Abstract
The production and handling of serotonin (5-HT) is an important determinant of colonic motility and has been reported to be altered in gastrointestinal (GI) disorders such as irritable bowel syndrome (IBS). Recent studies suggest that the intestinal microbiota and sex of the host can influence expression of genes involved in 5-HT biosynthesis and signaling. While expression of genes in serotonergic pathways has been shown to be variable, it is unclear whether genes within this pathway are co-regulated. As a first step in that direction, we investigated potential correlations in relative mRNA expression of serotonergic genes, in the proximal colon isolated from male and female mice in different states of microbial association: germ-free (GF), humanized (ex-germ-free colonized with human gut microbiota, HM), and conventionally raised (CR) mice. Among the ten pairwise comparisons conducted between five serotonergic transcripts, Tph1, Chga, Maoa, Slc6a4 and Htr4, we found a strong, positive correlation between colonic expression of Slc6a4 and Htr4 across different colonization states and sexes. We also identified a positive correlation between the expression of Tph1 and Chga; however, there were no correlations observed between any other tested pair of 5-HT-related transcripts. These data suggest that correlated expression of Slc6a4 and Htr4 likely involves co-regulation of genes located on different chromosomes which modulate serotonergic activity in the gut. Further work will need to be done to understand the pathways and cell types responsible for this correlated expression, given the important role of 5-HT in gastrointestinal physiology.
Keywords: Htr4, microbiota, SERT, Slc6a4, Tph1, visceral hypersensitivity
INTRODUCTION
Intestinally produced serotonin (5-hydroxytryptamine; 5-HT) plays an important role in gastrointestinal (GI) physiology and homeostasis. (1) Most 5-HT is synthesized by enterochromaffin (EC) cells (2) through the action of tryptophan hydroxylase 1 (Tph1), which catalyzes the rate-limiting step in 5-HT synthesis. (1) 5-HT is stored in secretory granules with chromogranin A (Chga) and its expression increases in response to several stimuli including bile and short chain fatty acids. (3, 4) 5-HT is expressed by and signals to a variety of cell types across the intestinal wall thereby affecting GI motility, secretion and sensation. (1, 2, 5-7) 5-HT is catabolized by monoamine oxidase A (Maoa) to 5-HIAA (5-hydroxyindoleacetic acid). In the lamina propria, 5-HT acts through several 5-HT receptors and can be taken up via the 5-HT transporter (Slc6a4, mouse ortholog of human SERT). (2) Htr4 (5-HT4 receptor), one of the 5-HT receptors, is expressed robustly by a subset of enteric neurons, as well as intestinal epithelial cells based on studies in transgenic rodents. (8)
The gut serotonergic pathway has been implicated in the pathogenesis of GI disorders such as irritable bowel syndrome (IBS). Pharmacological studies suggest that functions of Slc6a4 are important for normal GI motility and colonic sensitivity in mice. (9) Furthermore, evidence from rodent studies suggests that 5-HT4 receptors are important for initiation and generation of colonic migrating motor complexes (CMMCs), waves of motor activity which are critical for triggering colonic peristalsis. (10) The 5-HT4 receptor has been shown to accelerate propulsive motility in guinea pigs and inhibit visceral hypersensitivity in rats. (8)
Gut microbiota- and sex-dependent factors are known to be important in subsets of functional bowel disorders such as IBS (11-13) and may play a role in intestinal homeostasis of 5-HT by affecting 5-HT transporter and/or 5-HT receptor expression. The importance of gut microbiota in influencing functional bowel disorders (14) and modulating aspects of 5-HT biosynthesis and serotonergic signaling (such as 5-HT4 receptor) in the mammalian gut (3, 4) is being increasingly appreciated. We showed previously that both human- and mouse-derived microbiota significantly increase colonic Tph1 expression, compared to germ-free mouse colon. (4) We also showed that transcription of the gene encoding Slc6a4 is sex-dependent in conventionally raised mouse colon; reduced relative expression of Slc6a4 was detected in female mice. (4) Thus, both sex of the host and gut microbes may influence Slc6a4 and 5-HT signaling in the gut.
In this study, we used gene expression data to investigate correlations among the relative expression levels of some key colonic mRNA transcripts in the serotonergic pathway. We previously showed that Slc6a4 expression is sex-dependent in conventionally raised mouse colon and that colonic Tph1 expression depends on microbial colonization status; however, we had not examined the possibility that relative expression of functionally distinct serotonergic transcripts might be correlated in the mouse colon. Identifying correlation among these gene pairs is an important first step to delineating factors that determine localized 5-HT concentration in the colon.
In the current study, we conducted pairwise tests for correlation among the following transcripts associated with the serotonergic system: Tph1 (tryptophan hydroxylase 1), Slc6a4 (5-HT transporter), Chga (chromogranin A), Htr4 (5-HT4 receptor), and Maoa (monoamine oxidase A). As there is increasing evidence for the role of gut microbes and sex of the host in influencing the serotonergic pathway, we also determined whether a subset of correlations were influenced by either colonization state of the gut or sex of the host.
METHODS
Animals
Conventionally raised (CR) mice, associated with normal mouse microbiota from birth, and germ-free (GF) breeding pairs used to produce the GF and humanized (HM) mice in this study were obtained from Taconic Farms, Inc. (Germantown, NY). HM mice were generated (colonized) at 4-6 weeks of age by gavaging GF mice with 300 μL of a 1:1 suspension of pre-reduced PBS and fecal microbiota from a healthy 49-year-old male human. All HM mice were colonized with human microbiota for 4-5 weeks prior to sacrifice and all animals in these studies were 8-12 week old Swiss-Webster mice.
Following euthanasia by CO2 inhalation, fecal contents were expelled from the colon and a short, full-thickness segment (~2 mm long) was immediately resected, frozen in liquid nitrogen, and stored at −80°C until being subjected to qRT-PCR. All protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the Mayo Clinic.
Quantitative RT-PCR
Total RNA was extracted from colonic segments using a tissue homogenizer and RNeasy Mini Kit (Qiagen, Valencia, CA, USA) with on-column DNase I (Qiagen) treatment. 500-ng aliquots of total RNA were used to generate random hexamer-primed cDNA with the SuperScript™ III First-Strand Synthesis System (Invitrogen, Carlsbad, CA, USA) according to manufacturer instructions. qRT-PCR was performed in 25-μl reactions containing 1×LightCycler 480 SYBR Green I Master (Roche, Basel, Switzerland) and 900 nM gene-specific primers; 300 nM primer concentrations were used to quantify Rpl32 (L32) mRNA. Each sample was assayed in triplicate using a LightCycler 480 Instrument II (Roche) and relative mRNA expression was calculated following normalization to L32 mRNA (mouse colon) expression using the ΔΔCT analysis method. (15) All primer sequences used in this study have been published previously: L32 (16) , Tph1 (17), Chga (18), Maoa (19); Slc6a4 (PrimerBank ID: 7110639a1) and Htr4 (PrimerBank ID: 6680325a1) primers were obtained from PrimerBank. (20)
Statistics
Graphing and statistical tests were performed using GraphPad Prism (La Jolla, CA, USA). Expression values for Slc6a4 and Htr4 from one humanized female mouse was outside two standard deviations and was hence excluded.
RESULTS
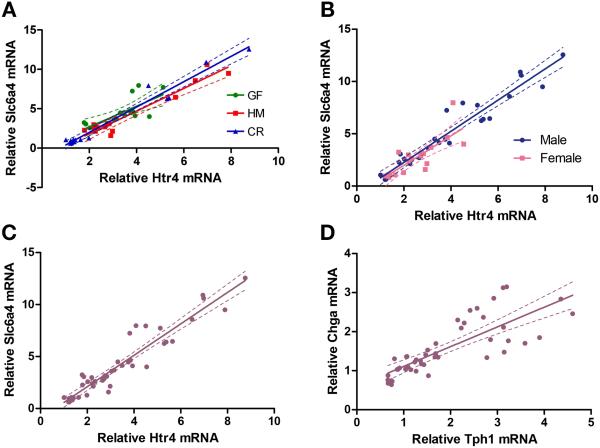
We initially examined the expression of Slc6a4 (5-HT transporter) and Htr4 (5-HT4 receptor) in conventionally raised (CR) male mice given the important role of their gene products in colonic 5-HT bioavailability and GI motility. We found a strong correlation between the two transcripts in male CR mice (R2=0.97, slope=1.60±0.08; n=16 mice). The 5-HT signaling pathway plays an important role in functional bowel disorders which exhibit female preponderance and a previous report showed significantly lower expression of Slc6a4 and Htr4 in female CR mice as compared to male CR mice. (4) Hence, we next examined if sex of the host has an effect on the correlated expression of Slc6a4 and Htr4. We also found a positive correlation between Slc6a4 and Htr4 in female CR mice (R2=0.62, slope=0.43±0.17; n=6 mice), and the slopes were not significantly different between male and female CR mice (F=0.18, p=0.68), suggesting that this correlation is independent of sex in CR mice.
Gut microbiota can alter expression of individual genes in the serotonergic pathway, but it is unclear if they affect correlated expression of genes within this pathway. Hence, we examined the effect of gut microbiota on correlation of Slc6a4 and Htr4 using both male and female mice. The two colonic transcripts showed coordinate expression in GF and microbiota-colonized mice (HM; ex-GF colonized with human microbiota and CR mice): GF (R2=0.53, slope=1.31±0.39; n=16), HM (R2=0.93, slope=1.44±0.12; n=13), and CR (R2=0.97, slope=1.61±0.08; n=16; Fig. 1a) and the slopes were not significantly different across the different colonization states (F=0.85, p=0.43). We then examined differences in male and female mice across the different colonization states (Supplementary Fig. 1) and found a positive correlation in both male (R2=0.93, slope 1.49±0.08; n=26) and female mice (R2=0.63, slope=1.41±0.26; n=19; Fig. 1b), with no significant difference between the slopes (F=0.09, p=0.76). Finally, we assessed all colonization states and male and female mice together and found that expression of Slc6a4 and Htr4 were correlated (R2=0.90, slope=1.51±0.08; n=45 mice; Fig. 1c). These data show a strong correlation between relative expression of Slc6a4 and Htr4 mRNA transcripts in the mouse colon, which is independent of sex and gut microbiota.
Figure 1. Relative mRNA expression of a subset of serotonergic genes in short segments of proximal colon from germ-free, humanized, and conventionally raised mice.
(A) Relative colonic mRNA expression (fold-difference) of solute carrier family 6, member 4 (Slc6a4, encoding an ortholog of SERT, 5-HT transporter) versus 5-HT4 receptor, in germ free and microbiota-colonized mice (n = 16 germ-free, GF; n = 13 humanized, HM; n = 16 conventionally raised, CR). (B) Similar to panel (A), Slc6a4 versus Htr4 mRNA expression in both males (n = 26) and females (n = 19). (C) Slc6a4 versus Htr4 mRNA expression in colonic segments from all mice showing coordinate expression (n = 45 mice). (D) Relative mRNA expression of tryptophan hydroxylase 1 (Tph1) versus chromogranin A (Chga) in mouse colonic segments from all three colonization states and both sexes (n = 46 mice).
We also performed a pairwise comparison of Tph1 (tryptophan hydroxylase 1) and Chga (chromogranin A), and found a positive correlation between the expression of Tph1 and Chga (R2=0.63, slope 0.51±0.06; n=46 mice; Fig. 1d), including all colonization states and both male and female mice. It is possible that these two transcripts are correlated due to their co-expression in EC cells and their related roles in 5-HT signaling. We performed pairwise comparisons of several 5-HT-related transcripts including male and female mice across different colonization states and found no correlation in any other pairs of mRNA transcripts (e.g., Tph1 vs Slc6a4, R2=0.17; Chga vs Slc6a4, R2=0.22; Tph1 vs Htr4, R2=0.16; Chga vs Htr4, R2=0.21; Tph1 vs Maoa, R2=0.00; Slc6a4 vs Maoa, R2=0.00; n=46 for all comparisons).
DISCUSSION
We provide evidence that expression of Slc6a4 and Htr4 are strongly correlated in short, full-thickness segments of proximal mouse colon and that this correlation is independent of sex or colonization status, as slopes of the resultant trend lines are similar between the two sexes and among the three assessed colonization states. We also found a positive correlation in the expression of Chga and Tph1. However, no significant correlations were observed among other 5-HT-related mRNA transcripts tested.
The strong correlation in expression of Slc6a4 and Htr4 raises questions regarding the co-regulation of these two important serotonergic genes that are located on different chromosomes (in humans on chromosome 17 and 5, respectively, but in mice on chromosome 11 and 18, respectively). It also raises questions about the cellular and physiological relationships between 5-HT transporter and 5-HT receptors in intestinal function given that, acting in concert, they can increase the relative abundance and biological activity of 5-HT. Both the 5-HT4 receptor and 5-HT transporter have been shown to play a role in the pathogenesis of visceral hypersensitivity by influencing local 5-HT abundance/availability. (21)
Because of the potential effect of sex and gut microbiota on expression of Htr4 (3), as well as the relevance of Htr4 in pathophysiology of intestinal disorders (21), we focused primarily on expression related to this receptor isoform, even though there are multiple families of 5-HT receptors are expressed in the gut. The origins of the gut microbiota (mouse- or human-derived) in CR and HM mice did not impact the correlated expression of Slc6a4 and Htr4; however, transcript levels are affected by interactions between host sex and gut microbial colonization (presence or absence), (4) emphasizing that host sex and colonization may co-regulate Slc6a4 and Htr4. Although the estrous cycle of female mice was not controlled in this study, it may be useful to examine its potential effects on expression of Htr4 and Slc6a4 in future studies. Given the importance of 5-HT transporter in human bowel disorders (22, 23), and the use of drugs targeting the 5-HT4 receptor in GI disorders such as IBS, additional studies are warranted to understand the signaling pathways responsible for driving expression of the transcripts encoding these factors. Future studies will be needed to determine the cell type(s) and the mediators responsible for the observed correlation between Slc6a4 and Htr4 expression in the colon, and to determine if this correlation is limited to the colon or may be seen in other areas, such as the brain.
We also report a positive correlation in the expression of Chga and Tph1, again located on different chromosomes, but largely expressed in EC cells, in addition to a few other cell types. Both genes have been shown to be increased in the presence of gut microbiota, independent of host sex, suggesting a potential role of gut microbiota. (4) However, further work is needed to elucidate the factors that drive the co-expression of these two genes related to biosynthesis and release of 5-HT.
These novel findings raise several important biological questions regarding the role of specific gene pairs which, in spite of being on different chromosomes, are likely co-regulated and may represent components of a pathway important for targeting disorders such as IBS associated with alterations in 5-HT homeostasis.
Supplementary Material
Key Points.
Serotonergic gene expression in the mouse colon depends on microbial colonization and sex of the host. However, correlations among expression of serotonergic genes have not been studied.
We identified a positive correlation between colonic Htr4 (5-HT4 receptor) and Slc6a4 (5-HT transporter) mRNAs, observed in both sexes and different states of microbial association: germ-free, humanized and conventionally raised.
Correlated expression of Slc6a4 and Htr4 suggests that 5-HT reuptake and 5-HT4 receptor activity may be coupled in colon, which is relevant in functional bowel disorders.
Acknowledgements
We thank Charles Salmonson for technical assistance and gnotobiotic mouse husbandry; we thank Kristy Zodrow for administrative assistance.
Funding
This work was made possible by funding from NIH K08 DK100638, as well as the Global Probiotic Council, Minnesota Partnership for Biotechnology and Genomics, and Center for Individualized Medicine (CIM; Mayo Clinic).
Non-standard abbreviations
- 5-HT
Serotonin, 5-hydroxytryptamine
- GF
Germ-free
- HM
Humanized
- CR
Conventionally raised
- Tph1
Tryptophan hydroxylase 1
- Chga
Chromogranin A
- Maoa
Monoamine oxidase A
- Slc6a4
Serotonin transporter (mouse)
- SERT
Serotonin transporter (human)
- Htr4
Serotonin receptor 5-HT4
- L32
60S ribosomal protein L32
- ACTB
Beta-actin
- IBS
Irritable bowel syndrome
- EC
Enterochromaffin
- GI
Gastrointestinal
Footnotes
Conflicts of Interest
The authors declare that no conflict of interest exists.
Authors Contributions
CSR and PCK designed the research study; CSR and PCK performed the research; DRL, JHS, JLS, and GF edited the manuscript, provided valuable advice, and essential reagents or tools; CSR and PCK wrote the manuscript; each author has read the final manuscript and approved it.
References
- 1.Li Z, Chalazonitis A, Huang YY, et al. Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/survival of enteric dopaminergic neurons. J Neurosci. 2011;31:8998–9009. doi: 10.1523/JNEUROSCI.6684-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397–414. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Yano JM, Yu K, Donaldson GP, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reigstad CS, Salmonson CE, Rainey JF, 3rd, et al. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. Faseb J. 2015;29:1395–1403. doi: 10.1096/fj.14-259598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bulbring E, Crema A. The action of 5-hydroxytryptamine, 5-hydroxytryptophan and reserpine on intestinal peristalsis in anaesthetized guinea-pigs. J Physiol. 1959;146:29–53. doi: 10.1113/jphysiol.1959.sp006176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bulbring E, Lin RC. The effect of intraluminal application of 5-hydroxytryptamine and 5-hydroxytryptophan on peristalsis; the local production of 5-HT and its release in relation to intraluminal pressure and propulsive activity. J Physiol. 1958;140:381–407. [PMC free article] [PubMed] [Google Scholar]
- 7.Mawe GM, Hoffman JM. Serotonin signalling in the gut--functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2013;10:473–486. doi: 10.1038/nrgastro.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffman JM, Tyler K, MacEachern SJ, et al. Activation of colonic mucosal 5-HT(4) receptors accelerates propulsive motility and inhibits visceral hypersensitivity. Gastroenterology. 2012;142:844–854.e844. doi: 10.1053/j.gastro.2011.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coates MD, Johnson AC, Greenwood-Van Meerveld B, Mawe GM. Effects of serotonin transporter inhibition on gastrointestinal motility and colonic sensitivity in the mouse. Neurogastroenterol Motil. 2006;18:464–471. doi: 10.1111/j.1365-2982.2006.00792.x. [DOI] [PubMed] [Google Scholar]
- 10.Kendig DM, Grider JR. Serotonin and colonic motility. Neurogastroenterol Motil. 2015;27:899–905. doi: 10.1111/nmo.12617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeffery IB, O'Toole PW, Ohman L, et al. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut. 2012;61:997–1006. doi: 10.1136/gutjnl-2011-301501. [DOI] [PubMed] [Google Scholar]
- 12.Mulak A, Tache Y, Larauche M. Sex hormones in the modulation of irritable bowel syndrome. World J Gastroenterol. 2014;20:2433–2448. doi: 10.3748/wjg.v20.i10.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tana C, Umesaki Y, Imaoka A, Handa T, Kanazawa M, Fukudo S. Altered profiles of intestinal microbiota and organic acids may be the origin of symptoms in irritable bowel syndrome. Neurogastroenterol Motil. 2010;22:512–519. doi: 10.1111/j.1365-2982.2009.01427.x. e114-515. [DOI] [PubMed] [Google Scholar]
- 14.Ohman L, Tornblom H, Simren M. Crosstalk at the mucosal border: importance of the gut microenvironment in IBS. Nature reviews Gastroenterology & hepatology. 2015;12:36–49. doi: 10.1038/nrgastro.2014.200. [DOI] [PubMed] [Google Scholar]
- 15.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 16.Sonnenburg JL, Chen CT, Gordon JI. Genomic and metabolic studies of the impact of probiotics on a model gut symbiont and host. PLoS biology. 2006;4:e413. doi: 10.1371/journal.pbio.0040413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu C, Maejima T, Wyler SC, Casadesus G, Herlitze S, Deneris ES. Pet-1 is required across different stages of life to regulate serotonergic function. Nat Neurosci. 2010;13:1190–1198. doi: 10.1038/nn.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu Y, Ippolito JE, Garabedian EM, Humphrey PA, Gordon JI. Molecular characterization of a metastatic neuroendocrine cell cancer arising in the prostates of transgenic mice. J Biol Chem. 2002;277:44462–44474. doi: 10.1074/jbc.M205784200. [DOI] [PubMed] [Google Scholar]
- 19.Lee AK, Mojtahed-Jaberi M, Kyriakou T, et al. Effect of high-fat feeding on expression of genes controlling availability of dopamine in mouse hypothalamus. Nutrition. 2010;26:411–422. doi: 10.1016/j.nut.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, Seed B. A PCR primer bank for quantitative gene expression analysis. Nucleic Acids Res. 2003;31:e154. doi: 10.1093/nar/gng154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan C, Xin-Guang L, Hua-Hong W, Jun-Xia L, Yi-Xuan L. Effect of the 5-HT4 receptor and serotonin transporter on visceral hypersensitivity in rats. Braz J Med Biol Res. 2012;45:948–954. doi: 10.1590/S0100-879X2012007500122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuan J, Kang C, Wang M, et al. Association study of serotonin transporter SLC6A4 gene with Chinese Han irritable bowel syndrome. PLoS One. 2014;9:e84414. doi: 10.1371/journal.pone.0084414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galligan JJ, Patel BA, Schneider SP, et al. Visceral hypersensitivity in female but not in male serotonin transporter knockout rats. Neurogastroenterol Motil. 2013;25:e373–381. doi: 10.1111/nmo.12133. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.