Abstract
Hyperthermia is a severe complication associated with the recreational use of 3,4-methylenedioxymethamphetamine (MDMA, Ecstasy). In this review, the clinical laboratory studies that tested the effects of MDMA on body temperature are summarized. The mechanisms that underlie the hyperthermic effects of MDMA in humans and treatment of severe hyperthermia are presented. The data show that MDMA produces an acute and dose-dependent rise in core body temperature in healthy subjects. The increase in body temperature is in the range of 0.2-0.8°C and does not result in hyperpyrexia (>40°C) in a controlled laboratory setting. However, moderately hyperthermic body temperatures >38.0°C occur frequently at higher doses, even in the absence of physical activity and at room temperature. MDMA primarily releases serotonin and norepinephrine. Mechanistic clinical studies indicate that the MDMA-induced elevations in body temperature in humans partially depend on the MDMA-induced release of norepinephrine and involve enhanced metabolic heat generation and cutaneous vasoconstriction, resulting in impaired heat dissipation. The mediating role of serotonin is unclear. The management of sympathomimetic toxicity and associated hyperthermia mainly includes sedation with benzodiazepines and intravenous fluid replacement. Severe hyperthermia should primarily be treated with additional cooling and mechanical ventilation.
Keywords: hyperthermia, hyperpyrexia, MDMA, norepinephrine, serotonin, treatment
Abbreviations
- MDMA
3,4-methylenedioxymethamphetamine
Introduction
Hyperpyrexia (body temperature >40°C) is the most important acute severe complication of the recreational use of 3,4-methylenedioxymethamphetamine (MDMA, Ecstasy). MDMA-induced hyperpyrexia is relatively rare and not observed in placebo-controlled studies in humans. However, moderate effects of MDMA on body temperature have been documented in several placebo-controlled laboratory studies in human subjects. This review summarizes the clinical studies on MDMA-induced hyperthermic effects and the potential pharmacological mechanisms that are involved in humans. Many preclinical studies (for review, see1) but relatively few clinical studies have evaluated the effects of MDMA on body temperature. The present review focuses on the findings from placebo-controlled studies that assessed MDMA in humans and addresses treatment options for hyperpyrexia caused by recreational Ecstasy use. The thermal effects of Ecstasy in dance clubbers have previously been described and summarized.2
Hyperthermia associated with Ecstasy use
The association between Ecstasy use and hyperpyrexia is well-established,3-6 and reports were systematically compiled by Grunau and colleagues.6 Hyperpyrexia is relatively rare, but if it occurs, then it typically leads to intravascular coagulation, rhabdomyolysis, and renal or other organ failure.2,4-10 In particular, body temperature >41°C can result in fatalities.4,6 Moderate forms of Ecstasy-induced hyperthermia (>38°C) are frequently observed. Increased body temperature (>37.1°C) was found in 19% of patients,9 and hyperthermia (>38°C) was found in 4% of patients5 who presented in emergency departments with Ecstasy-related medical problems.
Basic mechanisms involved in hyperthermia
Drug-induced hyperthermia resembles heat stroke. The basic mechanisms include increased heat production and/or decreased heat loss (Fig. 1). In heat stroke, heat dissipation is primarily impaired by a hot environment, and heat generation is often increased by exertion. In drug-induced hyperthermia, the drug exerts direct actions to increase metabolic heat generation and reduce heat dissipation as mostly studied in animals,7,11-13 whereas a hot environment and exertion may act as additional permissive factors.2,14 Additionally, in the case of drug-induced hyperthermia, central heat regulation mechanisms may be disturbed.12 Many psychotropic substances, including both therapeutic medications and recreational drugs, alter body and brain temperature15 and may cause hyperthermia.12 Serotonin syndrome, which includes increases in body temperature, may result when serotonergic drugs are used at high doses or in combinations.16 Neuroleptic malignant syndrome is an adverse hyperthermic reaction to antipsychotic drugs.12 MDMA releases serotonin and therefore may induce a serotonin syndrome, including increased body temperature.17 Additionally, MDMA has been shown to induce alterations in mitochondrial energy metabolism in animals, resulting in increased heat generation instead of metabolic energy carrier (i.e., adenosine triphosphate) production.11,18 Additionally, psychostimulants, including MDMA, constrict blood vessels via sympathetic stimulation, including adrenergic receptor stimulation, thereby reducing peripheral blood flow and heat dissipation by convection via the skin (Fig. 1).19 Furthermore, central thermoregulation may be dysregulated in the case of substances that act as direct or indirect serotonin receptor agonists, such as MDMA.20 Dopaminergic mechanisms have also been implicated in MDMA-induced hyperthermia in preclinical studies.21 Many permissive factors have been shown to enhance the thermogenic effects of MDMA, typically in preclinical studies, and have been summarized with regard to human MDMA use.2 Factors that increase the risk of MDMA-induced hyperthermia in animals and possibly also in humans include multiple dosing (booster) or high doses,2,22 high ambient temperature,7,23,24 reduced fluid intake,23 crowded conditions, physical activity,23 and social interaction.24,25 Several of these risk factors are typically present in dance clubs or party settings where MDMA and other stimulant drugs are consumed. Hyperthermia is also seen in human MDMA exposure outside “rave” party settings in the absence of physical activity.26 In placebo-controlled laboratory studies in humans, these conditions are mostly not present for safety reasons. In real-world studies of ecstasy users at dance clubs, increases in tympanic body temperature of +0.2 to +1.6°C have been measured in response to Ecstasy use (for a summary, see Parrott2).
Figure 1.
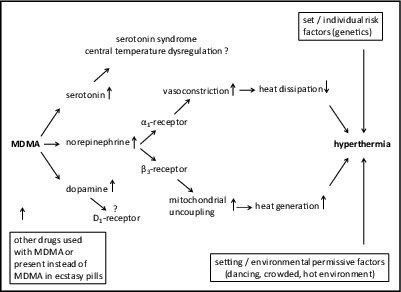
Basic mechanism involved in 3,4-methylenedioxymethamphetamine (MDMA)-induced hyperthermia.
Effects of MDMA on body temperature in experimental clinical studies
Clinical laboratory studies that investigated the effects of MDMA using a placebo-controlled study design are summarized in Table 1. MDMA was administered orally in all these studies but different doses were used. A series of small experimental studies (n < 20) assessed the thermogenic effects of MDMA in healthy subjects.27-40 These studies typically used axillary, oral, or tympanic temperature measurements. Body temperature was not the primary outcome measure in these studies, with one exception. Pooled analyses of the effects of MDMA have been reported using aggregated data from smaller studies with 27 subjects by da la Torre and colleagues,41 74 subjects by Liechti and colleagues,34 and 80 subjects by Hysek and Liechti.32 In the studies by Liechti and colleagues,42-46 which were conducted in Zurich, Switzerland, axillary body temperature was measured in a total of 54 men and 20 women after MDMA doses of 1.35-1.8 mg/kg.34 Peripheral body temperature was significantly increased by 0.4°C compared with placebo in male subjects, whereas MDMA-induced increases in body temperature did not reach statistical significance in the smaller group of female participants.34 All axillary body temperatures remained below 38°C.34 In our more recently published placebo-controlled studies performed in Basel, Switzerland, core body temperature was measured using an ear thermometer and repeatedly after the administration of MDMA at doses of 75 mg (n = 30) and 125 mg (n = 96) in a total of 126 healthy subjects.27-33 The effects of the 75 mg dose of MDMA on body temperature were reported by Schmid et al.47 The effects of the 125 mg dose of MDMA were reported in a pooled analysis by Hysek and Liechti (n = 80)32 and separately for 16 additional subjects.33,47 MDMA did not significantly alter body temperature at a dose of 75 mg compared with placebo in 30 subjects.47 The maximum increase after MDMA was 0.35 ± 0.06°C above pretreatment baseline compared with 0.25 ± 0.06°C after placebo.47 At a higher dose of 125 mg, MDMA robustly and significantly increased body temperature in several of our recent studies.27,28,30,31,33 The pooled data from 5 of these studies were presented in an analysis of the autonomic effects of MDMA in 80 healthy subjects32 and in a study of dose-response effects in the online supplemental material that accompanied the reported by Schmid et al.47 The increase in body temperature after 125 mg of MDMA was typically 0.3-0.8°C.32,47 As expected, a statistically significant MDMA dose-response effect on body temperature was observed.47 Maximal temperatures occurred 2-2.5 h after MDMA administration and after subjective effects, heart rate, or blood pressure had peaked, which was at 1.5 h.32 An analysis of the data from all 126 subjects showed a peak maximum body temperature of 37.5 ± 0.5°C. After the 75 mg dose of MDMA, tympanic temperatures remained below 38°C in all 30 subjects. However, after the 125 mg dose in a total of 96 subjects, tympanic temperatures were >38.0°C in 21 subjects (22%) and >38.5°C in 3 subjects (3%), reaching a maximum of 39.1°C in one subject (1%). Importantly, these body temperatures were measured with subjects at rest and at a mean room temperature of 22.7 ± 0.6°C. The time course of the increase in tympanic body temperature after MDMA administration at a dose of 125 mg in 96 subjects is shown in Figure 2. The figure shows the pooled data from all our 6 published studies using the 125 mg dose of MDMA.27-31,33 Significant elevations in body temperature were observed between 90 min and 4 h after MDMA administration.
Table 1.
Placebo-controlled studies that investigated effects of MDMA on body temperature
| Reference | other drugs in study | Sample size | oral doses | measurement | effect |
|---|---|---|---|---|---|
| 39 |
no |
n = 6 |
0.25-1.0 mg/kg |
oral |
no significant change |
| 42 |
no |
n = 13 |
1.7 mg/kg |
axillary |
no significant change |
| 35 |
amphetamine |
n = 8 |
75 and 125 mg |
oral |
no significant change |
|
a41 |
no |
n = 27, pooled |
50-150 mg |
oral |
increase after initial decrease |
| 43 |
citalopram |
n = 16 |
1.5 mg/kg |
axillary |
increase |
| 46 |
haloperidol |
n = 14 |
1.5 mg/kg |
axillary |
no significant change |
| 44 |
ketanserin |
n = 14 |
1.5 mg/kg |
axillary |
increase |
|
b34 |
no |
n = 74, pooled |
1.35-1.8 mg/kg |
axillary |
increase |
| 37 |
no |
n = 8 |
0.5 and 1.5 mg/kg |
finger and tympanic |
no significant change |
| 36 |
mCPP, amphetamine |
n = 12 |
1 and 2 mg/kg |
oral |
increase |
| 38 |
no |
n = 9 |
100 mg |
oral |
no significant change |
| 50 |
no |
n = 10 |
2 mg/kg |
ccore body and skin |
increase (core), trend-increase (skin) |
| 49 |
no |
n = 8 |
1.0 and 1.6 mg/kg |
tympanic |
no significant change |
| 40 |
tetrahydrocannabinol |
n = 16 |
100 mg |
tympanic |
increase |
| 27 |
reboxetine |
n = 16 |
125 mg |
tympanic |
increase |
| 48 |
methamphetamine |
n = 11 |
100 mg |
oral |
no significant change |
| 29 |
clonidine |
n = 16 |
125 mg |
tympanic |
no significant change |
| 28 |
duloxetine |
n = 16 |
125 mg |
tympanic |
increase |
| 30 |
carvedilol |
n = 16 |
125 mg |
tympanic |
increase |
| 31 |
doxazosin |
n = 16 |
125 mg |
tympanic |
increase |
|
d32 |
pooled analysis |
n = 80, pooled |
125 mg |
tympanic |
increase |
| 33 |
methylphenidate |
n = 16 |
125 mg |
tympanic |
increase |
| 47 | methylphenidate | n = 30 | 75 mg | tympanic | no significant change |
MDMA, 3,4-methylenedioxymethamphetamine; mCPP, metachlorophenylpiparazine.
apooled data including study 35 (n = 8) and an additional 19 subjects.
bpooled data including studies 42, 43, 46, and 44, and data from 16 subjects from study 45.
cingested radio-telemetry pill, chest, upper arm, thigh, and lower leg.
dpooled data including 27-31, and 33.
Figure 2.
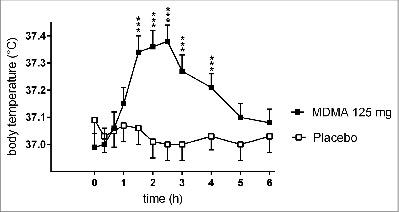
Effects of 3,4-methylenedioxymethamphetamine (MDMA, 125 mg orally) and placebo on core body (tympanic) temperature in healthy subjects. Absolute raw data values from all subjects who participated in our 6 placebo-controlled experimental studies using a dose of MDMA of 125 mg MDMA27-31,33 were pooled and are presented here as mean ± SEM of absolute tympanic temperature values in 96 healthy subjects (48 male and 48 female). MDMA significantly increased body temperature compared with placebo (repeated-measures analysis of variance: time × drug interaction: F10,940 = 19.72, P < 0.001). *** for P < 0.001 indicate significant differences compared with placebo for individual time points based on Tukey post hoc test. MDMA or placebo was administered at t = 0. MDMA was administered in a quiet hospital setting and the subjects were not physically active.
Several other smaller studies have also evaluated the thermogenic effects of MDMA. Oral temperature slightly increased after doses of 75 and 125 mg MDMA in 8 subjects, but no statistically significant differences were observed compared with placebo.35 In the same study, amphetamine at an oral dose of 40 mg was also without effects on oral temperature.35 A similar nonsignificant increase in oral temperature was found in another small study that included 9 subjects and a dose of 100 mg.38 The same group reported significant increases in oral temperature after MDMA administration at doses of 75, 100, and 125 mg from a pooled analysis of several studies that included doses of 50 mg (n = 2), 75 mg (n = 10), 100 mg (n = 13), 125 mg (n = 8), and 150 mg (n = 2).41 Other small studies by different research groups were also found. Harris and colleagues measured both skin (i.e., index finger) and core (i.e., tympanic) temperature after MDMA administration (0.5 and 1.5 mg/kg) in 8 subjects.37 Although skin temperature decreased 5.0 ± 4°C from pretreatment levels after 1.5 mg/kg, it was not significantly lower than in the placebo condition in this small study.37 The finding of reduced finger skin temperature is consistent with reports of cold extremities after MDMA administration and very likely reflects vasoconstriction in the periphery and reduced heat dissipation. Unfortunately, no other studies have measured finger temperature to confirm this finding in a larger sample. Kirkpatrick and colleagues found that MDMA at an oral dose of 100 mg had no effects on oral body temperature in 11 subjects.48 This study also found no effects of methamphetamine (40 mg, orally) on body temperature.48 Kolbrich and colleagues found nonsignificant elevations in tympanic temperature in 8 healthy subjects with MDMA doses of 1.0 and 1.6 mg/kg (46-150 mg).49 Tancer and Johanson showed that MDMA at a dose of 2 mg/kg significantly increased oral body temperature in 12 subjects.36 Significant increases in tympanic temperature of 0.3°C were also shown after 100 mg of MDMA in 16 subjects by Dumont and colleagues.40 A study by Freedman, Johanson, and Tancer provided a comprehensive evaluation of the effects of 2.0 mg/kg MDMA on core and skin temperature at low (18°C) and high (30°C) ambient temperatures.50 This also appears to be the only laboratory study of the effects of MDMA in humans in which body temperature was the primary outcome measure. In all of the other studies, body temperature was a secondary measure. Core body temperature was measured in 10 subjects using an ingested radiotelemetry pill.50 Skin temperature was measured at the chest, upper arm, thigh, and lower leg, and a weighted average was calculated. Absolute core temperatures were higher after MDMA in the warm environment compared with the cold environment. However, core temperature was also higher in the warm environment compared with the cold environment after placebo. Thus, MDMA similarly increased core temperature at the low and high ambient temperatures compared with placebo.50 These increases were related to increases in metabolic rate, measured by indirect calorimetry, in the same study. Skin temperature was markedly increased in the hot and decreased in the cold environment, and MDMA produced a near-significant increase in skin temperature under both temperature conditions and compared with placebo.50
Altogether, considering the pooled data analyses from our laboratory and those of the Freedman study, MDMA is well documented to produce an acute and dose-dependent elevation in core body temperature in healthy subjects. The increase in body temperature is also evidently rather small, in the range of 0.2-0.8°C, and does not result in hyperpyrexia (>40°C) in a controlled laboratory setting. Importantly, no laboratory study observed MDMA-induced hyperpyrexia in a controlled setting. However, moderately hyperthermic body temperatures >38.0°C were documented in a substantial number of our subjects (23% after a 125 mg dose in our sample), demonstrating that MDMA induces moderate hyperthermia even in the absence of any permissive factors and at room temperature (23°C).
Neurochemistry of the thermogenic properties of MDMA in humans
Several mechanistic studies assessed the effects of pharmacological pretreatments on the response to MDMA in healthy subjects to evaluate the mediating role of different neurotransmitters and receptors. These human studies also provide important information on the mechanisms involved in MDMA-induced increases in body temperature and likely also in the more severe hyperpyrexia associated with uncontrolled use. MDMA mainly releases serotonin and norepinephrine and to a lesser extent dopamine through the corresponding presynaptic monoamine transporters.28,51 MDMA also interacts directly with monoamine receptors52,53 but only at relatively high concentrations, likely making these effects less relevant in humans. Serotonin transporter inhibitors block the interaction between MDMA with the transporter to release serotonin.28 Transporter inhibitors, therefore, can be used as pharmacological tools to investigate the role of serotonin release in the mechanism of action of MDMA. Serotonin transporter inhibitors reduced the psychotropic and most physiological effects of MDMA in healthy humans,43,54-56 suggesting a mediating role for serotonin in most effects of MDMA in humans. Regarding the thermogenic effects of MDMA, serotonin transporter inhibition reduced MDMA-induced increases in oral55 but not axillary body temperature.43 The combined serotonin and norepinephrine transporter inhibitor duloxetine also tended to attenuate the MDMA-induced increase in body temperature in humans, but the effect was not significant.28 These findings are inconclusive but are consistent with the view that both serotonin and norepinephrine are involved in the effects of MDMA on body temperature. Interestingly, in animals, serotonin transporter inhibition reduced MDMA-induced hyperthermia in mice57 but not rats.21 Serotonin release is the major mediator of most of the clinical effects of MDMA, but its precise role in the thermogenic response in humans remains to be determined. The serotonin 5-HT2A receptor antagonist ketanserin reduced the MDMA-induced elevation in body temperature in humans,44 consistent with studies in rats.58 However, in humans, ketanserin alone also reduced body temperature compared with placebo, and the effects with MDMA were therefore mostly additive.44 Additionally, ketanserin has α1-adrenergic receptor-blocking properties59 and may reduce peripheral vascular resistance and body temperature. The serotonin 5-HT1A receptor antagonist pindolol did not alter the MDMA-induced increase in body temperature in humans,60 also consistent with preclinical data.58 Potential dopaminergic mediation of the effects of MDMA on body temperature has not been well-studied in humans. Preclinical data suggest a role for the dopamine D1 receptor in the mediation of hyperthermia associated with MDMA.21 Because no selective D1 antagonists are available for human use, the role of the D1 receptor has not been studied in humans. The interaction between MDMA and the dopamine D2 receptor antagonist haloperidol was examined in healthy subjects, but MDMA did not produce significant elevations in body temperature in that study to provide meaningful results.46 Studying the role of the dopamine transporter in MDMA-induced hyperthermia using pharmacological tools was also difficult because dopamine transporter inhibitors may not effectively block MDMA-induced dopamine release21 or may have effects on their own.61 In fact, the dopamine uptake inhibitor methylphenidate increased body temperature in healthy subjects when given alone but failed to alter the thermogenic response to MDMA when administered prior to MDMA.33,47 In contrast, MDMA did not produce an increase in body temperature in humans after pretreatment with the selective norepinephrine transporter blocker reboxetine.27 Additionally, reboxetine blocked MDMA-induced increases in norepinephrine plasma levels and elevations in heart rate and blood pressure,27 supporting the view that norepinephrine rather than serotonin is critical in the mediation of not only the cardiovascular but also hyperthermic response to MDMA. Further human studies examined the contributing role of different adrenergic receptors. β1-adrenergic receptors did not appear to be involved because the β1-receptor blocker pindolol did not alter the temperature response to MDMA in humans.60 Administration of the non-selective β-receptor antagonists propranolol or nadolol also had no effect on the thermogenic response to MDMA in rats.62 The α2-adrenergic receptor agonist and sympatholytic drug clonidine, which is expected to reduce central adrenergic output, lowered body temperature in humans when given alone but not the MDMA-induced elevations in body temperature.29 In contrast, the α1-adrenergic receptor antagonist doxazosin reduced the increase in body temperature induced by MDMA in humans.31 This is again consistent with the view that MDMA-induced increases in norepinephrine mediate the thermogenic effects of MDMA and that increases in body temperature are linked to cutaneous vasoconstriction that results in impaired heat dissipation, and doxazosin prevents α1-adrenergic receptor-mediated vasoconstriction. Peripheral vasoconstriction and improper heat dissipation have also been identified as critical mechanisms that underlie MDMA-induced core (brain) hyperthermia in rats treated with MDMA under conditions that simulate drug use in humans.24 Additionally, the combined α1- and β1-3-adrenergic receptor antagonist carvedilol effectively prevented temperature elevations in healthy subjects,30,63 consistent with preclinical data.62 This latter finding is particularly interesting because carvedilol is expected to block both MDMA-induced heat generation by mitochondrial uncoupling18,64, by blocking β3-adrenergic receptor- and α1-adrenergic receptor-mediated vasoconstriction. In fact, carvedilol more effectively reduced the hyperthermic response to MDMA compared with α1 receptor blockade in both animals62 and humans.30,31 Similarly, both the α1-antagonist prazosin and the β3-antagonists cyanopindolol only partly attenuated body temperature increases in skeletal muscle (cyanopindolol) or core (prazosin) after MDMA and the combination of the 2 completely blocked MDMA-induced hyperthermia (muscle and core).65 Additionally, carvedilol reduced MDMA-induced increases in both heart rate and blood pressure in healthy human subjects.30 Thus, carvedilol reduced several signs of sympathetic activation by MDMA including the cardiostimulant and the termogenic effects.
Dumont and colleagues studied the interactive effects of MDMA and tetrahydrocannabinol in healthy subjects. Tetrahydrocannabinol delayed the MDMA-induced increase in temperature, and the duration of the temperature elevation was prolonged, although the mean temperature increase was comparable to administration of MDMA alone.40 The results indicate that tetrahydrocannabinol is unlikely to protect against MDMA-induced hyperthermia, although cannabinoids decrease body temperature in rats66 and in contrast to previous hypotheses.67
Several experimental human studies also tested the effects of other psychostimulants with a slightly different pharmacology than MDMA on body temperature. Various psychostimulants, including MDMA, enhance noradrenergic neurotransmission, but their relative dopaminergic vs. serotonergic activity varies. For example, Tancer and Johanson assessed the effects of MDMA, D-amphetamine (mostly a dopamine and norepinephrine releaser), and metachlorophenylpiperazine (a serotonin inhibitor and releaser) on oral temperature in the same study.36 Both amphetamine and metachlorophenylpiperazine increased body temperature similarly to MDMA.36 The data suggest that psychotropics with either serotonergic (metachlorophenylpiperazine) or dopaminergic (amphetamine) properties increase body temperature. Similar to amphetamine, methylphenidate (a selective dopamine and norepinephrine transporter inhibitor with no serotonergic properties) also acutely increased body temperature in humans when given at a dose of 40 mg47 or 60 mg.33 Cocaine enhanced the progressive increase in core body temperature during passive heating.68 The elevation in body temperature was attributable to impaired heat dissipation, including reduced sweating and cutaneous vasodilation.68 Similar to MDMA, cocaine has serotonergic and noradrenergic properties.52 Importantly, all of these recreational drugs, whether serotonergic (MDMA or metachlorophenylpiperazine) or dopaminergic (amphetamine or methylphenidate) or both (cocaine), also enhance norepinephrine transmission. Therefore, norepinephrine likely contributes to the thermogenic effects of these substances, consistent with the reducing effects of carvedilol on the temperature response to MDMA.
A series of novel psychoactive substances with structural similarity to MDMA have been implicated in hyperpyrexia. In particular, para-methoxyamphetamine and para-methoxymethamphetamine, which are occasionally sold as Ecstasy,69 have been associated with an especially high risk of hyperthermia.69-72 Para-methoxyamphetamine and para-methoxymethamphetamine predominantly act on the serotonin system,73 consistent with the role of serotonin in hyperthermia. Four-Methylthioamphetamine is another serotonergic compound73 that has been linked to hyperthermia.74 Other novel substances, such as 3,4-methylendioxypyrovalerene, which potently inhibits dopamine and norepinephrine uptake52 and induces marked and prolonged agitation, has also been reported to induce hyperthermia.75 Notably, hyperthermia has also been described with the dopamine and norepinephrine transporter inhibitor methylphenidate.76
Altogether, the mechanistic studies in humans provide support for the conclusion that MDMA mainly increases body temperature via the release of norepinephrine, which then increases metabolic heat generation and impairs heat dissipation via vasoconstriction. Additionally, the release of serotonin may also contribute to the thermogenic effects of MDMA in humans.
Management of Ecstasy-induced hyperthermia in patients
The various treatments for hyperpyrexia induced by MDMA or other psychostimulants have not been systematically evaluated in the emergency room setting. Hyperthermic complications are relatively rare, and clinical trials are unlikely to be conducted. However, as described above, several placebo-controlled mechanistic experimental studies have been conducted with healthy subjects, which can inform us on the pharmacological mechanism of MDMA-induced hyperthermia in humans and potential effects of pharmacological treatments. As a limitation, the experimental studies used doses of pure MDMA in the range of 50-150 mg while recreational users of ecstasy pills may ingest MDMA at lager doses or repeated doses. For example, in an naturalistic observational study among 49 partying people, 34 used doses of MDMA of 0-150 mg while 15 took cumulative doses of 150-280 mg.77 An analysis of ecstasy pills from 5,786 recreational users found an average MDMA content in the ecstasy pills of 82.5 ± 35.2 mg.69 Approximately 20% of users presenting to emergency departments with medical problems report having ingested 2 or more pills.5,9 Additionally, MDMA is often used in combination with other substances5,9 many of which may also affect body temperature.
Paramedics and emergency department personnel must recognize hyperthermia in subjects with acute substance-induced disorders. It is not uncommon that agitated subjects die of hyperpyrexia because body temperature was not measured, and elevations in body temperature went unrecognized. Agitated subjects should not be restrained. Sedation with benzodiazepines and intravenous fluid replacement are the most important acute supportive care measures in patients with substance-induced sympathomimetic toxidromes and/or agitation.3,5,78 The management of hyperpyrexia includes cooling (fanning, water, ice packs, ice bath, cooling blankets) and mechanical ventilation.10 Dantrolene, which acts at skeletal muscles to inhibit the release of calcium, has also been used.6,10,79 However, dantrolene does not inhibit the thermogenic effects of MDMA,80 and the drug does not modulate the mechanism of MDMA-induced hyperthermia discussed above. Nevertheless, case reports support its benefits in cases of extreme hyperpyrexia (>42°C).6
Antipsychotics, such as haloperidol, should not be used as a routine treatment of drug-induced agitation or only with great care and after treatment with benzodiazepines. Antipsychotics are associated with hyperthermia in the context of neuroleptic malignant syndrome and some (e.g., phenothiazines) also act as anticholinergics and potentially reduce sweating and thus heat dissipation by evaporation. Additionally, haloperidol has been shown to enhance acute anxiety and the negative mood effects of MDMA46 and psilocybin.81 Thus, the use of this antipsychotic medication may actually enhance adverse drug effects, at least in certain situations. The atypical antipychotic clozapine has been shown to reverse hyperthermia and cutaneous vasoconstriction induced by MDMA in rats or rabbits82 but human data to support its clinical use are lacking. Hyperthermia is often associated with other signs of sympathomimetic stimulation, including agitation, tachycardia, and hypertension. Benzodiazepines are also beneficial in the treatment of these symptoms. However, additional hypertensive treatment with vasodilators, such as nitrates, may be needed. Adrenergic receptor antagonists can be useful. However, β blockade without α blockade should be avoided in drug-induced sympathomimetic toxicity because of the unopposed α-adrenergic receptor stimulation that enhances vasoconstriction83 and results in further increases in blood pressure60,84 and possibly body temperature. In contrast, α blockade reduced the blood pressure response to MDMA and elevations in body temperature,31 and combined α/β blockade reduced MDMA-induced increases in blood pressure, heart rate, and body temperature30,63 and blood pressure response to cocaine.85-87 Accordingly, α blockers (e.g., phentolamine) or α/β blockers (e.g., carvedilol) could be useful in situations of extreme sympathomimetic stimulation, although real-world clinical data are mostly lacking. Phentolamine was successfully used to treat a hypertensive emergency caused by amphetamine overdose.88
Besides from hyperthermia, brain edema is another severe complication of MDMA use. MDMA induces a syndrome of inappropriate secretion of antidiuretic hormone89-91 which may lead to symptomatic hyponatremia including brain edema in particular in women.92-94 Thus, excessive water consumption but also fluid treatment can be potentially dangerous in the prevention or the treatment of MDMA-induced hyperthermia. Additionally, animal studies indicate that the blood-brain barrier is disrupted during MDMA-induced hyperthermia also leading to brain edema.95
Conclusion
MDMA increases body temperature in humans. However, hyperpyrexia (>40°C) is not seen in controlled laboratory settings. The MDMA-induced elevations in body temperature in humans appear to depend on the MDMA-induced release of norepinephrine and involve cutaneous vasoconstriction and likely also enhanced metabolic heat generation. The role of serotonin needs further clarification. The management of overdose cases includes sedation treatment with benzodiazepines, intravenous fluid replacement, and additional cooling and mechanical ventilation in severe cases.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The author thanks Yasmin Schmid for data preparation and comments on the manuscript.
Funding
This work was supported by the Swiss National Science Foundation (no. 320030_149493/1).
References
- 1. Green AR, Mechan AO, Elliott JM, O’Shea E, Colado MI. The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”). Pharmacol Rev 2003; 55:463-508; PMID:12869661; http://dx.doi.org/ 10.1124/pr.55.3.3. [DOI] [PubMed] [Google Scholar]
- 2. Parrott AC. MDMA and temperature: a review of the thermal effects of ‘Ecstasy’ in humans. Drug Alcohol Depend 2012; 121:1-9; PMID:21924843; http://dx.doi.org/ 10.1016/j.drugalcdep.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 3. Halpern P, Moskovich J, Avrahami B, Bentur Y, Soffer D, Peleg K. Morbidity associated with MDMA (ecstasy) abuse: a survey of emergency department admissions. Hum Exp Toxicol 2011; 30:259-66; PMID:20488845; http://dx.doi.org/ 10.1177/0960327110370984. [DOI] [PubMed] [Google Scholar]
- 4. Henry JA, Jeffreys KJ, Dawling S. Toxicity and deaths from 3,4-methylenedioxymethamphetamine (“ecstasy”). Lancet 1992; 340:384-7; PMID:1353554; http://dx.doi.org/ 10.1016/0140-6736(92)91469-O. [DOI] [PubMed] [Google Scholar]
- 5. Liechti ME, Kunz I, Kupferschmidt H. Acute medical problems due to Ecstasy use: case-series of emergency department visits. Swiss Med Wkly 2005; 135:652-7; PMID:16380853. [DOI] [PubMed] [Google Scholar]
- 6. Grunau BE, Wiens MO, Brubacher JR. Dantrolene in the treatment of MDMA-related hyperpyrexia: a systematic review. CJEM 2010; 12:435-42; PMID:20880437. [DOI] [PubMed] [Google Scholar]
- 7. Docherty JR, Green AR. The role of monoamines in the changes in body temperature induced by 3,4-methylenedioxymethamphetamine (MDMA, ecstasy) and its derivatives. Br J Pharmacol 2010; 160:1029-44; PMID:20590597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rogers G, Elston J, Garside R, Roome C, Taylor R, Younger P, Zawada A, Somerville M. The harmful health effects of recreational ecstasy: a systematic review of observational evidence. Health Technol Assess 2009; 13:iii-iv, ix-xii, 1-315; PMID:19195429. [DOI] [PubMed] [Google Scholar]
- 9. Williams H, Dratcu L, Taylor R, Roberts M, Oyefeso A. “Saturday night fever:" ecstasy related problems in a London accident and emergency department. J Accid Emerg Med 1998; 15:322-6; PMID:9785160; http://dx.doi.org/ 10.1136/emj.15.5.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hall AP, Henry JA. Acute toxic effects of ‘Ecstasy’ (MDMA) and related compounds: overview of pathophysiology and clinical management. Br J Anaesth 2006; 96:678-85; PMID:16595612; http://dx.doi.org/ 10.1093/bja/ael078. [DOI] [PubMed] [Google Scholar]
- 11. Rusyniak DE, Tandy SL, Hekmatyar SK, Mills E, Smith DJ, Bansal N, MacLellan D, Harper ME, Sprague JE. The role of mitochondrial uncoupling in 3,4-methylenedioxymethamphetamine-mediated skeletal muscle hyperthermia and rhabdomyolysis. J Pharmacol Exp Ther 2005; 313:629-39; PMID:15644431; http://dx.doi.org/ 10.1124/jpet.104.079236. [DOI] [PubMed] [Google Scholar]
- 12. McAllen KJ, Schwartz DR. Adverse drug reactions resulting in hyperthermia in the intensive care unit. Crit Care Med 2010; 38:S244-52; PMID:20502177. [DOI] [PubMed] [Google Scholar]
- 13. Rusyniak DE, Sprague JE. Toxin-induced hyperthermic syndromes. Med Clin North Am 2005; 89:1277-96; PMID:16227063; http://dx.doi.org/ 10.1016/j.mcna.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 14. Green AR, O’Shea E, Colado MI. A review of the mechanisms involved in the acute MDMA (ecstasy)-induced hyperthermic response. Eur J Pharmacol 2004; 500:3-13; PMID:15464016; http://dx.doi.org/ 10.1016/j.ejphar.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 15. Kiyatkin EA. The hidden side of drug action: brain temperature changes induced by neuroactive drugs. Psychopharmacology (Berl) 2013; 225:765-80; PMID:23274506; http://dx.doi.org/ 10.1007/s00213-012-2957-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med 2005; 352:1112-20; PMID:15784664; http://dx.doi.org/ 10.1056/NEJMra041867. [DOI] [PubMed] [Google Scholar]
- 17. Silins E, Copeland J, Dillon P. Qualitative review of serotonin syndrome, ecstasy (MDMA) and the use of other serotonergic substances: hierarchy of risk. Aust N Z J Psychiatry 2007; 41:649-55; PMID:17620161; http://dx.doi.org/ 10.1080/00048670701449237. [DOI] [PubMed] [Google Scholar]
- 18. Mills EM, Banks ML, Sprague JE, Finkel T. Pharmacology: uncoupling the agony from ecstasy. Nature 2003; 426:403-4; PMID:14647371; http://dx.doi.org/ 10.1038/426403a. [DOI] [PubMed] [Google Scholar]
- 19. Mills EM, Rusyniak DE, Sprague JE. The role of the sympathetic nervous system and uncoupling proteins in the thermogenesis induced by 3,4-methylenedioxymethamphetamine. J Mol Med 2004; 82:787-99; PMID:15602689; http://dx.doi.org/ 10.1007/s00109-004-0591-7. [DOI] [PubMed] [Google Scholar]
- 20. Saadat KS, O’Shea E, Colado MI, Elliott JM, Green AR. The role of 5-HT in the impairment of thermoregulation observed in rats administered MDMA (‘ecstasy’) when housed at high ambient temperature. Psychopharmacology (Berl) 2005; 179:884-90; PMID:15650843; http://dx.doi.org/B 10.1007/s00213-004-2106-1. [DOI] [PubMed] [Google Scholar]
- 21. Mechan AO, Esteban B, O’Shea E, Elliott JM, Colado MI, Green AR. The pharmacology of the acute hyperthermic response that follows administration of 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’) to rats. Br J Pharmacol 2002; 135:170-80; PMID:11786492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schutte JK, Schafer U, Becker S, Oldewurtel C, Starosse A, Singler P, Richard A, Wappler F, Gerbershagen MU. Three,4-Methylenedioxymethamphetamine induces a hyperthermic and hypermetabolic crisis in pigs with and without a genetic disposition for malignant hyperthermia. Eur J Anaesthesiol 2013; 30:29-37; PMID:23138574; http://dx.doi.org/ 10.1097/EJA.0b013e32835a1127. [DOI] [PubMed] [Google Scholar]
- 23. Dafters RI. Hyperthermia following MDMA administration in rats: effects of ambient temperature, water consumption, and chronic dosing. Physiol Behav 1995; 58:877-82; PMID:8577883; http://dx.doi.org/ 10.1016/0031-9384(95)00136-7. [DOI] [PubMed] [Google Scholar]
- 24. Kiyatkin EA, Kim AH, Wakabayashi KT, Baumann MH, Shaham Y. Critical role of peripheral vasoconstriction in fatal brain hyperthermia induced by MDMA (Ecstasy) under conditions that mimic human drug use. J Neurosci 2014; 34:7754-62; PMID:24899699; http://dx.doi.org/ 10.1523/JNEUROSCI.0506-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brown PL, Kiyatkin EA. Brain hyperthermia induced by MDMA (ecstasy): modulation by environmental conditions. Eur J Neurosci 2004; 20:51-8; PMID:15245478; http://dx.doi.org/ 10.1111/j.0953-816X.2004.03453.x. [DOI] [PubMed] [Google Scholar]
- 26. Patel MM, Belson MG, Longwater AB, Olson KR, Miller MA. Methylenedioxymethamphetamine (ecstasy)-related hyperthermia. J Emerg Med 2005; 29:451-4; PMID:16243206; http://dx.doi.org/ 10.1016/j.jemermed.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 27. Hysek CM, Simmler LD, Ineichen M, Grouzmann E, Hoener MC, Brenneisen R, Huwyler J, Liechti ME. The norepinephrine transporter inhibitor reboxetine reduces stimulant effects of MDMA (“ecstasy”) in humans. Clin Pharmacol Ther 2011; 90:246-55; PMID:21677639; http://dx.doi.org/ 10.1038/clpt.2011.78. [DOI] [PubMed] [Google Scholar]
- 28. Hysek CM, Simmler LD, Nicola V, Vischer N, Donzelli M, Krähenbühl S, Grouzmann E, Hoener MC, Liechti ME. Duloxetine inhibits effects of MDMA (“ecstasy”) in vitro and in humans in a randomized placebo-controlled laboratory study. PLoS One 2012; 7:e36476; PMID:22574166; http://dx.doi.org/ 10.1371/journal.pone.0036476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hysek CM, Brugger R, Simmler LD, Bruggisser M, Donzelli M, Grouzmann E, Hoener MC, Liechti ME. Effects of the α2-adrenergic agonist clonidine on the pharmacodynamics and pharmacokinetics of 3,4-methylenedioxymethamphetamine in healthy volunteers. J Pharmacol Exp Ther 2012; 340:286-94; PMID:22034656; http://dx.doi.org/ 10.1124/jpet.111.188425. [DOI] [PubMed] [Google Scholar]
- 30. Hysek CM, Schmid Y, Rickli A, Simmler LD, Donzelli M, Grouzmann E, Liechti ME. Carvedilol inhibits the cardiostimulant and thermogenic effects of MDMA in humans. Br J Pharmacol 2012; 166:2277-88; PMID:22404145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hysek CM, Fink AE, Simmler LD, Donzelli M, Grouzmann E, Liechti ME. α-Adrenergic receptors contribute to the acute effects of MDMA in humans. J Clin Psychopharmacol 2013; 33:658-666; PMID:23857311; http://dx.doi.org/ 10.1097/JCP.0b013e3182979d32. [DOI] [PubMed] [Google Scholar]
- 32. Hysek CM, Liechti ME. Effects of MDMA alone and after pretreatement with reboxetine, duloxetine, clonidine, carvedilol, and doxazosin on pupillary light reflex. Psychopharmacology (Berl) 2012; 224:363-76; PMID:22700038; http://dx.doi.org/ 10.1007/s00213-012-2761-6. [DOI] [PubMed] [Google Scholar]
- 33. Hysek CM, Simmler LD, Schillinger N, Meyer N, Schmid Y, Donzelli M, Grouzmann E, Liechti ME. Pharmacokinetic and pharmacodynamic effects of methylphenidate and MDMA administered alone and in combination. Int J Neuropsychopharmacol 2014; 17:371-81; PMID:24103254; http://dx.doi.org/ 10.1017/S1461145713001132. [DOI] [PubMed] [Google Scholar]
- 34. Liechti ME, Gamma A, Vollenweider FX. Gender differences in the subjective effects of MDMA. Psychopharmacology (Berl) 2001; 154:161-8; PMID:11314678; http://dx.doi.org/ 10.1007/s002130000648. [DOI] [PubMed] [Google Scholar]
- 35. Mas M, Farre M, de la Torre R, Roset PN, Ortuno J, Segura J, Cami J. Cardiovascular and neuroendocrine effects and pharmacokinetics of 3,4-methylenedioxymethamphetamine in humans. J Pharmacol Exp Ther 1999; 290:136-45; PMID:10381769. [PubMed] [Google Scholar]
- 36. Tancer M, Johanson CE. Reinforcing, subjective, and physiological effects of MDMA in humans: a comparison with d-amphetamine and mCPP. Drug Alcohol Depend 2003; 72:33-44; PMID:14563541; http://dx.doi.org/ 10.1016/S0376-8716(03)00172-8. [DOI] [PubMed] [Google Scholar]
- 37. Harris DS, Baggott M, Mendelson JH, Mendelson JE, Jones RT. Subjective and hormonal effects of 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacol (Berl) 2002; 162:396-405; PMID:12172693; http://dx.doi.org/ 10.1007/s00213-002-1131-1. [DOI] [PubMed] [Google Scholar]
- 38. Farre M, de la Torre R, Mathuna BO, Roset PN, Peiro AM, Torrens M, Ortuno J, Pujadas M, Cami J. Repeated doses administration of MDMA in humans: pharmacological effects and pharmacokinetics. Psychopharmacol (Berl) 2004; 173:364-75; PMID:15071716; http://dx.doi.org/ 10.1007/s00213-004-1789-7. [DOI] [PubMed] [Google Scholar]
- 39. Grob CS, Poland RE, Chang L, Ernst T. Psychobiologic effects of 3,4-methylenedioxymethamphetamine in humans: methodological considerations and preliminary observations. Behav Brain Res 1996; 73:103-7; PMID:8788485; http://dx.doi.org/ 10.1016/0166-4328(96)00078-2. [DOI] [PubMed] [Google Scholar]
- 40. Dumont GJ, Kramers C, Sweep FC, Touw DJ, van Hasselt JG, de Kam M, van Gerven JM, Buitelaar JK, Verkes RJ. Cannabis coadministration potentiates the effects of “ecstasy” on heart rate and temperature in humans. Clin Pharmacol Ther 2009; 86:160-6; PMID:19440186; http://dx.doi.org/ 10.1038/clpt.2009.62. [DOI] [PubMed] [Google Scholar]
- 41. de la Torre R, Farre M, Roset PN, Lopez CH, Mas M, Ortuno J, Menoyo E, Pizarro N, Segura J, Cami J. Pharmacology of MDMA in humans. Ann N Y Acad Sci 2000; 914:225-37; PMID:11085324; http://dx.doi.org/ 10.1111/j.1749-6632.2000.tb05199.x. [DOI] [PubMed] [Google Scholar]
- 42. Vollenweider FX, Gamma A, Liechti ME, Huber T. Psychological and cardiovascular effects and short-term sequelae of MDMA (“ecstasy”) in MDMA-naive healthy volunteers. Neuropsychopharmacology 1998; 19:241-51; PMID:9718588; http://dx.doi.org/ 10.1016/S0893-133X(98)00013-X. [DOI] [PubMed] [Google Scholar]
- 43. Liechti ME, Vollenweider FX. The serotonin uptake inhibitor citalopram reduces acute cardiovascular and vegetative effects of 3,4-methylenedioxymethamphetamine (‘Ecstasy’) in healthy volunteers. J Psychopharmacol 2000; 14:269-74; PMID:11106307; http://dx.doi.org/ 10.1177/026988110001400313. [DOI] [PubMed] [Google Scholar]
- 44. Liechti ME, Saur MR, Gamma A, Hell D, Vollenweider FX. Psychological and physiological effects of MDMA (“Ecstasy”) after pretreatment with the 5-HT2 antagonist ketanserin in healthy humans. Neuropsychopharmacology 2000; 23:396-404; PMID:10989266; http://dx.doi.org/ 10.1016/S0893-133X(00)00126-3. [DOI] [PubMed] [Google Scholar]
- 45. Gamma A, Buck A, Berthold T, Liechti ME, Vollenweider FX. Three,4-Methylenedioxymethamphetamine (MDMA) modulates cortical and limbic brain activity as measured by [H215O]-PET in healthy humans. Neuropsychopharmacology 2000; 23:388-95; PMID:10989265; http://dx.doi.org/ 10.1016/S0893-133X(00)00130-5. [DOI] [PubMed] [Google Scholar]
- 46. Liechti ME, Vollenweider FX. Acute psychological and physiological effects of MDMA (“Ecstasy”) after haloperidol pretreatment in healthy humans. Eur Neuropsychopharmacol 2000; 10:289-295; PMID:10871712; http://dx.doi.org/ 10.1016/S0924-977X(00)00086-9. [DOI] [PubMed] [Google Scholar]
- 47. Schmid Y, Hysek CM, Simmler LD, Crockett MJ, Quednow BB, Liechti ME. Differential effects of MDMA and methylphenidate on social cognition. J Psychopharmacol 2014; 28:847-56; PMID:25052243; http://dx.doi.org/ 10.1177/0269881114542454 [DOI] [PubMed] [Google Scholar]
- 48. Kirkpatrick MG, Gunderson EW, Perez AY, Haney M, Foltin RW, Hart CL. A direct comparison of the behavioral and physiological effects of methamphetamine and 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacol (Berl) 2012; 219:109-22; PMID:21713605; http://dx.doi.org/ 10.1007/s00213-011-2383-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kolbrich EA, Goodwin RS, Gorelick DA, Hayes RJ, Stein EA, Huestis MA. Physiological and subjective responses to controlled oral 3,4-methylenedioxymethamphetamine administration. J Clin Psychopharmacol 2008; 28:432-40; PMID:18626271; http://dx.doi.org/ 10.1097/JCP.0b013e31817ef470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Freedman RR, Johanson CE, Tancer ME. Thermoregulatory effects of 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacol (Berl) 2005; 183:248-56; PMID:16163516; http://dx.doi.org/ 10.1007/s00213-005-0149-6. [DOI] [PubMed] [Google Scholar]
- 51. Verrico CD, Miller GM, Madras BK. MDMA (ecstasy) and human dopamine, norepinephrine, and serotonin transporters: implications for MDMA-induced neurotoxicity and treatment. Psychopharmacology 2007; 189:489-503; PMID:16220332; http://dx.doi.org/ 10.1007/s00213-005-0174-5. [DOI] [PubMed] [Google Scholar]
- 52. Simmler L, Buser T, Donzelli M, Schramm Y, Dieu LH, Huwyler J, Chaboz S, Hoener M, Liechti ME. Pharmacological characterization of designer cathinones in vitro. Br J Pharmacol 2013; 168:458-70; PMID:22897747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Battaglia G, Brooks BP, Kulsakdinun C, De Souza EB. Pharmacologic profile of MDMA (3,4-methylenedioxymethamphetamine) at various brain recognition sites. Eur J Pharmacol 1988; 149:159-63; PMID:2899513; http://dx.doi.org/ 10.1016/0014-2999(88)90056-8. [DOI] [PubMed] [Google Scholar]
- 54. Liechti ME, Baumann C, Gamma A, Vollenweider FX. Acute psychological effects of 3,4-methylenedioxymethamphetamine (MDMA, “Ecstasy”) are attenuated by the serotonin uptake inhibitor citalopram. Neuropsychopharmacology 2000; 22:513-21; PMID:10731626; http://dx.doi.org/ 10.1016/S0893-133X(99)00148-7. [DOI] [PubMed] [Google Scholar]
- 55. Farre M, Abanades S, Roset PN, Peiro AM, Torrens M, O’Mathuna B, Segura M, de la Torre R. Pharmacological interaction between 3,4-methylenedioxymethamphetamine (ecstasy) and paroxetine: pharmacological effects and pharmacokinetics. J Pharmacol Exp Ther 2007; 323:954-62; PMID:17890444; http://dx.doi.org/ 10.1124/jpet.107.129056. [DOI] [PubMed] [Google Scholar]
- 56. Tancer M, Johanson CE. The effects of fluoxetine on the subjective and physiological effects of 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology 2007; 189:565-73; PMID:17047932; http://dx.doi.org/ 10.1007/s00213-006-0576-z. [DOI] [PubMed] [Google Scholar]
- 57. O’Shea E, Esteban B, Camarero J, Green AR, Colado MI. Effect of GBR 12909 and fluoxetine on the acute and long term changes induced by MDMA (‘ecstasy’) on the 5-HT and dopamine concentrations in mouse brain. Neuropharmacology 2001; 40:65-74; PMID:11077072; http://dx.doi.org/ 10.1016/S0028-3908(00)00106-4. [DOI] [PubMed] [Google Scholar]
- 58. Nash JF, Jr, Meltzer HY, Gudelsky GA. Elevation of serum prolactin and corticosterone concentrations in the rat after the administration of 3,4-methylenedioxymethamphetamine. J Pharmacol Exp Ther 1988; 245:873-9; PMID:2898523. [PubMed] [Google Scholar]
- 59. Brogden RN, Sorkin EM. Ketanserin. a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in hypertension and peripheral vascular disease. Drugs 1990; 40:903-49; PMID:2079001; http://dx.doi.org/ 10.2165/00003495-199040060-00010. [DOI] [PubMed] [Google Scholar]
- 60. Hysek CM, Vollenweider FX, Liechti ME. Effects of a β-blocker on the cardiovascular response to MDMA (ecstasy). Emerg Med J 2010; 27:586-9; PMID:20378736; http://dx.doi.org/ 10.1136/emj.2009.079905. [DOI] [PubMed] [Google Scholar]
- 61. Simmler LD, Wandeler R, Liechti ME. Bupropion, methylphenidate, and 3,4-methylenedioxypyrovalerone antagonize methamphetamine-induced efflux of dopamine according to their potencies as dopamine uptake inhibitors: implications for the treatment of methamphetamine dependence. BMC Res Notes 2013; 6:220; PMID:23734766; http://dx.doi.org/ 10.1186/1756-0500-6-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sprague JE, Moze P, Caden D, Rusyniak DE, Holmes C, Goldstein DS, Mills EM. Carvedilol reverses hyperthermia and attenuates rhabdomyolysis induced by 3,4-methylenedioxymethamphetamine (MDMA, Ecstasy) in an animal model. Crit Care Med 2005; 33:1311-16; PMID:15942349. [DOI] [PubMed] [Google Scholar]
- 63. Hysek CM, Schmid Y, Rickli A, Liechti ME. Carvedilol inhibits the cardiostimulant and thermogenic effects of MDMA in humans: lost in translation. Br J Pharmacol 2013; 170:1273-75; PMID:24033079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sprague JE, Brutcher RE, Mills EM, Caden D, Rusyniak DE. Attenuation of 3,4-methylenedioxymethamphetamine (MDMA, Ecstasy)-induced rhabdomyolysis with α1- plus β3-adrenoreceptor antagonists. Br J Pharmacol 2004; 142:667-70; PMID:15159279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sprague JE, Banks ML, Cook VJ, Mills EM. Hypothalamic-pituitary-thyroid axis and sympathetic nervous system involvement in hyperthermia induced by 3,4-methylenedioxymethamphetamine (Ecstasy). J Pharmacol Exp Ther 2003; 305:159-66; PMID:12649364; http://dx.doi.org/ 10.1124/jpet.102.044982. [DOI] [PubMed] [Google Scholar]
- 66. Rawls SM, Cowan A, Tallarida RJ, Geller EB, Adler MW. N-methyl-D-aspartate antagonists and WIN 55212-2 [4,5-dihydro-2-methyl-4(4-morpholinylmethyl)-1-(1-naphthalenyl-carbonyl)-6H-pyrrolo[3,2,1-i,j]quinolin-6-one], a cannabinoid agonist, interact to produce synergistic hypothermia. J Pharmacol Exp Ther 2002; 303:395-402; PMID:12235276; http://dx.doi.org/ 10.1124/jpet.102.037473. [DOI] [PubMed] [Google Scholar]
- 67. Parrott AC, Milani RM, Gouzoulis-Mayfrank E, Daumann J. Cannabis and Ecstasy/MDMA (3,4-methylenedioxymethamphetamine): an analysis of their neuropsychobiological interactions in recreational users. J Neural Transm 2007; 114:959-68; PMID:17520319; http://dx.doi.org/ 10.1007/s00702-007-0715-7. [DOI] [PubMed] [Google Scholar]
- 68. Crandall CG. Vongpatanasin W, Victor RG. Mechanism of cocaine-induced hyperthermia in humans. Ann Intern Med 2002; 136:785-91; PMID:12044126. [DOI] [PubMed] [Google Scholar]
- 69. Brunt TM, Koeter MW, Niesink RJ, van den Brink W. Linking the pharmacological content of ecstasy tablets to the subjective experiences of drug users. Psychopharmacology (Berl) 2012; 220:751-62; PMID:21993879; http://dx.doi.org/ 10.1007/s00213-011-2529-4. [DOI] [PubMed] [Google Scholar]
- 70. Lurie Y, Gopher A, Lavon O, Almog S, Sulimani L, Bentur Y. Severe paramethoxymethamphetamine (PMMA) and paramethoxyamphetamine (PMA) outbreak in Israel. Clin Toxicol (Phila) 2012; 50:39-43; PMID:22148985; http://dx.doi.org/ 10.3109/15563650.2011.635148. [DOI] [PubMed] [Google Scholar]
- 71. Refstad S. Paramethoxyamphetamine (PMA) poisoning; a ‘party drug’ with lethal effects. Acta Anaesthesiol Scand 2003; 47:1298-9; PMID:14616331; http://dx.doi.org/ 10.1046/j.1399-6576.2003.00245.x. [DOI] [PubMed] [Google Scholar]
- 72. Johansen SS, Hansen AC, Muller IB, Lundemose JB, Franzmann MB. Three fatal cases of PMA and PMMA poisoning in Denmark. J Anal Toxicol 2003; 27:253-6; PMID:12820749; http://dx.doi.org/ 10.1093/jat/27.4.253. [DOI] [PubMed] [Google Scholar]
- 73. Simmler LD, Rickli A, Hoener MC, Liechti ME. Monoamine transporter and receptor interaction profiles of a new series of designer cathinones. Neuropharmacology 2014; 79:152-60; PMID:24275046; http://dx.doi.org/ 10.1016/j.neuropharm.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 74. De Letter EA, Coopman VA, Cordonnier JA, Piette MH. One fatal and seven non-fatal cases of 4-methylthioamphetamine (4-MTA) intoxication: clinico-pathological findings. Int J Legal Med 2001; 114:352-6; PMID:11508803; http://dx.doi.org/ 10.1007/s004140100204. [DOI] [PubMed] [Google Scholar]
- 75. Borek HA, Holstege CP. Hyperthermia and multiorgan failure after abuse of “bath salts” containing 3,4-methylenedioxypyrovalerone. Ann Emerg Med 2012; 60:103-5; PMID:22387085; http://dx.doi.org/ 10.1016/j.annemergmed.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 76. Peyre H, Delorme R. A case of severe hyperthermia after administration of methylphenidate. J Clin Psychopharmacol 2012; 32:299-300; PMID:22388166; http://dx.doi.org/ 10.1097/JCP.0b013e3182499677. [DOI] [PubMed] [Google Scholar]
- 77. Morefield KM, Keane M, Felgate P, White JM, Irvine RJ. Pill content, dose and resulting plasma concentrations of 3,4-methylendioxymethamphetamine (MDMA) in recreational ‘ecstasy’ users. Addiction 2011; 106:1293-300; PMID:21320226; http://dx.doi.org/ 10.1111/j.1360-0443.2011.03399.x. [DOI] [PubMed] [Google Scholar]
- 78. Dubin WR, Feld JA. Rapid tranquilization of the violent patient. Am J Emerg Med 1989; 7:313-20; PMID:2565724; http://dx.doi.org/ 10.1016/0735-6757(89)90179-4. [DOI] [PubMed] [Google Scholar]
- 79. Green AR, Cross AJ, Goodwin GM. Review of the pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA or “Ecstasy”). Psychopharmacology 1995; 119:247-60; PMID:7675958; http://dx.doi.org/ 10.1007/BF02246288. [DOI] [PubMed] [Google Scholar]
- 80. Rusyniak DE, Banks ML, Mills EM, Sprague JE. Dantrolene use in 3,4-methylenedioxymethamphetamine (ecstasy)-mediated hyperthermia. Anesthesiology 2004; 101:263; author reply 264; PMID:15220814; http://dx.doi.org/ 10.1097/00000542-200407000-00053. [DOI] [PubMed] [Google Scholar]
- 81. Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Babler A, Vogel H, Hell D. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport 1998; 9:3897-902; PMID:9875725; http://dx.doi.org/ 10.1097/00001756-199812010-00024. [DOI] [PubMed] [Google Scholar]
- 82. Blessing WW, Seaman B, Pedersen NP, Ootsuka Y. Clozapine reverses hyperthermia and sympathetically mediated cutaneous vasoconstriction induced by 3,4-methylenedioxymethamphetamine (ecstasy) in rabbits and rats. J Neurosci 2003; 23:6385-91; PMID:12867524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lange RA, Cigarroa RG, Flores ED, McBride W, Kim AS, Wells PJ, Bedotto JB, Danziger RS, Hillis LD. Potentiation of cocaine-induced coronary vasoconstriction by β-adrenergic blockade. Ann Intern Med 1990; 112:897-903; PMID:1971166. [DOI] [PubMed] [Google Scholar]
- 84. Ramoska E, Sacchetti AD. Propranolol-induced hypertension in treatment of cocaine intoxication. Ann Emerg Med 1985; 14:1112-3; PMID:4051280; http://dx.doi.org/ 10.1016/S0196-0644(85)80934-3. [DOI] [PubMed] [Google Scholar]
- 85. Boehrer JD, Moliterno DJ, Willard JE, Hillis LD, Lange RA. Influence of labetalol on cocaine-induced coronary vasoconstriction in humans. Am J Med 1993; 94:608-10; PMID:8506886; http://dx.doi.org/ 10.1016/0002-9343(93)90212-8. [DOI] [PubMed] [Google Scholar]
- 86. Sofuoglu M, Brown S, Babb DA, Pentel PR, Hatsukami DK. Effects of labetalol treatment on the physiological and subjective response to smoked cocaine. Pharmacol Biochem Behav 2000; 65:255-9; PMID:10672977; http://dx.doi.org/ 10.1016/S0091-3057(99)00201-4. [DOI] [PubMed] [Google Scholar]
- 87. Sofuoglu M, Brown S, Babb DA, Pentel PR, Hatsukami DK. Carvedilol affects the physiological and behavioral response to smoked cocaine in humans. Drug Alcohol Depend 2000; 60:69-76; PMID:10821991; http://dx.doi.org/ 10.1016/S0376-8716(99)00143-X. [DOI] [PubMed] [Google Scholar]
- 88. Spaziani ML, Schult RF, Wiegand TJ. Lisdexamfetamine ingestion resulting in hypertensive emergency treated with phentolamine. Clin Toxicol (Phila) 2014; 52:406; http://dx.doi.org/ 10.3109/15563650.2014.906213. [DOI] [Google Scholar]
- 89. Holden R, Jackson MA. Near-fatal hyponatraemic coma due to vasopressin over-secretion after “ecstasy” (3,4-MDMA). Lancet 1996; 347:1052; PMID:8606600; http://dx.doi.org/ 10.1016/S0140-6736(96)90196-8. [DOI] [PubMed] [Google Scholar]
- 90. Hartung TK, Schofield E, Short AI, Parr MJ, Henry JA. Hyponatraemic states following 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’) ingestion. QJM 2002; 95:431-437; PMID:12096147; http://dx.doi.org/ 10.1093/qjmed/95.7.431. [DOI] [PubMed] [Google Scholar]
- 91. Simmler LD, Hysek CM, Liechti ME. Sex differences in the effects of MDMA (ecstasy) on plasma copeptin in healthy subjects. J Clin Endocrinol Metab 2011; 96:2844-50; PMID:21715530; http://dx.doi.org/ 10.1210/jc.2011-1143. [DOI] [PubMed] [Google Scholar]
- 92. Rosenson J, Smollin C, Sporer KA, Blanc P, Olson KR. Patterns of ecstasy-associated hyponatremia in California. Ann Emerg Med 2007; 49:164-71; PMID:17084942; http://dx.doi.org/ 10.1016/j.annemergmed.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 93. Budisavljevic MN, Stewart L, Sahn SA, Ploth DW. Hyponatremia associated with 3,4-methylenedioxymethylamphetamine (“Ecstasy”) abuse. Am J Med Sci 2003; 326:89-93; PMID:12920440; http://dx.doi.org/ 10.1097/00000441-200308000-00006. [DOI] [PubMed] [Google Scholar]
- 94. Balmelli C, Kupferschmidt H, Rentsch K, Schneemann M. [Fatal brain edema after ingestion of ecstasy and benzylpiperazine]. Dtsch Med Wochenschr 2001; 126:809-11; PMID:11499262; http://dx.doi.org/ 10.1055/s-2001-15702. [DOI] [PubMed] [Google Scholar]
- 95. Sharma HS, Ali SF. Acute administration of 3,4-methylenedioxymethamphetamine induces profound hyperthermia, blood-brain barrier disruption, brain edema formation, and cell injury. Ann N Y Acad Sci 2008; 1139:242-58; PMID:18991870; http://dx.doi.org/ 10.1196/annals.1432.052. [DOI] [PubMed] [Google Scholar]


