Abstract
Thermoregulation is an essential homeostatic process in which critical mechanisms of heat production and dissipation are controlled centrally in large part by the hypothalamus and peripherally by activation of the sympathetic nervous system. Drugs that disrupt the components of this highly orchestrated multi-organ process can lead to life-threatening hyperthermia. In most cases, hyperthermic agents raise body temperature by increasing the central and peripheral release of thermoregulatory neurotransmitters that ultimately lead to heat production in thermogenic effector organs skeletal muscle (SKM) and brown adipose tissue (BAT). In many cases hyperthermic drugs also decrease heat dissipation through peripheral changes in blood flow. Drug-induced heat production is driven by the stimulation of mechanisms that normally regulate the adaptive thermogenic responses including both shivering and non-shivering thermogenesis (NST) mechanisms. Modulation of the mitochondrial electrochemical proton/pH gradient by uncoupling protein 1 (UCP1) in BAT is the most well characterized mechanism of NST in response to cold, and may contribute to thermogenesis induced by sympathomimetic agents, but this is far from established. However, the UCP1 homologue, UCP3, and the ryanodine receptor (RYR1) are established mediators of toxicant-induced hyperthermia in SKM. Defining the molecular mechanisms that orchestrate drug-induced hyperthermia will be essential in developing treatment modalities for thermogenic illnesses. This review will briefly summarize mechanisms of thermoregulation and provide a survey of pharmacologic agents that can lead to hyperthermia. We will also provide an overview of the established and candidate molecular mechanisms that regulate the actual thermogenic processes in heat effector organs BAT and SKM.
Keywords: non-shivering thermogenesis; uncoupling proteins; hyperthermia; 3,4-methylenedioxyamphetamine (MDMA; Ecstasy); UCP3
Abbreviations
- a1AR
a1-adrenoceptor
- b3AR
b3-adrenoceptor
- BAT
brown adipose tissue
- Ca2C
calcium ion
- FADH2
flavin adenine dinucleotide
- MAOIs
monoamine oxidase inhibitors
- LSD
lysergic acid diethylamide
- MDMA
ecstasy, methylenedioxyamphetamine
- NADH
nicotinamide adenine dinucleotide
- NST
non-shivering thermogenesis
- NE
norepinephrine
- RYR1
ryanodine receptor
- SSRIs
selective serotonin reuptake inhibitors
- SERCA
sarcoplasmic/endoplasmic reticulum-ATPase pump
- SKM
skeletal muscle
- SNS
sympathetic nervous system
- UCP1
uncoupling protein 1
- UCP3
uncoupling protein 3
- TCA
tricyclic antidpressant
Introduction to Toxicant-Induced Hyperthermia
The 2 broad categories of medical conditions that result in increased body temperature are hyperthermia and fever. Hyperthermia refers to a catastrophic rise in body temperature resulting from an imbalance between the accumulation of body heat (either from environmental temperature extremes and/or body heat generation) and the capacity to dissipate heat. Hyperthermia typically involves a failure of central hypothalamic autoregulatory body temperature control. In contrast, fever is a regulated process and presents as an adaptive, physiologic response to infection involving a rise in the hypothalamic body temperature “set point.” Although fever can be treated with non-steroidal anti-inflammatory agents (e.g. aspirin, acetaminophen), these drugs are ineffective in the therapeutic use for hyperthermia.1
A variety of pharmacologic and toxicologic agents can trigger hyperthermia, and in most cases, they do so in large part by altering hypothalamic and sympathetic nervous system (SNS) regulation of body temperature. As depicted in Table 1, the most well characterized drug-induced hyperthermic syndromes include neuroleptic malignant syndrome, serotonin syndrome, sympathomimetic syndrome, and malignant hyperthermia. Hyperthermia typically coincides with devastating medical complications, including hyperkalemia, renal failure, metabolic acidosis, liver failure, intravascular coagulation, rhabdomyolysis and death.2,3 Thus, it is essential to understand the molecular changes that are involved in order to effectively treat hyperthermic crises.4 In most cases, the precise mechanisms of drug-induced heat generation are either unknown or only beginning to be understood. Treatment options that target thermogenic responses are currently limited to only small fraction of drug-induced hyperthermic illnesses, and our existing approaches for the vast majority of cases focus mainly on providing supportive care, which involves, external cooling, anxiolytic drugs, and rehydration.2,3,5
Table 2.
Mechanisms of Obligatory and Facultative Thermogenesis
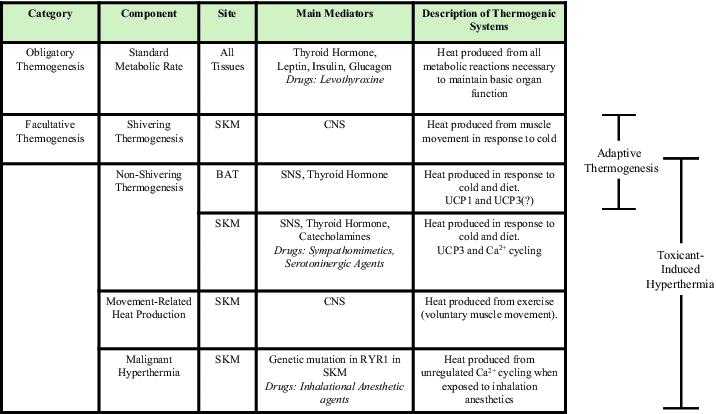 |
Table 1.
Overview of drug-induced hyperthermic conditions
| Drug-Induced Hyperthermia | Causative Agents | Implicated Molecular Mechanisms |
|---|---|---|
| Sympathomimetic Syndrome | Sympathomimetics: Amphetamine, Methamphetamine, Cocaine Antidepressants: MAOIs | Uncoupling proteins in BAT and SKM |
| Serotonin Syndrome | Antidepressants: ↑ Serotonin synthesis: L-Tryptophan ↓ Serotonin reuptake: SSRIs, TCAs ↓ Serotonin breakdown: MAOIs Sympathomimetics: ↑ Serotonin release: MDMA, Methamphetamine, Cocaine Serotonin agonists: Lithium, LSD Sumatriptan, Buspirone |
Uncoupling proteins in BAT and SKM |
| Malignant Hyperthermia | Inhalational anesthetics: Halothane, Sevoflurane, Desflurane Depolarizing neuromuscular agents: Succinylcholine, Decamethonium, Gallamine Triethiodide |
Mutations in ryanodine receptor (RYR1) in SKM |
| Neuroleptic Malignant Syndrome | Atypical antipsychotics: Olanzapine, Risperidone, Clozapine Dopamine antagonists: Haloperidol, Metoclopramide, Prochloperazine, Promethazine |
Unknown |
Abbreviations: Monoamine oxidase inhibitors (MAOIs), Lysergic acid diethylamide (LSD), Methylenedioxyamphetamine (MDMA, Ecstasy), Selective serotonin reuptake inhibitors (SSRIs), Tricyclic antidpressant (TCAs).
Mechanisms of Thermoregulation: The Thermogenic Effector Systems in BAT and SKM
At thermoneutrality, the temperature at which animals need not make “extra” body heat to conserve normal body temperature (∼37°C), basal temperature is maintained by the combined inefficiency of all exergonic cellular reactions.6 This is commonly referred to as obligatory thermogenesis. By contrast, in response to chronic cold exposure, feeding, and infection, endotherms can also rapidly increase thermogenesis to defend core body temperature or raise it through physiological heat-generating processes collectively referred to as facultative thermogenesis (see Table 2). The hypothalamus is the predominant, master controller of obligatory and facultative thermogenesis and also coordinates cooling mechanisms that dissipate heat, including sweating (in humans) and cutaneous vasodilation.7-9 A considerable body of work has defined many of the efferent and afferent neuroanatomical networks that signal within, to and from the hypothalamus, spinal cord, and periphery to control thermogenesis and heat dissipation. Although, the central and peripheral regulators of the neurochemical mechanisms that coordinate body temperature and thermogenesis are not the main focus of this manuscript, these pathways have been well-reviewed elsewhere.10,11
When considering the direct thermogenic effector mechanisms of body heat production, only a dramatic increase in cellular “work” (e.g., muscle contraction) or other exergonic biochemical reactions in organs of sufficient metabolic capacity (e.g., BAT, SKM) can increase body temperature. Rapid muscle contraction / shivering is a highly thermogenic mode of SKM facultative thermogenesis that mediates an early and temporary component of the adaptive response to cold and infection. However, shivering is energetically costly and impractical to sustain for extended periods of time. Therefore, endotherms have evolved alternative mechanisms of heat generation that are recruited to withstand prolonged periods of cold exposure without shivering, i.e. non-shivering thermogenesis (NST).12
The 2 predominant thermogenic organs are BAT and SKM. SNS stimulation of BAT mitochondrial uncoupling protein 1 (UCP1) is the prototypical mechanism of NST. The role of UCP1 (originally identified as thermogenin) in heat production was initially characterized in the 1980s.13 UCP1 is part of a highly conserved family of mitochondrial solute carriers that have the ability to dramatically increase mitochondrial respiration and uncouple oxidative phosphorylation from ATP production by dissipating the proton gradient.14 By allowing protons to leak across the mitochondrial inner membrane and circumvent the F1/F0-ATPase complex of the electron transport chain, UCP1 releases the energy stored in the electrochemical gradient in the form of heat. Mitochondrial proton leak sets up what is commonly referred to as a biochemical futile cycle where 2 metabolic pathways (proton extrusion and proton leak) run simultaneously in opposite directions. The thermogenic futile cycle induced by mitochondrial proton leak is simulated by the metabolic toxicant dinitrophenol, a weak acid that works by localizing to the mitochondrial inner membrane and inducing dose-dependent proton leak. Persons exposed to the drug, either unintentionally in munitions factories or during its brief stint as an anti-obesity medicine routinely developed hyperthermia and many died.15,16
Sarcoplasmic reticulum calcium extrusion and ATP dependent calcium uptake in SKM is another futile cycle that is implicated in adaptive NST as well as drug-induced hyperthermia (discussed below). Other examples of futile cycles that may be involved in thermoregulation include the simultaneous occurrence of protein synthesis and breakdown (especially in muscle), and leakage of the sodium-potassium ATPase pump.17 However, in general, UCP1-dependent mitochondrial proton leak is the most well characterized physiological mechanism of NST in mammals. The extent to which other futile cycles contribute to whole body thermogenesis is unclear.
In the canonical response to cold, the hypothalamus activates the SNS, triggering the release of norepinephrine (NE) from sympathetic neurons that densely innervate BAT tissue depots.18 NE binds to β3-adrenoreceptors (β3AR) on brown adipocytes, initiating a signaling cascade that leads to the generation of free fatty acids from cytoplasmic multilocular lipid droplets via the cyclic AMP-protein kinase A-hormone sensitive lipase signaling pathway.19,20 Free fatty acids are then transported into BAT mitochondria via the carnitine shuttle system, where they are used as substrates for β-oxidation, and activate UCP1-mediated thermogenesis.20,21 Studies in genetic mutant mouse models with defects in fatty acid transport and metabolism that exhibit cold-sensitivity phenotypes22 reinforce the physiological importance of fatty acids in thermogenesis. On a side note, NE can also interact with α1–adrenoceptors (α1AR) to stimulate vessel constriction and therefore block heat dissipation.23 Support for the classic pathway of β3AR –stimulated thermogenesis has been demonstrated in studies where lipolysis and BAT thermogenesis are blocked by administration of the PKA inhibitor, H-89,24 and by adrenergic blocking agents.25 Likewise, experiments in brown adipocytes show that treatment with fatty acids alone can simulate NE-induced thermogenesis, but only in the presence of UCP1.26 Recent elegant studies using mitochondrial patch clamping demonstrated that UCP1 requires long chain fatty acids to associate with and activate UCP1-driven thermogenic proton leak.27 Thus, it is the presence and activation of UCP1 by fatty acids in brown adipocytes that endows these cells the specialized ability to generate heat. Although it is unclear the extent to which UCP1 participates in toxicant- and drug-induced hyperthermia, unpublished work in our laboratory shows that UCP1-null mice harbor dramatically blunted thermogenic responses to methamphetamine. These findings are consistent with recent studies demonstrating that mice with surgically ablated or denervated BAT exhibited lower peak temperatures and shorter durations of MDMA-induced hyperthermia.28 Together, these findings validate the contribution of sympathetic regulation of BAT-UCP1 thermogenesis in drug-induced hyperthermia.
UCP1 is thought to be primarily, if not exclusively, expressed in BAT. Compared to rodents and newborns, only a fraction of adult humans have significant amounts of BAT.29,30 However, BAT tissue is not only activated by SNS stimulation, it can also increase in size in response to SNS activation.31-33 Nonetheless, it remains uncertain the extent to which BAT contributes to NST in adults.34 It is likely that alternative thermogenic systems in addition to BAT have evolved for the regulation of NST. This notion is supported by studies in animals that completely lack BAT (amphibians, reptiles, and birds) yet still exhibit SNS-dependent thermogenic responses.35-37 Furthermore, mice lacking UCP1 still exhibit some, albeit blunted NE-induced thermogenesis.38
Skeletal muscle (SKM) is an attractive site of NST because it is the largest and most highly metabolic organ in the body. However, the mechanisms involved in muscle NST are far less well characterized compared to BAT. Several studies have implied that thermogenic systems similar to the UCP1-regulated pathways may also exist in SKM.39-41 The idea that these SKM specific systems are regulated similarly to BAT thermogenesis through adrenergic stimulation is demonstrated in studies showing that NE administration in isolated muscle increases metabolism/thermogenesis.42 Interestingly, most if not all types of drug-induced hyperthermic conditions are associated with SKM damage.43 For example, malignant hyperthermia (MH) involves a well-defined pharmacogenetic mechanism of drug-induced hyperthermia that arises from muscle in individuals harboring mutations in the SKM ryanodine receptor calcium channel (RYR1). In these patients, in response to volatile anesthetic administration, a highly exergonic futile cycle is created by the excessive release of Ca2+ in to the cytoplasm, thus uncoupling the sarcoplasmic / endoplasmic reticulum-ATPase pump (SERCA) from ATP hydrolysis.44 Although it is not established whether RYR1 is a direct regulator of NST under normal, physiological circumstances, it is very likely that intra-myocyte calcium handling, in general, plays an important role in normal thermoregulation. This idea is corroborated by recent studies showing that mice lacking sarcolipin, a negative regulator of SERCA were unable to defend core body temperature in cold environments.41
The skeletal muscle-enriched UCP1 homolog, UCP3, has also been implicated in drug-induced hyperthermia45 and physiological NST responses to thyroid hormone.46,47 UCP3, shares more than 50% amino acid homology with UCP1 and has been shown to regulate proton flux in reconstituted systems in vitro.48,49 Compared to UCP1, UCP3 is more broadly expressed in heart, skeletal muscle, BAT,50 and as we recently demonstrated, skin.51 UCP3 knockout SKM mitochondria also show decreased proton leak (i.e., increased “coupling”) in some studies52,53 and transgenic mice that overexpress UCP3 exhibit enhanced SKM thermogenesis.54 Furthermore, UCP1-null mice exhibit skeletal muscle-specific, cold-induced adaptive thermogenesis that correlates with increased expression of UCP3.55 In addition, thyroid hormone, arguably the most important positive endocrine regulator of the majority of obligatory and facultative thermogenic processes, also modulates SKM metabolic efficiency and proton leak.46,56 Interestingly, thyrotoxicosis, a dangerous feature of hyperthyroid conditions, is often accompanied by hyperthermia and rhabdomyolysis, but is associated with decreased activity of BAT UCP1.56 Thus, the hyperthyroid hyperthermic crisis of thyrotoxicosis may preferentially involve SKM over BAT. The tenet that UCP1 homologues are mediators of thyroid thermogenesis is also supported with the finding that UCP3 is significantly increased at the protein level by thyroid hormone in rodents.57,58 Together, the data support the argument that thermogenic mechanisms in SKM, involving the sarcoplasmic reticulum RYR1 and mitochondrial UCP3, are strong candidate mediators of muscle thermogenesis.
It should be mentioned that controversy exists dealing with UCP3 as a physiological thermogenic regulator. Soon after its identification in 1997, UCP3-null mice were generated and shown to protect body temperature normally as compared to wild type mice when exposed to cold.57 As made evident in numerous subsequent reviews, this singular observation led the UCP field to conclude that UCP3 may not, unlike UCP1, mediate NST at least in response to cold exposure. However, the mere lack of a cold-phenotype does not rule out the involvement of UCP3 in thermogenesis by different mechanisms, or the possibility that a thermogenic compensatory process has fulfilled the heat requirements in the UCP3-null background. As mentioned above, thyroid hormone is a potent inducer of SKM UCP3 protein along with its upstream regulator β3AR.46,56 In a study using a severely cold intolerant, β1,2,3AR adrenergic receptor-deficient “β-less” mouse model, T3 administration upregulated skeletal muscle UCP3, and drove oxygen consumption in muscle in the β-less background, but not in the β-less UCP3-null background.59 Although UCP3 is not an established regulator of BAT NST, these results provide evidence that it is a key thermogenic effector in SKM, at least in response to certain types of drug- or hormonally-regulated stimuli.
Sympathomimetic-Induced Hyperthermia
Amphetamine-type drugs and their derivatives have long been associated with increased body heat production, hypermetabolism, catastrophic and lethal hyperthermia and skeletal muscle rhabdomyolysis.60 It is worth noting that amphetamine-type drugs and other sympathomimetics have different affinities for protein targets that affect the release and uptake of monoamines.61,62 For example, while cocaine and related analogs are selective, competitive inhibitors for dopamine monoamine transporters, MDMA acts as a competitive substrate for monoamine transporters and is relatively specific for serotonin.63 Therefore, these sympathomimetics have distinct neuropharmacological profiles that contribute to the central and peripheral effects of these agents, depending on their direct and indirect sympathomimetic actions. However, the final common pathway of SNS-hyperthermia through adrenergic mechanisms appears similar for sympathomimetics as a class.
3,4-methylenedioxymethamphetamine (MDMA, Ecstasy) is a prototypical sympathomimetic amine agent and a worldwide major drug of abuse in large part because of its euphoric and stimulant properties.64 MDMA increases body heat production via multiple mechanisms of thermoregulation. Unsurprisingly, even though catastrophic hyperthermia is a relatively rare event in MDMA use, one of the most commonly reported subjective effects of MDMA in humans is a feeling of “being hot”.65 The idea that MDMA disrupts central regulation of body temperature is supported by observations that environmental ambient temperature and physical activity can profoundly affect the variability of MDMA-hyperthermic responses.66,67 In rodent models, hyperthermia occurs when MDMA is administered at ambient temperatures of 24°C or greater,68 and the degree of hyperthermia positively correlates with increasing ambient temperature.69,70 In contrast, low environmental temperatures can block the hyperthermic effects of MDMA,71 and even result in hypothermic responses.68 These observations have important implications for MDMA users, where the drug is typically used in the setting of dance parties referred to as “raves” that increase the susceptibility to hyperthermia.
The challenge that arises from treating MDMA-induced hyperthermia stems from the complex and peripheral effects this agent can have on normal pathways of thermoregulation. MDMA administration is thought to cause a central deregulation of thermogenesis by altering the release of key monoamine neurotransmitters including serotonin, dopamine, and NE72-75 and by affecting reuptake and transport of these neurotransmitters. Neuronal activation in response to neurotransmitter release of specific hypothalamic pathways in the supraoptic and median preoptic nuclei has been demonstrated with c-fos expression staining.76 MDMA and amphetamine derivatives have been shown to increase NE release,77and also potentiate the effects of NE by blocking reuptake through the NE transporter.61,78-80 Furthermore, MDMA can act as substrate for the monoamine transporter that can be taken up into nerve terminals to cause the redistribution of cytoplasmic monoamine vesicles and lead to the reverse transport of neurotransmitters.63 These complex changes in neurotransmitter release and accumulation lead to alternations noreadrenergic singaling that contribute to the peripheral effects of MDMA by affecting cutaneous vasoconstriction of blood flow and stimulation of heat production in thermogenic effector organs SKM and BAT.62,81,82
UCP3 and Thyroid Hormone in MDMA-Induced Hyperthermia
Several lines of evidence support the hypothesis that SKM UCP3 is a major molecular mediator of sympathomimetic-induced hyperthermia. Foremost, the strongest evidence is that UCP3 knockout mice have severely blunted (80–100%) thermogenic responses to MDMA in vivo.45 Resistance to MDMA thermogenesis in UCP3 knockout mice also corresponds with complete protection from the lethal effects of the drug. Moreover, recent work showed that MDMA treatment in rodents led to a change in UCP3 protein phosphorylation and increased proton leak in the MDMA exposed animal SKM compared to controls.83 Consistent with the aforementioned study in β-less mice administered thyroid hormone, hyperthyroid and hypothyroid rats show dramatic increases or complete blunting, respectively, of MDMA hyperthermic responses, with body temperatures correlating in a near linear fashion with levels of UCP3 protein in isolated SKM mitochondria.84 Interestingly, a published case study showed that a woman that allegedly ingested 1–1.5 MDMA tablets (usually 150 mg MDMA) and died from hyperthermia had, on autopsy, subclinical hyperthyroidism.85 This suggests the likelihood that some of the idiosyncratic hyperthermic reactions to sympathomimetics could be thyroid hormone related.
The identification of UCP3 (and now also UCP1) as a thermogenic effector of sympathomimetic agents provides the opportunity to target these thermogenic proteins or their upstream mediators to mitigate in human cases of MDMA toxicity. After MDMA administration, the plasma levels of NE and sympathetic activity are elevated in human subjects.86 In rodents, NE increases by 35-fold in the bloodstream prior to the development of peak hyperthermia,25 as do levels of plasma free fatty acids.87 The absence of a concomitant rise in the NE metabolite dihydroxyphenyglycol following MDMA administration suggests that peripheral NE reuptake is also blocked.25 In response to increased plasma NE, triglyceride lipolysis leads to fatty acid release from white adipose tissue depots into the bloodstream. It is postulated that they serve as endocrine “thermokines” to activate UCP3 for SKM thermogenesis.87 Expression levels of α1- and β3-AR are relatively low in skeletal muscle.88-90 However, adrenergic actions may also be mediated directly in SKM, as made evident by observations from the Astrup lab that infusion of various adrenergic agonists and sympathomimetics can directly stimulate oxygen consumption in perfused muscle in rodents and humans.91,92 As depicted in Figure 1, the fatty acid activation model of SKM UCP3 contrasts with that of BAT, where the storage, lipolysis, liberation of fatty acid, and fatty acid activation of UCP1 each occurs within the brown adipocyte and its mitochondria.
Figure 1.
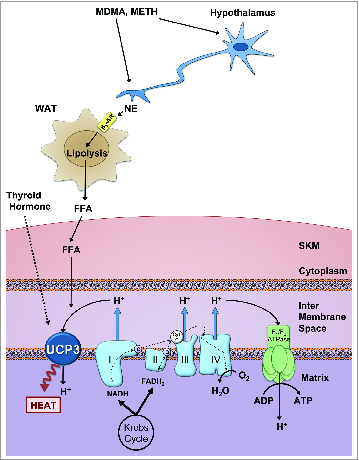
Hypothetical mechanism of MDMA-induced hyperthermia In response to treatment with amphetamine-like agents, hypothalamic neurons signal to the sympathetic nervous system causing the peripheral release of norepinephrine (NE). MDMA and other amphetamines also inhibit reuptake of NE at SNS synapses. NE binds to β3 adrenergic receptors on target tissues, notably white adipocytes, where it triggers lipolysis, resulting in an increase in circulating free fatty acids (FFA). FFA are taken up by skeletal muscle (SKM), where they are used as substrates for β-oxidation, and also directly activate uncoupling protein 3 (UCP3). UCP3 stimulates proton (H+) leak back into the mitochondrial matrix, circumventing ATP production and releasing the free energy from proton flux as heat. Thyroid hormone plays a contributory role in this model, through the regulation of UCP3 gene and protein expression in SKM. Abbreviations: Nicotinamide adenine dinucleotide (NADH), Flavin adenine dinucleotide (FADH2)
Owing to the central role of the SNS, NE, and β3AR, along with the α1AR induced vasoconstriction in sympathomimetic hyperthermia, treatment of rodents with experimental adrenergic blocking drugs prevents MDMA hyperthermia in rodents, and dramatically decreases SKM rhabdomyolysis.25 Similarly, in clinically relevant work, treatment of rats with the pan αAR / βAR antagonist carvedilol completely blocks MDMA hyperthermia, and importantly, fully and rapidly resolves peak hyperthermia back to pre-treatment baseline temperatures.25 Importantly, carvedilol is the only FDA approved drug that antagonizes β3AR. It is quite likely that this activity endows its treatment benefit in MDMA hyperthermia, because studies have shown in rodents that non-selective antagonists of β1–2AR fail to block MDMA hyperthermia in the least.25 Results from the carvedilol study using rodents were recently repeated in MDMA-exposed humans, where the MDMA-induced rise in body temperature was fully blocked, but not the euphoric effects.93 As a bonus, carvedilol is generally regarded as a safe and well tolerated cardiovascular medicine and may be easy to employ in those cases when other supportive cooling measures and benzodiazepines fail to cool the hyperthermic patient. However, it is worth noting that the carvedilol is only available as an oral drug and exhibits variability in bioavailability due to poor gut perfusion. Furthermore, due to ethical considerations in the aforementioned clinical studies, subjects were given relatively low doses of MDMA and experienced only moderate hyperthermic responses. Therefore, additional studies are crucial in characterizing the drug's clinical effectiveness in severe cases associated with MDMA toxicity.
Uncoupling Proteins and Molecular Mechanisms in Other Thermogenic Conditions
As mentioned above, many drug- and toxicant-induced hyperthermic conditions present with similar clinical symptoms when compared to sympathomimetic hyperthermia, and in most every case is thought to be associated with altered hypothalamic regulation of sympathetic outflow. As with MDMA, and as we have also shown with methamphetamine94 it is highly likely that activation of the uncoupling mechanism by UCP3 in SKM (and perhaps UCP1 & UCP3 in BAT) accounts for a significant portion of thermogenesis induced by most sympathomimetics. The exemplars in this class are amphetamines, cocaine, ephedrine, and a new series of drugs on the worldwide market referred to as “bath salts.”95 Serotonin syndrome is characterized by a variety of symptoms including cognitive, autonomic, neuromuscular dysfunction, and hyperthermia and can be induced by a variety of agents that have different actions involving serotonin.96-98 Along with sympathomimetics that increase serotonin release, other agents associated with serotonin syndrome that either act as agonists to serotonin or affect serotonin synthesis, reuptake, and breakdown respectively including LSD, L-tryptophan, tricyclic antidepressants, and MAOIs99 (Table 1). The tenet that the toxic thermogenic mechanisms associated with serotonin syndrome largely overlap with sympathomimetic syndrome is supported with experiments in animal models of serotonin syndrome showing enhanced levels of serotonin and norepinephrine in the anterior hypothalamus.100,101 It is likely that elevated serotonin levels increase central catecholamine release to alter hypothalamic regulation of the thyroid axis and peripheral release of NE.102,103 Unlike the hyperthermia observed with sympathomimetics, these central serotonin releasers are thought to have indirect effects on α1- and β3- adrenergic receptors, and that the potential uncoupling activity and heat generation in these conditions in BAT and SKM arises predominantly from NE release rather than a combined blockade of reuptake. Future work will be necessary to test the involvement of uncoupling proteins in response to non-sympathomimetic thermoregulation agents.
UCP Hyperthermia Versus RYR1 “Malignant Hyperthermia”
It has classically been assumed that MDMA hyperthermia must share a common mechanism with RYR1-mediated malignant hyperthermia, especially because the clinical and metabolic changes in both conditions are very similar. However, the literature indicates that the final heat-generating mechanisms of each syndrome are distinct, despite some potential upstream common regulatory features.104 As mentioned, MH is an autosomal dominant disorder caused by a mutation in RYR1, a major regulator of Ca2+ transport in SKM.105 It is triggered in susceptible patients by exposure to general anesthetic gases, and is clinically characterized by metabolic acidosis, hyperthermia, muscle rigidity, and rhabdomyolysis. The postulate that the mechanisms of MDMA induced hyperthermia are analogous to MH is reasonable based on observations that both conditions produce SKM damage, and alterations in NE and serotonin signaling.106–108 However, unlike sympathomimetic hyperthermia, where activation of the SNS is the primary culprit behind massive heat generation, whether or not SNS and serotonergic modulation is causal or merely correlative in MH is not clear. Similar to what has been observed with MDMA toxicity in rodent models, MH susceptible adrenalectomized pigs were also more resistant to MH toxicity,109 and treatment with thyroid hormone led to an earlier onset of MH in pigs,110 implicating activation of the hypothalamic-pituitary-adrenal and -thyroid axes in MH. Despite the similarities, a recent study done by Schutte et al.111 to investigate whether MDMA could trigger MH in susceptible pigs found that there were no metabolic differences between MH-susceptible pigs and MH-normal pigs following MDMA exposure. This study, along with the finding that MDMA increases intracellular Ca2+ through a mechanism independent of the ryanodine receptor pathway in SKM,112 strongly suggests that MDMA is not a classic trigger of MH and works through a distinct biochemical mechanism to produce heat. This is also supported by studies using the RYR1 inhibitor dantrolene, the established and effective MH antagonist.104 Because of the erroneous assumption that MDMA hyperthermia and MH arise from the same mechanism, dantrolene has been indicated for treatment of MDMA hyperthermia.113 However, clinicians should be cognizant of the fact that the molecular mechanisms underlying these 2 pathologies are significantly divergent and should consider the possibility that other strategies may provide greater therapeutic benefit compared to dantrolene (i.e. adrenergic blockade) in extreme cases of MDMA hyperthermia.
Concluding Remarks
As shown in studies with MDMA and methamphetamine, it is likely that the family of uncoupling proteins, chiefly UCP1 and UCP3 play the dominant roles in the generation of the hyperthermic and SKM toxic responses to the vast majority of drugs that increase SNS activity via hypothalamic, serotonergic and direct peripheral adrenergic mechanisms. If true, this would predict that the use of the drug carvedilol may not only be useful for MDMA poisoning, but also in response to overdose with the cadre of sympathomimetics, and perhaps the newest members of this class – the bath salts. Testing the involvement of UCPs in a variety of thermogenic responses to other drugs and toxins will be of obvious importance.
It should also be noted that very interesting recent work highlights the fact that it would be erroneous to assume all sympathetic activity is “the same” when considering thermogenesis and hyperthermia. Indeed, SNS activation by different stimuli can lead to diverse thermogenic responses. This was recently demonstrated in a clinical report showing that cold exposure activated human BAT, but systemic treatment with a thermogenic dose of the sympathomimetic drug ephedrine failed to do so.114 This highlights the fact that much is to be learned regarding the neuroanatomic distinctions that govern body temperature regulation to diverse SNS regulatory stimuli. Because so very few treatments exist, investigators will need to use strong mechanistic tools, tissue-targeted knockout mice, and neuro-tracing methodologies to provide a comparative assessment of the detailed mechanisms that distinguish the varied drug induced thermogenic syndromes. Recent clinical work identifying carvedilol as a potential sympathomimetic hyperthermia treatment is a step in the right direction.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Stitt JT. Fever versus hyperthermia. Fed Proc 1979; 38:39-43; PMID:759237 [PubMed] [Google Scholar]
- 2. Chan TC, Evans SD, Clark RF. Drug-induced hyperthermia. Crit Care Clin 1997; 13:785-808; PMID:9330841; http://dx.doi.org/ 10.1016/S0749-0704(05)70369-9 [DOI] [PubMed] [Google Scholar]
- 3. Musselman ME, Saely S. Diagnosis and treatment of drug-induced hyperthermia. Am J Health Syst Pharm 2013; 70:34-42; PMID:23261898; http://dx.doi.org/ 10.2146/ajhp110543 [DOI] [PubMed] [Google Scholar]
- 4. Olson KR, Benowitz NL. Environmental and drug-induced hyperthermia. Pathophysiology, recognition, and management. Emerg Med Clin North Am 1984; 2:459-74; PMID:6534737 [PubMed] [Google Scholar]
- 5. Rosenberg J, Pentel P, Pond S, Benowitz N, Olson K. Hyperthermia associated with drug intoxication. Crit Care Med 1986; 14:964-9; PMID:3769509 [DOI] [PubMed] [Google Scholar]
- 6. Silva JE. Thermogenic mechanisms and their hormonal regulation. Physiol Rev 2006; 86:435-64; PMID:16601266; http://dx.doi.org/ 10.1152/physrev.00009.2005 [DOI] [PubMed] [Google Scholar]
- 7. Charkoudian N. Skin blood flow in adult human thermoregulation: how it works, when it does not, and why. Mayo Clin Proc 2003; 78:603-12; PMID:12744548 [DOI] [PubMed] [Google Scholar]
- 8. Nielsen B. Heat acclimation-mechanisms of adaptation to exercise in the heat. Int J Sports Med 1998; 19 Suppl 2:S154-6; PMID:9694425 [DOI] [PubMed] [Google Scholar]
- 9. De Witte J. Perioperative shivering: Physiology and pharmacology. Anesthesiology 2002; 96:467-484. [DOI] [PubMed] [Google Scholar]
- 10. Morrison SF, Nakamura K. Central neural pathways for thermoregulation. Front Biosci (Landmark Ed) 2011; 16:74-104; PMID:21196160; http://dx.doi.org/ 10.2741/3677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rusyniak DE, Sprague JE. Toxin-induced hyperthermic syndromes. Med Clin North America 2005; 89:1277-96; PMID:16227063; http://dx.doi.org/ 10.1016/j.mcna.2005.06.002 [DOI] [PubMed] [Google Scholar]
- 12. Silva JE. Thermogenic mechanisms and their hormonal regulation. Physiol Rev 2006; 86:435-64; PMID:16601266; http://dx.doi.org/ 10.1152/physrev.00009.2005 [DOI] [PubMed] [Google Scholar]
- 13. Lin CS, Klingenberg M. Isolation of the uncoupling protein from brown adipose tissue mitochondria. FEBS Lett 1980; 113:299-303; PMID:7389900; http://dx.doi.org/ 10.1016/0014-5793(80)80613-2 [DOI] [PubMed] [Google Scholar]
- 14. Ricquier D, Lin C.-S, Klingenberg M. Isolation of the GDP binding protein from brown adipose tissue mitochondria of several animals and amino acid composition study in rat. Biochem Biophys Res Commun 106:582-9; PMID:6285923; http://dx.doi.org/ 10.1016/0006-291X(82)91150-0 [DOI] [PubMed] [Google Scholar]
- 15. Harper JA, Dickinson K, Brand MD. Mitochondrial uncoupling as a target for drug development for the treatment of obesity. Obes Rev 2001; 2:255-65; PMID:12119996; http://dx.doi.org/ 10.1046/j.1467-789X.2001.00043.x [DOI] [PubMed] [Google Scholar]
- 16. Colman E. Dinitrophenol and obesity: an early twentieth-century regulatory dilemma. Regul Toxicol Pharmacol 2007; 48:115-7; PMID:17475379; http://dx.doi.org/ 10.1016/j.yrtph.2007.03.006 [DOI] [PubMed] [Google Scholar]
- 17. She P, Reid TM, Bronson SK, Vary TC, Hajnal A, Lynch CJ, Hutson SM. Disruption of BCATm in mice leads to increased energy expenditure associated with the activation of a futile protein turnover cycle. Cell Metabolism 2007; 6:181-94; PMID:17767905; http://dx.doi.org/ 10.1016/j.cmet.2007.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Smith RE, Horwitz BA. Brown fat and thermogenesis. Physiol Rev 1969; 49:330-425; PMID:4888392 [DOI] [PubMed] [Google Scholar]
- 19. Nicholls DG, Locke RM. Thermogenic mechanisms in brown fat. Physiol Rev 1984; 64:1-64; PMID:6320232 [DOI] [PubMed] [Google Scholar]
- 20. Jezek P, Freisleben HJ. Fatty acid binding site of the mitochondrial uncoupling protein. Demonstration of its existence by EPR spectroscopy of 5-DOXYL-stearic acid. FEBS Lett 1994; 343:22-6; PMID:8163011; http://dx.doi.org/ 10.1016/0014-5793(94)80599-7 [DOI] [PubMed] [Google Scholar]
- 21. Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 2004; 84:277-359; PMID:14715917; http://dx.doi.org/ 10.1152/physrev.00015.2003 [DOI] [PubMed] [Google Scholar]
- 22. Guerra C, Koza RA, Walsh K, Kurtz DM, Wood PA, Kozak LP. Abnormal nonshivering thermogenesis in mice with inherited defects of fatty acid oxidation. J Clin Invest 1998; 102:1724-31; PMID:9802886; http://dx.doi.org/ 10.1172/JCI4532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhao J, Cannon B, Nedergaard J. alpha1-Adrenergic stimulation potentiates the thermogenic action of beta3-adrenoreceptor-generated cAMP in brown fat cells. J Biol Chem 1997; 272:32847-56; PMID:9407062; http://dx.doi.org/ 10.1074/jbc.272.52.32847 [DOI] [PubMed] [Google Scholar]
- 24. Fredriksson JM, Thonberg H, Ohlson KB, Ohba K, Cannon B, Nedergaard J. Analysis of inhibition by H89 of UCP1 gene expression and thermogenesis indicates protein kinase A mediation of beta(3)-adrenergic signalling rather than beta(3)-adrenoceptor antagonism by H89. Biochim Biophys Acta 2001; 1538:206-17; PMID:11336791 [DOI] [PubMed] [Google Scholar]
- 25. Sprague JE, Moze P, Caden D, Rusyniak DE, Holmes C, Goldstein DS, Mills EM. Carvedilol reverses hyperthermia and attenuates rhabdomyolysis induced by 3,4-methylenedioxymethamphetamine (MDMA, Ecstasy) in an animal model*. Crit Care Med 2005; 33:1311-6; PMID:15942349; http://dx.doi.org/ 10.1097/01.CCM.0000165969.29002.70 [DOI] [PubMed] [Google Scholar]
- 26. Nahrendorf M, Ohlson KB, Fredriksson JM, Jacobsson A, Nedergaard J, Cannon B. Thermogenic responses in brown fat cells are fully UCP1-dependent. UCP2 or UCP3 do not substitute for UCP1 in adrenergically or fatty scid-induced thermogenesis. J Biol Chem 2000; 275:25073-81; PMID:10825155; http://dx.doi.org/ 10.1074/jbc.M000547200 [DOI] [PubMed] [Google Scholar]
- 27. Fedorenko A, Lishko PV, Kirichok Y. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell 151:400-13; PMID:23063128; http://dx.doi.org/ 10.1016/j.cell.2012.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sanchez-Alavez M, Sanchez-Alavez M1, Conti B, Wood MR, Bortell N, Bustamante E, Saez E, Fox HS, Marcondes MC. ROS and sympathetically mediated mitochondria activation in brown adipose tissue contribute to methamphetamine-induced hyperthermia. Front Endocrinol (Lausanne) 2013; 4:44; PMID:23630518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 2009; 360:1509-17; PMID:19357406; http://dx.doi.org/ 10.1056/NEJMoa0810780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zingaretti MC, Crosta F, Vitali A, Guerrieri M, Frontini A, Cannon B, Nedergaard J, Cinti S. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J 2009; 23:3113-20; PMID:19417078; http://dx.doi.org/ 10.1096/fj.09-133546 [DOI] [PubMed] [Google Scholar]
- 31. Söderlund V, Larsson SA, Jacobsson H. Reduction of FDG uptake in brown adipose tissue in clinical patients by a single dose of propranolol. Eur J Nucl Med Mol Imaging 2007; 34:1018-22; PMID:17225118; http://dx.doi.org/ 10.1007/s00259-006-0318-9 [DOI] [PubMed] [Google Scholar]
- 32. Christensen CR, Clark PB, Morton KA. Reversal of hypermetabolic brown adipose tissue in F-18 FDG PET imaging. Clin Nucl Med 2006; 31:193-6; PMID:16550009; http://dx.doi.org/ 10.1097/01.rlu.0000204199.33136.05 [DOI] [PubMed] [Google Scholar]
- 33. Enerback S. Human brown adipose tissue. Cell Metab 2010; 11:248-52; PMID:20374955 [DOI] [PubMed] [Google Scholar]
- 34. Heaton JM. The distribution of brown adipose tissue in the human. J Anat 1972; 112:35-9; PMID:5086212 [PMC free article] [PubMed] [Google Scholar]
- 35. Hissa R, Pyörnilä A, Saarela S. Effect of peripheral noradrenaline on thermoregulation in temperature-acclimated pigeon. Comp Biochem Physiol C, Comp Pharmacol 1975; 51:243-7; PMID:241583; http://dx.doi.org/ 10.1016/0306-4492(75)90068-4 [DOI] [PubMed] [Google Scholar]
- 36. Harri M, Hedenstam R. Calorigenic effect of adrenaline and noradrenaline in the frog, Rana temporaria. Comp Biochem Physiol A Comp Physiol 1972; 41:409-19; PMID:4404317; http://dx.doi.org/ 10.1016/0300-9629(72)90071-0 [DOI] [PubMed] [Google Scholar]
- 37. Barre H, Rouanet JL. Calorigenic effect of glucagon and catecholamines in king penguin chicks. Am J Physiol 1983; 244:R758-63; PMID:6859289 [DOI] [PubMed] [Google Scholar]
- 38. Golozoubova V. UCP1 is essential for adaptive adrenergic nonshivering thermogenesis. Am J Physiol Endocrinol Metab 2006; 291:E350-7; PMID:16595854; http://dx.doi.org/ 10.1152/ajpendo.00387.2005 [DOI] [PubMed] [Google Scholar]
- 39. Silva JE. Physiological importance and control of non-shivering facultative thermogenesis. Front Biosci (Schol Ed) 2011; 3:352-71; PMID:21196381 [DOI] [PubMed] [Google Scholar]
- 40. Block B, Franzini-Armstrong C. The structure of the membrane systems in a novel muscle cell modified for heat production. J Cell Biol 1988; 107:1099-112; PMID:3417775; http://dx.doi.org/ 10.1083/jcb.107.3.1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bal NC, Maurya SK, Sopariwala DH, Sahoo SK, Gupta SC, Shaikh SA, Pant M, Rowland LA, Bombardier E, Goonasekera SA, et al. Sarcolipin is a newly identified regulator of muscle-based thermogenesis in mammals. Nat Med 2012; 18:1575-9; PMID:22961106; http://dx.doi.org/ 10.1038/nm.2897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Means of noradrenalin action during non-shivering thermogenesis in a single muscle. Int J Biometeorol 1971; 15:321-4; PMID:5146828 [DOI] [PubMed] [Google Scholar]
- 43. Rusyniak DE, Sprague JE. Hyperthermic syndromes induced by toxins. Clin Lab Med 2006; 26:165-84; PMID:16567230; http://dx.doi.org/ 10.1016/j.cll.2006.01.007 [DOI] [PubMed] [Google Scholar]
- 44. MacLennan DH, Duff C, Zorzato F, Fujii J, Phillips M, Korneluk RG, Frodis W, Britt BA, Worton RG. Ryanodine receptor gene is a candidate for predisposition to malignant hyperthermia. Nature 1990; 343:559-61; PMID:1967823; http://dx.doi.org/ 10.1038/343559a0 [DOI] [PubMed] [Google Scholar]
- 45. Mills EM, Banks ML, Sprague JE, Finkel T. Pharmacology: uncoupling the agony from ecstasy. Nature 2003; 426:403-4; PMID:14647371; http://dx.doi.org/ 10.1038/426403a [DOI] [PubMed] [Google Scholar]
- 46. Silva JE. Thyroid hormone control of thermogenesis and energy balance. Thyroid 1995; 5:481-92; PMID:8808101; http://dx.doi.org/ 10.1089/thy.1995.5.481 [DOI] [PubMed] [Google Scholar]
- 47. Lanni A, Moreno M, Lombardi A, Goglia F. Thyroid hormone and uncoupling proteins. FEBS Lett 2003; 543:5-10; PMID:12753895; http://dx.doi.org/ 10.1016/S0014-5793(03)00320-X [DOI] [PubMed] [Google Scholar]
- 48. Jaburek M, Varecha M, Gimeno RE, Dembski M, Jezek P, Zhang M, Burn P, Tartaglia LA, Garlid KD. Transport function and regulation of mitochondrial uncoupling proteins 2 and 3. J Biol Chem 1999; 274:26003-7; PMID:10473545; http://dx.doi.org/ 10.1074/jbc.274.37.26003 [DOI] [PubMed] [Google Scholar]
- 49. Boss O, Hagen T, Lowell BB. Uncoupling proteins 2 and 3: potential regulators of mitochondrial energy metabolism. Diabetes 2000; 49:143-56; PMID:10868929; http://dx.doi.org/ 10.2337/diabetes.49.2.143 [DOI] [PubMed] [Google Scholar]
- 50. Vidal-Puig A, Solanes G, Grujic D, Flier JS, Lowell BB. UCP3: an uncoupling protein homologue expressed preferentially and abundantly in skeletal muscle and brown adipose tissue. Biochem Biophys Res Commun 1997; 235:79-82; PMID:9196039; http://dx.doi.org/ 10.1006/bbrc.1997.6740 [DOI] [PubMed] [Google Scholar]
- 51. Lago C, Nowinski SM, Rundhaug JE, Pfeiffer ME, Kiguchi K, Hirasaka K, Yang X, Abramson EM, Bratton SB, Rho O, et al. Mitochondrial respiratory uncoupling promotes keratinocyte differentiation and blocks skin carcinogenesis 2012; 31:4725-31; PMID:22266853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gong DW, Monemdjou S, Gavrilova O, Leon LR, Marcus-Samuels B, Chou CJ, Everett C, Kozak LP, Li C, Deng C, et al. Lack of obesity and normal response to fasting and thyroid hormone in mice lacking uncoupling protein-3. J Biol Chem 2000; 275:16251-7; PMID:10748195; http://dx.doi.org/ 10.1074/jbc.M910177199 [DOI] [PubMed] [Google Scholar]
- 53. Vidal-Puig AJ, Grujic D, Zhang CY, Hagen T, Boss O, Ido Y, Szczepanik A, Wade J, Mootha V, Cortright R, et al. Energy metabolism in uncoupling protein 3 gene knockout mice. J Biol Chem 2000; 275:16258-66; PMID:10748196 [DOI] [PubMed] [Google Scholar]
- 54. Clapham JC, Arch JR, Chapman H, Haynes A, Lister C, Moore GB, Piercy V, Carter SA, Lehner I, Smith SA, et al. Mice overexpressing human uncoupling protein-3 in skeletal muscle are hyperphagic and lean. Nature 2000; 406:415-8; PMID:10935638; http://dx.doi.org/ 10.1038/35019082 [DOI] [PubMed] [Google Scholar]
- 55. Shabalina IG, Shabalina IG1, Hoeks J, Kramarova TV, Schrauwen P, Cannon B, Nedergaard J. Cold tolerance of UCP1-ablated mice: A skeletal muscle mitochondria switch toward lipid oxidation with marked UCP3 up-regulation not associated with increased basal, fatty acid- or ROS-induced uncoupling or enhanced GDP effects. BBA - Bioenergetics 2010; 1797:968-80 [DOI] [PubMed] [Google Scholar]
- 56. Reitman M, Gong DW. Thyroid hormone and other regulators of uncoupling proteins. International Journal of Obesity 1999; 23:S56-S59. [DOI] [PubMed] [Google Scholar]
- 57. Gong DW, He Y, Karas M, Reitman M. Uncoupling protein-3 is a mediator of thermogenesis regulated by thyroid hormone, beta3-adrenergic agonists, and leptin. J Biol Chem 1997; 272:24129-32; PMID:9305858; http://dx.doi.org/ 10.1074/jbc.272.39.24129 [DOI] [PubMed] [Google Scholar]
- 58. Larkin S, Mull E, Miao W, Pittner R, Albrandt K, Moore C, Young A, Denaro M, Beaumont K. Regulation of the third member of the uncoupling protein family, UCP3, by cold and thyroid hormone. Biochem Biophys Res Commun 1997; 240:222-7; PMID:9367914; http://dx.doi.org/ 10.1006/bbrc.1997.7636 [DOI] [PubMed] [Google Scholar]
- 59. Flandin P, Lehr L, Asensio C, Giacobino JP, Rohner-Jeanrenaud F, Muzzin P, Jimenez M. Uncoupling protein-3 as a molecular determinant of the action of 3,5,3’-triiodothyronine on energy metabolism. Endocr 2009; 36:246-54; PMID:19598006; http://dx.doi.org/ 10.1007/s12020-009-9217-8 [DOI] [PubMed] [Google Scholar]
- 60. Brown JM, Yamamoto BK. Effects of amphetamines on mitochondrial function: role of free radicals and oxidative stress. Pharmacol Ther 2003; 99:45-53; PMID:12804698; http://dx.doi.org/ 10.1016/S0163-7258(03)00052-4 [DOI] [PubMed] [Google Scholar]
- 61. Steele TD, Nichols DE, Yim GK. Stereochemical effects of 3,4-methylenedioxymethamphetamine (MDMA) and related amphetamine derivatives on inhibition of uptake of [3H]monoamines into synaptosomes from different regions of rat brain. Biochem Pharmacol 1987; 36:2297-303; PMID:2886126; http://dx.doi.org/ 10.1016/0006-2952(87)90594-6 [DOI] [PubMed] [Google Scholar]
- 62. Docherty JR, Green AR. The role of monoamines in the changes in body temperature induced by 3,4-methylenedioxymethamphetamine (MDMA, ecstasy) and its derivatives. Br J Pharmacol 2010; 160:1029-44; PMID:20590597; http://dx.doi.org/ 10.1111/j.1476-5381.2010.00722.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Torre GE, Gainetdinov RR, Caron MC. Plasma membrane monoamine transporters: structure, regulation and function. Nat Rev Neurosci 2003; 4:13-25; PMID:12511858; http://dx.doi.org/ 10.1038/nrn1008 [DOI] [PubMed] [Google Scholar]
- 64. Green AR, Mechan AO, Elliott JM, O’Shea E, Colado MI. The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’). Pharmacol Rev 2003; 55:463-508; PMID:12869661; http://dx.doi.org/ 10.1124/pr.55.3.3 [DOI] [PubMed] [Google Scholar]
- 65. Parrott AC, Lock J, Conner AC, Kissling C, Thome J. Dance clubbing on MDMA and during abstinence from EcstasyMDMA: prospective neuroendocrine and psychobiological changes. Neuropsychobiology 2008; 57:165-80; PMID:18654086; http://dx.doi.org/ 10.1159/000147470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Duarte JA, Leão A, Magalhães J, Ascensão A, Bastos ML, Amado FL, Vilarinho L, Quelhas D, Appell HJ, Carvalho F. Strenuous exercise aggravates MDMA-induced skeletal muscle damage in mice. Toxicology 2005; 206:349-58; PMID:15588925; http://dx.doi.org/ 10.1016/j.tox.2004.07.012 [DOI] [PubMed] [Google Scholar]
- 67. Hardinge MG, Peterson DI. The effects of exercise and limitation of movement on amphetamine toxicity. J Pharmacol Exp Ther 1963; 141:260-5; PMID:14060481 [PubMed] [Google Scholar]
- 68. Gordon CJ, Watkinson WP, O’Callaghan JP, Miller DB. Effects of 3,4-methylenedioxymethamphetamine on autonomic thermoregulatory responses of the rat. Pharmacol Biochem Behav 1991; 38:339-44; PMID:1676171 [DOI] [PubMed] [Google Scholar]
- 69. Malberg JE, Seiden LS. Small changes in ambient temperature cause large changes in 3,4-methylenedioxymethamphetamine (MDMA)-induced serotonin neurotoxicity and core body temperature in the rat. J Neurosci 1998; 18:5086-94; PMID:9634574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Miller DB, O’Callaghan JP. Elevated environmental temperature and methamphetamine neurotoxicity. Environ Res 2003; 92:48-53; PMID:12706754; http://dx.doi.org/ 10.1016/S0013-9351(02)00051-8 [DOI] [PubMed] [Google Scholar]
- 71. Ali SF, Newport GD, Holson RR, Slikker W, Bowyer JF. Low environmental temperatures or pharmacologic agents that produce hypothermia decrease methamphetamine neurotoxicity in mice. Brain Res 1994; 658:33-8; PMID:7530580; http://dx.doi.org/ 10.1016/S0006-8993(09)90007-5 [DOI] [PubMed] [Google Scholar]
- 72. Colado MI, O’Shea E, Green AR. Acute and long-term effects of MDMA on cerebral dopamine biochemistry and function. Psychopharmacology (Berl) 2004; 173:249-63; PMID:15083264 [DOI] [PubMed] [Google Scholar]
- 73. Rothwell NJ. CNS regulation of thermogenesis. Crit Rev Neurobiol 1994; 8:1-10; PMID:8124729 [PubMed] [Google Scholar]
- 74. Cox B, Lee TF. Further evidence for a physiological role for hypothalamic dopamine in thermoregulation in the rat. J Physiol 1980; 300:7-17; PMID:7381796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Mallick BN, Jha SK, Islam F. Presence of alpha-1 adrenoreceptors on thermosensitive neurons in the medial preoptico-anterior hypothalamic area in rats. Neuropharmacology 2002; 42:697-705; PMID:11985828; http://dx.doi.org/ 10.1016/S0028-3908(02)00016-3 [DOI] [PubMed] [Google Scholar]
- 76. Stephenson CP, Hunt GE, Topple AN, McGregor IS. The distribution of 3,4-methylenedioxymethamphetamine ‘Ecstasy’-induced c-fos expression in rat brain. Neuroscience 1999; 92:1011-23; PMID:10426541; http://dx.doi.org/ 10.1016/S0306-4522(99)00049-4 [DOI] [PubMed] [Google Scholar]
- 77. McDaid J, Docherty JR. Vascular actions of MDMA involve alpha1 and alpha2-adrenoceptors in the anaesthetized rat. Br J Pharmacol 2001; 133:429-37; PMID:11375260; http://dx.doi.org/ 10.1038/sj.bjp.0704094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Leonardi ET, Azmitia EC. MDMA (ecstasy) inhibition of MAO type A and type B: comparisons with fenfluramine and fluoxetine (Prozac). Neuropsychopharmacology 1994; 10:231-8; PMID:7945733; http://dx.doi.org/ 10.1038/npp.1994.26 [DOI] [PubMed] [Google Scholar]
- 79. Cleary L, Docherty JR. Actions of amphetamine derivatives and cathinone at the noradrenaline transporter. Eur J Pharmacol 2003; 476:31-4; PMID:12969746; http://dx.doi.org/ 10.1016/S0014-2999(03)02173-3 [DOI] [PubMed] [Google Scholar]
- 80. Fitzgerald JL, Reid JJ. Sympathomimetic actions of methylenedioxymethamphetamine in rat and rabbit isolated cardiovascular tissues. J Pharm Pharmacol 1994; 46:826-32; PMID:7699571; http://dx.doi.org/ 10.1111/j.2042-7158.1994.tb03738.x [DOI] [PubMed] [Google Scholar]
- 81. Blessing WW, Zilm A, Ootsuka Y. Clozapine reverses increased brown adipose tissue thermogenesis induced by 3,4-methylenedioxymethamphetamine and by cold exposure in conscious rats. Neuroscience 2006; 141:2067-73; PMID:16814930; http://dx.doi.org/ 10.1016/j.neuroscience.2006.05.050 [DOI] [PubMed] [Google Scholar]
- 82. Pedersen NP, Blessing WW. Cutaneous vasoconstriction contributes to hyperthermia induced by 3,4-methylenedioxymethamphetamine (ecstasy) in conscious rabbits. J Neurosci 2001; 21:8648-54; PMID:11606652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kellya OM, McNamarab YM, Manzkea LH, Meeganb MJ, Portera RK. The preservation of in vivo phosphorylated and activated uncoupling protein 3 (UCP3) in isolated skeletal muscle mitochondria following administration of 3,4-methylenedioxymethamphetamine (MDMA aka ecstasy) to ratsmice. Mitochondrion 2012; 12:110-9; PMID:21453795; http://dx.doi.org/ 10.1016/j.mito.2011.03.011 [DOI] [PubMed] [Google Scholar]
- 84. Sprague JE, Banks ML, Cook VJ, Mills EM. Hypothalamic-pituitary-thyroid axis and sympathetic nervous system involvement in hyperthermia induced by 3,4-methylenedioxymethamphetamine (Ecstasy). J Pharmacol Exp Ther 2003; 305:159-66; PMID:12649364; http://dx.doi.org/ 10.1124/jpet.102.044982 [DOI] [PubMed] [Google Scholar]
- 85. Martin TL, Chiasson DA, Kish SJ. Does hyperthyroidism increase risk of death due to the ingestion of ecstasy? J Forensic Sci 2007; 52:951-3; PMID:17524054; http://dx.doi.org/ 10.1111/j.1556-4029.2007.00463.x [DOI] [PubMed] [Google Scholar]
- 86. Stuerenburg HJ, Petersen K, Bäumer T, Rosenkranz M, Buhmann C, Thomasius R. Plasma concentrations of 5-HT, 5-HIAA, norepinephrine, epinephrine and dopamine in ecstasy users. Neuro Endocrinol Lett 2002; 23:259-61; PMID:12080289 [PubMed] [Google Scholar]
- 87. Mills EM, Weaver KL, Abramson E, Pfeiffer M, Sprague JE. Influence of dietary fats on Ecstasy-induced hyperthermia. Br J Pharmacol 2007; 151:1103-08; PMID:17533413; http://dx.doi.org/ 10.1038/sj.bjp.0707312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Martin WH, Tolley TK, Saffitz JE. Autoradiographic delineation of skeletal muscle alpha 1-adrenergic receptor distribution. Am J Physiol 1990; 259:H1402-8; PMID:1978576 [DOI] [PubMed] [Google Scholar]
- 89. Sillence MN, Moore NG, Pegg GG, Lindsay DB. Ligand binding properties of putative beta 3-adrenoceptors compared in brown adipose tissue and in skeletal muscle membranes. Br J Pharmacol 1993; 109:1157-63; PMID:8104645; http://dx.doi.org/ 10.1111/j.1476-5381.1993.tb13743.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Chamberlain PD, Jennings KH, Paul F, Cordell J, Berry A, Holmes SD, Park J, Chambers J, Sennitt MV, Stock MJ, et al. The tissue distribution of the human beta3-adrenoceptor studied using a monoclonal antibody: direct evidence of the beta3-adrenoceptor in human adipose tissue, atrium and skeletal muscle. Int J Obes Relat Metab Disord 1999; 23:1057-65; PMID:10557026; http://dx.doi.org/ 10.1038/sj.ijo.0801039 [DOI] [PubMed] [Google Scholar]
- 91. Astrup A, Breum L, Toubro S. Pharmacological and clinical studies of ephedrine and other thermogenic agonists. Obes Res 1995; 3 Suppl 4:537S-540S; PMID:8697055; http://dx.doi.org/ 10.1002/j.1550-8528.1995.tb00224.x [DOI] [PubMed] [Google Scholar]
- 92. Astrup A, Bülow J, Christensen NJ, Madsen J. Ephedrine-induced thermogenesis in man: no role for interscapular brown adipose tissue. Clin Sci 1984; 66:179-86; PMID:6692652 [DOI] [PubMed] [Google Scholar]
- 93. Hysek C, Schmid Y, Rickli A, Simmler LD, Donzelli M, Grouzmann E, Liechti ME. Carvedilol inhibits the cardiostimulant and thermogenic effects of MDMA in humans. Br J Pharmacol 2012; 166:2277-88; PMID:22404145; http://dx.doi.org/ 10.1111/j.1476-5381.2012.01936.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Sprague JE, Mallett NM, Rusyniak DE, Mills E. UCP3 and thyroid hormone involvement in methamphetamine-induced hyperthermia. Biochem Pharmacol 2004; 68:1339-43; PMID:15345323; http://dx.doi.org/ 10.1016/j.bcp.2004.03.049 [DOI] [PubMed] [Google Scholar]
- 95. Banks ML, Worst TJ, Rusyniak DE, Sprague JE. Synthetic cathinones (‘bath salts’). J Emerg Med 2014; 46:632-42; PMID:24565885; http://dx.doi.org/ 10.1016/j.jemermed.2013.11.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Iqbal MM, Basil MJ, Kaplan J, Iqbal T. Overview of serotonin syndrome. Ann Clin Psychiat 2012; 24:310-8; PMID:23145389 [PubMed] [Google Scholar]
- 97. Morley JE, Shafer RB, Elson MK, Slag MF, Raleigh MJ, Brammer GL, Yuwiler A, Hershman JM. Amphetamine-induced hyperthyroxinemia. Ann Intern Med 1980; 93:707-9; PMID:6782925; http://dx.doi.org/ 10.7326/0003-4819-93-5-707 [DOI] [PubMed] [Google Scholar]
- 98. Neuvonen PJ, Pohjola-Sintonen S, Tacke U, Vuori E. Five fatal cases of serotonin syndrome after moclobemide-citalopram or moclobemide-clomipramine overdoses. Lancet 1993; 342:1419; PMID:7901695; http://dx.doi.org/ 10.1016/0140-6736(93)92774-N [DOI] [PubMed] [Google Scholar]
- 99. Mason PJ, Morris V, Balcezak T. Serotonin syndrome. Presentation of 2 cases and review of the literature. Med Baltimore 2000; 79:201-9; PMID:10941349; http://dx.doi.org/ 10.1097/00005792-200007000-00001 [DOI] [PubMed] [Google Scholar]
- 100. Nisijima K, Yoshino T, Ishiguro T. Risperidone counteracts lethality in an animal model of the serotonin syndrome. Psychopharmacol (Berl.) 2000; 150:9-14; PMID:10867971; http://dx.doi.org/ 10.1007/s002130000397 [DOI] [PubMed] [Google Scholar]
- 101. Nisijima K, Shioda K, Yoshino T, Takano K, Kato S. Diazepam and chlormethiazole attenuate the development of hyperthermia in an animal model of the serotonin syndrome. Neurochem Int 2003; 43:155-64; PMID:12620284; http://dx.doi.org/ 10.1016/S0197-0186(02)00213-9 [DOI] [PubMed] [Google Scholar]
- 102. Swerdlow NR, Koob GF, Cador M, Lorang M, Hauger RL. Pituitary-adrenal axis responses to acute amphetamine in the rat. Pharmacol Biochem Behav 1993; 45:629-37; PMID:8392732; http://dx.doi.org/ 10.1016/0091-3057(93)90518-X [DOI] [PubMed] [Google Scholar]
- 103. Welch JE, Saphier D. Central and peripheral mechanisms in the stimulation of adrenocortical secretion by the 5-hydroxytryptamine2 agonist, (+-)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane. J Pharmacol Exp Ther 1994; 270:918-28; PMID:7932204 [PubMed] [Google Scholar]
- 104. Rusyniak DE, Banks ML, Mills EM, Sprague JE. Dantrolene use in 3,4-methylenedioxymethamphetamine (ecstasy)-mediated hyperthermia. Anesthesiology 2004; 101:263-author reply 264; PMID:15220816; http://dx.doi.org/ 10.1097/00000542-200407000-00053 [DOI] [PubMed] [Google Scholar]
- 105. Fujii J, Otsu K, Zorzato F, de Leon S, Khanna VK, Weiler JE, O'Brien PJ, MacLennan DH. Identification of a mutation in porcine ryanodine receptor associated with malignant hyperthermia. Science 1991; 253:448-51; PMID:1862346; http://dx.doi.org/ 10.1126/science.1862346 [DOI] [PubMed] [Google Scholar]
- 106. Fiege M, Wappler F, Weisshorn R, Gerbershagen MU, Menge M, Schulte Am Esch J. Induction of malignant hyperthermia in susceptible swine by 3,4-methylenedioxymethamphetamine (‘ecstasy’). Anesthesiology 2003; 99:1132-36; PMID:14576550; http://dx.doi.org/ 10.1097/00000542-200311000-00020 [DOI] [PubMed] [Google Scholar]
- 107. Häggendal J, Jönsson L, Carlsten J. The role of sympathetic activity in initiating malignant hyperthermia. Acta Anaesthesiologica Scandinavica 1990; 34:677-82; PMID:24843434; http://dx.doi.org/ 10.1111/j.1399-6576.1990.tb03172.x [DOI] [PubMed] [Google Scholar]
- 108. Williams CH, Dozier SE, Buzello W, Gehrke CW, Wong JK, Gerhardt KO. Plasma levels of norepinephrine and epinephrine during malignant hyperthermia in susceptible pigs. J Chromatogr 1985; 344:71-80; PMID:4086571 [DOI] [PubMed] [Google Scholar]
- 109. Lucke JN, Denny H, Hall GM, Lovell R, Lister D. Porcine malignant hyperthermia. VI: the effects of bilateral adrenalectomy and pretreatment with bretylium on the halothane-induced response. Br J Anaesth 1978; 50:241-6; PMID:637997; http://dx.doi.org/ 10.1093/bja/50.3.241 [DOI] [PubMed] [Google Scholar]
- 110. Shailesh Kumar MV, Carr RJ, Komanduri V, Reardon RF, Beebe DS, Iaizzo PA, Belani KG. Differential diagnosis of thyroid crisis and malignant hyperthermia in an anesthetized porcine model. Endocr Res 1999; 25:87-103; PMID:10098596 [DOI] [PubMed] [Google Scholar]
- 111. Schütte JK, Schäfer U, Becker S, Oldewurtel C, Starosse A, Singler P, Richard A, Wappler F, Gerbershagen MU. 3,4-Methylenedioxymethamphetamine induces a hyperthermic and hypermetabolic crisis in pigs with and without a genetic disposition for malignant hyperthermia. Eur J Anaesthesiol 2013; 30:29-37; PMID:23138574; http://dx.doi.org/ 10.1097/EJA.0b013e32835a1127 [DOI] [PubMed] [Google Scholar]
- 112. Klingler W, Heffron JJ, Jurkat-Rott K, O'sullivan G, Alt A, Schlesinger F, Bufler J, Lehmann-Horn F. 3,4-Methylenedioxymethamphetamine (ecstasy) activates skeletal muscle nicotinic acetylcholine receptors. J Pharmacol Exp Ther 2005; 314:1267-73; PMID:15947037; http://dx.doi.org/ 10.1124/jpet.105.086629 [DOI] [PubMed] [Google Scholar]
- 113. Grunau BE, Wiens MO, Brubacher JR. Dantrolene in the treatment of MDMA-related hyperpyrexia: a systematic review. CJEM 2010; 12:435-42; PMID:20880437 [DOI] [PubMed] [Google Scholar]
- 114. Cypess AM, Chen YC, Sze C, Wang K, English J, Chan O, Holman AR, Tal I, Palmer MR, Kolodny GM, et al. Cold but not sympathomimetics activates human brown adipose tissue in vivo. Proc Natl Acad Sci U S A 2012; 109:10001-5; PMID:22665804; http://dx.doi.org/ 10.1073/pnas.1207911109 [DOI] [PMC free article] [PubMed] [Google Scholar]


