Abstract
Trypanosoma brucei genes encoding putative fatty acid synthesis enzymes are homologous to those encoding type II enzymes found in bacteria and organelles such as chloroplasts and mitochondria. It was therefore not surprising that triclosan, an inhibitor of type II enoyl-acyl carrier protein (enoyl-ACP) reductase, killed both procyclic forms and bloodstream forms of T. brucei in culture with 50% effective concentrations (EC50s) of 10 and 13 μM, respectively. Triclosan also inhibited cell-free fatty acid synthesis, though much higher concentrations were required (EC50s of 100 to 200 μM). Unexpectedly, 100 μM triclosan did not affect the elongation of [3H]laurate (C12:0) to myristate (C14:0) in cultured bloodstream form parasites, suggesting that triclosan killing of trypanosomes may not be through specific inhibition of enoyl-ACP reductase but through some other mechanism. Interestingly, 100 μM triclosan did reduce the level of incorporation of [3H]myristate into glycosyl phosphatidylinositol species (GPIs). Furthermore, we found that triclosan inhibited fatty acid remodeling in a cell-free assay in the same concentration range required for killing T. brucei in culture. In addition, we found that a similar concentration of triclosan also inhibited the myristate exchange pathway, which resides in a distinct subcellular compartment. However, GPI myristoylation and myristate exchange are specific to the bloodstream form parasite, yet triclosan kills both the bloodstream and procyclic forms. Therefore, triclosan killing may be due to a nonspecific perturbation of subcellular membrane structure leading to dysfunction in sensitive membrane-resident biochemical pathways.
The surface of bloodstream form Trypanosoma brucei is cloaked with 10 million copies of a variant surface glycoprotein (VSG) that is glycosyl phosphatidylinositol (GPI) anchored to the plasma membrane (10). This GPI is unusual in that its fatty acyl moiety is exclusively myristate, a 14-carbon saturated fatty acid (11). The GPI precursor glycolipid A is initially synthesized in the endoplasmic reticulum (ER) in a form that possesses fatty acids that are longer than myristate (5, 20). Then, in a process known as fatty acid remodeling, these longer fatty acids are sequentially removed and replaced with myristate (21, 25). Glycolipid A is then linked to newly synthesized VSG upon its import into the ER. In addition, myristates can also be incorporated into VSG anchors already attached to VSG by myristate exchange, a biochemically distinct reaction localized in a post-ER compartment of the secretory pathway (3, 4). The existence of two different GPI myristoylation pathways, along with the bloodstream form’s selective toxicity to myristate analogs (7), emphasizes the importance of VSG GPI myristoylation to this parasite.
Myristate is relatively scarce in mammalian blood (9), and the parasite does not store significant amounts (6). Yet, with 10 million VSG molecules, each with two myristates in their GPI anchors, trypanosomes have an enormous need for this limited resource. Not surprisingly, T. brucei has evolved efficient means to scavenge myristate from host serum and to channel much of it into GPIs. Furthermore, T. brucei compensates for the scarcity of myristate by synthesizing it de novo (26). In fact, the fatty acid synthesis pathway in bloodstream form T. brucei appears to be specialized; its primary product is myristate, and most of the myristate is incorporated into the VSG GPI anchor. In contrast, procyclic trypanosomes, residing in the tsetse fly midgut, synthesize a range of fatty acids, the largest of which is probably stearate, an 18-carbon fatty acid. Interestingly, procyclic trypanosomes do not myristoylate their GPIs. For a review of T. brucei fatty acid synthesis, see reference 27.
Fatty acid synthesis occurs through the sequential addition of two carbon units onto an acyl primer in a repeating cycle of condensation, reduction, dehydration, and reduction reactions. Type I fatty acid synthases (FAS), found in the cytoplasm of eukaryotes, are large multifunctional enzymes. In contrast, type II FAS, found in prokaryotes and eukaryotic organelles, such as mitochondria and chloroplasts, are composed of multiple proteins, each of which catalyzes a different step of the pathway. Examination of the T. brucei genome database (http://www.genedb.org/) revealed that many of the putative FAS genes appeared to be type II (27). Supporting this idea is the observed sensitivity of T. brucei to thiolactomycin, a known type II inhibitor that targets the β-ketoacyl-acyl carrier protein (β-ketoacyl-ACP) synthase (26). The localization of T. brucei FAS is unknown, but some of the genes have a predicted mitochondrial targeting sequence, suggesting that the pathway may be mitochondrial.
There is currently great interest in developing FAS as a drug target in protozoan parasites (30). One candidate compound for antiprotozoal therapy is triclosan, a chlorinated bisphenol (see the structure in Fig. 1) widely used in topical formulations as a general biocide (1). Triclosan was originally thought to act nonspecifically by disrupting cellular membranes (29) but was subsequently shown to have a specific target, type II enoyl-ACP reductase (13, 23). Recently, triclosan was reported to inhibit the growth of two apicomplexan pathogens, Plasmodium falciparum and Toxoplasma gondii (22, 31), and it eliminated the parasitemia in Plasmodium-infected mice. These parasites possess a type II fatty acid synthesis pathway in a plastid organelle called the apicoplast (33), which is inhibited by triclosan (31). In addition, the crystal structure of P. falciparum enoyl-ACP reductase bound to triclosan has been recently solved (28). Following these findings, we examined the effects of triclosan on the bloodstream and procyclic forms of T. brucei.
FIG. 1.
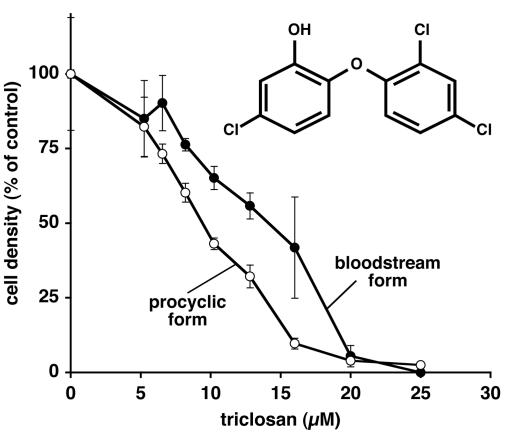
Inhibition of T. brucei growth in culture by triclosan. Cultures of T. brucei procyclic and bloodstream form trypanosomes were treated in duplicate for 48 h with either triclosan or NaOH as a solvent control, and cell density was assessed by hemocytometer counting. Cell densities are expressed as percentages of the density of mock-treated culture. The inset shows the structure of triclosan.
MATERIALS AND METHODS
Materials.
Triclosan was from KIC Chemicals, Inc. (Armonk, N.Y.). [2-14C]malonyl coenzyme A (CoA) and [11,12-3H]laurate (C12:0) were from American Radiolabeled Chemicals (St. Louis, Mo.). GDP-[2-3H]mannose, [9,10-3H]myristate (C14:0), [9,10-3H]palmitate (C16:0), and En3Hance were from PerkinElmer (Boston, Mass.). Serum Plus was from JRH Biosciences, Inc. (Lenexa, Kans.). Cell culture media and antibiotics were from Invitrogen Life Technologies, Inc. (Carlsbad, Calif.). All other reagents were from Sigma (St. Louis, Mo.). Organic solvents were high-pressure liquid chromatography grade. Thin-layer chromatography (TLC) plates were from AnalTech (Newark, Del.).
Trypanosome strains and cell culture.
T. brucei brucei procyclic form 427 was grown in SDM-79 medium (2) supplemented with 10% fetal bovine serum (FBS) in a 28°C, 5% CO2 incubator. T. b. brucei bloodstream form 427 was grown in HMI-9 medium (16) supplemented with 10% FBS and 10% Serum Plus in a 37°C, 5% CO2 incubator. The T. b. brucei bloodstream form GPI-phospholipase C (GPI-PLC) null mutant was kindly provided by George Cross (17). To obtain large amounts of bloodstream parasites, T. b. brucei bloodstream form ILTat1.3 or the GPI-PLC null trypanosomes were grown in rats and harvested from the blood at a parasitemia level of 1 ×108 to 2 × 108 cells/ml (8).
Cell-free reactions.
Cell-free fatty acid synthesis assays were performed with a membrane fraction from hypotonic cell lysates (26). Briefly, washed membranes (3 × 107 to 5 × 107 cell equivalents) were incubated at 37°C for 30 min with a final concentration of 2 mM NADPH, 1 mM dithiothreitol (DTT), 5 mM MnCl2, 0.8 μg of tunicamycin/μl, 50 μM butyryl-CoA, and 50 μM [14C]malonyl-CoA (55 mCi/mmol; 0.0415 μCi) in HKML (50 mM HEPES-KOH [pH 7.4], 25 mM KCl, 5 mM MgCl2, and 1 μg of leupeptin/ml) (final volume, 15 μl). For cell-free fatty acid elongation, 50 μM concentrations of various acyl-CoAs were substituted for butyryl-CoA. For triclosan inhibition experiments, the washed membranes were preincubated at 37°C for 30 min with various concentrations of freshly prepared triclosan or an equivalent volume of 0.1 M NaOH-0.16% Triton X-100 as a solvent control.
Cell-free GPI core glycan synthesis and fatty acid remodeling assays were performed as described previously (25), using a membrane fraction from hypotonic lysates prepared from GPI-PLC null trypanosomes. To synthesize the GPI core glycan, washed membranes (3 × 107 to 4 × 107 cell equivalents) were incubated at 37°C for 8 min with a final concentration of 1 mM DTT, 0.8 μg of tunicamycin/μl, 5 mM MnCl2, 1 mM UDP-GlcNAc, and 0.87 μM [3H]GDP-mannose (20 Ci/mmol; 1.2 μCi) in HKML (final volume, 50 μl), followed by a 5-min chase with 1 mM nonradioactive UDP-mannose. After core glycan synthesis, membranes were washed twice in HKML and then incubated for 17 min at 37°C with 5 μM myristoyl-CoA to initiate remodeling. Cell-free myristate exchange reactions were performed as described previously (4), using a membrane fraction from hypotonic lysates prepared from GPI-PLC null trypanosomes. Briefly, membranes (3 × 107 to 4 × 107 cell equivalents) were incubated for 15 min at 37°C with a solution containing 1 μM [3H]myristate (53 Ci/mmol; 4.5 μCi), 200 μM ATP, 200 μM CoA, 1 mM DTT, 5 mM MnCl2, and 0.8 μg of tunicamycin/μl in HKML (final volume, 100 μl).
For triclosan inhibition of core glycan synthesis and myristate exchange, membranes were preincubated at 0°C for 30 min with various concentrations of freshly prepared triclosan or the equivalent volume of dimethyl sulfoxide as a solvent control. For triclosan inhibition of fatty acid remodeling, core glycan synthesis was allowed to proceed in the absence of triclosan. After the chase with nonradioactive UDP-mannose, membranes were incubated at 0°C for 30 min with triclosan or with dimethyl sulfoxide as a solvent control. After the triclosan incubation on ice, fatty acid remodeling was initiated by the addition of myristoyl-CoA and incubation at 37°C, as described above.
In vivo metabolic labeling and triclosan treatment.
Cultured bloodstream form 427 trypanosomes were preincubated with 100 μM triclosan or an equivalent volume of 1 M NaOH as a solvent control for 5.25 h and then centrifuged and resuspended to a density of 108 cells/ml in 1 ml of 100% FBS containing 100 μM triclosan (or a solvent control) and 100 μCi of [3H]myristate (53 Ci/mmol) or [3H]laurate (60 Ci/mmol). After a 45-min labeling in a 37°C, 5% CO2 incubator, the trypanosomes were washed four times in 1 ml of 50 mM Na-Bicine-50 mM NaCl-5 mM KCl-70 mM glucose (pH 8.0) to remove unincorporated radioactivity prior to lipid extraction.
Extraction and analysis of lipids.
Extraction and analysis of lipids were performed as described previously (25, 26). Briefly, bulk lipids were extracted from cells or reaction mixtures in chloroform-methanol-water (10:10:3, vol/vol/vol), followed by analysis of the organic phase on Kieselgel 60 silica TLC plates with chloroform-methanol-water (10:10:3, vol/vol/vol) as the mobile phase. To analyze glycolipids, chloroform-methanol-water organic phases were dried under N2 and then further subjected to four rounds of butanol-water (1:1, vol/vol) partitioning. The pooled butanol phases were dried under N2, resuspended in chloroform-methanol-water (10:10:3, vol/vol/vol), and separated on Kieselgel 60 silica TLC plates with chloroform-methanol-water (10:10:3, vol/vol/vol) as the mobile phase. For myristate exchange samples, chloroform-methanol extracts were dried under N2 and boiled for 5 min in 1 mM DTT-30 mM MnCl2 to cleave unreacted [3H]myristoyl-CoA, which comigrates with some glycolipids on TLC (34). To analyze fatty acid chain lengths, lipids in chloroform-methanol-water extracts were converted to their fatty acid methyl esters, followed by hexane extraction as described previously (26). Hexane phases were separated by C18 reverse-phase high-performance TLC using chloroform-methanol-water (5:15:1, vol/vol/vol) as the mobile phase. After separation, TLC plates were sprayed with En3Hance and exposed to Kodak MR film at −80°C. Densitometric quantitation of TLC data was performed on scans of appropriately exposed autoradiographic films analyzed with MacBAS software.
RESULTS
Triclosan inhibits growth of T. brucei in culture.
We first examined whether triclosan (Fig. 1, inset) affected trypanosome growth and viability in culture. Late-log-phase cultures of bloodstream form 427 cells (at 1 × 106 to 2 × 106 cells/ml) and procyclic 427 cells (at 1 × 107 to 2 × 107 cells/ml) were diluted 100-fold into fresh medium containing various amounts of triclosan or solvent control. We monitored growth after 48 h by determining the cell density of each culture by hemocytometer counting. Triclosan inhibited the growth of both bloodstream form and procyclic trypanosomes, with the effective concentrations at which the cell density is 50% of that of the solvent-treated control (EC50s) being 13 and 10 μM, respectively (Fig. 1). The cultures treated with solvent alone grew to the same density as untreated cultures (data not shown). Light microscopy of triclosan-inhibited cultures revealed dead and dying cells, indicating that triclosan treatment was killing the trypanosomes.
Triclosan did not ameliorate T. brucei infections in mice.
We tested the efficacy of triclosan in an in vivo mouse model of T. brucei infection. Although we used up to 50 mg of triclosan/kg of body weight/day and a variety of vehicles and dosage routes, we observed no reduction in the level of parasitemia or prolonged life beyond that of the controls (data not shown). These results contrast with the observation that a single injection of 38 mg of triclosan/kg, a concentration well below the acute toxicity level (1), cured Plasmodium berghei-infected mice (31). The basis for this difference in levels of in vivo effectiveness against P. berghei and T. brucei is unknown but may reflect their differing intrinsic sensitivities to triclosan. Therefore, triclosan may not be a good candidate drug for the treatment of African sleeping sickness.
Triclosan inhibits cell-free fatty acid synthesis.
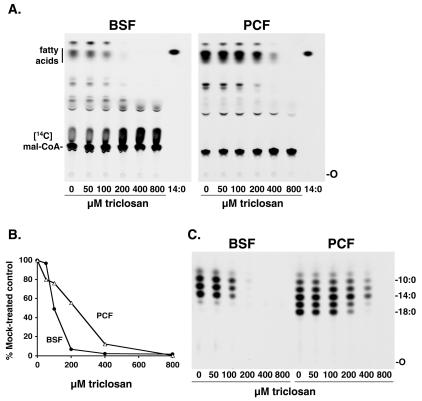
Using a standard cell-free fatty acid synthesis assay (26), we preincubated bloodstream form or procyclic trypanosome membranes with various concentrations of triclosan for 30 min. This treatment inhibited fatty acid synthesis with an EC50 of ∼100 μM for bloodstream forms and ∼200 μM for procyclic forms (Fig. 2A and B). This is a 10- to 20-fold-higher concentration of triclosan than that required to kill T. brucei in culture. We also assessed the chain lengths of fatty acid products after triclosan inhibition by analysis of their methyl esters by using reverse-phase TLC (Fig. 2C). Triclosan inhibited the synthesis of all of the observed fatty acid species.
FIG. 2.
Inhibition of cell-free fatty acid synthesis by triclosan. Bloodstream form (BSF) and procyclic form (PCF) membranes were preincubated with various concentrations of triclosan and assayed for fatty acid synthesis. (A) Lipids were extracted and analyzed by TLC. 14:0, [3H]myristate marker; O, origin; Mal-CoA, malonyl-CoA. (B) Densitometric quantitation of fatty acids from panel A. (C) Chain length analysis of the fatty acids shown in panel A. Fatty acid methyl esters were prepared and analyzed by reverse-phase TLC. The labels 10:0, 14:0, and 18:0 indicate the positions of [methyl-3H]decanoate, [methyl-3H]myristate, and [methyl-3H]stearate, respectively.
We also tested the effect of triclosan on fatty acid elongation by substituting octanoyl (C8:0)-CoA, decanoyl (C10:0)-CoA, and lauroyl (C12:0)-CoA for butyryl-CoA in the cell-free assay. For both bloodstream form and procyclic form membranes, the elongation of these acyl-CoA substrates was inhibited with the same EC50s as those observed when butyryl-CoA was used as the primer (data not shown). These triclosan data contrast with the effect of cerulenin, an inhibitor of both type I and type II ketoacyl-ACP synthases which selectively inhibited the elongation of decanoate (C10:0) (see Fig. 4 in reference 26). Thus, triclosan similarly affects both the cerulenin-insensitive pathway that synthesizes decanoate from butyrate and the cerulenin-sensitive pathway that elongates decanoate to myristate.
FIG. 4.
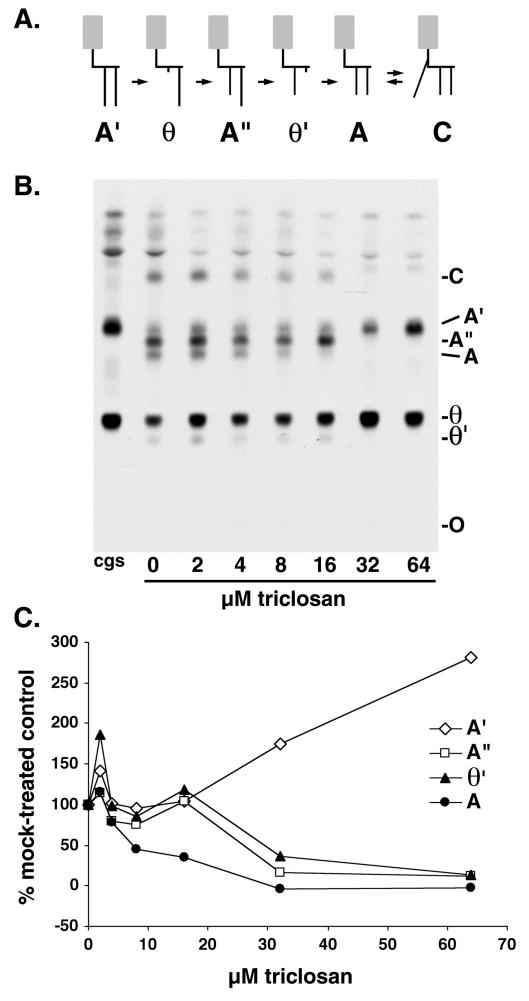
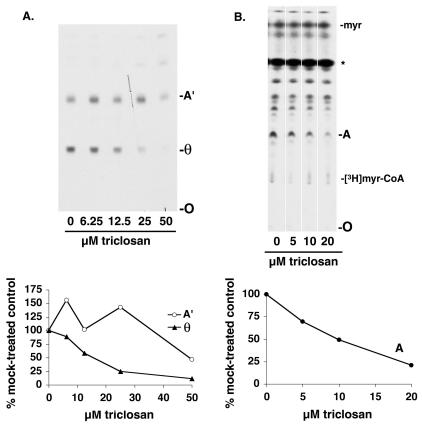
Inhibition of cell-free fatty acid remodeling by triclosan. (A) Scheme of fatty acid remodeling pathway of the VSG GPI anchor. The dimyristoylated GPI anchor precursor glycolipid A is generated from glycolipid A′ (whose fatty acids are longer than myristate) through two rounds of deacylation and myristoylation. See reference 25 for an alternative remodeling pathway involving inositol-acylated GPIs. Gray boxes represent the glycan portion of the GPI, short black vertical lines represent myristate, and long black vertical lines represent fatty acids longer than myristate. (B) Bloodstream form membranes were incubated with GDP-[3H]mannose to generate radiolabeled glycolipids A′ and θ, the substrates for fatty acid remodeling (see core glycan synthesis [cgs] lane). The lysates were then incubated for 30 min on ice with various amounts of triclosan prior to the addition of myristoyl-CoA. Glycolipids were extracted and analyzed by normal-phase TLC. The faster-migrating bands above glycolipid A′ are intermediates in core glycan synthesis and inositol-acylated species. O, origin. (C) Quantitation of the results of TLC shown in panel B, obtained by densitometry. Values are expressed as a percentage of the value obtained with a 0 μM triclosan control reaction.
Triclosan did not inhibit the elongation of laurate in vivo.
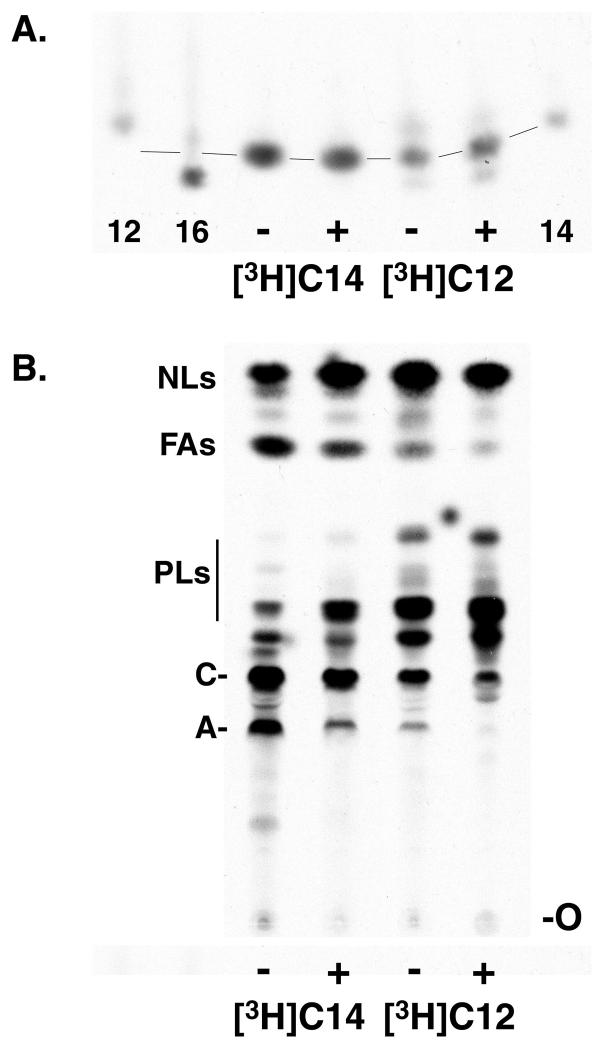
Because killing cultured bloodstream form T. brucei requires 13 μM triclosan, but a 100 μM concentration is needed to block cell-free fatty acid synthesis, we tested the effect of triclosan on fatty acid synthesis in vivo. For these studies, we could not use the preferred precursor [3H]butyrate, as its uptake is very inefficient (26). There is also little or no uptake of acetate, another potential precursor for in vivo fatty acid synthesis (26). However, longer precursors, such as decanoate and laurate, are taken up by trypanosomes and elongated to myristate. Thus, we studied the in vivo effect of triclosan on myristate synthesis using a [3H]laurate precursor. Mid-log-phase cultures of bloodstream trypanosomes were preincubated with 100 μM triclosan (about eight times the EC50 for inhibition of growth in culture for 48 h) for 5 to 6 h before metabolic labeling with either [3H]myristate or [3H]laurate. Total cellular fatty acids were converted to their methyl esters, and the chain lengths of the esters were analyzed by reverse-phase TLC (Fig. 3A). As expected, triclosan pretreatment had no effect on the chain length of [3H]myristate, since under the labeling conditions used, myristate undergoes little elongation (6, 26). However, 100 μM triclosan also had no effect on the fate of [3H]laurate, which was almost quantitatively elongated to myristate, with a small amount of palmitate (C16:0) also being formed. Interestingly, unlike with [3H]laurate, no elongation of [3H]myristate to palmitate was observed, suggesting that the [3H]myristate may not have entered the fatty acid synthesis pathway at all.
FIG. 3.
Inhibition of metabolic labeling of glycolipids A and C. (A) Cultured bloodstream trypanosomes were metabolically labeled in the absence (−) or presence (+) of 100 μM triclosan with [3H]myristate ([3H]C14), or [3H]laurate ([3H]C12). Lipids were extracted, fatty acid methyl esters were prepared, and fatty acid chain lengths were analyzed by reverse-phase TLC. Only a portion of the TLC is shown; the rest of the TLC was blank. The labels 12, 14, and 16 indicate [methyl-3H]laurate, [methyl-3H]myristate, and [methyl-3H]palmitate, respectively. Lines indicate the pattern of “smiling” on the TLC. (B) Total lipid extracts from the same cells analyzed for panel A were analyzed by normal-phase TLC. NLs, neutral lipids; FAs, free fatty acids; PLs, phospholipids; C, glycolipid C; A, glycolipid A; O, origin.
Triclosan inhibits metabolic labeling of GPI precursors.
The fatty acyl chain length analysis (Fig. 3A) revealed that triclosan had no effect on the elongation of [3H]laurate to myristate or on the chain length of [3H]myristate. Therefore, under both labeling conditions, there should be a pool of [3H]myristate free to enter the GPI fatty acid remodeling pathway (see Fig. 4A for a cartoon of the remodeling pathway). Thus, we expected to see no effect of triclosan upon the incorporation of these labeled fatty acids into the GPI precursors glycolipid A and glycolipid C (an inositol-acylated form of glycolipid A). Instead, when we examined the labeling of glycolipids in the same cells used previously and shown in Fig. 3A, we found that 100 μM triclosan inhibited the incorporation of both [3H]laurate and [3H]myristate into glycolipids A and C (Fig. 3B). In the same experiment, we also examined the incorporation of these labeled fatty acids into the mature VSG protein and found comparable levels of inhibition, with the extent of labeling reflecting the availability of labeled glycolipid precursors (data not shown).
Triclosan inhibits cell-free fatty acid remodeling.
Because triclosan caused a reduction in the labeling of glycolipids A and C by [3H]myristate and/or [3H]laurate under conditions in which laurate elongation was unaffected, we considered the possibility that triclosan affects fatty acid remodeling of GPIs. We therefore assessed the effect of triclosan on fatty acid remodeling in a cell-free assay. In the absence of triclosan, a membrane fraction was first allowed to undergo GPI core glycan synthesis, which generates glycolipid A′, and the first deacylation step, which generates glycolipid θ. These reaction products were then incubated on ice with various concentrations of triclosan prior to the initiation of fatty acid remodeling by the addition of myristoyl-CoA. Triclosan led to a reduction in the formation of the VSG GPI anchor precursor glycolipid A and the fatty acid remodeling intermediates θ′ and A" (Fig. 4B and C). Furthermore, the accumulation of some of the remodeling intermediates upon triclosan treatment suggests that this compound may have a differential effect upon the different steps in the pathway, with the later steps being more sensitive. For example, 8 μM triclosan appears to inhibit the synthesis of glycolipid A (and maybe θ′), while 32 μM triclosan inhibits all synthesis except the synthesis of θ from A′. At 64 μM, the entire pathway appears to be inhibited, since the levels of A′ and θ are similar to the starting levels generated in the core glycan synthesis reaction (Fig. 4B, lane cgs). The EC50s for the various steps in fatty acid remodeling ranged from 10 to ∼30 μM, a range similar to that required for triclosan to kill T. brucei in culture.
In addition, we tested the effect of triclosan on the enzymes involved in GPI core glycan synthesis and the first deacylation step by preincubating the membranes with triclosan. Although triclosan had only a small effect on the formation of glycolipid A′ (a 20 to 25% reduction was found with 50 μM triclosan), indicating that these ER enzymes were relatively resistant, it more strongly inhibited the first deacylation step that generates glycolipid θ (EC50 of 17 μM) (Fig. 5A).
FIG. 5.
Inhibition of θ formation and myristate exchange by triclosan. (A, upper panel) Bloodstream form membranes were preincubated with various amounts of triclosan and then incubated with GDP-[3H]mannose to generate radiolabeled glycolipids A′ and θ, the substrates for fatty acid remodeling. Glycolipids were extracted and analyzed by normal-phase TLC. (Lower panel) Quantitation by densitometry of the results of TLC shown in the upper panel. Values are expressed as a percentage of the value obtained with a 0 μM triclosan control reaction. A′ and θ, glycolipids A′ and θ; O, origin. (B, upper panel) Bloodstream form membranes were preincubated with various amounts of triclosan and assayed for myristate exchange by the addition of [3H]myristate, ATP, and CoA. Glycolipids were extracted and analyzed by normal-phase TLC. The species marked with an asterisk (*) is formed by boiling the samples (see reference 34). myr, myristate; A, glycolipid A; O, origin. The other unidentified species labeled with [3H]myristate are composed of neutral lipids, phospholipids, and other glycolipid species. (Lower panel) Quantitation by densitometry of the results of TLC shown in the upper panel. Values are expressed as a percentage of the value obtained with a 0 μM triclosan control reaction.
Triclosan inhibits myristate exchange.
Because triclosan inhibited fatty acid remodeling, we were curious about the effect of triclosan on myristate exchange, a second GPI myristoylation pathway catalyzed by a distinct set of enzymes that reside in a different compartment (probably the Golgi apparatus), separate from the site of GPI biosynthesis. Intriguingly, preincubation with triclosan also inhibited the incorporation of [3H]myristate into glycolipid A via myristate exchange, with an EC50 of 9 μM (Fig. 5B).
DISCUSSION
In this study, we demonstrated that triclosan kills T. brucei in culture, and we have explored the mechanism of its action in these parasites. The expected target of triclosan was the enoyl-ACP reductase enzyme that participates in fatty acid synthesis. However, the concentration of triclosan required to inhibit T. brucei cell-free fatty acid synthesis (EC50s of 100 to 200 μM) was 50- to 100-fold higher than the concentration required to inhibit Escherichia coli and P. falciparum FAS (EC50s of ∼2 μM) (15, 31). The high concentration of triclosan required for inhibition indicates that the T. brucei fatty acid synthesis pathway may be refractory to this drug. In support of this idea, 100 μM triclosan failed to inhibit the elongation of [3H]laurate to myristate in vivo, even though this concentration of triclosan was equivalent to the EC50 of the cell-free assay and eight times the EC50 for killing T. brucei in culture. In addition, we looked for triclosan-generated intermediates of fatty acid synthesis (i.e., β-hydroxy or trans-2,3 enoyl species) by TLC and high-pressure liquid chromatography and did not detect these intermediates either in living cells or in the cell-free assay (data not shown). Thus, these data suggest that the enoyl-ACP reductase of the T. brucei fatty acid synthesis pathway is relatively resistant to the effects of triclosan.
One possible reason for the relative resistance of the T. brucei enoyl-ACP reductase to triclosan is that it is structurally different from the conventional type II enoyl-ACP reductase enzyme, FabI. For example, mutations in E. coli or Staphylococcus aureus FabI resulted in 8- to 60-fold-higher MICs than those for the wild-type strains (12, 15, 23). Furthermore, the possession of unconventional enoyl-ACP reductases (FabK and FabL) renders some bacterial species, such as Bacillus subtilis and Streptococcus pneumoniae, more resistant to triclosan (14, 19). Extensive searches of the T. brucei database revealed no homolog to the bacterial FabI, FabK, or FabL gene, though a FabI homolog could be found in the related organism Leishmania major. However, the T. brucei genome possesses three putative homologs to yeast organellar type II enoyl reductases, which have no sequence similarity to FabI, FabK, or FabL. Although the sensitivity of this class of enoyl reductases to triclosan is unknown and their role in fatty acid synthesis in T. brucei needs to be determined, it is tempting to speculate that the unconventional nature of these candidate enoyl reductases may be the reason why fatty acid synthesis in T. brucei is more resistant to triclosan than it is in E. coli and the apicomplexan parasites.
Although T. brucei fatty acid synthesis is relatively resistant to the effects of triclosan, both in cultured cells and in a cell-free assay, the parasite itself is killed by triclosan. Procyclic forms and bloodstream forms were killed by triclosan, though the triclosan EC50s of 10 and 13 μM for T. brucei were higher than those for E. coli (∼2 μM) and the apicomplexan parasites P. falciparum (1 μM) and T. gondii (0.2 μM) (15, 22, 31). Interestingly, T. brucei is killed by triclosan concentrations 10 to 20 times lower than that required to inhibit cell-free fatty acid synthesis. Therefore, it is unlikely that killing by triclosan is due to the inhibition of fatty acid synthesis. Consistent with this idea is our inability to rescue triclosan-inhibited cultures by adding exogenous fatty acids to the medium (data not shown). One clue to the mechanism of triclosan inhibition came from attempts to inhibit the elongation of [3H]laurate to myristate in vivo. Although pretreatment of cells with triclosan did not inhibit elongation of laurate to myristate, it did inhibit another pathway, GPI myristoylation. Indeed, we found that triclosan inhibited multiple steps of the in vitro fatty acid remodeling pathway in the same concentration range required to kill trypanosomes in culture. We also showed that a similar concentration of triclosan inhibited myristate exchange, a second, biochemically distinct myristoylation pathway.
It seems unlikely that the enzymes catalyzing GPI myristoylation reactions represent a novel target of triclosan. For example, triclosan kills both bloodstream form and procyclic trypanosomes in culture at similar EC50s, but only bloodstream trypanosomes myristoylate their GPIs. A more likely possibility is that the fatty acid remodeling and myristate exchange pathways are being nonspecifically inhibited by triclosan, perhaps by perturbation of the membrane structure. Triclosan, being very hydrophobic, has been shown to incorporate into bacterial and eukaryotic membranes and to alter the physicochemical properties of artificial lipid bilayers and liposomes in concentrations comparable to those used in this study (18, 24, 32) and likely affects other essential pathways in the membranes of both bloodstream and procyclic trypanosomes. However, triclosan-induced changes in the organellar membrane would have to be subtle, as an examination of BiP (an ER luminal protein) and lipoamide dehydrogenase (a mitochondrial matrix protein) immunofluorescence in cells treated with triclosan (5 to 20 μM for 48 h or 100 μM for 5.25 h) showed no change in ER or mitochondrial morphology, respectively (data not shown). Interestingly, the enzymes that generate glycolipid A′ from phosphatidylinositol (core glycan synthesis) were barely affected by this concentration of triclosan. This difference in sensitivity may be due to the fact that core glycan synthesis takes place in the aqueous phase some distance from the membrane surface. In contrast, GPI myristoylation reactions occur on the acyl chains of the phosphatidylinositol moiety, which reside in the lipid bilayer.
Taken together, these data suggest that in T. brucei, triclosan acts upon cellular membranes, perturbing their physical properties and thereby disrupting the function of some membrane-resident enzymes. We have shown here that two bloodstream form-specific pathways are inhibited by triclosan but that other essential membrane pathways are likely to be inhibited, as triclosan kills both bloodstream and procyclic trypanosomes. This result contrasts with the well-established idea that enoyl-ACP reductase is the site of action of triclosan in bacteria and the more recent idea that triclosan action occurs at the same site in some apicomplexan parasites. Our findings reflect the results of earlier work on triclosan that hypothesized a nonspecific membrane target for triclosan action against bacteria, although the early hypotheses suggested that triclosan caused serious membrane damage resulting in cell lysis rather than the more subtle effects that we propose here (24, 29).
Acknowledgments
We thank the members of our laboratory and Sean Prigge for helpful discussions and critical reading of the manuscript.
This work was supported by NIH grants AI21334 (P.T.E.) and AI17340 (C.J.B.).
REFERENCES
- 1.Bhargava, H. N., and P. A. Leonard. 1996. Triclosan: applications and safety. Am. J. Infect. Control 24:209-218. [DOI] [PubMed] [Google Scholar]
- 2.Brun, R., and M. Shonenberger. 1979. Cultivation and in vitro cloning of procyclic culture forms of Trypanosoma brucei in a semi-defined medium. Acta Trop. 36:289-292. [PubMed] [Google Scholar]
- 3.Buxbaum, L. U., K. G. Milne, K. A. Werbovetz, and P. T. Englund. 1996. Myristate exchange on the Trypanosoma brucei variant surface glycoprotein. Proc. Natl. Acad. Sci. USA 93:1178-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buxbaum, L. U., J. Raper, F. R. Opperdoes, and P. T. Englund. 1994. Myristate exchange: a second glycosyl phosphatidylinositol myristoylation reaction in African trypanosomes. J. Biol. Chem. 269:30212-30220. [PubMed] [Google Scholar]
- 5.Doering, T. L., M. S. Pessin, G. W. Hart, D. M. Raben, and P. T. Englund. 1994. The fatty acids in unremodeled trypanosome glycosyl phosphatidylinositols. Biochem. J. 299:741-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doering, T. L., M. S. Pessin, E. F. Hoff, G. W. Hart, D. M. Raben, and P. T. Englund. 1993. Trypanosome metabolism of myristate, the fatty acid required for the variant surface glycoprotein membrane anchor. J. Biol. Chem. 268:9215-9222. [PubMed] [Google Scholar]
- 7.Doering, T. L., J. Raper, L. U. Buxbaum, S. P. Adams, J. I. Gordon, G. W. Hart, and P. T. Englund. 1991. An analog of myristic acid with selective toxicity for African trypanosomes. Science 252:1851-1854. [DOI] [PubMed] [Google Scholar]
- 8.Doering, T. L., J. Raper, L. U. Buxbaum, G. W. Hart, and P. T. Englund. 1990. Biosynthesis of glycosyl phosphatidylinositol protein anchors. Methods 1:288-296. [Google Scholar]
- 9.Edelstein, C. 1986. General properties of plasma lipoproteins and apolipoproteins, p. 495-505. In A. M. Scanu and A. A. Spector (ed.), Biochemistry and biology of plasma lipoproteins. Marcel Dekker, Inc., New York, N.Y.
- 10.Ferguson, M. A. 1999. The structure, biosynthesis and functions of glycosylphosphatidylinositol anchors, and the contributions of trypanosome research. J. Cell Sci. 112:2799-2809. [DOI] [PubMed] [Google Scholar]
- 11.Ferguson, M. A., and G. A. M. Cross. 1984. Myristylation of the membrane form of a Trypanosoma brucei variant surface glycoprotein. J. Biol. Chem. 259:3011-3015. [PubMed] [Google Scholar]
- 12.Heath, R. J., J. Li, G. E. Roland, and C. O. Rock. 2000. Inhibition of the Staphylococcus aureus NADPH-dependent enoyl-acyl carrier protein reductase by triclosan and hexachlorophene. J. Biol. Chem. 275:4654-4659. [DOI] [PubMed] [Google Scholar]
- 13.Heath, R. J., J. R. Rubin, D. R. Holland, E. Zhang, M. E. Snow, and C. O. Rock. 1999. Mechanism of triclosan inhibition of bacterial fatty acid synthesis. J. Biol. Chem. 274:11110-11114. [DOI] [PubMed] [Google Scholar]
- 14.Heath, R. J., N. Su, C. K. Murphy, and C. O. Rock. 2000. The enoyl-[acyl-carrier-protein] reductases FabI and FabL from Bacillus subtilis. J. Biol. Chem. 275:40128-40133. [DOI] [PubMed] [Google Scholar]
- 15.Heath, R. J., Y. T. Yu, M. A. Shapiro, E. Olson, and C. O. Rock. 1998. Broad spectrum antimicrobial biocides target the FabI component of fatty acid synthesis. J. Biol. Chem. 273:30316-30320. [DOI] [PubMed] [Google Scholar]
- 16.Hirumi, H., and K. Hirumi. 1989. Continuous cultivation of Trypanosoma brucei blood stream forms in a medium containing a low concentration of serum protein without feeder cell layers. J. Parasitol. 75:985-989. [PubMed] [Google Scholar]
- 17.Leal, S., A. Acosta-Serrano, Y. S. Morita, P. T. Englund, U. Bohme, and G. A. Cross. 2001. Virulence of Trypanosoma brucei strain 427 is not affected by the absence of glycosylphosphatidylinositol phospholipase C. Mol. Biochem. Parasitol. 114:245-247. [DOI] [PubMed] [Google Scholar]
- 18.Lygre, H., G. Moe, R. Skalevik, and H. Holmsen. 2003. Interaction of triclosan with eukaryotic membrane lipids. Eur. J. Oral Sci. 111:216-222. [DOI] [PubMed] [Google Scholar]
- 19.Marrakchi, H., W. E. Dewolf, Jr., C. Quinn, J. West, B. J. Polizzi, C. Y. So, D. J. Holmes, S. L. Reed, R. J. Heath, D. J. Payne, C. O. Rock, and N. G. Wallis. 2003. Characterization of Streptococcus pneumoniae enoyl-(acyl-carrier protein) reductase (FabK). Biochem. J. 370:1055-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masterson, W. J., T. L. Doering, G. W. Hart, and P. T. Englund. 1989. A novel pathway for glycan assembly: biosynthesis of the glycosyl-phosphatidylinositol anchor of the trypanosome variant surface glycoprotein. Cell 56:793-800. [DOI] [PubMed] [Google Scholar]
- 21.Masterson, W. J., J. Raper, T. L. Doering, G. W. Hart, and P. T. Englund. 1990. Fatty acid remodeling: a novel reaction sequence in the biosynthesis of trypanosome glycosyl phophatidylinositol membrane anchors. Cell 62:73-80. [DOI] [PubMed] [Google Scholar]
- 22.McLeod, R., S. P. Muench, J. B. Rafferty, D. E. Kyle, E. J. Mui, M. J. Kirisits, D. G. Mack, C. W. Roberts, B. U. Samuel, R. E. Lyons, M. Dorris, W. K. Milhous, and D. W. Rice. 2001. Triclosan inhibits the growth of Plasmodium falciparum and Toxoplasma gondii by inhibition of apicomplexan Fab I. Int. J. Parasitol. 31:109-113. [DOI] [PubMed] [Google Scholar]
- 23.McMurry, L. M., M. Oethinger, and S. B. Levy. 1998. Triclosan targets lipid synthesis. Nature 394:531-532. [DOI] [PubMed] [Google Scholar]
- 24.Meincke, B. E., R. G. Kranz, and D. L. Lynch. 1980. Effect of irgasan on bacterial growth and its adsorption into the cell wall. Microbios 28:133-147. [PubMed] [Google Scholar]
- 25.Morita, Y. S., A. Acosta-Serrano, L. U. Buxbaum, and P. T. Englund. 2000. Glycosyl phosphatidylinositol myristoylation in African trypanosomes. New intermediates in the pathway for fatty acid remodeling. J. Biol. Chem. 275:14147-14154. [DOI] [PubMed] [Google Scholar]
- 26.Morita, Y. S., K. S. Paul, and P. T. Englund. 2000. Specialized fatty acid synthesis in African trypanosomes: myristate for GPI anchors. Science 288:140-143. [DOI] [PubMed] [Google Scholar]
- 27.Paul, K. S., D. Jiang, Y. S. Morita, and P. T. Englund. 2001. Fatty acid synthesis in African trypanosomes: a solution to the myristate mystery. Trends Parasitol. 17:381-387. [DOI] [PubMed] [Google Scholar]
- 28.Perozzo, R., M. Kuo, A. S. Sidhu, J. T. Valiyaveettil, R. Bittman, W. R. Jacobs, Jr., D. A. Fidock, and J. C. Sacchettini. 2002. Structural elucidation of the specificity of the antibacterial agent triclosan for malarial enoyl acyl carrier protein reductase. J. Biol. Chem. 277:13106-13114. [DOI] [PubMed] [Google Scholar]
- 29.Regos, J., and H. R. Hitz. 1974. Investigations on the mode of action of Triclosan, a broad spectrum antimicrobial agent. Zentbl. Bakteriol. Orig. A 226:390-401. [PubMed] [Google Scholar]
- 30.Roberts, C. W., R. McLeod, D. W. Rice, M. Ginger, M. L. Chance, and L. J. Goad. 2003. Fatty acid and sterol metabolism: potential antimicrobial targets in apicomplexan and trypanosomatid parasitic protozoa. Mol. Biochem. Parasitol. 126:129-142. [DOI] [PubMed] [Google Scholar]
- 31.Surolia, N., and A. Surolia. 2001. Triclosan offers protection against blood stages of malaria by inhibiting enoyl-ACP reductase of Plasmodium falciparum. Nat. Med. 7:167-173. [DOI] [PubMed] [Google Scholar]
- 32.Villalain, J., C. R. Mateo, F. J. Aranda, S. Shapiro, and V. Micol. 2001. Membranotropic effects of the antibacterial agent Triclosan. Arch. Biochem. Biophys. 390:128-136. [DOI] [PubMed] [Google Scholar]
- 33.Waller, R. F., P. J. Keeling, R. G. Donald, B. Striepen, E. Handman, N. Lang-Unnasch, A. F. Cowman, G. S. Besra, D. S. Roos, and G. I. McFadden. 1998. Nuclear-encoded proteins target to the plastid in Toxoplasma gondii and Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 95:12352-12357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Werbovetz, K. A., and P. T. Englund. 1996. Lipid metabolism in Trypanosoma brucei: utilization of myristate and myristoyllysophosphatidylcholine for myristoylation of glycosyl phosphatidylinositols. Biochem. J. 318:575-581. [DOI] [PMC free article] [PubMed] [Google Scholar]