Abstract
Cyclin D2 (CCND2) is a member of the D-type cyclins, which plays a pivotal role in cell cycle regulation, differentiation and malignant transformation. However, its expression status and relative regulation mechanism remains unclear in renal cell cancer (RCC). In our study, the mRNA expression level of CCND2 is down-regulated in 22/23 paired RCC tissues (p<0.05). In addition, its protein expression level is also decreased in 43/43 RCC tumor tissues compared with its corresponding non-malignant tissues (p<0.001). We further detected that CCND2 was down-regulated or silenced in 6/7 RCC cell lines, but expressed in “normal” human proximal tubular (HK-2) cell line. Subsequently, MSP and BGS results showed that the methylation status in CCND2 promoter region is closely associated with its expression level in RCC cell lines. Treatment with 5-Aza with or without TSA restored CCND2 expression in several methylated RCC cell lines. Among the 102 RCC tumors, methylation of CCND2 was detected in 29/102 (28%) cases. Only 2/23 (8.7%) adjacent non-malignant tissues showed methylation. We then analyzed the correlation of clinical features and its promoter methylation. Collectively, our data suggested that loss of CCND2 expression is closely associated with the promoter aberrant methylation.
Introduction
In mammals, loss control of cell cycle is a distinctive feature of human carcinogenesis. The regulation of cell cycle mainly associated with the orderly change of phase-specific protein, such as cyclins, cyclin-dependent kinases (CDKs) and CDK inhibitors [1]. Among these cyclins, the D-type cyclins (D1, D2, D3) are mainly involved in regulation of transition from G1 to S phase during the cell cycle [2]. Their critical function is to associate with CDKs and phosphorylates for activity, then leading to the phosphorylation of the retinoblastoma tumor suppressor protein (RB), a critical negative regulator of G1/S transition [3]. The phosohorylation of RB inactivates it and leads to the activation of transcription factors such as E2F suppressed by RB, which then increased the activation of transcription of genes involved in DNA synthesis and induced cells to enter S phase [4]. Owing to their pivotal role in cell cycle regulation, the abnormal and untimely expression of these proteins is likely to disturb cell cycle and promote the carcinoma transformation [5,6].
Cyclin D2/CCND2, located at chromosome 12p13, is a member of the D-type cyclin family and mainly mediates the extracellular signaling environment with cell cycle progression [7]. CCND2 was generally considered as a protooncogene in various tumors, overexpression of CCND2 has been reported in testicular germ cell tumor cell lines [8], colon cancer [9] and gastric cancer correlating with tumor progression and poor prognosis [5,10]. Although well known for its proliferation-promoting function, D-type cyclins were also shown to have the ability of growth-inhibitory function by inducing a senescence-like phenotype [11] and inhibiting cell proliferation [12]. Several studies have found that loss of CCND2 expression was observed in breast [6,13–15], lung [15], prostate [16], pancreatic [17] and gastric cancer [18]. CCND2 has also been reported to be the only one of D-type cyclins, being up-regulated in the conditions of growth arrest, ectopic expression of CCND2 blocked the progression of cell cycle [12], suggesting that CCND2 might function as a tumor suppressor gene in a cancer-type dependent manner.
Aberrant promoter methylation of tumor suppressor gene is a common feature of renal cell cancer [19–21] and a promising tool for early diagnosis [21]. Precious research has explained that hypermethylation of promoter region within CpG ialands is associated with the transcriptional silence of several genes in multiple kinds of tumors [22]. An increasing number of studies have demonstrated that a large number of TSGs (tumor suppressive genes) have been found to be inactivated by promoter hypermethylation [19–21]. Indeed, reduced expression of CCND2 has been reported in various cancers and the mechanism underlying CCND2 silencing in these mentioned cases is the aberrant promoter methylation [6,14–18]. As is reported in prostate cancer, CCND2 promoter methylation was more frequently observed in high Gleason score tumors [23] and was associated with tumor development in prostate [24].However, the expression of CCND2 and the regulation mechanism underlying renal cell cancer has not been reported before.
Owing to the controversial role of CCND2 in different type of tumors, we aimed to assess the expression of CCND2 and the status of promoter methylation in RCC cell lines, tumors and adjacent non-malignant tissues. Our results showed that CCND2 was down-regulated in RCC cell lines and primary RCC tissues. Moreover, the methylation status of CCND2 was more frequently observed in RCC tissues than adjacent non-malignant tissues. Demethylation treatment of RCC cell lines restored CCND2 expression, which suggested that epigenetic alteration might be the mainly regulated manner of CCND2 expression.
Methods and Materials
The experimental methods and usage of clinical samples were approved by the Ethics Committee of the Peking University First Hospital. All these specimens were diagnosed by two urological pathological pathology physicians with patients’ written consent.
Samples and cell lines
The RCC cell lines 786-O, A498, CAKI-1, CAKI-2, OSRC, 769P, KOTO3 and HK-2 (a “normal” human proximal tubular cell line) were originally obtained (American Type Culture Collection, VA, USA). These cell lines were routinely maintained in RPMI1640 or DMEM medium with 10% fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA), 1% penicillin G, and 1% streptomycin at 37°C in humidified CO2 (5%) incubator.
All the RCC samples (fresh tissues and paraffine section tissues) were obtained from the Urology Department, Peking University First Hospital, Beijing, People’s Republic of China. All the RCC tissues and corresponding non-malignant tissues were collected from radical surgical resection without adjuvant therapy. All these specimens were diagnosed by two urological pathology physicians with patients’ written consent. A 2002 AJCC TNM stage and a Fuhrman nuclear grade were used for the classification of the tumor histopathology.
DNA / RNA extraction and Bisulfite treatment
For RNA extraction, fresh frozen tissues and cell lines were homogenized in TRI Reagent (Molecular Research Center, Cincinnati, OH) and isolated as previously described [25]. Total genomic DNA of RCC tissues and cell lines was extracted according to the manufacturer’s instruction supplied by QIAamp DNA Mini Kit (Qiagen GmbH, Hilden, Germany). Then sodium bisulfite modification of genomic DNA was carried out as described previously [26].
Reverse transcriptional PCR and Real-time PCR
To evaluate the mRNA expression of CCND2 in RCC cell lines, reverse transcription PCR (RT-PCR) with GoTaq® Green Master Mix (Promega, Madison, WI, USA) and Real-time PCR with (HT7500 system, Applied Biosystems) were used according to the manufacturer’s protocol. Then we used Real-time PCR to examine CCND2 mRNA status in 23 paired RCC tumors and adjacent non-malignant tissues. The primers were: CCND2-F: CATGGAGCTGCTGTGCCACG; CCND2-R: CCGACCTACCTCCAGCATCC. The RT-PCR reaction condition was 35 cycles (94°C for 30 s, 55°C for 30 s, and 72°C for 30 s). Real-time PCR was done for 45 cycles (94°C for 30 s, 55°C for 30 s, and 72°C for 30 s). GAPDH primers were: GAPDH-F: TCCTGTGGCATCCACGAAACT; GAPDH-R: GAAGCATTTGCGGTGGACGAT, which was used as an internal control.
Demethylation treatment
Six RCC cells were primarily seeded at a density of 1x106 cells/ml in six-well plate, after overnight culture, cells were treated with 10μM demethylation agent 5-Aza (5-aza-2’-deoxycytidine) for 96 hours, some RCC cell lines were further treated with 100 nmol/L histone deacetylase inhibitor TSA (trichostatin A) for additional 24 hours. After the treatment, the cells were collected for DNA and RNA extractions.
Methylation specific PCR (MSP) and bisulfite genomic sequencing (BGS)
MSP and BGS were performed on the bisulfite-modified DNA samples using primer sets targeting the CpG-rich region in CCND2 promoter to indentify the CCND2 promoter methylation status. The methylated and unmethylated primers of CCND2 were CCND2-m1: TACGTGTTAGGGTCGATCG; CCND2-m2: CGAAATATCTACGCTAAA-CG (276-bp product); CCND2-u1: GTTATGTTATGTTTGTTGTATG; CCND2-u2: TAAAATCCACCAACACAATCA (223-bp-product). The MSP was conducted in a 12.5μl reaction solution with GoTaq® Green Master Mix (Promega, Madison, WI, USA) for 35 cycles (94°C for 30 s, 55°C for 30 s, and 72°C). PCR products were analysed in 2% agarose gels. To further confirm our MSP results, BGS was used to analyze the detailed methylation status. The BGS primers were CCND2-BGS1: GGAGGAAGGAGGTGAAGAA; CCND2-BGS2: CCCCTACATCTACTAACAAAC. 40 cycles/58°C annealing temperature of PCR were performed using GoTaq® Green Master Mix. The PCR products were connected into the pEASY-T5 zero vectors (TransGen Biotech Co., Ltd, Beijing), then 5–9 colonies were randomly selected and sequenced.
Immunohistochemistry staining
Immunohistochemistry was carried out on forty-three paraffin-embedded RCC tissues and paired adjacent non-malignant tissues microarray with the primary antibody CCND2 (NOVUS, NBP2-14460) in 1:250 dilution. All the photographs were taken randomly and measured using Image Pro Plus (IPP, version 6.0, Media Cybernatics, Silver Spring, MD, USA).
Cell transfection
pLVX-IRES-ZsGreen1 and pLVX-IRES-ZsGreen1-CCND2 vectors were bought from company (YouBio, Chang Sha). Then 786-O and OSRC cells were transfected with pLVX-IRES-ZsGreen1 and pLVX-IRES-ZsGreen1-CCND2 using Lip 3000 kit (Invitrogen) according to the manufacturer’s instruction.
Cell proliferation assay
Cells transfected with CCND2 and vector were harvested and then plated in 96-well plate at a density of 2000 per well and incubated overnight at 37°C. The Cell Counting Kit-8 (Dojindo, Kumamoto, Japan) was used to analyze the cell proliferation. 10μL CCK-8 solution was added into each well with 100μL serum-free optional medium and incubated for 40 minutes. The absorbance was measured at 450nm. All the results were obtained from three independent experiments.
Statistical analysis
Data were presented as mean ± standard deviation (SD). Student’s t test, Fisher’s exact test and Chi-square test were used. Statistical analyzed using the SAS version 9.0 software. A p-value was considered significant when less than 0.05.
Results
Decreased expression of CCND2 in RCC patients
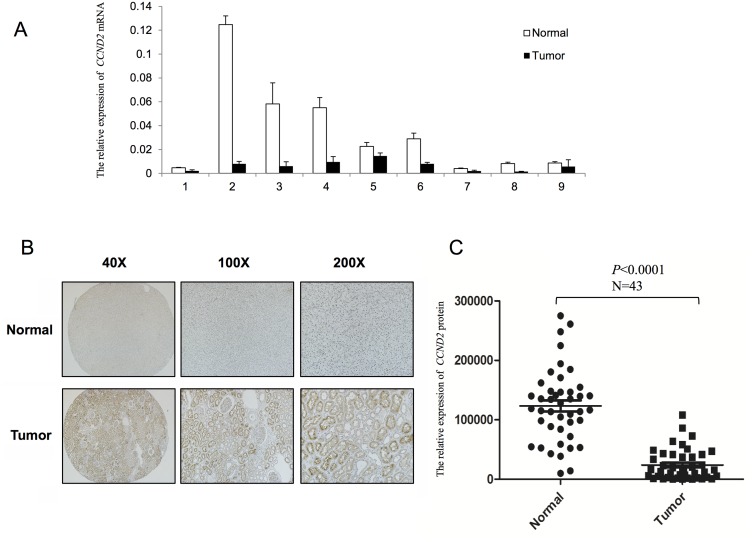
Serial analysis of gene expression (http://www.proteinatlas.org/) had previously revealed that, CCND2 expression level was significantly lower in RCC tissues. To check the validity of these findings, we performed Real-time PCR to evaluate the mRNA status of CCND2 in 23 paired RCC tumors and adjacent non-malignant tissues. The expression of CCND2 was noted in 22/23 adjacent non-malignant tissues. In contrast, only 1/23 adjacent non-malignant tissue showed slightly decreased expression of CCND2 compared with RCC tumor tissue (Fig 1A, p<0.05). Moreover, we used immunohistochemical staining to examine the CCND2 protein expression in 43 RCC tumors paired with adjacent non-malignnat tissues. We found that CCND2 was downregulated in RCC tissues inordinately. Interestingly, CCND2 protein expression was decreased in 43/43 RCC tumors compared with the corresponding adjacent non-malignant tissues (Fig 1B). By analyzing the immunohistochemistry staining results with Image Pro-Plus and statistical analysis, CCND2 expression showed a significant difference between tumors and adjacent non-malignant tissues (Fig 1C, p<0.0001). Thus, in both mRNA and protein status, specific loss of CCND2 was observed.
Fig 1. Evaluation of CCND2 expression in RCC samples.
(A) Real-time PCR revealed the mRNA expression level of CCND2 in paired RCC primary tumor tissues (black column) compared with the adjacent non-malignnat tissues (white column), p<0.05; 1–9 represents the patient number (selected randomly from the 23 paired RCC patients). (B) Immunohistochemistry staining showed a significant decrease of CCND2 protein expression in 43 paired RCC samples in 40X, 100X, 200X magnification. (C) Quantitative analysis of CCND2 protein expression status with Image Pro Plus software, p<0.001.
Hypermethylation of CCND2 is associated with transcriptional silencing in RCC cell lines
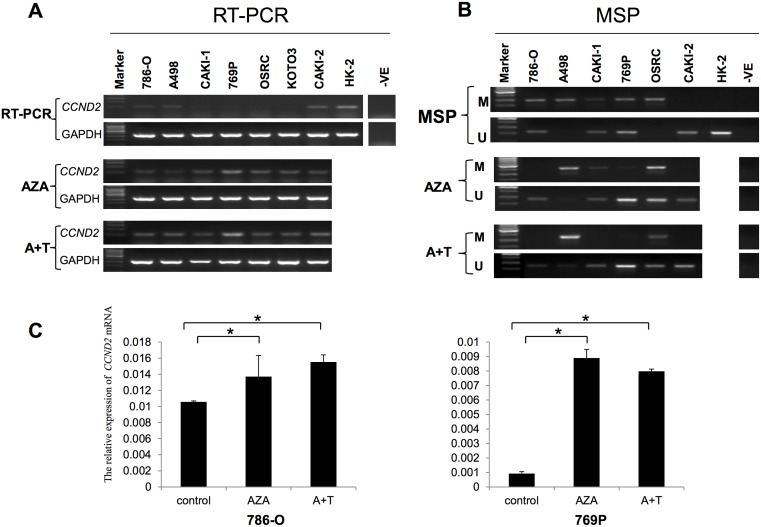
To check the expression of CCND2 in 7 RCC cell lines and one “normal” human proximal tubular (HK-2) cell line, RT-PCR was used with GAPDH as an internal control. As is shown in Fig 2A, CCND2 was significantly down-regulated in 6/7 RCC cell lines and weakly decreased in CAKI-2 cell line comparing with HK-2 cell line. This is accordance with the results of RCC clinical samples. As is known to all, both genetic and epigenetic aberrant alteration influence the expression of genes. By analyzing the promoter region, we found two typical CpG islands in CCND2 promoter region (Fig 3A, http://cpgislands.usc.edu/). To further assess the relationship between epigenetic regulation and CCND2 expression, methylation specific PCR (MSP) was performed to evaluate the methylation of CCND2 promoter. Hypermethylation at CpG-rich region with no mRNA expression of CCND2 was detected in 5/6 RCC cell lines (786-O, A498, CAKI-1, 769P, OSRC), but was not detected in CAKI-2 and HK-2 with CCND2 mRNA expression (Fig 2B). This implied that promoter methylation might be the mainly mechanism that regulated CCND2 expression.
Fig 2. CCND2 expression and promoter methylation in RCC cell lines before and after demethylation treament.
(A) Reverse transcription PCR (RT-PCR) was used to analysis CCND2 expression in RCC cell lines and HK-2 cell with or without demethylation drugs, AZA: 5-aza-2’-deoxycytidine; A+T: AZA + TSA (trichostatin A); -ve represents negative control. (B) Methylation specific PCR detected the corresponding methylation status in RCC cell lines. (C) Qualitative analysis of CCND2 mRNA expression in 786-O and 769P cells after demethylation treatment by Real-time PCR, * represents p<0.05.
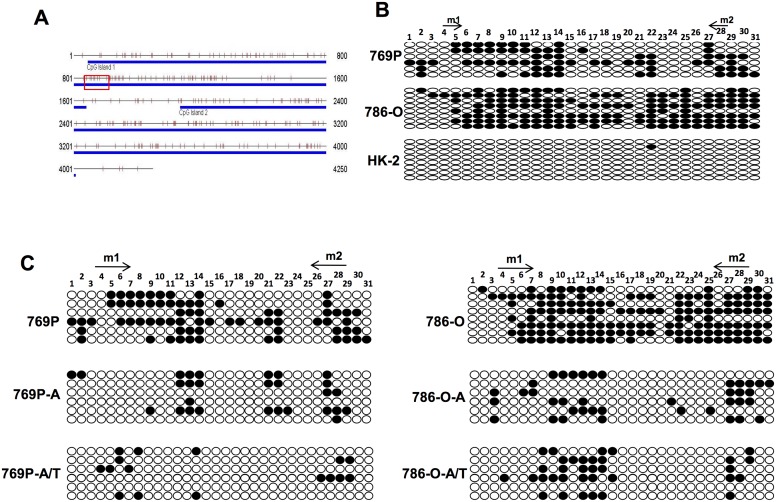
Fig 3. Bisulfite genome sequencing (BGS) analysis of the promoter CpG sites.
(A) Two CpG islands were found within CCND2 promoter region, the red square marked MSP tested sites. (B) Bisulfite genomic sequencing of each CpG site (oval) in CCND2 promoter region was performed in 769P, 786-O and HK-2 cell lines. Arrows: MSP primers binding sites; Solid oval: methylated CpG site; Hollow oval: unmethylated CpG site. (C) BGS analyzed the methylation of CpG sites after drug treatment in RCC cell lines.
Whether the methylation mainly mediated CCND2 expression needs further exploration. If the down-regulation/silence of CCND2 was regulated by promoter methylation, then demethylation of the gene by exposure to DNA methyltransferase inhibitor 5-Aza-2'-deoxycytidine (5-Aza) or treatment with histone deacetylase inhibitor trichostatin A (TSA) should result in the up-regulation of CCND2. Indeed, when six RCC cell lines were treated with 5-Aza with or without TSA, five out of six cell lines showed a differently increased expression of CCND2 (as analyzed by RT-PCR and Real-time PCR, Fig 2A and 2C) and partially demethylated (as analyze by MSP, Fig 2B).
Bisulfite genome sequencing (BGS) analysis of CCND2 promoter CpG sites
To verify the MSP findings and to explore the extent of promoter methylation, bisulfite genomic sequencing (BGS) was performed. The CpG-rich region of CCND2 promoter from the nucleotides -1507 to -1130 was sequenced after bisulfite modification. As shown in Fig 3B, BGS was performed in three RCC cell lines: one with CCND2 expression (HK-2), two with low CCND2 expression (769-P, 786-O). 769P and 786-O showed higher methylation status than HK-2 cell line, which is consistent with the previously results of MSP. From this part we concluded that hypermethylation may lead to the CCND2 silencing in RCC cell lines. Meanwhile, BGS results were consistant with the MSP result before or after pharmacological demethylation treatment (Fig 3C). These results implied that aberrant methylation of CCND2 suppressed its expression in RCC.
Analyzing the methylation status in primary RCC samples and adjacent non-malignant tissues
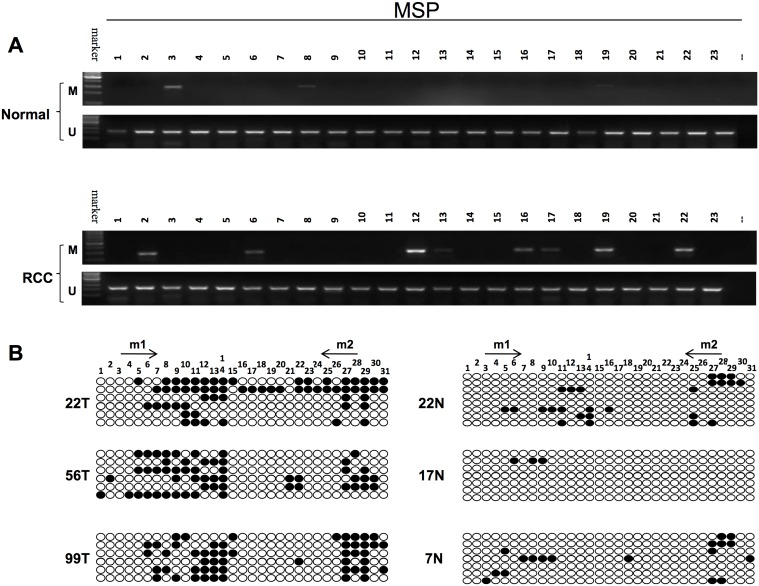
To determine the methylation of CCND2 promoter, MSP was used to examine the methylation status in 102 RCC tumors and 23 adjacent non-malignant tissues. As is shown in Table 1, the methylation of CCND2 was observed in 29/102 (28%) of RCC tumors, but only 2/23 (8.7%) in adjacent non-malignant tissues (Table 1), which suggested that CCND2 methylation was more likely to occur in tumor tissues, as is shown in Fig 4A. To verify the MSP results, BGS was subsequently done (Fig 4B). Then we analyzed the relationship of CCND2 methylation with clinicopathological features of these patients. As is listed in Table 2, there was no significant difference between CCND2 methylation and gender, age, tumor site, TNM stage, nuclear grade or histological classification by statistical evaluation. We found the percentage of CCND2 methylation decreased as the nuclear grade increased, suggesting CCND2 methylation occurs in a relatively early period in carcinogenesis. In conclusion, CCND2 methylation is a frequent event in RCC tumorigenesis.
Table 1. The percentage of methylation status in RCC samples and adjacent non-malignant tissues.
| RCC samples | CCND2 promoter | Methylation percentage | |
|---|---|---|---|
| Methylation | Unmethylation | ||
| Tumor | 29 | 73 | 28% |
| Non-malignant | 2 | 21 | 8.7% |
The methylation of CCND2 was observed in 29/102 (28%) of RCC, but only 2/23 (8.7%) in adjacent non-malignant tissues.
Fig 4. The methylation of CCND2 in primary RCC samples.
(A) MSP analyzed the CCND2 promoter methylation status in RCC tissues and adjacent non-malignnat tissues, M: metyhlated; U: unmethylated. (B) BGS analysis of CCND2 promoter CpG sites in RCC patients.
Table 2. The correlation of clinicopathological features and CCND2 methylation status.
| Clinicopathological Features | Methylated NO. (%) | Unmethylated NO. (%) | p value | |
|---|---|---|---|---|
| Age | 57.34+13.09 | 55.45+11.9 | 0.517 | |
| Gender | Male | 21(33%) | 43(67%) | 0.2586 |
| Female | 8(21%) | 30(79%) | ||
| Side | Left | 11(22%) | 40(78%) | 0.1874 |
| Right | 18(35%) | 33(65%) | ||
| TNM classification | pT1 | 20(30%) | 46(70%) | 0.0674 |
| pT2 | 3(33%) | 6(67%) | ||
| pT3 | 4(16%) | 21(84%) | ||
| pT4 | 2(100%) | 0(0) | ||
| Nuclear grade | G1 | 5(38%) | 8(62%) | 0.6537 |
| G2 | 20(27%) | 52(73%) | ||
| G3 | 4(23%) | 13(77%) | ||
| Pathological types | ccRCC | 26(30%) | 60(70%) | 0.49 |
| Chromophobe RCC | 2(33%) | 4(67%) | ||
| Papillary RCC | 1(20%) | 4(80%) | ||
| others | 0(0) | 5(100%) | ||
Correlation of CCND2 methylation and gender, age, tumor site, TNM stage, nuclear grade and histological classification, ccRCC: Clear Cell RCC.
Ectopic expression of CCND2 inhibits tumor cell proliferation
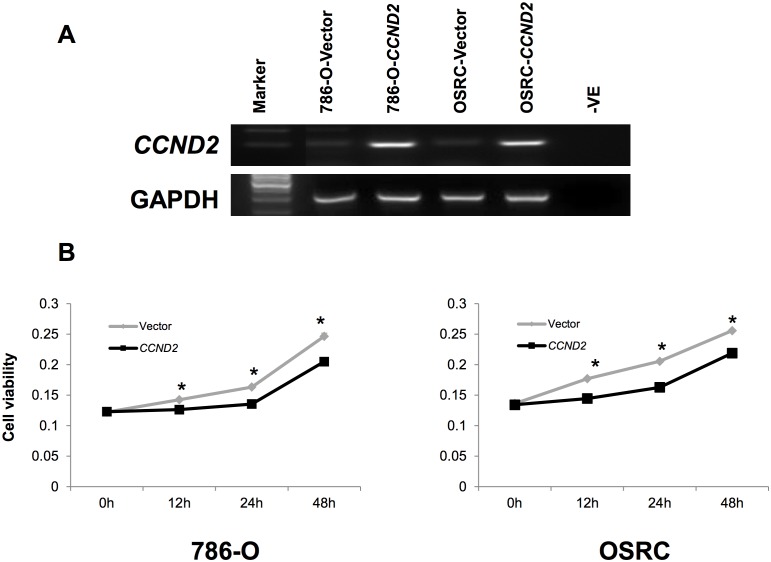
We investigated the effect of ectopic CCND2 expression on cell proliferation of 786-O and OSRC cells. As the results showed, CCND2 suppressed the proliferation ability of cells in CCND2 transfected 786-O and OSRC cells, compared with vector-transfected control cells (Fig 5B). Re-expression of CCND2 in transient transfected cell lines was evidenced by RT-PCR (Fig 5A). These results implied that CCND2 could to some extent inhibit the proliferation of tumor cells.
Fig 5. Effect of ectopic CCND2 expression on tumor growth.
(A) Re-expression of CCND2 in 786-O and OSRC cells was confirmed by RT-PCR. (B) Cell growth curve was inhibited by CCND2 in 786-O and OSRC cells. The experiments were repeated three times in triplicate. h represents hour Data are mean ± s.d; *p<0.01.
Discussion
Renal cell cancer is the most common urological cancer which account for about 90% kidney cancer cases in adults with a steady increasing incidence [27]. Even though many advanced methods have been found for RCC diagnosis and therapy, many patients still presented with an aggressive phenotype, including frequent metastasis to distant organs and less sensitive to chemotherapy and radiotherapy [28]. Therefore, elucidation of molecular mechanism underlying RCC and acquisition of specific biomarkers for early diagnosis is urgently needed.
In this study, we found that both RNA and protein expression levels of CCND2 were decreased in RCC tissues compared to adjacent non-malignant tissues. Moreover, the methylation level of CCND2 promoter was higher in RCC cell lines than HK-2 cell. It was interesting to found that drug demethylation treatment restored its expression and decreased the methylation level in RCC cell lines. Which suggest that promoter methylation could be the mainly mechanism underlying the loss of CCND2 expression in both RCC cell lines as well as primary RCC samples.
The cyclin D1-3 protein are generally recognized as regulators that lead to transition of cells from G1 to DNA synthesis by phosphorylation and inactivation of the retinoblastoma protein and activation of cyclin E. Besides for their function in cell cycle, it has been reported that D-type cyclins implicated in the differentiation and carcinogenesis. In renal cell cancer, CCND1 was absent in normal kidney samples but overexpressed in RCC. High CCND1 expression was related to good clinical outcome and to most known favorable prognostic factors [29]. Moreover, high CCND3 protein was observed in 16% of the tumors and was significantly associated with high TNM stage, high nuclear grade, high proliferation and young age [30]. The function and mechanism of CCND2 has been reported that CCND2 promote cell cycle progression through phosphorylation of retinoblastoma [3]. On the contrary, CCND2 could also contribute to the induction and/or maintenance of a non-proliferative state by sequestration of CDK2 catalytic subunit [12]. In spite of this, the role of CCND2 in renal cell cancer was less thoroughly investigated. Therefore, to assess the CCND2 expression and the regulation mechanism in RCC is helpful for further understanding the pathogenesis of RCC.
As previously reported, overexpression of CCND2 has been noted in gastric cancer, as the results showed overexpression of CCND2 closely correlates with cancer progression and supposed to be an independent prognostic factor [5]. It was also noted that CCND2 was over-expressed in various kinds of tumors [8,9]. In recent years, several groups have reported the reduced or lack of expression of CCND2 in breast, lung [6,13–15] and prostate cancers [16]. The different expression of CCND2 in different type of tumor indicated that CCND2 may function as an oncogene or TSG in a tumor type-dependent manner. Indeed, overexpression of CCND2 was linked with increased cell proliferation [31], but it was also reported that CCND2 could negatively regulate cell growth. Previous studies demonstrated that overexpression of CCND2 in fibroblast cell efficiently blocked the early G0 and G1 phase [12]. Thus, we showed diminished CCND2 mRNA expression in 23 RCC samples compared with adjacent non-malignant tissues (p<0.05). CCND2 mRNA was also decreased in 6/7 RCC cell lines as the RT-PCR results showed. Furthermore, lack of protein expression was also observed in 43 paired RCC samples (p<0.001). After ectopic expression of CCND2, we found the ability of tumor growth was inhibited. These findings may indicate a tumor suppressive role of CCND2 in RCC, and its down-regulation may be related to tumorigenesis.
Multiple mechanisms underlying inactivation of genes have been reported in various types of cancers, such as loss of heterozygosity, point mutations, homozygous deletions, and aberrant promoter methylation [32]. Aberrant methylation of CpG islands was identified as epigenetic mechanism that regulate the inactivation of TSGs in many cancer types, the percentage of methylated genes in cancers is estimated to be high [33,34]. Multiple TSGs has been reported to be down-regulated by promoter methylation in RCC [19,20]. The transcriptional silencing of CCND2 by aberrant promoter methylation has been reported in many kinds of tumors. In prostate cancer, the frequency of CCND2 methylation status was much higher in prostate cancer samples than in non-malignant prostate tissues (p<0.005). By analyzing the clinicopathological correlations, greater CCND2 methylation frequency was found to occur in high Gleason score group and in patients with higher mean PSA value. In addition, methylation of CCND2 correlates with prostate poor prognosis [23]. Likewise, it was reported that majority of primary breast carcinomas lack expression of CCND2 mRNA (18 of 24) and protein (10 of 13). In contrast, CCND2 was expressed in normal luminal and myoepithelial breast tissues. The silencing of CCND2 expression was associated with its promoter aberrant methylation by MSP. The methylation of CCND2 promoter was also detected in ductal carcinoma in situ, which suggested that loss of CCND2 is an early event in tumorigenesis and may associated with the evolution of breast cancer [6]. The absence of CCND2 expression by promoter methylation is also observed in gastric cancer [18]. To search for a mechanism underlying the consistent loss of CCND2 in renal cell cancer, we then test the methylation of CCND2 promoter as a possible cause that lead to the silence of this gene.
As the results showed, the methylation level of CCND2 was significantly higher in RCC tissues than in adjacent non-malignant samples (Table 2). Moreover, drug demethylation treatment restored CCND2 expression in RCC cell lines, indicating epigenetic regulation mainly controlled the expression of CCND2 and CCND2 promoter methylation may play a role in tumorigenesis. We also analyzed the correlation between CCND2 methylation and clinicopathological features such as gender, tumor diameters, pathological stage, nuclear grade and histological classification. No association was observed between CCND2 promoter methylation status and the clinicopathological features (gender, tumor diameters, pathological stage, nuclear grade and histological classification) of RCC patients. Many genes are known to be aberrantly methylated in RCC [19,21,35,36], according to our results, methylated CCND2 may be an useful part of the panel of markers that could be used for clinical diagnosis. Further exploration is needed to verify our previous results.
Conclusion
In conclusion, the result that CCND2 is down-regulated in renal cell cancer strongly suggests that the function of CCND2 is not only limited to its role in cell cycle transition from G1 to S. The cancer-specific loss of CCND2 in renal carcinoma and suppression of RCC cell growth provide clues for investigating a possible role of CCND2 in carcinogenesis in kidney and the methylation of its promoter may function as an optional biomarker for clinical application.
Data Availability
All relevant data are within the paper.
Funding Statement
This study was supported by grants from the National Natural Science Foundation (No. 81272290, No. 81572510, No. 81370858 and No. 81570683), Beijing Municipal Science and Technology Commission (No. Z131107002213130) and Beijing Natural Science Foundation (No. 7142161).
References
- 1.Tsihlias J, Kapusta L, Slingerland J (1999) The prognostic significance of altered cyclin-dependent kinase inhibitors in human cancer. Annual review of medicine 50: 401–423. [DOI] [PubMed] [Google Scholar]
- 2.Sherr CJ (1995) D-type cyclins. Trends in biochemical sciences 20: 187–190. [DOI] [PubMed] [Google Scholar]
- 3.Weinberg RA (1995) The retinoblastoma protein and cell cycle control. Cell 81: 323–330. [DOI] [PubMed] [Google Scholar]
- 4.Zhang P (1999) The cell cycle and development: redundant roles of cell cycle regulators. Current opinion in cell biology 11: 655–662. [DOI] [PubMed] [Google Scholar]
- 5.Takano Y, Kato Y, Masuda M, Ohshima Y, Okayasu I (1999) Cyclin D2, but not cyclin D1, overexpression closely correlates with gastric cancer progression and prognosis. The Journal of pathology 189: 194–200. [DOI] [PubMed] [Google Scholar]
- 6.Evron E, Umbricht CB, Korz D, Raman V, Loeb DM, et al. (2001) Loss of cyclin D2 expression in the majority of breast cancers is associated with promoter hypermethylation. Cancer research 61: 2782–2787. [PubMed] [Google Scholar]
- 7.Zhang Q, Sakamoto K, Wagner KU (2014) D-type Cyclins are important downstream effectors of cytokine signaling that regulate the proliferation of normal and neoplastic mammary epithelial cells. Molecular and cellular endocrinology 382: 583–592. 10.1016/j.mce.2013.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sicinski P, Donaher JL, Geng Y, Parker SB, Gardner H, et al. (1996) Cyclin D2 is an FSH-responsive gene involved in gonadal cell proliferation and oncogenesis. Nature 384: 470–474. [DOI] [PubMed] [Google Scholar]
- 9.Mermelshtein A, Gerson A, Walfisch S, Delgado B, Shechter-Maor G, et al. (2005) Expression of D-type cyclins in colon cancer and in cell lines from colon carcinomas. British journal of cancer 93: 338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takano Y, Kato Y, van Diest PJ, Masuda M, Mitomi H, et al. (2000) Cyclin D2 overexpression and lack of p27 correlate positively and cyclin E inversely with a poor prognosis in gastric cancer cases. The American journal of pathology 156: 585–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyyappan M, Atadja PW, Riabowol KT (1996) Regulation of gene expression and transcription factor binding activity during cellular aging. Biological signals 5: 130–138. [DOI] [PubMed] [Google Scholar]
- 12.Meyyappan M, Wong H, Hull C, Riabowol KT (1998) Increased expression of cyclin D2 during multiple states of growth arrest in primary and established cells. Molecular and cellular biology 18: 3163–3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buckley MF, Sweeney KJ, Hamilton JA, Sini RL, Manning DL, et al. (1993) Expression and amplification of cyclin genes in human breast cancer. Oncogene 8: 2127–2133. [PubMed] [Google Scholar]
- 14.Fackler MJ, McVeigh M, Evron E, Garrett E, Mehrotra J, et al. (2003) DNA methylation of RASSF1A, HIN-1, RAR-beta, Cyclin D2 and Twist in in situ and invasive lobular breast carcinoma. International journal of cancer 107: 970–975. [DOI] [PubMed] [Google Scholar]
- 15.Virmani A, Rathi A, Heda S, Sugio K, Lewis C, et al. (2003) Aberrant methylation of the cyclin D2 promoter in primary small cell, nonsmall cell lung and breast cancers. International journal of cancer 107: 341–345. [DOI] [PubMed] [Google Scholar]
- 16.Hsu A, Wong CP, Yu Z, Williams DE, Dashwood RH, et al. (2011) Promoter de-methylation of cyclin D2 by sulforaphane in prostate cancer cells. Clinical epigenetics 3: 3 10.1186/1868-7083-3-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsubayashi H, Sato N, Fukushima N, Yeo CJ, Walter KM, et al. (2003) Methylation of cyclin D2 is observed frequently in pancreatic cancer but is also an age-related phenomenon in gastrointestinal tissues. Clinical cancer research: an official journal of the American Association for Cancer Research 9: 1446–1452. [PubMed] [Google Scholar]
- 18.Yu J, Leung WK, Ebert MP, Leong RW, Tse PC, et al. (2003) Absence of cyclin D2 expression is associated with promoter hypermethylation in gastric cancer. British journal of cancer 88: 1560–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu B, Zhang L, Luo C, Qi Y, Cui Y, et al. (2015) Hypermethylation of the 16q23.1 tumor suppressor gene ADAMTS18 in clear cell renal cell carcinoma. International journal of molecular sciences 16: 1051–1065. 10.3390/ijms16011051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Q, Zhang L, Li L, Wang Z, Ying J, et al. (2014) Interferon regulatory factor 8 functions as a tumor suppressor in renal cell carcinoma and its promoter methylation is associated with patient poor prognosis. Cancer letters 354: 227–234. 10.1016/j.canlet.2014.07.040 [DOI] [PubMed] [Google Scholar]
- 21.Zhang Q, Ying J, Zhang K, Li H, Ng KM, et al. (2007) Aberrant methylation of the 8p22 tumor suppressor gene DLC1 in renal cell carcinoma. Cancer letters 249: 220–226. [DOI] [PubMed] [Google Scholar]
- 22.Esteller M (2008) Epigenetics in cancer. The New England journal of medicine 358: 1148–1159. 10.1056/NEJMra072067 [DOI] [PubMed] [Google Scholar]
- 23.Padar A, Sathyanarayana UG, Suzuki M, Maruyama R, Hsieh JT, et al. (2003) Inactivation of cyclin D2 gene in prostate cancers by aberrant promoter methylation. Clinical cancer research: an official journal of the American Association for Cancer Research 9: 4730–4734. [PubMed] [Google Scholar]
- 24.Henrique R, Costa VL, Cerveira N, Carvalho AL, Hoque MO, et al. (2006) Hypermethylation of Cyclin D2 is associated with loss of mRNA expression and tumor development in prostate cancer. Journal of molecular medicine 84: 911–918. [DOI] [PubMed] [Google Scholar]
- 25.Zhang L, Zhang Q, Li L, Wang Z, Ying J, et al. (2015) DLEC1, a 3p tumor suppressor, represses NF-kappaB signaling and is methylated in prostate cancer. Journal of molecular medicine 93: 691–701. 10.1007/s00109-015-1255-5 [DOI] [PubMed] [Google Scholar]
- 26.Tao Q, Swinnen LJ, Yang J, Srivastava G, Robertson KD, et al. (1999) Methylation status of the Epstein-Barr virus major latent promoter C in iatrogenic B cell lymphoproliferative disease. Application of PCR-based analysis. The American journal of pathology 155: 619–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siegel R, Naishadham D, Jemal A (2013) Cancer statistics, 2013. CA: a cancer journal for clinicians 63: 11–30. [DOI] [PubMed] [Google Scholar]
- 28.Uchida K, Miyao N, Masumori N, Takahashi A, Oda T, et al. (2002) Recurrence of renal cell carcinoma more than 5 years after nephrectomy. International journal of urology: official journal of the Japanese Urological Association 9: 19–23. [DOI] [PubMed] [Google Scholar]
- 29.Lima MS, Pereira RA, Costa RS, Tucci S, Dantas M, et al. (2014) The prognostic value of cyclin D1 in renal cell carcinoma. International urology and nephrology 46: 905–913. 10.1007/s11255-013-0602-0 [DOI] [PubMed] [Google Scholar]
- 30.Hedberg Y, Roos G, Ljungberg B, Landberg G (2002) Cyclin D3 protein content in human renal cell carcinoma in relation to cyclin D1 and clinico-pathological parameters. Acta oncologica 41: 175–181. [DOI] [PubMed] [Google Scholar]
- 31.Sinclair AJ, Palmero I, Holder A, Peters G, Farrell PJ (1995) Expression of cyclin D2 in Epstein-Barr virus-positive Burkitt's lymphoma cell lines is related to methylation status of the gene. Journal of virology 69: 1292–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shapiro GI, Park JE, Edwards CD, Mao L, Merlo A, et al. (1995) Multiple mechanisms of p16INK4A inactivation in non-small cell lung cancer cell lines. Cancer research 55: 6200–6209. [PubMed] [Google Scholar]
- 33.Baylin SB, Herman JG, Graff JR, Vertino PM, Issa JP (1998) Alterations in DNA methylation: a fundamental aspect of neoplasia. Advances in cancer research 72: 141–196. [PubMed] [Google Scholar]
- 34.Costello JF, Fruhwald MC, Smiraglia DJ, Rush LJ, Robertson GP, et al. (2000) Aberrant CpG-island methylation has non-random and tumour-type-specific patterns. Nature genetics 24: 132–138. [DOI] [PubMed] [Google Scholar]
- 35.Du Z, Li L, Huang X, Jin J, Huang S, et al. (2016) The epigenetic modifier CHD5 functions as a novel tumor suppressor for renal cell carcinoma and is predominantly inactivated by promoter CpG methylation. Oncotarget 7: 21618–21630. 10.18632/oncotarget.7822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Q, Ying J, Li J, Fan Y, Poon FF, et al. (2010) Aberrant promoter methylation of DLEC1, a critical 3p22 tumor suppressor for renal cell carcinoma, is associated with more advanced tumor stage. The Journal of urology 184: 731–737. 10.1016/j.juro.2010.03.108 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.