Abstract
Problem Identification
Oral anticancer medication (OAM) use has been steadily increasing, leading to several patient benefits. A notable challenge for nurses is accurate monitoring of patient OAM regimens because nonadherence is associated with poor health outcomes and decreased survival. Currently, no gold standard measure of OAM adherence exists. The authors conducted a systematic review of the association between objective and patient-reported measures of OAM adherence.
Literature Search
A systematic electronic literature search was conducted using PubMed, EMBASE, Scopus, PsycINFO®, Cochrane Library, Web of Science, and CINAHL® databases through November 2014.
Data Evaluation
Articles were independently reviewed to determine whether they included an original characterization of the level of association between objective and patient-reported measures of OAM adherence.
Synthesis
From a total of 11,135 articles retrieved, eight studies met inclusion criteria. Objective adherence was primarily assessed using pill counts or Medication Event Monitoring System (MEMSCap™). Patient-reported adherence was most commonly assessed using study-specific questionnaires. Significant positive correlations were observed between objective and patient-reported adherence across most studies, with three studies reporting higher rates of adherence via patient reporting.
Conclusions
Despite variation in the OAMs and measures used, patient-reported adherence rates were equal to or higher than objective adherence measures across studies. Social desirability bias may be a concern; however, given the significant concordance observed, using patient-reported methods in future studies of OAM adherence may be justified.
Implications for Nursing
This review provides evidence to support nursing use of patient-reported measures to accurately monitor OAM adherence and potentially improve the quality of patient–provider communication.
Keywords: medication adherence, neoplasms, oral medicine, patient outcome assessment
The use of oral anticancer medication (OAM) has been steadily on the rise to treat a variety of cancer types (Bedell, 2003; Moore, 2007; O'Neill & Twelves, 2002). OAMs allow patients to administer their own treatments from the privacy of their homes, decreasing the necessity for frequent clinic visits. As a result, OAMs have been shown to be a patient-preferred option, related primarily to the convenience of administration, as well as the perceived reduction in interference with everyday life (Foulon, Schoffski, & Wolter, 2011). The preference for oral over IV medications may also be related, in part, to an increase in perceived effectiveness and the perceived reduced toxicity of this method (Borner et al., 2002; Fallowfield et al., 2006; Thanki, Gangwal, Sangamwar, & Jain, 2013).
Despite the perceived benefits of OAM, adherence (defined, in this context, as the extent to which patients take their medications as prescribed by their healthcare providers either as part of clinical trial participation or routine care) has been frequently reported as suboptimal (Osterberg & Blaschke, 2005; Verbrugghe, Verhaeghe, Lauwaert, Beeckman, & Van Hecke, 2013). Poor adherence to OAMs is an important factor in providing care because it can negatively affect providers' abilities to determine treatment efficacy and effectiveness (Ruddy, Mayer, & Partridge, 2009). In addition, poor adherence contributes to increased healthcare costs and can lead to worsening of disease and decreased overall patient survival (Bestvina et al., 2014; Servick, 2014; Soria et al., 2011). Rates of adherence to OAMs are often less than 80%, with 10% of patients with cancer documented as not having refilled their OAM prescriptions (Kavookjian & Wittayanukorn, 2015; Puts et al., 2014; Spoelstra & Given, 2011; Streeter, Schwartzberg, Husain, & Johnsrud, 2011).
Several factors have contributed to poor OAM adherence. From a patient perspective, barriers to proper adherence include (a) challenging administration schedules, (b) beliefs that the medication may not be effective or outweigh the risks of side effects, and (c) patients' forgetfulness or drowsiness, leading to missed doses (Palmieri & Barton, 2007; Verbrugghe et al., 2013; Verma, Madarnas, Sehdev, Martin, & Bajcar, 2011). Adverse events have also been implicated in treatment discontinuation and missed or held doses (Deutsch, Koerner, Miller, Craft, & Fancher, 2016). Systemic barriers, such as lack of health insurance reimbursement for medication costs and proper re-filling of prescriptions, have also contributed to poor adherence rates. The number of perceived and actual barriers associated with oral medication adherence in cancer can be discouraging, particularly because OAMs have become increasingly popular and, when taken properly, are associated with positive clinical outcomes (Darkow et al., 2007; Ibrahim et al., 2011; Marin et al., 2010). From an oncology nursing perspective, the direct relationship between OAM regimen adherence and the therapeutic outcome of cancer treatment (Servick, 2014) places nurses in a position as essential stakeholders in helping to improve OAM adherence. Therefore, investigating best practices for capturing treatment-related adherence information is important.
Currently, no gold standard measure of OAM adherence exists. Approaches documented in the oncology literature have included patient self-report, pill counts, pharmacy refill rates, and the Medication Event Monitoring System (MEMSCap™) (Macintosh, Pond, Pond, Leung, & Siu, 2007; Walter et al., 2013). Each method has potential limitations, including errors made during pill counting, failure to properly document dosage modifications in the medical record, and issues related to patients being delayed in receiving their OAM. Despite variation in the approach used, few studies actually compare and test the effectiveness of subjective versus objective adherence measures. The authors of the current study conducted a systematic review of the available research evidence to assess the association between patient-reported and objective measures of OAM adherence in patients with cancer.
Methods
A systematic search of articles published in peer-reviewed journals was performed using PubMed, EMBASE, Scopus, Cochrane Library, Web of Science, PsycINFO®, and CINAHL®. Four categories of terms were searched: (a) cancer, (b) oral administration, (c) medication, and (d) medication adherence. No date or language restrictions were used, and each database was searched in its entirety through November 2014.
In PubMed and Cochrane Library, Medical Subject Headings (MeSH) were used in addition to key words. In EMBASE, Emtree terms were exploded in addition to key words. In Scopus, Web of Science, PsycINFO, and CINAHL, only key words were used. In addition, a keyword search was completed in the following grey literature sources: the National Cancer Institute, Can-cerCare, the American Cancer Society, the Oncology Nursing Society, and the Association of Community Cancer Centers. A complete list of MeSH and keyword terms used can be found in Figure 1. Studies were deemed eligible for inclusion if they included an original characterization of the level of association between patient-reported and objective OAMs in adult (i.e., aged 18 years or older) patients with cancer.
Figure 1. Medical Subject Heading (MeSH) and Keyword Terms for the Literature Search.
Initially, all titles were independently reviewed for eligibility by two coauthors. Records identified during the title review were then randomly assigned to a different pair of coauthors for full abstract screening. Articles moved forward for full-text review if both coauthors reached consensus on eligibility. In instances of disagreement, a third coauthor served as an arbitrator. For the full-text review phase, author teams were randomly assigned, and they consisted of a primary reviewer and a secondary reviewer for the purposes of verification and quality assurance. Both reviewers independently completed standardized coding forms to extract the predetermined information from each potentially eligible article. All reviewers then met as a group and compared full-text article reviews to resolve any discrepancies and make final decisions regarding article inclusion. Each author searched references from the included full-text articles to determine whether they should also be considered for inclusion. Study quality was assessed using a modified version of the Downs and Black (1998) study quality checklist.
Results
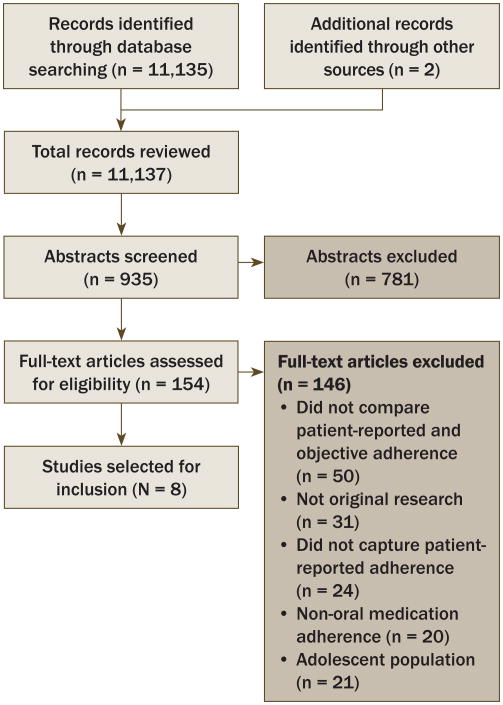
The initial electronic literature search yielded a total of 11,135 titles. Two additional titles were added through hand searching. Following the process of title screening, two of the primary authors independently reviewed each of the 935 unique article abstracts; 154 (110 full-text articles and 44 conference proceeding abstracts) were retained for the full-text review. Reasons for article exclusion during the full-text review phase included that they (a) did not compare patient-reported and objective adherence (n = 50), (b) did not capture patient-reported adherence (n = 24), (c) did not assess adherence for oral anticancer medication (n = 20), (d) were reviews or did not include original research findings (n = 31), or (e) included adolescent populations (n = 21). A total of eight articles met eligibility criteria and were included in this review (see Figure 2). Inter-rater agreement was high (Cohen's kappa = 0.82). Each of the eight articles possessed at least 80% of the relevant quality indicators from a modified version of the Downs and Black (1998) study quality checklist.
Figure 2. PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) Flow Chart.
Study Characteristics
Table 1 includes a summary of patient demographics and clinical characteristics of the eight included studies. Patients were of mixed cancer types, including chronic myeloid leukemia, as well as breast, gastrointestinal, hematologic, and lung cancers. OAM type was variable across studies and included capecitabine (Xeloda®), erlotinib (Tarceva®), exemestane (Aromasin®), gemcitabine (Gemzar®), imatinib (Gleevec®), sunitinib (Sutent®), tamoxifen (Nolvadex®), and temozolomide (Temodar®). Only two studies reported race, with the majority of those patients identifying as Caucasian (Gebbia et al., 2013; Schneider, Adams, & Gosselin, 2014). Ethnicity was not reported in any of the eight studies.
Table 1. Characteristics of Included Studies.
| Study | Sample | Objective Assessment | Patient-Reported Assessment | Reported Association |
|---|---|---|---|---|
| Addeo et al., 2015 | 285 female patients with breast cancer who were taking exemestane | Medication possession ratio, pill count | Self-report survey | 88% of women self-reported taking all of their OAM; 12% reported that they took nearly all of their OAM. Objective assessment (medication possession ratio) showed significantly lower adherence rates (78% across six months of treatment). |
| Daouphars et al., 2013 | 46 patients with chronic myeloid leukemia (50% female) with a mean age of 59 years (range = 29–89) who were taking imatinib | Medication possession ratio | Self-report survey | Patient-reported adherence and objective assessment (medication possession ratio) data were significantly positively associated (r = 0.68, p < 0.001). |
| Gebbia et al., 2013 | 150 patients with lung cancer (33% female) with a median age of 66 years who were taking erlotinib | Visual analog scale, pill count | Basel Assessment of Adherence Scale | No statistically significant correlation was found between adherence to erlotinib, as evaluated by patient-reported and objective measures (p = 0.067). |
| Schneider et al., 2014 | 48 patients with breast and gastrointestinal cancers and hematologic malignancies (65% female) with a mean age of 60 years (range = 35–86 years) who were taking capecitabine or tamoxifen | Pharmacy fill rates | Self-report survey | Pharmacy fill rates were significantly lower than patient-reported adherence. Patient-reported adherence was 86% and 89% during two and four months, respectively. Individuals who had adequate supply of medication to take their medication as prescribed was 73% and 71% at two and four months, respectively. Positive association (p = 0.0048) was found between patient-reported adherence and pharmacy fill rates at two months. |
| Timmers et al., 2014 | 177 patients with hemato-logic malignancies (44% female) with a mean age of 59 years (range = 22–86) who were taking capecitabine, dasatinib, erlotinib, imatinib, nilotinib, sunitinib, temozolomide, or thalidomide | Pill count, pharmacy fill rates | Medication adherence rating scale | Significant positive association was found between objective assessment and patient-reported assessment (optimal adherence [medication adherence rating scale ≥ 25] versus suboptimal adherence [medication adherence rating scale < 25]; p = 0.006). |
| Timmers et al., 2015 | 62 patients with lung cancer (47% female) with a median age of 64 years who were taking erlotinib | MEMSCap | Medication adherence rating scale | Significant association was found between suboptimal patient-reported adherence (medication adherence rating scale < 25) and suboptimal intake relative to time-points of a meal, as captured by MEMSCap (odds ratio = 4.83, p = 0.042). |
| Walter et al., 2013 | 19 patients with gastrointestinal malignancies (53% female) with a median age of 57 years (range = 39–76) who were taking XELIRI (with or without bevacizumab), XELOX, gemcitabine, or capecitabine | MEMSCap, pill count | Short standard questionnaire | Patient-reported adherence rate had a significant positive association with objective adherence captured by pill count but not via MEMSCap. |
| Waterhouse et al., 1993 | 26 patients with breast cancer (100% female) with a median age of 59 years (range = 42–86) who were taking tamoxifen | MEMSCap, pill count | Self-report survey | Patient-reported adherence was significantly higher than objective adherence (pill count [p = 0.008], MEMSCap [p = 0.005]). |
OAM—oral anticancer medication; MEMSCap™—Medication Event Monitoring System; XELIRI—capecitabine and irinotecan; XELOX—capecitabine and oxaliplatin
In the majority of the included articles (n = 5), study-specific assessment measures were used to capture patient-reported OAM adherence. Two studies captured patient-reported OAM adherence using the Medication Adherence Rating Scale (MARS) (Timmers et al., 2014, 2015), whereas one employed the Basel Assessment of Adherence Scale (BAAS) (Gebbia et al., 2013).
Objective measures used to capture OAM adherence were much more variable and included pharmacy fill rates, pill counts, medication possession ratio (MPR), visual analog scale (VAS), and MEMSCap. MPR was calculated as the total days' supply of an OAM dispensed during a follow-up period, adjusted for dosage reductions (Addeo et al., 2015; Daouphars et al., 2013). The VAS ranged from 0 (no adherence) to 100 (perfect adherence) (Gebbia et al., 2013). MEMSCap recorded adherence through documenting each medication bottle opening as a dose (Timmers et al., 2015; Walter et al., 2013; Waterhouse, Calzone, Mele, & Brenner, 1993).
Patient-Reported and Objective Oral Anticancer Medication Adherence
A significant positive relationship between patient-reported and objective OAM adherence was reported in four studies. MPR and patient self-rated adherence were significantly correlated (r = 0.68, p < 0.001) in a study of patients with chronic myeloid leukemia (Daouphars et al., 2013). Two separate studies of patients with hematologic malignancies and lung cancer conducted by Timmers et al. (2014, 2015) demonstrated a significant association between patient-reported OAM adherence, as captured by MARS, and objective assessments. Walter et al. (2013) found, in a study of patients with gastrointestinal malignancies, that patient-reported OAM adherence had a significant positive association with objective pill counts, but not MEMSCap.
Patient-reported and objective OAM adherence was only directly compared in three studies; however, in each case, patient-reported OAM adherence was significantly higher than objective OAM adherence. A study of women with breast cancer found that 88% self-reported completing all of their prescribed OAM during six months of treatment; objective assessment resulted in only 78% adherence (Addeo et al., 2015). In a study of patients with mixed cancer types, patient-reported OAM adherence was documented at 86% and 89% for the two- and four-month follow-ups, respectively. Pharmacy fill rates in this patient population indicated that 73% and 71% of patients had an adequate supply of medication to take their OAM as prescribed at the two- and four-month follow-ups, respectively (Schneider et al., 2014). An additional study of women with breast cancer demonstrated that patient-reported OAM adherence, as captured by a study-specific self-report survey, was significantly higher than that captured by pill counts (p < 0.008) or MEMSCap (p < 0.005) (Waterhouse et al., 1993). The remaining study reported no significant correlation between patient-reported (BAAS) and objective (VAS, pill counts) OAM measures (Gebbia et al., 2013).
Discussion
The authors' primary objective with this systematic review was to examine associations between patient-reported and objective measures of OAM adherence in patients with cancer. Findings suggest moderate agreement between patient-reported and objective measures of OAM adherence. Of the eight studies reviewed, four found positive correlations between patient-reported and objective measures, and only three studies explicitly compared measures. In the three studies that directly compared adherence methods, patient-reported measures yielded higher adherence rates. These higher rates are possibly an overestimation of the patients' true adherence rates. In addition, the lack of standardized use of subjective and objective adherence measures across studies makes comparison difficult.
Although these high rates may be indicative of social desirability patterns, where patients feel the need to be a “good patient,” leading to overinflation of adherence rates, the associations found among measures suggest that patient-reported adherence is a valid method for eliciting this important information. Psychometric research on the efficacy and validity of patient-reported outcomes further supports this viewpoint (Sadahiro et al., 2000; Shi et al., 2010). Future research that specifically evaluates the interaction between adherence rates and methods on clinical outcomes is needed to determine best practices when measuring adherence, particularly in clinical trials. These findings also suggest the need for a multicenter research collaboration addressing adherence to OAM using uniform patient-reported and objective measures.
All studies reviewed showed some degree of OAM medication nonadherence. Nonadherence is problematic for several reasons. Treatment adherence is one of the most salient predictors of patient survival, and poor adherence can result in drug resistance (Kavookjian & Wittayanukorn, 2015), low response rate (Marin et al., 2010), earlier and more frequent disease progression (Kavookjian & Wittayanukorn, 2015), and, ultimately, a greater risk of death (Richardson, Shelton, Krailo, & Levine, 1990). Nonadherence may also negatively affect healthcare providers' ability to determine treatment safety and efficacy (Ruddy et al., 2009). Poor adherence not only affects patient-related outcomes, but also can negatively influence system-related outcomes, including increased healthcare costs. Specifically, 33%–66% of hospital admissions are directly attributable to poor medication adherence, resulting in about $100 billion in healthcare costs each year (Osterberg & Blaschke, 2005).
Limitations
The current review is strengthened by its comprehensive and systematic approach to the examination of the available literature on OAM adherence but is limited by several factors. First, the review only focused on anticancer medications, limiting the ability to examine how adherence may be affected by other medications typically prescribed to patients with cancer. Recognizing the complexity of treatment regimens, which also include other types of oral medications, future research may address the impact of drug combinations on overall adherence and clinical outcomes. Second, the majority of the studies included in this review did not report effect sizes, so the authors were not able to employ meta-analytic techniques to report an overall effect. Lastly, a minority of studies directly compared patient-reported and objective measures of OAM adherence. Future research is needed to further examine the concordance and efficacy of these widely used assessments of adherence.
Implications for Practice and Research
Several factors contribute to poor OAM adherence, but one of the primary barriers is a lack of effective communication between patients and providers. One recommendation to increase OAM is to improve the quality of patient–provider communication through the increased use of nursing-centered patient education. The nursing role in medication teaching may mitigate the disconnect in communication between patients and providers, maximizing medication adherence. Oncology nurses are at the forefront of a patient's healthcare team and are in an ideal position to optimize patient–provider communication and help patients gain improved knowledge about the importance of OAM adherence. A number of trials have investigated the effectiveness of various nursing-led interventions to improve adherence rates, showing great promise for the role of nurses in improving adherence and, ultimately, long-term outcomes (Boucher, Lucca, Hooper, Pedulla, & Berry, 2015; Schneider et al., 2014; Schneider, Hess, & Gosselin, 2011). In addition, the use of smartphone and other mobile health technology may help to reduce communication gaps between nurses and patients, as has been preliminarily suggested in a review by Park, Howie-Esquivel, and Dracup (2014). For example, if patient nonadherence to their OAM is because of adverse events that are being attributed to the medication, the oncology nurse could be sent an electronic real-time notification, with this information emphasized for discussion at the next patient visit. In cases of extreme adverse events, the oncology nurse could give this patient-reported information to the patient's disease management team to inform a potential dosage modification.
Conclusion
Patient-reported OAM adherence rates were equal to or higher than objective OAM adherence measures across the majority of studies. Given the moderate concordance between patient-reported measures and objective measures, future research or clinical trials that aim to assess adherence to OAM may use patient-reported measures as a cost-effective and high-quality alternative to objective methods.
Knowledge Translation
Patient-reported measures appear to capture oral anticancer medication (OAM) adherence as well as traditional, objective methods, such as pill count or pharmacy fill rates.
Patient-reported measures of OAM adherence may be viable for use as a cost-effective and reliable alternative to objective capture of this information.
Patient-centered monitoring of OAM usage can potentially facilitate communication with nurses about the therapeutic importance of adhering to prescribed treatments.
Acknowledgments
This project was funded by a National Institutes of Health Research Training Grant (T32 CA009461-25) and a National Institutes of Health Support Grant (P30 CA08748-49), which provides partial support for the Behavioral Research Methods Core Facility used in conducting this investigation. Mention of specific products and opinions related to those products do not indicate or imply endorsement by the Oncology Nursing Forum or the Oncology Nursing Society.
Footnotes
Atkinson, Rodríguez, Gordon, Avildsen, Emanu, Anselmi, and Ginex contributed to the conceptualization and design. Atkinson, Rodríguez, Gordon, Avildsen, Emanu, and Jewell completed the data collection. Atkinson, Rodríguez, Gordon, Avildsen, Emanu, Jewell, and Ginex provided the analysis. All authors contributed to the manuscript preparation.
Contributor Information
Thomas M. Atkinson, Memorial Sloan Kettering Cancer Center in New York, NY.
Vivian M. Rodríguez, Memorial Sloan Kettering Cancer Center in New York, NY.
Mallorie Gordon, Memorial Sloan Kettering Cancer Center in New York, NY.
Isabelle K. Avildsen, Memorial Sloan Kettering Cancer Center in New York, NY.
Jessica C. Emanu, Memorial Sloan Kettering Cancer Center in New York, NY.
Sarah T. Jewell, Rutgers University in New Brunswick, NJ.
Kimberly A. Anselmi, Memorial Sloan Kettering Cancer Center.
Pamela K. Ginex, Memorial Sloan Kettering Cancer Center.
References
- Addeo R, Iodice P, Maiorino L, Febbraro A, Incoronato P, Pisano A, et al. Del Prete S. Acceptance and adherence of oral endocrine therapy in women with metastatic breast cancer: Exacampania group study. Breast Journal. 2015;21:326–328. doi: 10.1111/tbj.12409. [DOI] [PubMed] [Google Scholar]
- Bedell CH. A changing paradigm for cancer treatment: The advent of new oral chemotherapy agents. Clinical Journal of Oncology Nursing. 2003;7(Suppl):5–9. doi: 10.1188/03.CJON.S6.5-9. [DOI] [PubMed] [Google Scholar]
- Bestvina CM, Zullig LL, Rushing C, Chino F, Samsa GP, Altomare I, et al. Zafar SY. Patient-oncologist cost communication, fnancial distress, and medication adherence. Journal of Oncology Practice. 2014;10:162–167. doi: 10.1200/JOP.2014.001406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borner MM, Schoffski P, de Wit R, Caponigro F, Comella G, Sulkes A, et al. Fumoleau P. Patient preference and pharmacokinetics of oral modulated UFT versus intravenous fuorouracil and leucovorin: A randomised crossover trial in advanced colorectal cancer. European Journal of Cancer. 2002;38:349–358. doi: 10.1016/s0959-8049(01)00371-9. [DOI] [PubMed] [Google Scholar]
- Boucher J, Lucca J, Hooper C, Pedulla L, Berry DL. A structured nursing intervention to address oral chemotherapy adherence in patients with non-small cell lung cancer. Oncology Nursing Forum. 2015;42:383–389. doi: 10.1188/15.ONF.383-389. [DOI] [PubMed] [Google Scholar]
- Daouphars M, Ouvry M, Lenain P, Rouvet J, Jardin F, Bubenheim M, Varin R. Preliminary validation of self-assessment tool to measure imatinib adherence in patients with chronic myeloid leukemia. Pharmacotherapy. 2013;33:152–156. doi: 10.1002/phar.1174. [DOI] [PubMed] [Google Scholar]
- Darkow T, Henk HJ, Thomas SK, Feng W, Baladi JF, Goldberg GA, et al. Cortes J. Treatment interruptions and non-adherence with imatinib and associated healthcare costs: A retrospective analysis among managed care patients with chronic myelogenous leukaemia. PharmacoEconomics. 2007;25:481–496. doi: 10.2165/00019053-200725060-00004. [DOI] [PubMed] [Google Scholar]
- Deutsch S, Koerner P, Miller RT, Craft Z, Fancher K. Utilization patterns for oral oncology medications in a specialty pharmacy cycle management program. Journal of Oncology Pharmacy Practice. 2016;22:68–75. doi: 10.1177/1078155214547664. [DOI] [PubMed] [Google Scholar]
- Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. Journal of Epidemiology and Community Health. 1998;52:377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallowfield L, Atkins L, Catt S, Cox A, Coxon C, Langridge C, et al. Price M. Patients' preference for administration of endocrine treatments by injection or tablets: Results from a study of women with breast cancer. Annals of Oncology. 2006;17:205–210. doi: 10.1093/annonc/mdj044. [DOI] [PubMed] [Google Scholar]
- Foulon V, Schoffski P, Wolter P. Patient adherence to oral anticancer drugs: An emerging issue in modern oncology. Acta Clinica Belgica. 2011;66:85–96. doi: 10.1179/ACB.66.2.2062525. [DOI] [PubMed] [Google Scholar]
- Gebbia V, Bellavia M, Banna GL, Russo P, Ferraù F, Tralongo P, Borsellino N. Treatment monitoring program for implementation of adherence to second-line erlotinib for advanced non-small-cell lung cancer. Clinical Lung Cancer. 2013;14:390–398. doi: 10.1016/j.cllc.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Ibrahim AR, Eliasson L, Apperley JF, Milojkovic D, Bua M, Szydlo R, et al. Marin D. Poor adherence is the main reason for loss of CCyR and imatinib failure for chronic myeloid leukemia patients on long-term therapy. Blood. 2011;117:3733–3736. doi: 10.1182/blood-2010-10-309807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavookjian J, Wittayanukorn S. Interventions for adherence with oral chemotherapy in hematological malignancies: A systematic review. Research in Social and Administrative Pharmacy. 2015;11:303–314. doi: 10.1016/j.sapharm.2014.08.006. [DOI] [PubMed] [Google Scholar]
- Macintosh PW, Pond GR, Pond BJ, Leung V, Siu LL. A comparison of patient adherence and preference of packaging method for oral anticancer agents using conventional pill bottles versus daily pill boxes. European Journal of Cancer Care. 2007;16:380–386. doi: 10.1111/j.1365-2354.2006.00758.x. [DOI] [PubMed] [Google Scholar]
- Marin D, Bazeos A, Mahon FX, Eliasson L, Milojkovic D, Bua M, et al. Khorashad JS. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. Journal of Clinical Oncology. 2010;28:2381–2388. doi: 10.1200/JCO.2009.26.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S. Facilitating oral chemotherapy treatment and compliance through patient/family-focused education. Cancer Nursing. 2007;30:112–122. doi: 10.1097/01.NCC.0000265009.330.53.2d. [DOI] [PubMed] [Google Scholar]
- O'Neill VJ, Twelves CJ. Oral cancer treatment: Developments in chemotherapy and beyond. British Journal of Cancer. 2002;87:933–937. doi: 10.1038/sj.bjc.6600591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterberg L, Blaschke T. Adherence to medication. New England Journal of Medicine. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- Palmieri FM, Barton DL. Challenges of oral medications in patients with advanced breast cancer. Seminars in Oncology Nursing. 2007;23(Suppl):S17–S22. doi: 10.1016/j.soncn.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Park LG, Howie-Esquivel J, Dracup K. A quantitative systematic review of the effcacy of mobile phone interventions to improve medication adherence. Journal of Advanced Nursing. 2014;70:1932–1953. doi: 10.1111/jan.12400. [DOI] [PubMed] [Google Scholar]
- Puts MT, Tu HA, Tourangeau A, Howell D, Fitch M, Springall E, Alibhai SM. Factors infuencing adherence to cancer treatment in older adults with cancer: A systematic review. Annals of Oncology. 2014;25:564–577. doi: 10.1093/annonc/mdt433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson JL, Shelton DR, Krailo M, Levine AM. The effect of compliance with treatment on survival among patients with hematologic malignancies. Journal of Clinical Oncology. 1990;8:356–364. doi: 10.1200/JCO.1990.8.2.356. [DOI] [PubMed] [Google Scholar]
- Ruddy K, Mayer E, Partridge A. Patient adherence and persistence with oral anticancer treatment. CA: A Cancer Journal for Clinicians. 2009;59:56–66. doi: 10.3322/caac.20004. [DOI] [PubMed] [Google Scholar]
- Sadahiro S, Ohki S, Yamaguchi S, Takahashi T, Otani Y, Tsukikawa S, et al. Makuuchi H. Feasibility of a novel weekday-on/weekend-off oral UFT schedule as postoperative adjuvant chemotherapy for colorectal cancer. UFT Complicance Study Group, Kanagawa, Japan. Cancer Chemotherapy and Pharmacology. 2000;46:180–184. doi: 10.1007/s002800000146. [DOI] [PubMed] [Google Scholar]
- Schneider SM, Adams DB, Gosselin T. A tailored nurse coaching intervention for oral chemotherapy adherence. Journal of the Advanced Practitioner in Oncology. 2014;5:163–172. [PMC free article] [PubMed] [Google Scholar]
- Schneider SM, Hess K, Gosselin T. Interventions to promote adherence with oral agents. Seminars in Oncology Nursing. 2011;27:133–141. doi: 10.1016/j.soncn.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servick K. Biomedicine. ‘Nonadherence’: A bitter pill for drug trials. Science. 2014;346:288–289. doi: 10.1126/science.346.6207.288. [DOI] [PubMed] [Google Scholar]
- Shi L, Liu J, Koleva Y, Fonseca V, Kalsekar A, Pawaskar M. Concordance of adherence measurement using self-reported adherence questionnaires and medication monitoring devices. PharmacoEconomics. 2010;28:1097–1107. doi: 10.2165/11537400-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Soria JC, Blay JY, Spano JP, Pivot X, Coscas Y, Khayat D. Added value of molecular targeted agents in oncology. Annals of Oncology. 2011;22:1703–1716. doi: 10.1093/annonc/mdq675. [DOI] [PubMed] [Google Scholar]
- Spoelstra SL, Given CW. Assessment and measurement of adherence to oral antineoplastic agents. Seminars in Oncology Nursing. 2011;27:116–132. doi: 10.1016/j.soncn.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Streeter SB, Schwartzberg L, Husain N, Johnsrud M. Patient and plan characteristics affecting abandonment of oral oncolytic prescriptions. Journal of Oncology Practice. 2011;7(Suppl):46s–51s. doi: 10.1200/JOP.2011.000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanki K, Gangwal RP, Sangamwar AT, Jain S. Oral delivery of anticancer drugs: Challenges and opportunities. Journal of Controlled Release. 2013;170:15–40. doi: 10.1016/j.jconrel.2013.04.020. [DOI] [PubMed] [Google Scholar]
- Timmers L, Boons CC, Kropff F, van de Ven PM, Swart EL, Smit EF, et al. Hugtenburg JG. Adherence and patients' experiences with the use of oral anticancer agents. Acta Oncologica. 2014;53:259–267. doi: 10.3109/0284186X.2013.844353. [DOI] [PubMed] [Google Scholar]
- Timmers L, Boons CC, Moes-Ten Hove J, Smit EF, van de Ven PM, Aerts JG, et al. Hugtenburg JG. Adherence, exposure and patients' experiences with the use of erlotinib in non-small cell lung cancer. Journal of Cancer Research and Clinical Oncology. 2015;141:1481–1491. doi: 10.1007/s00432-015-1935-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbrugghe M, Verhaeghe S, Lauwaert K, Beeckman D, Van Hecke A. Determinants and associated factors infuencing medication adherence and persistence to oral anticancer drugs: A systematic review. Cancer Treatment Reviews. 2013;39:610–621. doi: 10.1016/j.ctrv.2012.12.014. [DOI] [PubMed] [Google Scholar]
- Verma S, Madarnas Y, Sehdev S, Martin G, Bajcar J. Patient adherence to aromatase inhibitor treatment in the adjuvant setting. Current Oncology. 2011;18(Suppl. 1):S3–S9. doi: 10.3747/co.v18i0.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter T, Wang L, Chuk K, Ng P, Tannock IF, Krzyzanowska MK. Assessing adherence to oral chemotherapy using different measurement methods: Lessons learned from capecitabine. Journal of Oncology Pharmacy Practice. 2013;20:249–256. doi: 10.1177/1078155213501100. [DOI] [PubMed] [Google Scholar]
- Waterhouse DM, Calzone KA, Mele C, Brenner DE. Adherence to oral tamoxifen: A comparison of patient self-report, pill counts, and microelectronic monitoring. Journal of Clinical Oncology. 1993;11:1189–1197. doi: 10.1200/JCO.1993.11.6.1189. [DOI] [PubMed] [Google Scholar]