Abstract
Fatal hyperthermia after administration of various amphetamines is well-known clinical phenomenon, however, there is no consistent theory explaining its etiology and/or pathogenesis. Dose-dependence of temperature responses to methamphetamine is intricate. Recently, using mathematical modeling it was suggested that delicate interplay of excitatory and inhibitory mechanisms underlies this complexity.
Keywords: body temperature, methamphetamine, modeling, neural control
Temperature dynamics evoked by amphetamines has multiple phases, can include both hypothermic and hyperthermic components, and depend on ambient temperature, dose, and previous exposure to the drug. Amphetamines engage multiple neuromediator systems. Body temperature by itself is controlled by multiple mechanisms and complex neuronal circuitry. To simplify data interpretation most of the research focuses on simplified experimental settings, and, as a result, often has little real-life prognostic potential. It has become clear that the complexity of phenomena under consideration requires a unified theoretical framework accounting for an extensive set of known experimental facts. As a first step in this direction, we developed a mathematical model that explains non-trivial dose-dependence of temperature responses to methamphetamine (Meth).1
At room temperature (24°C) Meth evokes 1–2°C temperature increase on a time scale of several hours depending on the dose administered. Low doses of Meth (∼1 mg/kg) cause a rapid increase in body temperature up to approximately 38°C, which lasts about 2 hours (Fig 1A). As the dose increases, the temperature curve transforms in a non-trivial manner. After doses between 1 and 5 mg/kg the temperature peaks at similar levels but the peak time progressively shifts. After 1 mg/kg the temperature peaks at 60–100 min while after 5 mg/kg the peak occurs at ∼180 minutes. However, after higher doses of Meth (10 mg/kg and above) temperature rapidly rises immediately after injection with a maximum between 60 and 90 min at about 39°C. The peak is followed by a plateau lasting up to 5 h after injection.
Figure 1.
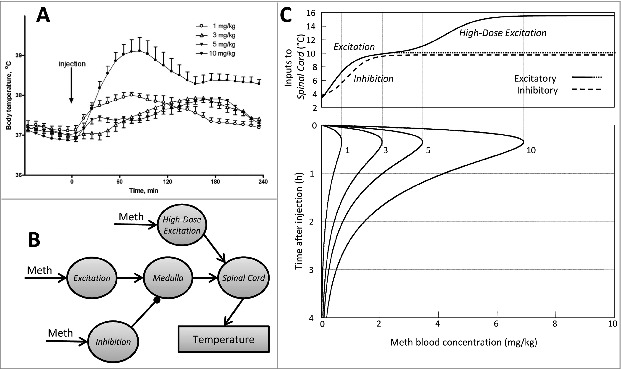
(A). Dose-dependence of temperature responses to Meth. Injections were performed i.p. at t = 0 min in a volume of 1 ml/kg. (B). Model schematic. Each circle represents a neural population. Meth-sensitive populations (marked by arrow with label “Meth”) are modeled as a formal neuron with sigmoid activation function applied to its input. The circuitry compiles the literature data: Excitation represents dorsomedial hypothalamus and other supramedullary excitatory inputs, Inhibition is an aggregate inhibitory node, High-Dose Excitation is a high-dose activated excitatory node, Medulla stands for raphe pallidus, and Spinal Cord represents sympathetic preganglionic neurons which provide thermogenic output. (C). Upper panel: the excitatory (solid line) and inhibitory (dashed line) components of the thermogenic activity as functions of blood concentration of Meth. Lower panel: time courses of Meth blood concentration for 4 doses of Meth: 1, 3, 5 and 10 mg/kg. Vertical lines show where the maximal Meth blood concentrations for each dose fall on the graph above. Adapted from Molkov et al. 20141 with permission.
The neuronal circuitry underlying those responses is not entirely unknown. Dorsomedial hypothalamus was identified as a key effector region whose activation plays an important role in generating fever and stress responses.2 Responses to low dose of Meth strongly resemble those induced by the stimulation of neurons in dorsomedial hypothalamus in conscious rats. This includes tachycardia, mild hypertension, hyperthermia, and activation of locomotion.3 In turn, inhibition of dorsomedial hypothalamus with microinjections of muscimol significantly attenuates responses to another amphetamine derivative, ecstasy.4 Peripheral responses mediated by dorsomedial hypothalamus, including thermoregulatory ones, are predominantly transmitted through the raphe pallidus in brainstem.2
The fact that the early phase response to intermediate dose of Meth is weaker than the one to the low dose suggests a presence of the inhibitory component. Blocking synaptic inhibition in dorsomedial hypothalamus and raphe pallidus evokes thermogenic responses.3 Therefore, both areas have inhibitory inputs.5 Meth-induced inhibition may also be mediated by activation of inhibitory subtypes of dopaminergic, adrenergic, or serotonergic receptors.6
Formalizing the model (Fig 1B) we refer to dorsomedial hypothalamus (and other supramedullary excitatory inputs) as the aggregate excitatory node (Excitation). Its projection to the medullary node (Medulla) competes for activation of the latter with the aggregate inhibitory drive (Inhibition). The high-dose node is the second excitatory component which bypasses Inhibition. It enters the thermogenic pathway at the inframedullary sympathetic preganglionic node (Spinal Cord) whose activity defines thermogenesis.
The Spinal Cord activity is defined by the balance between excitatory and inhibitory components for various Meth blood concentrations. Figure 1C visualizes how dose-dependence of temperature responses to Meth is formed. The lowest dose (1 mg/kg) activates neither Inhibition nor High-Dose Excitation. Thermal output at this dose depends on a single multisynaptic pathway from Excitation through Medulla to Spinal Cord. Activation of Excitation follows the blood concentration of Meth, thus driving up the body temperature. Increasing the dose to 3 mg/kg is insufficient to activate High-Dose Excitation, but it exceeds the threshold of activation of Inhibition. Output of Medulla is defined by the difference of excitatory and inhibitory drives (Fig 1C, see dotted line and dashed curve), which becomes very small to the right from 3 mg/kg vertical line.
Previous experimental studies have shown that inhibition of the medullary relay does not allow hypothalamic excitation to pass through.7 Similarly in our model the inhibitory drive successfully competes with the supramedullary excitatory drive, so at 3 mg/kg there is no immediate increase of body temperature. Although the absence of initial response may look like a delay, it is, in fact, a result of competition between excitatory and inhibitory components which slightly differ in sensitivity to Meth. As time progresses the blood level of Meth eventually drops below the threshold which allows keeping Inhibition active, yet remains sufficient to activate Excitation.
The highest dose activates High-Dose Excitation to full extent. In fact, this node provides remarkably stronger activation of Spinal Cord in comparison with supramedullary component. Interestingly, Excitation alone provides input of similar magnitude (Fig 1C), but its effect on Spinal Cord is significantly attenuated by the inhibitory counterpart. Accordingly the rate of the temperature rise in the beginning of the response to the highest dose is greater than for lower doses.
In conclusion, our study identifies the essential mechanism of Meth-evoked temperature fluctuations as the balance of excitatory and inhibitory drives, both of which are activated by Meth. This study has several important implications. First, any significant imbalance of excitatory and inhibitory components may cause life-threatening hyperthermia in response to even moderate doses of Meth. Second, we can consider reconstruction of activity of the circuitry nodes provided by our model as a prediction of activation patterns of their anatomical prototypes. Direct experimental verification of this prediction would proof our ability to estimate the activity of functional neuronal populations in intact freely moving animals using body temperature as easily accessible physiological end-point. Third, using the model we can analyze changes in the circuitry evoked by various interventions, such as repeated administration of the drug, application of pharmacological agents etc. Because of complexity of the system, changes in physiological responses do not allow for unequivocal conclusions about mechanisms involved. In contrast, statistical inference of changes in model parameters can be instrumental. For example, the fact that after administration of an antagonist of some receptors the model predicts a significant reduction of a particular projection in the network suggests that this projection is mediated by a corresponding neuromediator. Similarly, progressive changes in relevant model parameters in response to repeated consumption of the drug can shed light on such addiction related mechanisms as sensitization and tolerance.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Molkov YI, Zaretskaia MV, Zaretsky DV. Meth math: modeling temperature responses to methamphetamine. Am J Physiol Regul Integr Comp Physiol 2014; 306:R552-66; PMID:24500434; http://dx.doi.org/ 10.1152/ajpregu.00365.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dimicco JA, Zaretsky DV. The dorsomedial hypothalamus: a new player in thermoregulation. Am J Physiol Regul Integr Comp Physiol 2007; 292:R47-63; PMID:16959861; http://dx.doi.org/ 10.1152/ajpregu.00498.2006 [DOI] [PubMed] [Google Scholar]
- 3. Zaretskaia MV, Zaretsky DV, Shekhar A, DiMicco JA. Chemical stimulation of the dorsomedial hypothalamus evokes non-shivering thermogenesis in anesthetized rats. Brain Res 2002; 928:113-25; PMID:11844478; http://dx.doi.org/ 10.1016/S0006-8993(01)03369-8 [DOI] [PubMed] [Google Scholar]
- 4. Rusyniak DE, Zaretskaia MV, Zaretsky DV, DiMicco JA. Microinjection of muscimol into the dorsomedial hypothalamus suppresses MDMA-evoked sympathetic and behavioral responses. Brain Res 2008; 1226:116-23; PMID:18586013; http://dx.doi.org/ 10.1016/j.brainres.2008.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hunt JL, Zaretsky DV, Sarkar S, Dimicco JA. Dorsomedial hypothalamus mediates autonomic, neuroendocrine, and locomotor responses evoked from the medial preoptic area. Am J Physiol Regul Integr Comp Physiol 2010; 298:R130-40; PMID:19923355; http://dx.doi.org/ 10.1152/ajpregu.00574.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rusyniak DE, Zaretskaia MV, Zaretsky DV, DiMicco JA. 3,4-Methylenedioxymethamphetamine- and 8-hydroxy-2-di-n-propylamino-tetralin-induced hypothermia: role and location of 5-hydroxytryptamine 1A receptors. J Pharmacol Exp Ther 2007; 323:477-87; PMID:17702902; http://dx.doi.org/ 10.1124/jpet.107.126169 [DOI] [PubMed] [Google Scholar]
- 7. Samuels BC, Zaretsky DV, DiMicco JA. Tachycardia evoked by disinhibition of the dorsomedial hypothalamus in rats is mediated through medullary raphe. J Physiol 2002; 538:941-6; PMID:11826177; http://dx.doi.org/ 10.1113/jphysiol.2001.013302 [DOI] [PMC free article] [PubMed] [Google Scholar]


