of selected biochemical or physiological effects mediated by hyperthermia that are associated with AMPH and METH neurotoxicity. The reader can use these tables as adjunct references where they pertain to what is being presented in the text.
Figure 1.
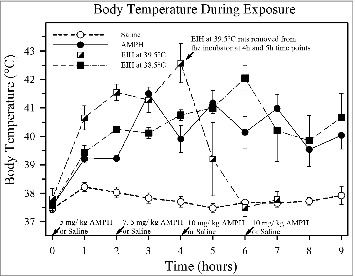
The effect of amphetamine (AMPH) on body temperature compared to environmentally-induced hyperthermia (EIH) and normothermic controls. The results of one of the more recent studies in the authors’ laboratory compares the hyperthermia observed during neurotoxic exposures to AMPH with that produced by EIH, which is very similar to heat stroke. The temperature profiles of animals given either 4 doses of either AMPH (n = 10) or normal saline (normothermic controls n = 9) s.c. at an environmental temperature of 22.5°C are shown. Their temperature profiles are compared to 2 groups of animals given 4 doses of saline in an infant incubator held at either 38°C to 39°C (ave. ≈38.5, n = 6) or 39°C to 40°C (ave. ≈39.5, n = 4) which induced hyperthermia (EIH). Animals at the higher incubator temperature became hyperthermic much more rapidly and as a group had slightly higher peak temperatures. They all had ataxia and hind limb dysfunction for 2 to 8 h after cooling. The second group of EIH animals at the lower 38.5°C temperature had a temperature profile almost identical to the AMPH group. The variability of the body temperatures of the AMPH group and the 38.5°C EIH group after the 3 h time point was due to cooling on ice to prevent death. The 39.5°C EIH group was not subjected to any further hyperthermia after 4 h since it would have been lethal (previous studies in the authors’ laboratory).
Table 1.
Effects of hyperthermia alone (EIH) compared to the toxicity of AMPH or METH exposures that are produced when hyperthermia occurs
| Exposure Group | |||
|---|---|---|---|
| Physiological or Pathological Effect | EIH | AMPH or METH with 40°C ≤ Body Temp. < 41°C | AMPH or METH with 41.0°C ≤ Body Temp. < 43.0°C |
| Dopamine Terminal Damage in Striatum | None | 50% < Depletion ≤ 80% | 80% < Depletion ≤ 95% |
| **Parietal Cortex Neurodegeneration | None | Present but diffuse | More prevalent at these body temperature ranges |
| Limbic Cortex Neurodegeneration | minimal | Present but diffuse | Extensive if seizures occur |
| Thalamus Neurodegeneration | None | Present but diffuse | More extensive at these body temp. ranges |
| Hippocampal Neurodegeneration | None | Minimal in rats but can be | *Extensive if motor seizure activity occurs |
| Convulsions/ Behavioral Seizures | **None | Convulsions often occur in mice but not in rats | Convulsions and status epilepticus |
| BBB disruption | Yes | Not in rat *but possibly in mice | *Yes |
| Choroid Plexus Dysfunction/ Damage | Yes | Not determined | ≤ EIH |
| aMAV Dysfunction/ Damage | Yes | Not determined | > EIH |
| Elevated Serum **Myoglobin | Increase < 2-fold | 2-fold < Increase < 3-fold | 3-fold < Increase < 10-fold |
| Elevated Serum **Bound Urea Nitrogen | Increase ≈ 2-fold | Increase < 2-fold | 2-fold < Increase < 3-fold |
| Elevated Serum **Alanine Transaminase | ***1-fold < Increase < 10-fold | < 2-fold | Increase ≈ 4-fold |
| Blood Glucose | ≈ Normal 100 to 150 mg/ dL | 60 to 100 mg/ dL | 30 to 80 mg/ dL |
| Peripheral Immune System Changes | Yes | Yes | Yes |
Note that this table represents a summary of the findings of the authors’ laboratory or (in a few instances) several other investigators. The damage estimates shown in the table for the given peak body temperature ranges with AMPH or METH are when these body temperatures are maintained for a duration of ≈ 3 h or more. Limbic cortex areas evaluated are piriform and the amygdala cortices.
a MAV is an abbreviation for meninges and associated cerebral cortical vasculature (includes all major cortical surface vasculature as well as pial arterioles).
*Damage is seizure dependent and is more prevalent with very high (> 20 mg/ kg) doses of AMPH or METH. It should be noted that overt motor seizures/ convulsions may not be necessary for neurodegeneration but that electrographic signs of epileptoid activity without convulsions is sufficient (from communications with Dr. Denson Fujikawa).
**Pertains to rat data.
***Effects strain dependent.
Table 2.
The role of selected biochemical or physiological effects mediated by hyperthermia that are associated with AMPH and METH neurotoxicity
| Adverse Biochemical or Physiological Effects Associated with Amphetamine or Methamphetamine Neurotoxicity |
|||||||
|---|---|---|---|---|---|---|---|
| Brain Region and Effect | Reactive Oxidative Stress | Elevated Extracellular Glutamate | Hyperactivity of Dopamine Transporter | Blood-Brain Barrier Disruption | Significant Seizure Activity | Meninges and Associated Vasculature | Neuro-inflammation |
| ↓Striatal Dopamine Axons/ Terminals | Strong Supporting Data | Significant Supporting Data | Strong Supporting Data | Not Necessary | Not Necessary | Not Necessary | **Not Necessary? |
| Striatal Neurodegeneration | Supporting Data | Supporting Data | *Indirect | Sparse Supporting Data | Unknown | Not Necessary | Unknown |
| Parietal Neurodegeneration | Supporting Data | Supporting Data | *Indirect | Not Necessary | Not Necessary | Some Supporting Data | Unknown |
| Piriform Neurodegeneration | Some Supporting Data | Indirect Supporting Data | *Indirect | Supporting Data | Supporting Data | Some Supporting Data | Unknown |
| Thalamic Neurodegeneration | Some Supporting Data | Unknown | *Indirect | Supporting Data | Not Necessary | Unknown | Unknown |
| Amygdala Neurodegeneration | Some Supporting Data | Indirect Supporting Data | *Indirect | Supporting Data | Supporting Data | Unknown | Unknown |
| Hippocampal Neurodegeneration | Some Supporting Data | Indirect Supporting Data | *Indirect | Supporting Data | Significant Supporting Data | Unknown | Unknown |
All the adverse biochemical or physiological effects listed are temperature dependent or greatly exacerbated by hyperthermia. The neurotoxicities listed are those that have been characterized histologically. The conclusions shown in the table are opinions of the authors derived from the literature. Most of which are supported to some extent by citation in the text.
Even though systemic immune system activation and peripheral organ damage all precede and correlate very positively with AMPH and METH toxicity, their roles at this point are still being investigated and controversial.
aDopamine plasma membrane (a.k.a. DAT, Slc6a)
*Almost all of the hyperthermic and neurotoxic effects of AMPH and METH are either directly or indirectly due to very high synaptic extracellular levels of dopamine or norepinephrine.
**See text for clarification.
Striatal dopamine terminal damage
Starting in the early 1960s, clinical reports and research in laboratory animals began to point to the important role that hyperthermia had in exacerbating the adverse physiological and lethal effects induced by AMPH and METH in humans 3-5 and laboratory animals.4,6,7 Approximately 10 years later, reports of METH damaging dopaminergic terminals were published.8-13 However, it wasn't until the early 1990s that the role of hyperthermia in METH and AMPH neurotoxicity began to be elucidated. At that time, the link between dopamine terminal degeneration in the striatum and pronounced hyperthermia (≥40°C) was reported in both rat 14-16 and mouse.17,18 It is now clear that when animals remain normothermic during exposures to very high doses of AMPH or METH, more transient depletions of striatal dopamine (decreases of 40 to 60% in mouse and 45 to 65% in rat) lasting for 1 month or so result along with rare sporadic occurrences of neurodegeneration in the parietal and piriform cortex.19-22 Under these normothermic conditions there is minimal or no neuroimmune response in the striatum. However, it was noted that pronounced hyperthermia alone would not produce this neurotoxicity. Under favorable conditions (waking cycle and in a 23.5°C environment) and with 8 h exposures, plasma concentrations of AMPH or METH as low as 3 μM are capable of producing hyperthermia and neurotoxicity.2,23 Further research in non-human primates has helped substantiate the clinical relevance of the hyperthermia in regards to METH producing dopamine terminal degeneration.24,25
METH-induced hyperthermia and excessive plasma membrane transporter (DAT) activity appear to be primary factors in the production of this striatal neurotoxicity.26 However, reactive metabolites of dopamine,27,28 vesicular monoamine transporter-2 damage,29 and elevated glutamate levels30-34 have also been implicated as exacerbating factors in METH toxicity. Increases in body and brain temperatures during AMPH exposure do appear to correlate with increased striatal dopamine and amygdala 5HT levels.15,29,35,36 Finally, the great swelling and loss of Fluoro-Ruby labeled axons of dopaminergic neurons in the striatum indicate that they are being destroyed by a “necrotic”-like effect.37 The exact mechanism of the apparent dopaminergic axonal and terminal destruction is unknown but is not due to classic apoptosis or necrosis since the loss of dopaminergic neurons in the substantia nigra is minimal at best (≤20%) compared to the ≥80% loss of striatal dopamine terminals.38
There are several mechanisms by which hyperthermia could potentiate AMPH and METH toxicity to dopamine terminals that have also been implicated in other types of neuronal degeneration. METH-induced hyperthermia directly increases ROS levels in striatum and causes a dramatic increase in ROS-induced gene expression.39-42 Interestingly, hyperthermia (EIH) alone produces equivalent increases in genes up-regulated by ROS in many brain regions.43 Concomitant with large increases in ROS and heat-shock protein induction is the dysfunction of proteins due to misfolding produced by pronounced hyperthermia.44,45 Such protein alterations or changes in lipid membranes could lead to mitochondrial46 and ion channel dysfunction.47-52 The tremendous increase in DAT activity/ transport produced by AMPH or METH may be sufficient to produce detectable depolarization due to concomitant Na+ influx with either AMPH or METH amphetamine into dopamine terminals and possibly alter glutamate activity.33,53 These affects will lead to large increases in sodium into the terminal that will require extensive amounts of energy to transport it out of the terminal. This alone is not sufficient to produce terminal degeneration without hyperthermia. However, we postulate that the occurrence of hyperthermia in the presence of AMPH or METH further compromise ion channels, mitochondrial function and damage to other import cellular components by ROS leading to terminal damage and/ or death.
Neuronal degeneration in forebrain
Specialized histological techniques and the knowledge that hyperthermia during exposures to amphetamines was necessary for dopamine terminal damage were applied to laboratory animal research starting in the mid-1990s. These studies resulted in the identification of brain regions where METH and AMPH produced cell body/ somatic neurodegeneration. The use of Fluoro-Jade to label degenerating neurons after AMPH and METH exposure in histological preparations of forebrain was key for the success of these studies in the author's and other laboratories.54-57 Areas of the somatosensory parietal cortex (vibrissae input) and limbic system (piriform and amygdala cortex and tenia tecta) were most sensitive. However, in animals in which the most pronounced (≥ 41.5°C) and prolonged hyperthermia was produced by AMPH, neurodegeneration was more extensive in the intralaminar regions of the thalamus and striatum.54 Hippocampal degeneration was minimal unless seizures occurred in animals. Neurotoxicity in the striatum, neocortex, and limbic regions of human brain has been reported with METH abuse.58-64 For a more complete description of how neurotoxic dosing regimens of AMPH or METH interact with body temperature and seizures to produce neurodegeneration in various brain regions, see Bowyer et al. 2008.1 It is still not known the degree to which body temperatures must be increased by AMPH or METH to produce dopamine terminal damage and neurodegeneration in humans.
The neurodegeneration observed in AMPH or METH animal studies65 using lower doses which do not usually produce seizure activity was restricted to the parietal cortex, and it was significantly less than that produced by systemic administration of either kainic or domoic acid (limbic cortex, hippocampus and cortex)66-68 or 3-nitroproprionic acid (striatum and thalamus).69-71 However, starting in 1998 more pronounced types of neurodegeneration found in limbic cortex, hippocampus and thalamus were observed in rats given multiple doses of 15 mg/kg AMPH 54 and particularly mice given single high doses (40 mg/ kg) of METH.72 The most likely explanation at the time was that these types of AMPH or METH exposures produced high/ neurotoxic levels of glutamate in the synaptic cleft or that ion channel dysfunction occurred due to METH-induced hyperthermia. However, there are other explanations.
Role of the Brain Vasculature in Neurodegeneration Produced by AMPH and METH
Overt BBB disruption and vascular leakage
We observed in 1998 that vasculature damage might be related to some types of AMPH-induced neurodegeneration. In some instances, exposure to multiple doses of AMPH over an 8 h period could, in conjunction with extreme hyperthermia, produce perivascular neurodegeneration in the thalamus and hippocampus.54 Subsequently, the studies by Deng et al. 72 indicated that a single very high dose of METH in mice could produce a much more pronounced neurodegeneration in the hippocampus and striatum than that observed in previous experiments evaluating METH and AMPH neurotoxicity. We speculated that vascular damage and repetitive seizures (status epilepticus) might play a role in such a very high dose effect, and later experiments bore this out to be true with METH and AMPH.1,19 These single, very high doses produced a rapid and pronounced onset of hyperthermia and subsequently status epilepticus resulting in a consistent breakdown of the BBB in the amygdala, hippocampus and, in some instances, the striatum. The neurodegeneration that subsequently occurs in these areas approaches that produced by systemic kainic or domoic acid in regards to the number (thousands) of degenerating neurons observable in a 30 to 40 μm coronal sections seen in these regions.
Others have reported that more moderate doses of METH and AMPH could produce localized damage to the BBB in several brain regions when hyperthermia occurred, and indicated that hyperthermia induced by EIH (in a 38° to 40°C environment) alone could also result in BBB disruption and neurotoxicity.73-75 As well, the combination of stress and METH appear to exacerbate more moderate types of damage to vasculature.76 Our research involving AMPH and METH has consistently indicated that dopamine terminal damage could not be produced by EIH alone.1,14,54 As well, diffuse neurodegeneration (somatic) in areas of the brain, such as parietal and piriform cortex, were not observed with EIH. Although studies in our laboratory indicate that hyperthermia alone (EIH) produces BBB disruption, we have observed that the pattern of the neurodegeneration and magnitude that accompanies the disruption is much less than that seen with AMPH and METH.1 In summary, there is substantial evidence that, when METH and AMPH produced extreme hyperthermia, regional BBB breakdowns can occur, which greatly enhance neurotoxicity. Such a disruption would likely prevent the regulation of the composition of the extracellular constituents surrounding neurons in affected regions, which could enhance seizure activity and neurodegeneration by most mechanisms proposed to be involved in neurotoxicity (e.g., ROS and excitotoxicity). It should be noted that the BBB disruption that occurs after either AMPH or METH are normally rapidly reversed, within 2 h after the end of hyperthermia (≤ 40°C), except in cases where extensive hippocampal neurodegeneration has occurred.1,19,77
Adverse effects of hyperthermia and amphetamines on choroid plexus, meninges and cerebral surface vasculature: a role in the exacerbation of neurotoxicity
One mechanism by which AMPH and METH may exacerbate neurodegeneration observed in the cortical regions (e.g., parietal or piriform cortex) of rat is through ischemia (decrease in cerebral blood flow, CBF) produced by vasoconstriction. This would result from AMPH and METH directly releasing norepinephrine from the noradrenergic innervation regulating the α1 noradrenergic receptors on pial arteries,78-80 and the resulting vasoconstriction reducing CBF in the cortex. The locus coeruleus is the other major noradrenergic input associated with brain that directly innervates cortex.81,82 This input has been shown to play a role in regulating cortical blood flow,83 and has often been associated with global decreases in CBF,84-86 which when combined with pial artery constriction would further increase the likelihood of cortical ischemia. However, more recent studies indicate that locus coeruleus input into parietal cortex may actually increase CBF through α and β noradrenergic receptors COX-2 and GABAergic cortical neurons and reduce ischemia.87
Thus, the overall net effect of AMPH and METH on CBF under neurotoxic and vasculotoxic conditions might not be easily predicted. None the less, one animal study clearly showed that METH was clearly capable of suppressing CBF during METH exposure and even after METH levels had subsided.88 Furthermore humans abusing amphetamines can develop cerebral vascular accidents and have worse outcomes than those not abusing amphetamines.89-91 Hypoxia lasting 24 h after exposure is also induced in laboratory animals by high doses (8 mg/ kg i.v.) of METH 92; however, the degree of hyperthermia and convulsive activity was not reported. Clearly, AMPH- and METH-induced vasospasms and the ischemia thus produce would be a factor that could contribute to cortical neurodegeneration.
There is indication at the mRNA transcript level that regulation of vascular tone, and possibly damage, in the striatum and parietal cortex is somewhat altered by more moderate neurotoxic exposures to AMPH and METH (those not producing repetitive seizures or BBB leakage).43,93 The slight (<2-fold) increases in mRNA for endothelial nitric oxide synthase (Nos3) and endothelin 1 (Edn1) would be expected if ischemia or vascular endothelial damage was occurring. The increase in Nos3 may be a response mechanism to produce more nitric oxide and reverse any maladaptive vasoconstriction present. The insult produced by either AMPH or EIH to vasculature present in the choroid plexus and the meninges and associated cerebral vasculature (MAV) appears to be significantly greater.43,93 This effect could be reflected in humans by the interaction of METH abuse and the development of meningitis, which has been reported.94
Also, there are many more pronounced increases in genes related to the immune system and inflammation in the choroid plexus and MAV (even more so) after AMPH or EIH. The gene expression changes in the MAV indicate that the lingering effects of AMPH damage coincide and may induce the vasospasms and prolonged decrease in CBF in the cortex discussed in the previous paragraph. Large increases in lipopolysaccharide (LPS) binding protein mRNA (Lbp) are observed in the choroid plexus and MAV at 1 day after AMPH but not EIH. Lbp increases are not seen in the parietal cortex and striatum after AMPH. Thus, in MAV and choroid plexus, Lbp increases are a unique immune response, which may be related to vascular damage to the MAV.43 LPS binding protein is an important part of the innate immune response, is a biomarker for sepsis and has been reported to bind LPS from bacteria.95,96 Whether the dramatic increase of Lbp in MAV after neurotoxic exposures to AMPH indicates an increased presence of bacteria (presages sepsis?) or activation of the innate immune system by other mechanisms remains to be determined.
In regards to the choroid plexus, previous studies indicate that AMPH does not appear to affect it, with regards to vascular and secretory cell damage, but that EIH is very detrimental to the choroid plexus.97,98 Data from our laboratory showed a greater effect of AMPH on the choroid plexus then that reported by others.43 Differences seen between the physiological effects of AMPH in our study with those reported in the earlier study, may explain the greater adverse effects. That is, more prolonged neurotoxic exposure to AMPH produced both severe hyperthermia and hypertension which was not observed in the earlier study with a single dose of AMPH.98 In addition, adverse effects produced by hyperthermia alone (EIH) are likely as great, or greater, than AMPH.43 Damage to the vascular and secretory cells present in the choroid plexus in a previous study involving hyperthermia or hypertension have resulted in neurotoxicity involving some neurodegeneration.97 However, our findings over the years have found that extreme, even to a greater degree than that produced by AMPH or METH; hyperthermia alone (EIH) does not produce the histological signs of neurodegeneration resembling AMPH or METH neurotoxicity.
Indirect Adverse Effects on Vasculature Due to the Muscle, Liver in Kidney Damage Produced by Hyperthermia and Amphetamines
The linkage between amphetamines and rhabdomyolosis, which produces muscle damage, goes back over 40 years.99-102 Also, the correlation between the magnitude of hyperthermia, serum myoglobin levels (resulting from muscle damage) and neurotoxicity produced by AMPH is very strong 103 as is the correlation between myoglobin and kidney damage.104,105 However, this is not proof that myoglobin levels are necessarily a causative effect in AMPH neurotoxicity. An equivalent hyperthermia produced by EIH resulted in a lesser non-statistically significant increase in myoglobin. When hemoglobin is released during hemolysis it can cause vascular toxicity.106,107 One would suspect that myoglobin, which like hemoglobin is heme containing and binds oxygen, may also be vasculotoxic. However, there is surprisingly little in the literature regarding the relationship between myoglobin in the circulating blood and toxicity to vasculature endothelium.
Also, there is some correlation between neurotoxic AMPH exposures with respect to blood nitrogen (BUN) but EIH can also produce similar significant increases in BUN indicating it is necessarily dependent on exposure to neurotoxic doses of AMPH.103 The muscle damage produced by neurotoxic doses of AMPH, when hyperthermia occurs, also results in increased circulating concentrations of other enzymes such as creatine kinase.103 Both the creatine kinase and myoglobin increases (5- to 6-fold control) were more pronounced than BUN levels (2- to 3-fold control). Although the increase in myoglobin and creatine kinase in blood could be due to renal damage, we found no evidence of renal damage histologically. Therefore, it is a plausible that significant increases in many types of muscle-related proteins, in addition to myoglobin and creatine kinase, appear in blood when amphetamine produces pronounced hyperthermia. Thus, we speculate that some vascular damage could be produced in the MAV, choroid plexus and the remaining brain vasculature as a result of proteins released by muscle during neurotoxic exposures to amphetamines. The exact mechanism by which this vascular damage may occur through muscle-derived serum proteins has yet to be explored.
Neurotoxic doses of METH can produce liver necrosis and elevate blood levels of ammonia.108 However, this is very likely due to hyperthermia since very high doses have been reported not to produce histopathology when conducted under normothermic conditions. When histopathology and adverse liver enzyme changes present in blood serum are compared, EIH and neurotoxic exposures to AMPH have similar adverse effects.103,109 Furthermore, liver necrosis is not necessary for either dopamine terminal damage or neurodegeneration but lesser perturbations in liver function still may exacerbate such processes.103 Finally, although AMPH can significantly elevate liver-specific alanine transaminase, and ammonia, in some strains of Sprague-Dawley rats,108 it minimally elevates this enzyme in other strains when AMPH produces neurotoxicity.103 One mechanism by which AMPH disruption of liver function may influence neurotoxicity is through liver glycogen depletion.103 This glycogen depletion may be behind the low blood glucose levels (70 to 40 mg/ dL) that normally occur as a result of neurotoxic exposures to AMPH and always precede body tremors and death (levels lower than 25 mg/ dL) (Bowyer, unpublished data).
Amphetamine and Hyperthermia Activation of the Circulating Immune System and Neurotoxicity
Neuroinflammation is a primary factor in neurological diseases such as multiple sclerosis.110-113 Neuroimmune system dysfunction more recently has also been implicated to varying extents in the pathogenesis of Alzheimer's as well as being a hindrance to treating the disease.114-117 Also, neuroinflammation has been implicated in Parkinson's disease.118-120 Neuroinflammation (microglial activation and astrocytosis) is known to occur in brain regions where terminal damage and neurodegeneration is found when METH and/ or AMPH produce pronounced hyperthermia.18,54,121-126 On the other hand, hyperthermia alone (EIH) produces minimal or no neuroinflammation in these brain regions. The importance of these effects in humans has been exemplified by the exacerbation of HIV neuropathology and drug addiction.127-129 However, the research to date has not supported that neuroinflammation exacerbates the neurotoxicity to dopaminergic terminals produced by amphetamines but that neuroinflammation instead results secondarily to neurotoxicity.123,130 Little research information is available as to whether neuroinflammation exacerbates the neurodegeneration seen in the various brain regions produced by AMPH or METH.
Neurotoxic exposures to AMPH produce greater immune responses in the MAV than other brain regions and choroid plexus.43,93 Hyperthermia has a significantly lesser effect on the expression of genes related to inflammation in MAV. Somewhat unexpectedly, the immune response in the choroid plexus to AMPH was much less than the MAV and was not that different from the immune response produced by EIH. In the case of the MAV, AMPH produces increases in the transcripts for the cell determinant protein Cd14 and Lbp, which are genes relatively specific for microglia.43 It is not yet known whether this translates into an increase in the number of macrophages present or an increased expression within individual macrophages. Additionally, it is not known whether the changes are occurring in the unique macrophages resident on the meningeal surfaces or are circulatory macrophages adhering to the endothelial cells in the lumen in damage areas of the vasculature in the MAV. It remains to be determined whether or not the increased immune response in MAV evoked by AMPH influences neurodegeneration within the underlying cortex.
Less is known about how neurotoxic exposures to amphetamines and hyperthermia affect the immune response tissues outside the brain and whether these changes influence the neurotoxic effects of amphetamines but information is emerging.131 Initial published results and ongoing studies indicate that the significant immune responses in the circulating blood evoked by neurotoxic exposures to AMPH and hyperthermia alone (EIH) are often pronounced just prior to the onset of neurotoxicity to at least 1 day after exposure103 (elevated protein levels IL-6, IL-10; and mRNA for IL-1b, Cd8a, Cxcr2, Itgam, and Tnfrsf1a unpublished data in GEO, NCBI; data file GSE29733). Tumor necrosis factor α levels were elevated 2-fold compared to control 1 day post AMPH. AMPH was observed to produce significantly higher levels of myoglobin in the serum than EIH indicating a greater damage to muscle. The release of proteins from muscle could well serve as damage associated molecular proteins (DAMPs) and activate the immune system.132,133 DAMPs appear to play an important role in the inflammation process in pancreatitis.134 It is interesting to note that AMPH, but not EIH, can trigger a tremendous increase in the expression of mRNA (Reg3a and Reg3b, biomarkers for pancreatic inflammation) in the MAV (but not blood, cortex, striatum or choroid plexus).43 It is not known how the cell types expressing these genes are affecting meningeal function and whether it exacerbates cortical neurodegeneration.
Activation of the immune system could also occur through damage to the liver which can be produced by hyperthermia (EIH) as well as AMPH.103 Regardless of how the immune response evoked by amphetamines in the circulating blood occurs, almost nothing is known as to how this alters the neurotoxic effect in the brain. There are reports that activating the immune system with LPS can exacerbate damage to dopaminergic systems by neurotoxins but this does not appear always to be the case with amphetamines.135 It is not known whether this is the case for other regions of the brain, such as parietal cortex, thalamus and hippocampus, where amphetamines can produce neurodegeneration. Finally, very little is known as to whether activation of the immune response in circulating blood and the periphery plays a part in the transient psychosis that can occur with the abuse or prolonged use of amphetamines.136-138
Summary
In animal models that evaluate the neurotoxicity of AMPH and METH, it is quite clear that hyperthermia is one of the essential components necessary for the production of histological signs of dopamine terminal damage and neurodegeneration in cortex, striatum, thalamus and hippocampus. When animals remain normothermic during AMPH or METH exposure, only transient depletions of striatal dopamine occur (1 month or less) along with rare sporadic occurrences of neurodegeneration in the parietal and piriform cortex. The dopamine terminal damage and neurodegeneration that occur when amphetamines produce hyperthermia are likely due, in part, directly to hyperthermia increasing ROS, protein misfolding/ dysfunction and altering ion channel permeability in the affected neurons. Hyperthermia can also indirectly enhance neurodegeneration produced by amphetamines through the triggering of repetitive seizure activity. The generation of repetitive seizures and status epilepticus that can be produced by AMPH or METH is likely due to a breakdown in the BBB in the amygdala and hippocampus. Growing information also implicates that hyperthermia during exposure to amphetamines may affect the neurodegeneration produced indirectly through MAV dysfunction (vasospasm and ischemia) and damage to the choroid plexus (adverse effects on CSF function). Finally, it is possible that muscle and liver damage that are exacerbated by hyperthermia play significant roles in the neurotoxicity of amphetamines through releasing cellular proteins and other toxic substances into the circulation and/ or activation of the systemic immune system which might subsequently exacerbate neuroinflammation.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Material
Supplemental Material may be downloaded here: publisher's website
Disclaimer
The findings and conclusions in this article have not been formally disseminated by the Food and Drug Administration and should not be construed to represent any Agency determination or policy.
References
- 1. Bowyer JF, Thomas MT, Schmued LC, Ali SF. Brain region-specific neurodegenerative profiles showing the relative importance of amphetamine dose, hyperthermia, seizures and the blood-brain barrier. Ann NY Acad Sci 2008; 1139:127-39; PMID:18991857; http://dx.doi.org/ 10.1196/annals.1432.005 [DOI] [PubMed] [Google Scholar]
- 2. Levi MS, Divine B, Hanig JP, Doerge DR, Vanlandingham MM, George NI, Twaddle NC, Bowyer JF. A comparison of methylphenidate-, amphetamine-, and methamphetamine-induced hyperthermia and neurotoxicity in male Sprague-Dawley rats during the waking (lights off) cycle. Neurotoxicol Teratol 2012; 34:253-62; PMID:22289608; http://dx.doi.org/ 10.1016/j.ntt.2012.01.007 [DOI] [PubMed] [Google Scholar]
- 3. Kalant H, Kalant OJ. Death in amphetamine users: causes and rates. Can Med Assoc J 1975; 112:299-304; PMID:1089034 [PMC free article] [PubMed] [Google Scholar]
- 4. Zalis EG, Lundberg GD, Knutson RA. The pathophysiology of acute amphetamine poisoning with pathologic correlation. J Pharmacol Exp Ther 1967; 158:115-27; PMID:6054070 [PubMed] [Google Scholar]
- 5. Zalis EG, Parmley LF, Jr. Fatal Amphetamine Poisoning. Arch Intern Med 1963; 112:822-6; PMID:14056847; http://dx.doi.org/ 10.1001/archinte.1963.03860060060004 [DOI] [PubMed] [Google Scholar]
- 6. Davis WM, Bedford JA, Buelke JL, Guinn MM, Hatoum HT, Waters IW, Wilson MC, Braude MC. Acute toxicity and gross behavioral effects of amphetamine, four methoxyamphetamines, and mescaline in rodents, dogs, and monkeys. Toxicol Appl Pharmacol 1978; 45:49-62; PMID:99845; http://dx.doi.org/ 10.1016/0041-008X(78)90027-3 [DOI] [PubMed] [Google Scholar]
- 7. Davis WM, Hatoum HT, Waters IW. Interactions of catecholaminergic receptor blockers with lethal doses of amphetamine or substituted amphetamines in mice. Res Commun Chem Pathol Pharmacol 1978; 21:27-36; PMID:687391 [PubMed] [Google Scholar]
- 8. Lewander T. Urinary excretion and tissue levels of catecholamines during chronic amphetamine intoxication. Psychopharmacologia 1968; 13:394-407; PMID:5706269; http://dx.doi.org/ 10.1007/BF00404954 [DOI] [PubMed] [Google Scholar]
- 9. Fibiger HC, Mogeer EG. Effect of acute and chronic methamphetamine treatment on tyrosine hydroxylase activity in brain and adrenal medulla. Eur J Pharmacol 1971; 16:176-80; PMID:4405780; http://dx.doi.org/ 10.1016/0014-2999(71)90008-2 [DOI] [PubMed] [Google Scholar]
- 10. Koda LY, Gibb JW. Adrenal and striatal tyrosine hydroxylase activity after methamphetamine. J Pharmacol Exp Ther 1973; 185:42-8; PMID:4144311 [PubMed] [Google Scholar]
- 11. Buening MK, Gibb JW. Influence of methamphetamine and neuroleptic drugs on tyrosine hydroxylase activity. Eur J Pharmacol 1974; 26:30-4; PMID:4151642; http://dx.doi.org/ 10.1016/0014-2999(74)90070-3 [DOI] [PubMed] [Google Scholar]
- 12. Ellinwood EH, Jr, Escalante O. Behavior and histopathological findings during chronic methedrine intoxication. Biol Psych 1970; 2:27-39; PMID:5414902 [PubMed] [Google Scholar]
- 13. Seiden LS, Fischman MW, Schuster CR. Long-term methamphetamine induced changes in brain catecholamines in tolerant rhesus monkeys. Drug Alcohol Depend 1976; 1:215-9; PMID:828106; http://dx.doi.org/ 10.1016/0376-8716(76)90030-2 [DOI] [PubMed] [Google Scholar]
- 14. Bowyer JF, Davies DL, Schmued L, Broening HW, Newport GD, Slikker W, Jr, Holson RR. Further studies of the role of hyperthermia in methamphetamine neurotoxicity. J Pharmacol Exp Ther 1994; 268:1571-80; PMID:8138969 [PubMed] [Google Scholar]
- 15. Bowyer JF, Gough B, Slikker W, Jr, Lipe GW, Newport GD, Holson RR. Effects of a cold environment or age on methamphetamine-induced dopamine release in the caudate putamen of female rats. Pharmacol, Biochem, Behav 1993; 44:87-98; PMID:8094252; http://dx.doi.org/ 10.1016/0091-3057(93)90284-Z [DOI] [PubMed] [Google Scholar]
- 16. Bowyer JF, Tank AW, Newport GD, Slikker W, Jr, Ali SF, Holson RR. The influence of environmental temperature on the transient effects of methamphetamine on dopamine levels and dopamine release in rat striatum. J Pharmacol Exp Ther 1992; 260:817-24; PMID:1346646 [PubMed] [Google Scholar]
- 17. Miller DB, O’Callaghan JP. Environment-, drug- and stress-induced alterations in body temperature affect the neurotoxicity of substituted amphetamines in the C57BL6J mouse. J Pharmacol Exp Ther 1994; 270:752-60; PMID:8071868 [PubMed] [Google Scholar]
- 18. O’Callaghan JP, Miller DB. Neurotoxicity profiles of substituted amphetamines in the C57BL6J mouse. J Pharmacol Exp Ther 1994; 270:741-51 [PubMed] [Google Scholar]
- 19. Bowyer JF, Ali S. High doses of methamphetamine that cause disruption of the blood-brain barrier in limbic regions produce extensive neuronal degeneration in mouse hippocampus. Synapse 2006; 60:521-32; PMID:16952162; http://dx.doi.org/ 10.1002/syn.20324 [DOI] [PubMed] [Google Scholar]
- 20. Bowyer JF, Harris AJ, Delongchamp RR, Jakab RL, Miller DB, Little AR, O’Callaghan JP. Selective changes in gene expression in cortical regions sensitive to amphetamine during the neurodegenerative process. Neurotoxicology 2004; 25:555-72; PMID:15183010; http://dx.doi.org/ 10.1016/j.neuro.2003.08.005 [DOI] [PubMed] [Google Scholar]
- 21. Bowyer JF, Holson RR, Miller DB, O’Callaghan JP. Phenobarbital and dizocilpine can block methamphetamine-induced neurotoxicity in mice by mechanisms that are independent of thermoregulation. Brain Res 2001; 919:179-83; PMID:11689178; http://dx.doi.org/ 10.1016/S0006-8993(01)03051-7 [DOI] [PubMed] [Google Scholar]
- 22. Bowyer JF, Pogge AR, Delongchamp RR, O’Callaghan JP, Patel KM, Vrana KE, Freeman WM. A threshold neurotoxic amphetamine exposure inhibits parietal cortex expression of synaptic plasticity-related genes. Neuroscience 2007; 144:66-76; PMID:17049170; http://dx.doi.org/ 10.1016/j.neuroscience.2006.08.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Clausing P, Gough B, Holson RR, Slikker W, Jr, Bowyer JF. Amphetamine levels in brain microdialysate, caudateputamen, substantia nigra and plasma after dosage that produces either behavioral or neurotoxic effects. J Pharmacol Exp Ther 1995; 274:614-21; PMID:7636721 [PubMed] [Google Scholar]
- 24. Ricaurte GA, McCann UD. Recognition and management of complications of new recreational drug use. Lancet 2005; 365:2137-45; PMID:15964451; http://dx.doi.org/ 10.1016/S0140-6736(05)66737-2 [DOI] [PubMed] [Google Scholar]
- 25. Yuan J, Hatzidimitriou G, Suthar P, Mueller M, McCann U, Ricaurte G. Relationship between temperature, dopaminergic neurotoxicity, and plasma drug concentrations in methamphetamine-treated squirrel monkeys. J Pharmacol Exp Ther 2006; 316:1210-8; PMID:16293712; http://dx.doi.org/ 10.1124/jpet.105.096503 [DOI] [PubMed] [Google Scholar]
- 26. Yuan J, Darvas M, Sotak B, Hatzidimitriou G, McCann UD, Palmiter RD, Ricaurte GA. Dopamine is not essential for the development of methamphetamine-induced neurotoxicity. J Neurochem 2010; 114:1135-42; PMID:20533999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Berman SB, Hastings TG. Inhibition of glutamate transport in synaptosomes by dopamine oxidation and reactive oxygen species. J Neurochem 1997; 69:1185-95; PMID:9282942; http://dx.doi.org/ 10.1046/j.1471-4159.1997.69031185.x [DOI] [PubMed] [Google Scholar]
- 28. LaVoie MJ, Hastings TG. Dopamine quinone formation and protein modification associated with the striatal neurotoxicity of methamphetamine: evidence against a role for extracellular dopamine. J Neurosci: Off J Soc Neurosci 1999; 19:1484-91; PMID:9952424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Volz TJ, Farnsworth SJ, Rowley SD, Hanson GR, Fleckenstein AE. Age-dependent differences in dopamine transporter and vesicular monoamine transporter-2 function and their implications for methamphetamine neurotoxicity. Synapse 2009; 63:147-51; PMID:19021208; http://dx.doi.org/ 10.1002/syn.20580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brown JM, Quinton MS, Yamamoto BK. Methamphetamine-induced inhibition of mitochondrial complex II: roles of glutamate and peroxynitrite. J Neurochem 2005; 95:429-36; PMID:16086684; http://dx.doi.org/ 10.1111/j.1471-4159.2005.03379.x [DOI] [PubMed] [Google Scholar]
- 31. Nash JF, Yamamoto BK. Methamphetamine neurotoxicity and striatal glutamate release: comparison to 3,4-methylenedioxymethamphetamine. Brain Res 1992; 581:237-43; PMID:1356579; http://dx.doi.org/ 10.1016/0006-8993(92)90713-J [DOI] [PubMed] [Google Scholar]
- 32. Sonsalla PK, Nicklas WJ, Heikkila RE. Role for excitatory amino acids in methamphetamine-induced nigrostriatal dopaminergic toxicity. Science 1989; 243:398-400; PMID:2563176; http://dx.doi.org/ 10.1126/science.2563176 [DOI] [PubMed] [Google Scholar]
- 33. Underhill SM, Wheeler DS, Li M, Watts SD, Ingram SL, Amara SG. Amphetamine modulates excitatory neurotransmission through endocytosis of the glutamate transporter EAAT3 in dopamine neurons. Neuron 2014; 83:404-16; PMID:25033183; http://dx.doi.org/ 10.1016/j.neuron.2014.05.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Northrop NA, Smith LP, Yamamoto BK, Eyerman DJ. Regulation of glutamate release by alpha7 nicotinic receptors: differential role in methamphetamine-induced damage to dopaminergic and serotonergic terminals. J Pharmacol Exp Ther 2011; 336:900-7; PMID:21159748; http://dx.doi.org/ 10.1124/jpet.110.177287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Clausing P, Bowyer JF. Time course of brain temperature and caudateputamen microdialysate levels of amphetamine and dopamine in rats after multiple doses of d-amphetamine. Ann New York Acad Sci 1999; 890:495-504; PMID:10668455; http://dx.doi.org/ 10.1111/j.1749-6632.1999.tb08031.x [DOI] [PubMed] [Google Scholar]
- 36. Tor-Agbidye J, Yamamoto B, Bowyer JF. Seizure activity and hyperthermia potentiate the increases in dopamine and serotonin extracellular levels in the amygdala during exposure to d-amphetamine. Toxicol Sci 2001; 60:103-11; PMID:11222877; http://dx.doi.org/ 10.1093/toxsci/60.1.103 [DOI] [PubMed] [Google Scholar]
- 37. Bowyer JF, Schmued LC. Fluoro-Ruby labeling prior to an amphetamine neurotoxic insult shows a definitive massive loss of dopaminergic terminals and axons in the caudate-putamen. Brain Res 2006; 1075:236-9; PMID:16458862; http://dx.doi.org/ 10.1016/j.brainres.2005.12.062 [DOI] [PubMed] [Google Scholar]
- 38. Bowyer JF, Frame LT, Clausing P, Nagamoto-Combs K, Osterhout CA, Sterling CR, Tank AW. Long-term effects of amphetamine neurotoxicity on tyrosine hydroxylase mRNA and protein in aged rats. J Pharmacol Exp Ther 1998; 286:1074-85; PMID:9694971 [PubMed] [Google Scholar]
- 39. Cadet JL, Krasnova IN, Jayanthi S, Lyles J. Neurotoxicity of substituted amphetamines: molecular and cellular mechanisms. Neurotoxicity Res 2007; 11:183-202; PMID:17449459; http://dx.doi.org/ 10.1007/BF03033567 [DOI] [PubMed] [Google Scholar]
- 40. Krasnova IN, Ladenheim B, Jayanthi S, Oyler J, Moran TH, Huestis MA, Cadet JL. Amphetamine-induced toxicity in dopamine terminals in CD-1 and C57BL6J mice: complex roles for oxygen-based species and temperature regulation. Neuroscience 2001; 107:265-74; PMID:11731100; http://dx.doi.org/ 10.1016/S0306-4522(01)00351-7 [DOI] [PubMed] [Google Scholar]
- 41. Cadet JL, Brannock C. Free radicals and the pathobiology of brain dopamine systems. Neurochem Int 1998; 32:117-31; PMID:9542724; http://dx.doi.org/ 10.1016/S0197-0186(97)00031-4 [DOI] [PubMed] [Google Scholar]
- 42. Imam SZ, el-Yazal J, Newport GD, Itzhak Y, Cadet JL, Slikker W, Jr, Ali SF. Methamphetamine-induced dopaminergic neurotoxicity: role of peroxynitrite and neuroprotective role of antioxidants and peroxynitrite decomposition catalysts. Ann New York Acad Sci 2001; 939:366-80; PMID:11462792; http://dx.doi.org/ 10.1111/j.1749-6632.2001.tb03646.x [DOI] [PubMed] [Google Scholar]
- 43. Bowyer JF, Patterson TA, Saini UT, Hanig JP, Thomas M, Camacho L, George NI, Chen JJ. Comparison of the global gene expression of choroid plexus and meninges and associated vasculature under control conditions and after pronounced hyperthermia or amphetamine toxicity. BMC Genomics 2013; 14:147; PMID:23497014; http://dx.doi.org/ 10.1186/1471-2164-14-147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lindquist S, Craig EA. The heat-shock proteins. Ann Rev Genet 1988; 22:631-77; PMID:2853609; http://dx.doi.org/ 10.1146/annurev.ge.22.120188.003215 [DOI] [PubMed] [Google Scholar]
- 45. Li GC, Mivechi NF, Weitzel G. Heat shock proteins, thermotolerance, and their relevance to clinical hyperthermia. Int J Hyperther: Off J Eur Soc Hyperther Oncol, North Am Hyperther Group 1995; 11:459-88; PMID:7594802; http://dx.doi.org/ 10.3109/02656739509022483 [DOI] [PubMed] [Google Scholar]
- 46. Wang Z, Cai F, Chen X, Luo M, Hu L, Lu Y. The role of mitochondria-derived reactive oxygen species in hyperthermia-induced platelet apoptosis. PloS One 2013; 8:e75044; PMID:24023970; http://dx.doi.org/ 10.1371/journal.pone.0075044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Howells J, Czesnik D, Trevillion L, Burke D. Excitability and the safety margin in human axons during hyperthermia. J Physiol 2013; 591:3063-80; PMID:23613528; http://dx.doi.org/ 10.1113/jphysiol.2012.249060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Thomas EA, Hawkins RJ, Richards KL, Xu R, Gazina EV, Petrou S. Heat opens axon initial segment sodium channels: a febrile seizure mechanism? Ann Neurol 2009; 66:219-26; PMID:19743470; http://dx.doi.org/ 10.1002/ana.21712 [DOI] [PubMed] [Google Scholar]
- 49. Ouardouz M, Lema P, Awad PN, Di Cristo G, Carmant L. N-methyl-D-aspartate, hyperpolarization-activated cation current (Ih) and gamma-aminobutyric acid conductances govern the risk of epileptogenesis following febrile seizures in rat hippocampus. Eur J Neurosci 2010; 31:1252-60; PMID:20345922; http://dx.doi.org/ 10.1111/j.1460-9568.2010.07159.x [DOI] [PubMed] [Google Scholar]
- 50. Mashimo T, Ohmori I, Ouchida M, Ohno Y, Tsurumi T, Miki T, Wakamori M, Ishihara S, Yoshida T, Takizawa A, et al. . A missense mutation of the gene encoding voltage-dependent sodium channel (Nav1.1) confers susceptibility to febrile seizures in rats. J Neurosci: Off J Soc Neurosci 2010; 30:5744-53; PMID:20410126; http://dx.doi.org/ 10.1523/JNEUROSCI.3360-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Brederson JD, Kym PR, Szallasi A. Targeting TRP channels for pain relief. Eur J Pharmacol 2013; 716:61-76; PMID:23500195; http://dx.doi.org/ 10.1016/j.ejphar.2013.03.003 [DOI] [PubMed] [Google Scholar]
- 52. Hunt RF, Hortopan GA, Gillespie A, Baraban SC. A novel zebrafish model of hyperthermia-induced seizures reveals a role for TRPV4 channels and NMDA-type glutamate receptors. Exp Neurol 2012; 237:199-206; PMID:22735490; http://dx.doi.org/ 10.1016/j.expneurol.2012.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sonders MS, Zhu SJ, Zahniser NR, Kavanaugh MP, Amara SG. Multiple ionic conductances of the human dopamine transporter: the actions of dopamine and psychostimulants. J Neurosci: Off J Soc Neurosci 1997; 17:960-74; PMID:8994051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bowyer JF, Peterson SL, Rountree RL, Tor-Agbidye J, Wang GJ. Neuronal degeneration in rat forebrain resulting from D-amphetamine-induced convulsions is dependent on seizure severity and age. Brain Res 1998; 809:77-90; PMID:9795148; http://dx.doi.org/ 10.1016/S0006-8993(98)00846-4 [DOI] [PubMed] [Google Scholar]
- 55. Schmued LC, Bowyer JF. Methamphetamine exposure can produce neuronal degeneration in mouse hippocampal remnants. Brain Res 1997; 759:135-40; PMID:9219871; http://dx.doi.org/ 10.1016/S0006-8993(97)00173-X [DOI] [PubMed] [Google Scholar]
- 56. Schmued LC, Stowers CC, Scallet AC, Xu L. Fluoro-Jade C results in ultra high resolution and contrast labeling of degenerating neurons. Brain Res 2005; 1035:24-31; PMID:15713273; http://dx.doi.org/ 10.1016/j.brainres.2004.11.054 [DOI] [PubMed] [Google Scholar]
- 57. Eisch AJ, Schmued LC, Marshall JF. Characterizing cortical neuron injury with Fluoro-Jade labeling after a neurotoxic regimen of methamphetamine. Synapse 1998; 30:329-33; PMID:9776136; http://dx.doi.org/ 10.1002/(SICI)1098-2396(199811)30:3%3c329::AID-SYN10%3e3.0.CO;2-V [DOI] [PubMed] [Google Scholar]
- 58. Sekine Y, Ouchi Y, Sugihara G, Takei N, Yoshikawa E, Nakamura K, Iwata Y, Tsuchiya KJ, Suda S, Suzuki K, et al. . Methamphetamine causes microglial activation in the brains of human abusers. J Neurosci: Off J Soc Neurosci 2008; 28:5756-61; PMID:18509037; http://dx.doi.org/ 10.1523/JNEUROSCI.1179-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, Lee JY, Toga AW, Ling W, London ED. Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci: Off J Soc Neurosci 2004; 24:6028-36; PMID:15229250; http://dx.doi.org/ 10.1523/JNEUROSCI.0713-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. McCann UD, Kuwabara H, Kumar A, Palermo M, Abbey R, Brasic J, Ye W, Alexander M, Dannals RF, Wong DF, et al. . Persistent cognitive and dopamine transporter deficits in abstinent methamphetamine users. Synapse 2008; 62:91-100; PMID:17992686; http://dx.doi.org/ 10.1002/syn.20471 [DOI] [PubMed] [Google Scholar]
- 61. Bartzokis G, Beckson M, Lu PH, Edwards N, Rapoport R, Wiseman E, Bridge P. Age-related brain volume reductions in amphetamine and cocaine addicts and normal controls: implications for addiction research. Psychiatry Res 2000; 98:93-102; PMID:10762735; http://dx.doi.org/ 10.1016/S0925-4927(99)00052-9 [DOI] [PubMed] [Google Scholar]
- 62. Ernst T, Chang L, Leonido-Yee M, Speck O. Evidence for long-term neurotoxicity associated with methamphetamine abuse: a 1H MRS study. Neurology 2000; 54:1344-9; PMID:10746608; http://dx.doi.org/ 10.1212/WNL.54.6.1344 [DOI] [PubMed] [Google Scholar]
- 63. Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Wong C, et al. . Higher cortical and lower subcortical metabolism in detoxified methamphetamine abusers. Am J Psychiat 2001; 158:383-9; PMID:11229978; http://dx.doi.org/ 10.1176/appi.ajp.158.3.383 [DOI] [PubMed] [Google Scholar]
- 64. Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, et al. . Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiat 2001; 158:377-82; PMID:11229977; http://dx.doi.org/ 10.1176/appi.ajp.158.3.377 [DOI] [PubMed] [Google Scholar]
- 65. Commins DL, Seiden LS. alpha-Methyltyrosine blocks methylamphetamine-induced degeneration in the rat somatosensory cortex. Brain Res 1986; 365:15-20; PMID:3947981; http://dx.doi.org/ 10.1016/0006-8993(86)90717-1 [DOI] [PubMed] [Google Scholar]
- 66. Olney JW. Kainic acid and other excitotoxins: a comparative analysis. Adv Biochem Psychopharmacol 1981; 27:375-84; PMID:7004119 [PubMed] [Google Scholar]
- 67. Olney JW. Excitotoxin-mediated neuron death in youth and old age. Prog Brain Res 1990; 86:37-51; PMID:1982368; http://dx.doi.org/ 10.1016/S0079-6123(08)63165-9 [DOI] [PubMed] [Google Scholar]
- 68. Scallet AC, Schmued LC, Johannessen JN. Neurohistochemical biomarkers of the marine neurotoxicant, domoic acid. Neurotoxicol Teratol 2005; 27:745-52; PMID:16203121; http://dx.doi.org/ 10.1016/j.ntt.2005.06.018 [DOI] [PubMed] [Google Scholar]
- 69. Beal MF, Brouillet E, Jenkins BG, Ferrante RJ, Kowall NW, Miller JM, Storey E, Srivastava R, Rosen BR, Hyman BT. Neurochemical and histologic characterization of striatal excitotoxic lesions produced by the mitochondrial toxin 3-nitropropionic acid. J Neurosci: Off J Soc Neurosci 1993; 13:4181-92; PMID:7692009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Coles CJ, Edmondson DE, Singer TP. Inactivation of succinate dehydrogenase by 3-nitropropionate. J Biol Chem 1979; 254:5161-7; PMID:447637 [PubMed] [Google Scholar]
- 71. Hamilton BF, Gould DH. Nature and distribution of brain lesions in rats intoxicated with 3-nitropropionic acid: a type of hypoxic (energy deficient) brain damage. Acta Neuropathol 1987; 72:286-97; PMID:3564909; http://dx.doi.org/ 10.1007/BF00691103 [DOI] [PubMed] [Google Scholar]
- 72. Deng X, Wang Y, Chou J, Cadet JL. Methamphetamine causes widespread apoptosis in the mouse brain: evidence from using an improved TUNEL histochemical method. Brain Res Mol Brain Res 2001; 93:64-9; PMID:11532339; http://dx.doi.org/ 10.1016/S0169-328X(01)00184-X [DOI] [PubMed] [Google Scholar]
- 73. Kiyatkin EA, Brown PL, Sharma HS. Brain edema and breakdown of the blood-brain barrier during methamphetamine intoxication: critical role of brain hyperthermia. Eur J Neurosci 2007; 26:1242-53; PMID:17767502; http://dx.doi.org/ 10.1111/j.1460-9568.2007.05741.x [DOI] [PubMed] [Google Scholar]
- 74. Sharma HS, Ali SF. Alterations in blood-brain barrier function by morphine and methamphetamine. Ann New York Acad Sci 2006; 1074:198-224; PMID:17105918; http://dx.doi.org/ 10.1196/annals.1369.020 [DOI] [PubMed] [Google Scholar]
- 75. Sharma HS, Sjoquist PO, Ali SF. Drugs of abuse-induced hyperthermia, blood-brain barrier dysfunction and neurotoxicity: neuroprotective effects of a new antioxidant compound H-29051. Curr Pharm Des 2007; 13:1903-23; PMID:17584116; http://dx.doi.org/ 10.2174/138161207780858375 [DOI] [PubMed] [Google Scholar]
- 76. Northrop NA, Yamamoto BK. Persistent neuroinflammatory effects of serial exposure to stress and methamphetamine on the blood-brain barrier. J Neuroimmune Pharmacol: Off J Soc NeuroImmune Pharmacol 2012; 7:951-68; PMID:22833424; http://dx.doi.org/ 10.1007/s11481-012-9391-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Bowyer JF, Tranter KM, Sarkar S, Raymick J, Hanig JP, Schmued LC. Systemic administration of fluoro-gold for the histological assessment of vascular structure, integrity and damage. Curr Neurovasc Res 2014; 11:31-47; PMID:24274907; http://dx.doi.org/ 10.2174/1567202610666131124235011 [DOI] [PubMed] [Google Scholar]
- 78. Burnstock G. Neurogenic control of cerebral circulation. Cephalalgia 1985; 5 Suppl 2):25-33; PMID:2410133 [DOI] [PubMed] [Google Scholar]
- 79. Hamel E. Perivascular nerves and the regulation of cerebrovascular tone. J Appl Physiol 2006; 100:1059-64; PMID:16467392; http://dx.doi.org/ 10.1152/japplphysiol.00954.2005 [DOI] [PubMed] [Google Scholar]
- 80. Kulik T, Kusano Y, Aronhime S, Sandler AL, Winn HR. Regulation of cerebral vasculature in normal and ischemic brain. Neuropharmacology 2008; 55:281-8; PMID:18541276; http://dx.doi.org/ 10.1016/j.neuropharm.2008.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Foote SL, Aston-Jones G, Bloom FE. Impulse activity of locus coeruleus neurons in awake rats and monkeys is a function of sensory stimulation and arousal. Proc Nat Acad Sci U S A 1980; 77:3033-7; PMID:6771765; http://dx.doi.org/ 10.1073/pnas.77.5.3033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Foote SL, Bloom FE, Aston-Jones G. Nucleus locus ceruleus: new evidence of anatomical and physiological specificity. Physiol Rev 1983; 63:844-914; PMID:6308694 [DOI] [PubMed] [Google Scholar]
- 83. Cohen Z, Molinatti G, Hamel E. Astroglial and vascular interactions of noradrenaline terminals in the rat cerebral cortex. J Cereb Blood Flow Metab: Off J Int Soc Cereb Blood Flow Metab 1997; 17:894-904; PMID:9290587; http://dx.doi.org/ 10.1097/00004647-199708000-00008 [DOI] [PubMed] [Google Scholar]
- 84. de la Torre JC. Evidence for central innervation of intracerebral blood vessels: local cerebral blood flow measurements and histofluorescence analysis by the sucrose-phosphate-glyoxylic acid (SPG) method. Neuroscience 1976; 1:455-7; PMID:11370237; http://dx.doi.org/ 10.1016/0306-4522(76)90096-8 [DOI] [PubMed] [Google Scholar]
- 85. Goadsby PJ, Duckworth JW. Low frequency stimulation of the locus coeruleus reduces regional cerebral blood flow in the spinalized cat. Brain Res 1989; 476:71-7; PMID:2914215; http://dx.doi.org/ 10.1016/0006-8993(89)91537-0 [DOI] [PubMed] [Google Scholar]
- 86. Raichle ME, Hartman BK, Eichling JO, Sharpe LG. Central noradrenergic regulation of cerebral blood flow and vascular permeability. Proc Nat Acad Sci U S A 1975; 72:3726-30; PMID:810805; http://dx.doi.org/ 10.1073/pnas.72.9.3726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Toussay X, Basu K, Lacoste B, Hamel E. Locus coeruleus stimulation recruits a broad cortical neuronal network and increases cortical perfusion. J Neurosci: Off J Soc Neurosci 2013; 33:3390-401; PMID:23426667; http://dx.doi.org/ 10.1523/JNEUROSCI.3346-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Polesskaya O, Silva J, Sanfilippo C, Desrosiers T, Sun A, Shen J, Feng C, Polesskiy A, Deane R, Zlokovic B, et al. . Methamphetamine causes sustained depression in cerebral blood flow. Brain Res 2011; 1373:91-100; PMID:21156163; http://dx.doi.org/ 10.1016/j.brainres.2010.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Moon K, Albuquerque FC, Mitkov M, Ducruet AF, Wilson DA, Crowley RW, Nakaji P, McDougall CG. Methamphetamine use is an independent predictor of poor outcome after aneurysmal subarachnoid hemorrhage. J Neurointerventional Surg 2014; PMID:24780822; http://dx.doi.org/ 10.1136/neurintsurg-2014-011161 [DOI] [PubMed] [Google Scholar]
- 90. Beadell NC, Thompson EM, Delashaw JB, Cetas JS. The deleterious effects of methamphetamine use on initial presentation and clinical outcomes in aneurysmal subarachnoid hemorrhage. J Neurosurg 2012; 117:781-6; PMID:22920957; http://dx.doi.org/ 10.3171/2012.7.JNS12396 [DOI] [PubMed] [Google Scholar]
- 91. Kousik SM, Graves SM, Napier TC, Zhao C, Carvey PM. Methamphetamine-induced vascular changes lead to striatal hypoxia and dopamine reduction. Neuroreport 2011; 22:923-8; PMID:21979424; http://dx.doi.org/ 10.1097/WNR.0b013e32834d0bc8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Weaver J, Yang Y, Purvis R, Weatherwax T, Rosen GM, Liu KJ. In vivo evidence of methamphetamine induced attenuation of brain tissue oxygenation as measured by EPR oximetry. Toxicol Appl Pharmacol 2014; 275:73-8; PMID:24412707; http://dx.doi.org/ 10.1016/j.taap.2013.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Thomas M, George NI, Patterson TA, Bowyer JF. Amphetamine and environmentally induced hyperthermia differentially alter the expression of genes regulating vascular tone and angiogenesis in the meninges and associated vasculature. Synapse 2009; 63:881-94; PMID:19582783; http://dx.doi.org/ 10.1002/syn.20661 [DOI] [PubMed] [Google Scholar]
- 94. Heninger M, Collins KA. Acute bacterial meningitis with coincident methamphetamine use: a case report and review of the literature. J Forensic Sci 2013; 58:1088-91; PMID:23601243; http://dx.doi.org/ 10.1111/1556-4029.12176 [DOI] [PubMed] [Google Scholar]
- 95. Park BS, Lee JO. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp Mol Med 2013; 45:e66; PMID:24310172; http://dx.doi.org/ 10.1038/emm.2013.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Schumann RR. Old and new findings on lipopolysaccharide-binding protein: a soluble pattern-recognition molecule. Biochem Soc Trans 2011; 39:989-93; PMID:21787335; http://dx.doi.org/ 10.1042/BST0390989 [DOI] [PubMed] [Google Scholar]
- 97. Sharma HS, Johanson CE. Blood-cerebrospinal fluid barrier in hyperthermia. Prog Brain Res 2007; 162:459-78; PMID:17645933; http://dx.doi.org/ 10.1016/S0079-6123(06)62023-2 [DOI] [PubMed] [Google Scholar]
- 98. Murphy VA, Johanson CE. Adrenergic-induced enhancement of brain barrier system permeability to small nonelectrolytes: choroid plexus versus cerebral capillaries. J Cereb Blood Flow Metab: Off J Int Soc Cereb Blood Flow Metab 1985; 5:401-12; PMID:3928638; http://dx.doi.org/ 10.1038/jcbfm.1985.55 [DOI] [PubMed] [Google Scholar]
- 99. Penn AS, Rowland LP, Fraser DW. Drugs, coma, and myoglobinuria. Arch Neurol 1972; 26:336-43; PMID:5015592; http://dx.doi.org/ 10.1001/archneur.1972.00490100066006 [DOI] [PubMed] [Google Scholar]
- 100. Kendrick WC, Hull AR, Knochel JP. Rhabdomyolysis and shock after intravenous amphetamine administration. Ann Internal Med 1977; 86:381-7; PMID:848798; http://dx.doi.org/ 10.7326/0003-4819-86-4-381 [DOI] [PubMed] [Google Scholar]
- 101. Chan P, Chen JH, Lee MH, Deng JF. Fatal and nonfatal methamphetamine intoxication in the intensive care unit. J Toxicol Clin Toxicol 1994; 32:147-55; PMID:8145354; http://dx.doi.org/ 10.3109/15563659409000444 [DOI] [PubMed] [Google Scholar]
- 102. Sperling LS, Horowitz JL. Methamphetamine-induced choreoathetosis and rhabdomyolysis. Ann Internal Med 1994; 121:986; PMID:7978734; http://dx.doi.org/ 10.7326/0003-4819-121-12-199412150-00019 [DOI] [PubMed] [Google Scholar]
- 103. Levi MS, Patton RE, Hanig JP, Tranter KM, George NI, James LP, Davis KJ, Bowyer JF. Serum myoglobin, but not lipopolysaccharides, is predictive of AMPH-induced striatal neurotoxicity. Neurotoxicology 2013; 37C:40-50; PMID:23608161; http://dx.doi.org/ 10.1016/j.neuro.2013.04.003 [DOI] [PubMed] [Google Scholar]
- 104. Ishigami A, Tokunaga I, Gotohda T, Kubo S. Immunohistochemical study of myoglobin and oxidative injury-related markers in the kidney of methamphetamine abusers. Legal Med 2003; 5:42-8; PMID:12935649; http://dx.doi.org/ 10.1016/S1344-6223(03)00005-1 [DOI] [PubMed] [Google Scholar]
- 105. Holt S, Moore K. Pathogenesis of renal failure in rhabdomyolysis: the role of myoglobin. Exp Nephrol 2000; 8:72-6; PMID:10729745; http://dx.doi.org/ 10.1159/000020651 [DOI] [PubMed] [Google Scholar]
- 106. Reeder BJ. The redox activity of hemoglobins: from physiologic functions to pathologic mechanisms. Antioxidants Redox Signaling 2010; 13:1087-123; PMID:20170402; http://dx.doi.org/ 10.1089/ars.2009.2974 [DOI] [PubMed] [Google Scholar]
- 107. Vinchi F, Tolosano E. Therapeutic approaches to limit hemolysis-driven endothelial dysfunction: scavenging free heme to preserve vasculature homeostasis. Oxidative Med Cell Longevity 2013; 2013:396527; PMID:23781294; http://dx.doi.org/ 10.1155/2013/396527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Halpin LE, Yamamoto BK. Peripheral ammonia as a mediator of methamphetamine neurotoxicity. J Neurosci: Off J Soc Neurosci 2012; 32:13155-63; PMID:22993432; http://dx.doi.org/ 10.1523/JNEUROSCI.2530-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Maruta T, Nihira M, Tomita Y. [Histopathological study on acute poisoning of methamphetamine, morphine or cocaine]. Nihon Arukoru Yakubutsu Igakkai zasshi = JPN J Alcohol Stud Drug Depen 1997; 32:122-38; PMID:9168637 [PubMed] [Google Scholar]
- 110. Zhao S, Li F, Leak RK, Chen J, Hu X. Regulation of neuroinflammation through programed death-1programed death ligand signaling in neurological disorders. Front Cell Neurosci 2014; 8:271; PMID:25232304; http://dx.doi.org/ 10.3389/fncel.2014.00271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Jons D, Sundstrom P, Andersen O. Targeting Epstein-Barr virus infection as an intervention against multiple sclerosis. Acta Neurologica Scandinavica 2014; PMID:25208981; http://dx.doi.org/ 10.1111/ane.12294 [DOI] [PubMed] [Google Scholar]
- 112. Sperlagh B, Illes P. P2´7 receptor: an emerging target in central nervous system diseases. Trends Pharmacol Sci 2014; 35:537-47; PMID:25223574; http://dx.doi.org/ 10.1016/j.tips.2014.08.002 [DOI] [PubMed] [Google Scholar]
- 113. Ben-Nun A, Kaushansky N, Kawakami N, Krishnamoorthy G, Berer K, Liblau R, Hohlfeld R, Wekerle H. From classic to spontaneous and humanized models of multiple sclerosis: impact on understanding pathogenesis and drug development. J Autoimmun 2014; S0896-8411:00101-2; PMID:25175979; http://dx.doi.org/ 10.1016/j.jaut.2014.06.004 [DOI] [PubMed] [Google Scholar]
- 114. Fuller JP, Stavenhagen JB, Teeling JL. New roles for Fc receptors in neurodegeneration-the impact on Immunotherapy for Alzheimer's Disease. Front Neurosci 2014; 8:235; PMID:25191216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Mosher KI, Wyss-Coray T. Microglial dysfunction in brain aging and Alzheimer's disease. Biochem Pharmacol 2014; 88:594-604; PMID:24445162; http://dx.doi.org/ 10.1016/j.bcp.2014.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Canton J, Neculai D, Grinstein S. Scavenger receptors in homeostasis and immunity. Nat Rev Immunol 2013; 13:621-34; PMID:23928573; http://dx.doi.org/ 10.1038/nri3515 [DOI] [PubMed] [Google Scholar]
- 117. Needham E, Zandi MS. Recent advances in the neuroimmunology of cell-surface CNS autoantibody syndromes, Alzheimer's disease, traumatic brain injury and schizophrenia. J Neurol 2014; 261:2037-42; PMID:25182699; http://dx.doi.org/ 10.1007/s00415-014-7473-x [DOI] [PubMed] [Google Scholar]
- 118. Kannarkat GT, Boss JM, Tansey MG. The role of innate and adaptive immunity in Parkinson's disease. J Parkinson's Dis 2013; 3:493-514; PMID:24275605; http://dx.doi.org/ 10.3233/JPD-130250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Rodrigues MC, Sanberg PR, Cruz LE, Garbuzova-Davis S. The innate and adaptive immunological aspects in neurodegenerative diseases. J Neuroimmunol 2014; 269:1-8; PMID:24161471; http://dx.doi.org/ 10.1016/j.jneuroim.2013.09.020 [DOI] [PubMed] [Google Scholar]
- 120. Greggio E, Civiero L, Bisaglia M, Bubacco L. Parkinson's disease and immune system: is the culprit LRRKing in the periphery? J Neuroinflammation 2012; 9:94; PMID:22594666; http://dx.doi.org/ 10.1186/1742-2094-9-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Hess A, Desiderio C, McAuliffe WG. Acute neuropathological changes in the caudate nucleus caused by MPTP and methamphetamine: immunohistochemical studies. J Neurocytol 1990; 19:338-42; PMID:1975269; http://dx.doi.org/ 10.1007/BF01188403 [DOI] [PubMed] [Google Scholar]
- 122. JP OC, DB M. Neurotoxic effects of substituted amphetamines in rats and mice. In: Massaro EJ, ed. Handbook of Neurotoxicology. Totowa: Humana Press Inc, 2002; 269-301. [Google Scholar]
- 123. O’Callaghan JP, Sriram K, Miller DB. Defining “neuroinflammation”. Ann New York Acad Sci 2008; 1139:318-30; PMID:18991877; http://dx.doi.org/ 10.1196/annals.1432.032 [DOI] [PubMed] [Google Scholar]
- 124. Sriram K, Miller DB, O’Callaghan JP. Minocycline attenuates microglial activation but fails to mitigate striatal dopaminergic neurotoxicity: role of tumor necrosis factor-alpha. J Neurochem 2006; 96:706-18; PMID:16405514; http://dx.doi.org/ 10.1111/j.1471-4159.2005.03566.x [DOI] [PubMed] [Google Scholar]
- 125. Thomas DM, Kuhn DM. MK-801 and dextromethorphan block microglial activation and protect against methamphetamine-induced neurotoxicity. Brain Res 2005; 1050:190-8; PMID:15987631; http://dx.doi.org/ 10.1016/j.brainres.2005.05.049 [DOI] [PubMed] [Google Scholar]
- 126. Thomas DM, Walker PD, Benjamins JA, Geddes TJ, Kuhn DM. Methamphetamine neurotoxicity in dopamine nerve endings of the striatum is associated with microglial activation. J Pharmacol Exp Ther 2004; 311:1-7; PMID:15163680; http://dx.doi.org/ 10.1124/jpet.104.070961 [DOI] [PubMed] [Google Scholar]
- 127. Mahajan SD, Hu Z, Reynolds JL, Aalinkeel R, Schwartz SA, Nair MP. Methamphetamine modulates gene expression patterns in monocyte derived mature dendritic cells: implications for HIV-1 pathogenesis. Mol Diagn Ther 2006; 10:257-69; PMID:16884330; http://dx.doi.org/ 10.1007/BF03256465 [DOI] [PubMed] [Google Scholar]
- 128. Mahajan SD, Aalinkeel R, Sykes DE, Reynolds JL, Bindukumar B, Fernandez SF, Chawda R, Shanahan TC, Schwartz SA. Tight junction regulation by morphine and HIV-1 tat modulates blood-brain barrier permeability. J Clin Immunol 2008; 28:528-41; PMID:18574677; http://dx.doi.org/ 10.1007/s10875-008-9208-1 [DOI] [PubMed] [Google Scholar]
- 129. Mahajan SD, Aalinkeel R, Sykes DE, Reynolds JL, Bindukumar B, Adal A, Qi M, Toh J, Xu G, Prasad PN, et al. . Methamphetamine alters blood brain barrier permeability via the modulation of tight junction expression: implication for HIV-1 neuropathogenesis in the context of drug abuse. Brain Res 2008; 1203:133-48; PMID:18329007; http://dx.doi.org/ 10.1016/j.brainres.2008.01.093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. O’Callaghan JP, Kelly KA, VanGilder RL, Sofroniew MV, Miller DB. Early activation of STAT3 regulates reactive astrogliosis induced by diverse forms of neurotoxicity. PloS One 2014; 9:e102003; PMID:Can't; http://dx.doi.org/ 10.1371/journal.pone.0102003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Peerzada H, Gandhi JA, Guimaraes AJ, Nosanchuk JD, Martinez LR. Methamphetamine administration modifies leukocyte proliferation and cytokine production in murine tissues. Immunobiology 2013; 218:1063-8; PMID:23518444; http://dx.doi.org/ 10.1016/j.imbio.2013.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Hirsiger S, Simmen HP, Werner CM, Wanner GA, Rittirsch D. Danger signals activating the immune response after trauma. Mediat Inflamm 2012; 2012:315941; PMID:22778496; http://dx.doi.org/ 10.1155/2012/315941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Tang D, Kang R, Coyne CB, Zeh HJ, Lotze MT. PAMPs and DAMPs: signal 0s that spur autophagy and immunity. Immunol Rev 2012; 249:158-75; PMID:22889221; http://dx.doi.org/ 10.1111/j.1600-065X.2012.01146.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Kang R, Lotze MT, Zeh HJ, Billiar TR, Tang D. Cell death and DAMPs in acute pancreatitis. Mol Med 2014; PMID:25105302; http://dx.doi.org/ 10.2119/molmed.2014.00117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Lai YT, Tsai YP, Cherng CG, Ke JJ, Ho MC, Tsai CW, Yu L. Lipopolysaccharide mitagates methamphetamine-induced striatal dopamine depletion via modulating local TNF-alpha and dopamine transporter expression. J Neural Trans 2009; 116:405-15; PMID:19271121; http://dx.doi.org/ 10.1007/s00702-009-0204-2 [DOI] [PubMed] [Google Scholar]
- 136. Bramness JG, Gundersen OH, Guterstam J, Rognli EB, Konstenius M, Loberg EM, Medhus S, Tanum L, Franck J. Amphetamine-induced psychosis–a separate diagnostic entity or primary psychosis triggered in the vulnerable? BMC Psychiat 2012; 12:221; PMID:23216941; http://dx.doi.org/ 10.1186/1471-244X-12-221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Cortese S, Holtmann M, Banaschewski T, Buitelaar J, Coghill D, Danckaerts M, Dittmann RW, Graham J, Taylor E, Sergeant J, et al. . Practitioner review: current best practice in the management of adverse events during treatment with ADHD medications in children and adolescents. J Child Psychol Psychiat Allied Disciplines 2013; 54:227-46; PMID:23294014; http://dx.doi.org/ 10.1111/jcpp.12036 [DOI] [PubMed] [Google Scholar]
- 138. Degenhardt L, Hall W. Extent of illicit drug use and dependence, and their contribution to the global burden of disease. Lancet 2012; 379:55-70; PMID:22225671; http://dx.doi.org/ 10.1016/S0140-6736(11)61138-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


