Abstract
Saccharomyces cerevisiae Yaf9p and the mammalian leukemia-associated protein ENL share a high degree of similarity. To investigate the biological function of Yaf9p, this protein was used to search for interacting proteins in a two-hybrid system. Here, we demonstrate that Yaf9p binds directly to Swc4p, the yeast homolog of the mammalian DNA-methyltransferase-associated protein 1. Yaf9p and Swc4p associate through C-terminal domains, and both proteins coprecipitate in vitro in pull-down experiments and in vivo by immunoprecipitation. In living cells, Swc4p is present in a megadalton protein complex that shows a fractionation behavior in gel filtration similar to that of Esa1p, the histone acetyltransferase of the NuA4 complex. Recruitment of Yaf9p to DNA leads to promoter-specific transcriptional activation that can be inhibited by dominant negative Swc4p lacking the Yaf9p binding domain. Interference with Swc4p function also increases sensitivity to the microtubule toxin benomyl, a trait that corresponds to the known phenotype of a yaf9− knockout strain. In summary, the results suggest that Yaf9p and Swc4p form a protein pair that has a role in chromatin modification with possible implications also for the function of their mammalian counterparts.
Genetic traits are not exclusively encoded in the sequence of DNA. A wide variety of so-called epigenetic alterations contribute to the storage of information. Other than methylation of the DNA itself, a phenomenon absent in budding Saccharomyces cerevisiae, a wide variety of conserved covalent attachments to N-terminal histone tails has been identified. Often catalyzed by multiprotein complexes, histones can become acetylated, methylated, phosphorylated, ubiquitinilated, and also linked to SUMO, a small ubiquitin-like protein (4, 14). In addition to having a direct influence on the higher packing order of chromatin, these modifications also create sites that are recognized by distinct functional domains present in a multitude of other proteins. Prime examples are the chromobox (16), which binds to methylated histones, and the bromodomain (17), which has a specific affinity for acetylated histones. It is therefore not surprising that chromatin modification has been implicated in almost all aspects of DNA metabolism, foremost in transcription regulation but also in replication, repair, and recombination (10, 15).
A perturbation of these processes has serious consequences, and it has become increasingly clear that missing or incorrect histone modifications can promote cellular transformation and therefore cancer. A paradigm for this pathology is mixed-lineage leukemia. This aggressive disease is characterized by chromosomal aberrations, mostly translocations, that affect the genomic locus of the MLL (mixed-lineage leukemia) gene at 11q23 (1, 6, 9). The corresponding MLL protein contains a SET domain that is responsible for a histone H3 lysine-4 methylase activity (20). Like its fly homolog TRX (trithorax), MLL is a member of a large multiprotein complex that ensures the maintenance of preestablished transcription patterns within the cellular memory system (22). Due to the translocation event, the C-terminal portion of MLL is deleted and fused in frame to a variety of different partner genes. This leads to the production of a chimeric oncoprotein, where the SET domain of MLL is replaced by a new function brought in by the fusion partner. For MLL-ENL (eleven-nineteen-leukemia protein), one of the best-studied MLL fusion proteins, this creates a novel transcriptional activator that exerts its transforming activity by constitutive transactivation of members of the homeobox gene family (25, 26, 32). This newly acquired transactivation function likely involves chromatin modification as it can be replaced only by other strong transactivators that are known to be composites of domains that simultaneously recruit chromatin remodeling complexes and RNA polymerase II (27, 33). In line with this reasoning is the finding that ENL and AF9, another frequent MLL fusion partner, share an intriguing homology with the yeast proteins Sas5p (YOR213C), Taf14p (YPL129W; also called Taf30p, Tfg3p, and Anc1p), and Yaf9p (YNL107W), which are all involved in chromatin remodeling complexes.
Sas5p is a member of the SAS (something about silencing) histone acetyltransferase (HAT) complex that is necessary for silencing at telomeres and the mating-type loci (23, 28). Taf14p can be found in the SWI/SNF chromatin remodeling complex and also as a subunit of the general transcription factors TFIID and TFIIF (3). Yaf9p, finally, has been suggested based on a high-throughput screen to be associated with the NuA4 HAT complex (12, 13, 29), an assumption that was corroborated recently by an independent approach (19). The NuA4 acetylating activity is also present in mammalian cells, where it corresponds to the Tip60 complex (8). In yeast cells as well as in mammals, this megadalton assembly targets histone H4 and H2A. Functionally, NuA4 activity has been implicated in transcriptional regulation, DNA repair, and cell cycle control (reviewed in reference 30).
In order to learn more about the transforming mechanism of MLL-ENL and to complement our search for ENL-interacting proteins in mammalian cells, we intended to exploit the exceptional evolutionary conservation between ENL and Yaf9p to check for a possible homology that extends beyond structure. To this end, we wanted to investigate in detail the molecular interactions of yeast Yaf9p with respect to direct protein-protein binding that might also be conserved in higher eukaryotes.
Here, we present evidence that Yaf9p interacts directly in vitro and in vivo with yeast Swc4p. Swc4p is highly homologous to the mammalian DNA-methyltransferase-associated protein 1 (DMAP1) that was originally identified in a two-hybrid screen with DNA-methyltransferase as a bait (24). Apart from the physical association, Swc4p also influences the capability of Yaf9p for promoter-specific transactivation. The detection of this interaction opens up new aspects for the function of Yaf9 homologues in higher eukaryotes.
MATERIALS AND METHODS
Plasmids, strains, media, inhibitors, and antibodies.
The coding sequences of Yaf9p and Swc4p were amplified by PCR with genomic DNA from yeast strain K699 as a template and inserted into different vector systems. All cloning products were verified by sequencing. For two-hybrid purposes, the GAL4 fusion plasmids pGADT7 and pGBKT7 (BD Biosciences Clontec, Palo Alto, Calif.) were used. Galactose-inducible expression of epitope-tagged protein versions was done with the pESC vector series (Stratagene, La Jolla, Calif.), and for constitutive expression the inserts were transferred to pVT102 (31).
The reporter yeast strains AH109 (MATa trp1-901 leu2-3,112 ura3-52 his3-200 gal4Δ gal80Δ LYS2::GAL1UAS-GAL1TATA-HIS3 GAL2UAS-GAL2TATA-ADE2 URA3::MEL1UAS-MEL1TATA-lacZ) and PJ69-4A (MATa trp1-901 leu2-3,112 ura3-52 his3-200 gal4Δ gal80Δ GAL2-ADE2 LYS2::GAL1-HIS3 met2::GAL7-lacZ) were obtained from Clontech (Palo Alto, Calif.). Wild-type K699 (MATa ade2-1 trp1-1 can1-100 leu2-3,112 his3-11,15 ura3 GAL [psi+]) was a laboratory stock. K699 yaf9::Trp1 was constructed by a PCR-mediated one-step disruption strategy, replacing the complete reading frame of YAF9 by the TRP1 gene. Successful targeting was controlled by PCR and Southern blotting (results not shown).
Standard yeast genetic techniques and media were applied. lacZ assays were performed exactly according to the protocol outlined by the manufacturer (Clontech yeast protocols handbook). Benomyl was purchased from Sigma-Aldrich (Taufkirchen, Germany). Monoclonal antibodies directed against GAL4-DNA binding domain (BD) and GAL4 activation domain (AD), as well as polyclonal anti-myc and anti-Esa1 antibodies, were obtained from Santa Cruz Biotechnology (Santa Cruz, Calif.). Monoclonal anti-Flag (M2) was from Sigma-Aldrich.
Yeast two-hybrid analysis.
For the yeast two-hybrid screening, the baits and target plasmids were cotransfected into yeast strain AH109 (Clontech) according to the instructions of the manufacturer (Clontech yeast protocols handbook).
Protein production, GSTpull-down experiments.
All proteins used for the in vitro interaction experiments were produced either by in vitro transcription-translation with the TNT System (Promega, Madison, Wis.) or by expression and purification of glutathione S-transferase (GST) fusion proteins in yeast cells (pYEX System; Clontech) according to the manufacturers' instructions. The GST pull-down assay has been previously described (11).
Yeast extracts, immunoprecipitation, and gel filtration.
To generate protein extracts, yeast cells from 50 ml of exponentially growing culture were harvested and bead milled at 4°C with two to three times the pellet volume of lysis buffer (20 mM Tris-HCl [pH 7.5], 150 mM NaCl, 0.2% Triton X-100, 10% glycerol, 2 mM dithiothreitol [DTT], 1 mM phenylmethylsulfonylfluoride, and 10 μl of complete proteinase inhibitor solution for yeast [Sigma-Aldrich]/ml).
Immunoprecipitation was done from extracts corresponding to 25 ml of yeast culture with 5 μg of either a polyclonal antibody directed against the GAL4-DNA BD or the same amount of a polyclonal anti-myc antibody. After binding, extensive washes were done with buffer W (20 mM Tris-HCl [pH 7.5], 400 mM NaCl, 0.25% bovine serum albumin, 5 mM DTT, and 0.4% Triton X-100). The precipitates were analyzed by immunoblotting with either a monoclonal anti-GAL4 AD or a monoclonal anti-Flag antibody according to standard protocols.
For gel filtration, extracts were prepared in standard phosphate-buffered saline supplemented with 2 mM DTT, 1 mM phenylmethylsulfonylfluoride, and 10 μl of complete protease inhibitors (Sigma-Aldrich)/ml. Two-milliliter aliquots were run on a Sephadex 200 column equilibrated with the same buffer. The column was calibrated with substances of known molecular weight (highmolecular weight gel filtration calibration kit; Amersham Biosciences, Little Chalfont, United Kingdom) according to the instructions of the manufacturer. The substances and proteins used were blue dextran (2,000 kDa), thyroglobulin (669 kDa), ferritin (440 kDa), catalase (232 kDa), aldolase (158 kDa), serum albumin (65 kDa), and ovalbumin (41 kDa).
RESULTS
Yaf9p is the closest yeast homolog of ENL.
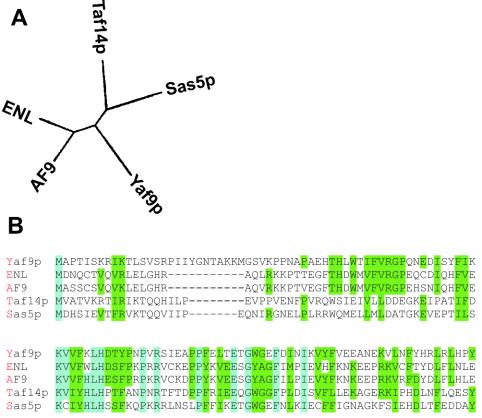
Three proteins in S. cerevisiae show a significant homology to human ENL. The region with the highest similarity between these proteins has been named the YEATS domain after its occurrence in Yaf9p, ENL, AF9, Taf14p, and Sas5p (2). In order to identify the yeast protein most closely related to ENL, the N-terminal YEATS motifs of these five proteins were compared in a multiple-sequence alignment. An analysis with the CLUSTALW program (5) grouped Yaf9p closer to the mammalian ENL and AF9 proteins than to Taf14p or Sas5p (Fig. 1). Because of this result and since little was known about this protein, we decided to study this molecule more in detail.
FIG. 1.
Multiple sequence alignment. (A) Dendrogram depicting the relationship of YEATS domain proteins; (B) alignment of the YEATS domain with amino acids given in one-letter codes.
Yaf9p interacts with Swc4p through a C-terminal motif separate from the YEATS domain.
To preferentially identify interactions with YAF9p that were conserved during evolution, a cross-species screen was conducted. For this purpose, full-length S. cerevisiae Yaf9p was used as bait in a two-hybrid experiment to interrogate a target library from Schizosaccaromyces pombe. Positive primary clones were identified, and then the corresponding homologs from budding yeast cells were cross-checked for binding to Yaf9p. A screen of 1.5 × 106 independent library clones yielded 72 candidates that scored positive, allowing the test strain AH109 to overcome histidine as well as adenine auxotrophy. Within this group, 18 clones contained the S. pombe homolog of the budding yeast gene SWC4 (YGR002C; this gene has been also named GOD1 and EAF2 [www.yeastgenome.org]) (8). Also, authentic S. cerevisiae Swc4p showed a strong interaction with Yaf9p in a two-hybrid setup (Fig. 2A). This interaction was specific, as an unrelated bait (human lamin C) or the empty bait vector alone did not give a positive readout (Fig. 2A and data not shown).
FIG. 2.
Analysis of the Yaf9p/Swc4p interaction in the two-hybrid system. (A) The yeast two-hybrid reporter strain AH109 was transformed with combinations of bait and prey plasmids encoding the following S. cerevisiae proteins: (i) Snf1p as bait and Snf4p as prey (+, positive control for interaction), (ii) full-length Yaf9p as bait and full-length Swc4p as prey, and (iii) human lamin C as bait with Swc4p as prey. Except for the positive control, two independent transformants are shown. Growth was assessed on dropout medium plates lacking tryptophan and leucine (−trp/−leu), histidine (−his), or adenine (−ade). (B) Protein expression of bait (Yaf9p) and prey (Swc4p) derivatives. Various deletion mutants of Yaf9p and Swc4p (numbers indicate respective cDNA positions) were fused either to the DNA BD (GAL4-BD) or the transactivation domain (GAL4-AD) of GAL4 as indicated. Yeast strain AH109 was transformed with the resulting plasmids, and the protein expression was verified by immunoblotting with either an anti-GAL4-DNA BD or an anti-GAL4 AD antibody. (C) Schematic representation of the two-hybrid results obtained with Yaf9p and Swc4p mutants. The bars are drawn to scale. The conserved YEATS domain is indicated by shading. +, growth on double-dropout medium lacking adenine and histidine; −, no growth on selective medium.
A series of Yaf9p and Swc4p deletion mutants was used to delineate the extension of the protein surfaces that participate in the mutual interaction. All Yaf9p constructs were fused to the GAL4-DNA BD, whereas the Swc4p derivatives were joined with the GAL4 transactivation domain. Immunoblotting of cellular proteins extracted from the respective yeast transformants verified the proper expression and correct size of the resulting GAL4 fusions (Fig. 2B). In further two-hybrid experiments, these mutants were each tested with the corresponding full-length protein as a counterpart and with unrelated bait or prey proteins as controls. In Yaf9p as well as in Swc4p, C-terminal portions were responsible for the mutual interaction. The last 100 amino acids of Swc4p were sufficient to bind specifically to full-length Yaf9p. A 44-amino-acid region in the C-terminal half of Yaf9p was capable of binding independently to Swc4p (Fig. 2C). No sequences within the YEATS homology domain of Yaf9p were involved in the interaction with Swc4p.
Swc4p interacts directly with Yaf9p, and it is present in a high-molecular-weight complex in vivo.
To investigate whether the interaction of Yaf9p and Swc4p is direct, an in vitro pull-down assay was carried out. Glutathione-coated beads were loaded either with recombinant GST-Yaf9p fusion protein or with GST protein only. After being extensively washed, an aliquot of the charged beads was boiled in sodium dodecyl sulfate (SDS)-gel electrophoresis buffer, and the resulting extract was examined by gel electrophoresis followed by Coomassie staining to prove the proper loading with the respective proteins (Fig. 3A, left panel). Swc4p was labeled with 35S by coupled in vitro transcription-translation and incubated with equal amounts of GST and GST-Yaf9p-bearing beads. After being washed, the proteins retained by both types of solid support were analyzed by SDS-gel electrophoresis and autoradiography. As an indication for a direct interaction between the two proteins, Swc4p bound specifically to GST-Yaf9p but not to the GST control (Fig. 3B, right panel).
FIG. 3.
In vitro and in vivo interaction between Yaf9p and Swc4p. (A) GST pull-down experiment. Recombinant GST or a fusion of GST with full-length Yaf9p was loaded onto glutathione beads, and contaminating proteins were removed by thorough washing. To ensure proper binding, an aliquot of the charged beads was extracted in SDS sample buffer and analyzed by SDS-gel electrophoresis (left). The prepared beads were incubated with radioactive 35S-labeled Swc4p produced by in vitro transcription and translation. After being washed, the proteins still attached to the beads were eluted, separated by gel electrophoresis, and visualized by autoradiography. As a control, 10% of the input transcription-translation reaction mixture was run alongside the proteins. The positions of molecular mass marker standards are given. (B) Immunoprecipitation (i.p.). Protein extracts from the two-hybrid strain containing GAL4 fusions of Yaf9p (GAL4 BD) and Swc4p (GAL4-AD) were precipitated either with an anti GAL4-DNA BD antibody or with an unspecific control antibody. Proteins in the precipitate were examined for the presence of GAL4 AD-Swc4p by immunoblotting. As a control, an aliquot of protein extract before immunoprecipitation was used (left panel). In a second approach, expression vectors for Flag-tagged Yaf9p were transfected either alone or in combination with a plasmid coding for myc-tagged Swc4p into yeast strain K699, as indicated. Precipitation with an anti-myc antibody pulled down Flag-tagged Yaf9p only if myc-tagged Swc4p was present, indicating a specific interaction between these proteins (right panel). (C) Gel filtration experiment. Native protein extracts from K699 expressing myc-tagged Swc4p were separated on a Superdex 200 sizing column that was calibrated by proteins with a known molecular mass. Outflow fractions were scored for the presence of myc-Swc4p and Esa1p by immunoblotting. The elution positions for blue dextran (molecular mass, 2 MDa) and calibration proteins (see Materials and Methods) are indicated together with the molecular masses of the protein extracts.
To add further proof for a Swc4p-Yaf9p contact within living cells, two different coimmunoprecipitation experiments were conducted. First, the two-hybrid strain AH109 containing the corresponding GAL4 fusion proteins was used. In extracts from these cells, GAL4 AD-Swc4p could be coprecipitated by an antibody directed against GAL4-DNA BD-Yaf9p but not by an unspecific control antibody (Fig. 3B, left panel). To exclude any artifacts that might arise from the inclusion of the large GAL4 moiety, a second immunoprecipitation experiment was done with proteins that were fused to smaller epitope tags. Flag-tagged Yaf9p was expressed in the K699 yeast strain either alone or in the presence of myc-tagged Swc4p. As expected, an anti-myc antibody pulled down Flag-tagged Yaf9p only in the presence of myc-Swc4p, thus demonstrating a specific contact between Swc4p and Yaf9p (Fig. 3B, right panel).
Yaf9p has been found to be associated with the NuA4 HAT complex, a megadalton protein assembly. If the Yaf9p-Swc4p link is operative within cells, Swc4p should also be found in the high-molecular-mass fraction of a yeast extract. To verify this assumption, myc-tagged Swc4p was expressed in yeast strain K699, and soluble proteins were eluted under native conditions. The protein preparation was separated on a size exclusion column that had been calibrated by substances with known molecular weights. The analysis of the outflow fractions by immunoblotting (Fig. 3C) revealed a bipartite elution profile of Swc4p with the majority of the protein present in fractions indicative of a molecular mass of more than 1 MDa. A minor peak of myc-Swc4p was detectable with an apparent molecular mass of approximately 50 to 60 kDa. This finding fits with the calculated relative molecular mass (56 kDa) for a myc-tagged Swc4p monomer within the resolution limits of the chromatography method. For comparison, the same fractions were probed for the presence of the central NuA4 complex component Esa1p. In these experiments, Esa1p showed a very similar distribution. Most of the protein eluted in the high-molecular-mass range of >1 MDa. In addition, low levels of Esa1p were detectable in all later-eluting fractions with a small elution peak corresponding to the 50-kDa molecular mass of the monomeric protein.
Yaf9p can act as a transcriptional activator that depends on proper Swc4p function.
During the two-hybrid experiments, it was noticed that under certain circumstances Yaf9p was able to activate a reporter gene in the absence of any target GAL4 AD construct. Upon further examination of this phenomenon, it became clear that Yaf9p possesses a promoter-specific transactivation capability. When tethered to DNA by the GAL4-DNA BD, Yaf9p autonomously activated the lacZ reporter (Fig. 4A, left panel) but not the HIS1 reporter (right panel). In the yeast strain (PJ69-4A) used for this experiment, lacZ is under control of a GAL7 upstream activating region whereas HIS1 is driven by a GAL1 promoter. Both reporters were functioning properly as they responded to a positive control, an interacting pair of the proteins Snf1p and Snf4p (Fig. 4A). Activation by Yaf9p was not an intrinsic property of the lacZ reporter, as the same gene was unresponsive to Yaf9p in a different two-hybrid strain (AH109) where lacZ is under the control of a MEL1 promoter.
FIG. 4.
Functional interaction of Yaf9p and Swc4p. (A) Promoter-specific transactivation capacity of Yaf9p. Full-length Yaf9p fused to the GAL4-DNA BD was transformed into the indicated yeast reporter strains (Yaf9). An empty bait vector (−) or a known positive two-hybrid interaction between the Snf1p and Snf4p proteins (+) served as a negative or positive control, respectively. lacZ and HIS1 reporter gene activation was measured either by determination of β-galactosidase activity or by monitoring growth of the transformants in histidine dropout media. The upstream activation sequences driving reporter expression are indicated for each gene. lacZ units are the means and standard deviation of triplicate samples. For growth assessment, optical density (O.D.) at 600 nm was recorded at the indicated time points. (B) Perturbation of the Yaf9p/Swc4p interaction can interfere with Yaf9p transcriptional activity. A deletion mutant of Swc4p that has lost the C-terminal domain necessary for Yaf9p binding (Swc4Δ) or Swc4p itself (Swc4) was constitutively expressed in the two-hybrid strain PJ69-4A together with GAL4 fusions of either Yaf9p, Gal4p, or Gcn4p as indicated. The expression of the Swc4 proteins was verified by Western blotting (upper left panel). An empty expression vector or a construct producing unrelated GST protein was used as a control. The function of the respective transcriptional activators in the presence ofSwc4Δ or Swc4 was judged by determination of lacZ activity derived from the endogenous GAL7-lacZ reporter. Basal activity in the absence of additional proteins was set at 100%. The means and standard deviations of results of triplicate experiments are shown. A schematic representation of the principles of dominant negative and squelching mechanisms is depicted. (C) Perturbation of the Yaf9p/Swc4p interaction can interfere with endogenous Yaf9p function. Yaf9p has been shown to be necessary for resistance against the spindle toxin benomyl (19). Wild-type S. cerevisiae (strain K699, a W303 derivative) was transformed with an empty expression vector (pESC) or with constructs that allowed the overexpression of Swc4Δp and Swc4p under the control of a galactose-inducible promoter. A genomic yaf9− mutant in the same genetic background was used as an additional control. Three independent transformants were each plated on medium, with the following results.(Left plate) Galactose medium in the absence of benomyl allows for protein production and controls for a general influence of Swc4p/Swc4Δp on growth. (Center plate) Glucose represses expression of ectopic Swc4p/Swc4Δp, and the addition of benomyl reveals the intrinsic sensitivity of the strains towards spindle stress. (Right plate) Galactose induction of Swc4p/Swc4Δp in the presence of benomyl increases the sensitivity of the respective transformants to spindle stress compared to that of cells treated with vector only.
Transcriptional activation by Yaf9p most likely involves the NuA4 histone acetylating activity. Since strains with deletions of SWC4 or ESA1, which encodes the NuA4 central HAT, are not viable, a direct test of NuA4 involvement in Yaf9p transactivation is difficult. However, if aYaf9p/Swc4p interaction is involved in NuA4 recruitment by Yaf9p, a dominant negative Swc4p mutant (Swc4Δp) that has lost the Yaf9p BD should be able to inhibit Yaf9p-mediated transactivation. Furthermore, a gross perturbation of the Yap9p/Swc4p stoichiometry by overexpression of excess Swc4p might also lead to a squelching of Yaf9p-mediated transactivation (Fig. 4B). To test these predictions, a Swc4p deletion mutant (Swc41-1281) that has lost the C-terminal domain necessary for Yaf9p binding or Swc4p itself was constitutively expressed in the two-hybrid strain PJ69-4A together with a GAL4-DNA BD-Yaf9p construct. The production of Swc4p and Swc4Δp was verified by Western blotting (Fig. 4B). The empty expression vector and a construct directing the synthesis of GST protein served as controls to exclude any unspecific effects that might occur due to ectopic protein expression. In these experiments, the presence of dominant negative Swc4p reduced the autonomous Yaf9p transactivation ability to about 65% of its basal level. Overexpression of wild-type Swc4p also had a consistent but minor effect. The inhibition of transactivation by Swc4Δp was specific for Yaf9p, as the transcriptional activation by Gal4p and Gcn4p was unaltered in the same experimental setup (Fig. 4B).
No endogenous target genes of Yaf9p have been identified with certainty. However, it is known that Yaf9p is most likely involved in the execution of the spindle checkpoint as Yaf9 mutants are oversensitive to benomyl, a microtubule-destabilizing drug (19). Therefore, we wanted to know with respect to benomyl sensitivity if interference with the Yaf9p/Swc4p association produces a phenocopy of a yaf9− knockout strain. To this purpose, wild-type yeast cells (strain K699, a W303 derivative) were transfected with constructs that allowed the overexpression of Swc4Δp and Swc4p under the control of a galactose-inducible promoter in the expression vector pESC. As controls, K699 and a K699 yaf9− mutant were transfected with vector only. When plated on galactose inducer medium in the absence of benomyl, all transformants showed identical growth, indicating that the overexpression of Swc4Δp or Swc4p has no effect on proliferation per se (Fig. 4C). On glucose plates under repressive conditions, only the yaf9− strain displayed a higher sensitivity towards benomyl, whereas the Swc4Δp/Swc4p transformants behaved exactly like the wild type (Fig. 4C). Once protein expression was induced on galactose medium and benomyl was added, however, the Swc4Δp/Swc4p-expressing yeast cells became moderately but consistently and reproducibly more sensitive towards this spindle poison, which suggested a partial loss of Yaf9p function.
DISCUSSION
S. cerevisiae has become an indispensable tool for studying the molecular basis of cellular function on a comprehensive level. Many essential biochemical processes have been conserved during evolution from yeast to humans. Therefore, we attempted to identify interaction partners of yeast Yaf9p, a close homolog of the mammalian ENL protein that plays an instrumental role in leukemia. Here, we present evidence that Yaf9p interacts directly and functionally with Swc4p, which in turn has a conserved mammalian counterpart, DNA-cytosine-methyltransferase-associated protein 1.
Despite the availability of several high-throughput two-hybrid screens, this interaction has not been identified before. The PathCalling Yeast Interaction Database (www.portal.curagen.com/pathcalling_portal/index.htm) has no entry for either Yaf9p or Swc4p. The Yeast Resource Center Informatics Platform (www.yeastrc.org) records several two-hybrid interactions for Swc4p, but Yaf9p is not included among them. Finally, the Biomolecular Interaction Network Database (www.blueprint.org) describes an association of Yaf9p with the ceramide biosynthesis protein Lac1p.
In contrast, support for our results comes from attempts to identify yeast protein complexes on a global scale by TAP tag purification combined with mass spectrometry. By its very nature, however, this method does not distinguish between direct binding and indirect association through other proteins. Tagged Swc4p was found by this strategy to precipitate all known core proteins of the NuA4 complex, including Yaf9p and the central HAT Esa1p (www.yeastrc.org) (12, 13). More evidence for a Yaf9p/Swc4p association and therefore for a role of Swc4p as a NuA4 complex constituent came from the recent purification of the mammalian counterpart of the NuA4 assembly, the Tip60 complex (8). Eleven of 12 proteins that were associated with Tip60 had a corresponding yeast homolog in the NuA4 complex. Among these, the mammalian Swc4p homolog DMAP1 was found to copurify with Tip60. In contrast to this finding and despite the structural homologies of the corresponding yeast proteins, we could not detect a direct interaction between human ENL and human DMAP1 (data not shown). Since the Yaf9p/Swc4p interaction surface is outside of the conserved YEATS domain, this might be a hint that another mammalian protein with even higher similarity to Yaf9p exists. Indeed, Coté and colleagues have found GAS41 another YEATS domain protein in the human Tip60 complex (8). GAS41 has approximately the same size as Yaf9p, and the two proteins are also homologous outside of the YEATS motif. Interestingly, GAS41 has also been implicated in leukemias that are caused by MLL fusion proteins. The MLL fusion partner AF10 binds GAS41 in two-hybrid and coprecipitation experiments (7). This supports a specific role for YEATS proteins in cellular transformation and cancer. It will be interesting to see if ENL also possesses a hitherto unknown connection to histone modification.
The link between chromatin and YEATS proteins was strengthened recently by an exciting new development. Confirming our results that Yaf9p and Swc4p form a closely interacting physical and functional unit, both proteins have been found in a complex (SWR-C) that has a Snf2 family-related ATPase core component (18, 21). SWR-C executes a completely novel mode of chromatin alteration. The complex catalyzes the exchange of histone H2A against the histone variant Htz1 in transcriptionally active chromatin. This process seems to aid transcriptional activation, preferentially for genes that are in telomeric regions. In the absence of SWR-C, these genes are affected by encroaching telomeric heterochromatin, leading to a repression of transcription.
It is very likely that a closer investigation of the YEATS proteins in mammals, supported by the knowledge that was derived from yeast studies, will uncover new mechanisms of transcriptional control that play a role in the etiology of cancer.
Acknowledgments
We are grateful to Renate Zimmermann for technical assistance, Christian Betz for advice on yeast techniques, and Georg Fey for continuous support. We thank Francis Stewart for critical reading of the manuscript.
This work was supported in part by DFG grants SL27/6-1 and SFB473/B10 to R.K.S. and by a stipend of GRK592 to D.T.Z. R.K.S. is the recipient of a Ria Freifrau-von-Fritsch Stiftung career development award.
REFERENCES
- 1.Ayton, P. M., and M. L. Cleary. 2001. Molecular mechanisms of leukemogenesis mediated by MLL fusion proteins. Oncogene 20:5695-5707. [DOI] [PubMed] [Google Scholar]
- 2.Bateman, A., L. Coin, R. Durbin, R. D. Finn, V. Hollich, S. Griffiths-Jones, A. Khanna, M. Marshall, S. Moxon, E. L. Sonnhammer, D. J. Studholme, C. Yeats, and S. R. Eddy. 2004. The Pfam protein families database. Nucleic Acids Res. 32:D138-D141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cairns, B. R., N. L. Henry, and R. D. Kornberg. 1996. TFG/TAF30/ANC1, a component of the yeast SWI/SNF complex that is similar to the leukemogenic proteins ENL and AF-9. Mol. Cell. Biol. 16:3308-3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carrozza, M. J., R. T. Utley, J. L. Workman, and J. Cote. 2003. The diverse functions of histone acetyltransferase complexes. Trends Genet. 19:321-329. [DOI] [PubMed] [Google Scholar]
- 5.Chenna, R., H. Sugawara, T. Koike, R. Lopez, T. J. Gibson, D. G. Higgins, and J. D. Thompson. 2003. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 31:3497-3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins, E. C., and T. H. Rabbitts. 2002. The promiscuous MLL gene links chromosomal translocations to cellular differentiation and tumour tropism. Trends Mol. Med. 8:436-442. [DOI] [PubMed] [Google Scholar]
- 7.Debernardi, S., A. Bassini, L. K. Jones, T. Chaplin, B. Linder, D. R. de Bruijn, E. Meese, and B. D. Young. 2002. The MLL fusion partner AF10 binds GAS41, a protein that interacts with the human SWI/SNF complex. Blood 99:275-281. [DOI] [PubMed] [Google Scholar]
- 8.Doyon, Y., W. Selleck, W. S. Lane, S. Tan, and J. Cote. 2004. Structural and functional conservation of the NuA4 histone acetyltransferase complex from yeast to humans. Mol. Cell. Biol. 24:1884-1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ernst, P., J. Wang, and S. J. Korsmeyer. 2002. The role of MLL in hematopoiesis and leukemia. Curr. Opin. Hematol. 9:282-287. [DOI] [PubMed] [Google Scholar]
- 10.Galarneau, L., A. Nourani, A. A. Boudreault, Y. Zhang, L. Heliot, S. Allard, J. Savard, W. S. Lane, D. J. Stillman, and J. Cote. 2000. Multiple links between the NuA4 histone acetyltransferase complex and epigenetic control of transcription. Mol. Cell 5:927-937. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Cuellar, M. P., S. A. Schreiner, M. Birke, M. Hamacher, G. H. Fey, and R. K. Slany. 2000. ENL, the MLL fusion partner in t(11;19), binds to the c-Abl interactor protein 1 (ABI1) that is fused to MLL in t(10;11). Oncogene 19:1744-1751. [DOI] [PubMed] [Google Scholar]
- 12.Gavin, A. C., M. Bosche, R. Krause, P. Grandi, M. Marzioch, A. Bauer, J. Schultz, J. M. Rick, A. M. Michon, C. M. Cruciat, M. Remor, C. Hofert, M. Schelder, M. Brajenovic, H. Ruffner, A. Merino, K. Klein, M. Hudak, D. Dickson, T. Rudi, V. Gnau, A. Bauch, S. Bastuck, B. Huhse, C. Leutwein, M. A. Heurtier, R. R. Copley, A. Edelmann, E. Querfurth, V. Rybin, G. Drewes, M. Raida, T. Bouwmeester, P. Bork, B. Seraphin, B. Kuster, G. Neubauer, and G. Superti-Furga. 2002. Functional organization of the yeast proteome by systematic analysis of protein complexes. Nature 415:141-147. [DOI] [PubMed] [Google Scholar]
- 13.Hazbun, T. R., L. Malmstrom, S. Anderson, B. J. Graczyk, B. Fox, M. Riffle, B. A. Sundin, J. D. Aranda, W. H. McDonald, C. H. Chiu, B. E. Snydsman, P. Bradley, E. G. Muller, S. Fields, D. Baker, J. R. Yates III, and T. N. Davis. 2003. Assigning function to yeast proteins by integration of technologies. Mol. Cell 12:1353-1365. [DOI] [PubMed] [Google Scholar]
- 14.Iizuka, M., and M. M. Smith. 2003. Functional consequences of histone modifications. Curr. Opin. Genet. Dev. 13:154-160. [DOI] [PubMed] [Google Scholar]
- 15.Ikura, T., V. V. Ogryzko, M. Grigoriev, R. Groisman, J. Wang, M. Horikoshi, R. Scully, J. Qin, and Y. Nakatani. 2000. Involvement of the TIP60 histone acetylase complex in DNA repair and apoptosis. Cell 102:463-473. [DOI] [PubMed] [Google Scholar]
- 16.Jones, D. O., I. G. Cowell, and P. B. Singh. 2000. Mammalian chromodomain proteins: their role in genome organisation and expression. Bioessays 22:124-137. [DOI] [PubMed] [Google Scholar]
- 17.Kouzarides, T. 2000. Acetylation: a regulatory modification to rival phosphorylation? EMBO J. 19:1176-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krogan, N. J., M. C. Keogh, N. Datta, C. Sawa, O. W. Ryan, H. Ding, R. A. Haw, J. Pootoolal, A. Tong, V. Canadien, D. P. Richards, X. Wu, A. Emili, T. R. Hughes, S. Buratowski, and J. F. Greenblatt. 2003. A Snf2 family ATPase complex required for recruitment of the histone H2A variant Htz1. Mol. Cell 12:1565-1576. [DOI] [PubMed] [Google Scholar]
- 19.Le Masson, I., D. Y. Yu, K. Jensen, A. Chevalier, R. Courbeyrette, Y. Boulard, M. M. Smith, and C. Mann. 2003. Yaf9, a novel NuA4 histone acetyltransferase subunit, is required for the cellular response to spindle stress in yeast. Mol. Cell. Biol. 23:6086-6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milne, T. A., S. D. Briggs, H. W. Brock, M. E. Martin, D. Gibbs, C. D. Allis, and J. L. Hess. 2002. MLL targets SET domain methyltransferase activity to Hox gene promoters. Mol. Cell 10:1107-1117. [DOI] [PubMed] [Google Scholar]
- 21.Mizuguchi, G., X. Shen, J. Landry, W. H. Wu, S. Sen, and C. Wu. 2004. ATP-driven exchange of histone H2AZ variant catalyzed by SWR1 chromatin remodeling complex. Science 303:343-348. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura, T., T. Mori, S. Tada, W. Krajewski, T. Rozovskaia, R. Wassell, G. Dubois, A. Mazo, C. M. Croce, and E. Canaani. 2002. ALL-1 is a histone methyltransferase that assembles a supercomplex of proteins involved in transcriptional regulation. Mol. Cell 10:1119-1128. [DOI] [PubMed] [Google Scholar]
- 23.Osada, S., A. Sutton, N. Muster, C. E. Brown, J. R. Yates III, R. Sternglanz, and J. L. Workman. 2001. The yeast SAS (something about silencing) protein complex contains a MYST-type putative acetyltransferase and functions with chromatin assembly factor ASF1. Genes Dev. 15:3155-3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rountree, M. R., K. E. Bachman, and S. B. Baylin. 2000. DNMT1 binds HDAC2 and a new co-repressor, DMAP1, to form a complex at replication foci. Nat. Genet. 25:269-277. [DOI] [PubMed] [Google Scholar]
- 25.Schreiner, S. A., M. P. Garcia-Cuellar, G. H. Fey, and R. K. Slany. 1999. The leukemogenic fusion of MLL with ENL creates a novel transcriptional transactivator. Leukemia 13:1525-1533. [DOI] [PubMed] [Google Scholar]
- 26.Slany, R. K., C. Lavau, and M. L. Cleary. 1998. The oncogenic capacity of HRX-ENL requires the transcriptional transactivation activity of ENL and the DNA binding motifs of HRX. Mol. Cell. Biol. 18:122-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.So, C. W., and M. L. Cleary. 2003. Common mechanism for oncogenic activation of MLL by forkhead family proteins. Blood 101:633-639. [DOI] [PubMed] [Google Scholar]
- 28.Sutton, A., W. J. Shia, D. Band, P. D. Kaufman, S. Osada, J. L. Workman, and R. Sternglanz. 2003. Sas4 and Sas5 are required for the histone acetyltransferase activity of Sas2 in the SAS complex. J. Biol. Chem. 278:16887-16892. [DOI] [PubMed] [Google Scholar]
- 29.Uetz, P., L. Giot, G. Cagney, T. A. Mansfield, R. S. Judson, J. R. Knight, D. Lockshon, V. Narayan, M. Srinivasan, P. Pochart, A. Qureshi-Emili, Y. Li, B. Godwin, D. Conover, T. Kalbfleisch, G. Vijayadamodar, M. Yang, M. Johnston, S. Fields, and J. M. Rothberg. 2000. A comprehensive analysis of protein-protein interactions in Saccharomyces cerevisiae. Nature 403:623-627. [DOI] [PubMed] [Google Scholar]
- 30.Utley, R. T., and J. Cote. 2003. The MYST family of histone acetyltransferases. Curr. Top. Microbiol. Immunol. 274:203-236. [DOI] [PubMed] [Google Scholar]
- 31.Vernet, T., D. Dignard, and D. Y. Thomas. 1987. A family of yeast expression vectors containing the phage f1 intergenic region. Gene 52:225-233. [DOI] [PubMed] [Google Scholar]
- 32.Zeisig, B. B., T. Milne, M. P. Garcia-Cuellar, S. Schreiner, M. E. Martin, U. Fuchs, A. Borkhardt, S. K. Chanda, J. Walker, R. Soden, J. L. Hess, and R. K. Slany. 2004. Hoxa9 and Meis1 are key targets for MLL-ENL-mediated cellular immortalization. Mol. Cell. Biol. 24:617-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeisig, B. B., S. Schreiner, M. P. Garcia-Cuellar, and R. K. Slany. 2003. Transcriptional activation is a key function encoded by MLL fusion partners. Leukemia 17:359-365. [DOI] [PubMed] [Google Scholar]