Abstract
Multiple endocrine neoplasia (MEN) type 1 (MEN1) and 2 (MEN2) rarely co-exist in one case. Here we report a patient with features of both syndromes. The patient presented with typical MEN1 features plus pheochromocytoma and thickened corneal nerves. She had a germline 1132delG frameshift mutation in MEN1, no mutation in CDKN1B (p27) and no RET mutation, but had both RET polymorphisms Gly691Ser and Arg982Cys. This is the first case report of a combination of typical clinical findings of MEN1 harboring a germline MEN1 mutation and the MEN2-like phenotype with negative full RET gene analysis of pathogenic variants. Possible explanations include a previously unrecognized phenotype–genotype association or the influence of potential phenotypic modifying RET variants. Furthermore, the combination observed in this patient may point to a single molecular pathway, and supports the possibility of as yet unrecognized connections between the molecular pathways for MEN1/menin protein and MEN2/RET protein.
Keywords: 1132delG, G691S, MEN1, MEN2, MEN4, MENX, multiple endocrine neoplasias, R982C
Background
Multiple endocrine neoplasia type 1 (MEN1) is a syndrome with autosomal dominant (AD) transmission; it is defined as the presence of endocrine tumors in two out of three of its main affected tissues (parathyroid, anterior pituitary and enter-opancreatic neuroendocrine tissues); it results usually from germline inactivation of the MEN1 tumor suppressor gene [1]. Multiple endocrine neoplasia type 2 (MEN2) is defined as one of two clinical syndromes (MEN2A and MEN2B) of an AD disorder with medullary thyroid cancer, each due to germline activating mutation of the RET proto-oncogene [1,2]. Both MEN1 and MEN2 are rare, each has a prevalence of about 1 in 30,000. Although there is partial overlap in the tumor types, it is unclear if there is any interaction between the important but incompletely understood molecular pathways involving the products of MEN1 and RET genes.
Rare germline mutations of cyclin-dependent kinase inhibitor (CDKI) genes have also been implicated in a MEN1-like syndrome of man [3]. Franklin et al. reported that combined knockout of p27 and p18 CDKI genes in mice caused neoplasia with the tissue specificity simultaneously of MEN1 plus MEN2 [4]. Pellegata et al. reported homozygous inactivation of p27 in a rat strain manifesting tumors of both MEN1 and MEN2 [5]; they also reported a human kindred with a MEN1-like syndrome and a p27 germline mutation [6]. Agarwal et al. reported rare MEN1-like families with mutation of p27 or other CDKI genes (p21, p18, p15) but no MEN1 mutation [3]. The MEN1-like syndrome attributed to these mutations was later named MENX (in rats) or MEN4 (in humans) [6]. Unlike in rodents, none of the reported human families with MEN1 syndrome and CDKI mutations has shown features of MEN2.
Here we report a patient with a germline mutation in the MEN1 gene and features of MEN1 and MEN2. An earlier report of eleven cases with features of both MEN1 and MEN2 that included our case [7] preceded the mapping and availability of MEN1 or RET testing, so it is uncertain if those other patients had mutations of either or both genes.
Case report
The index case of this family initially presented at age 23 with hypercalcemia and subsequently underwent a total of three surgeries for control of hyperparathyroidism [7]. At the age of 29 she presented with Cushing’s syndrome, including hypertension. She underwent bilateral adrenalectomy, showing left adrenal cortical adenoma and right adrenal pheochromocytoma; she also underwent subtotal pancreatectomy for multiple pancreatic islet tumors. Subsequently, she was treated for Zollinger–Ellison syndrome (ZES) (including gastrectomy), pituitary adenoma and bronchial carcinoid. She subsequently died at the age of 58 likely from complications of metastatic neuroendocrine tumor. Other features of MEN1 in this patient included subcentimeter esophageal leiomyomas seen at autopsy, multiple uterine fibroids, cutaneous lipomas and angiofibromas. She had thickened corneal nerves by slit-lamp examination, which was confirmed on multiple examinations throughout her years of follow-up at NIH. Genetic testing revealed a germline frameshift mutation 1132delG (NM_130799.2) in MEN1 (also called c.1039delG (NM_000244.3) and described at the protein level as p.Ala347Argfs*26). No RET mutation was identified after full gene sequencing. She was found to be heterozygous for the Gly691Ser polymorphism in RET exon 11 and the Arg982Cys polymorphism in exon 18, as well as several more common synonymous polymorphisms in the gene. Pheochromocytoma tumor tissue was unavailable for further genetic or immunohistochemistry testing, as her surgery had occurred at an outside institution many years back.
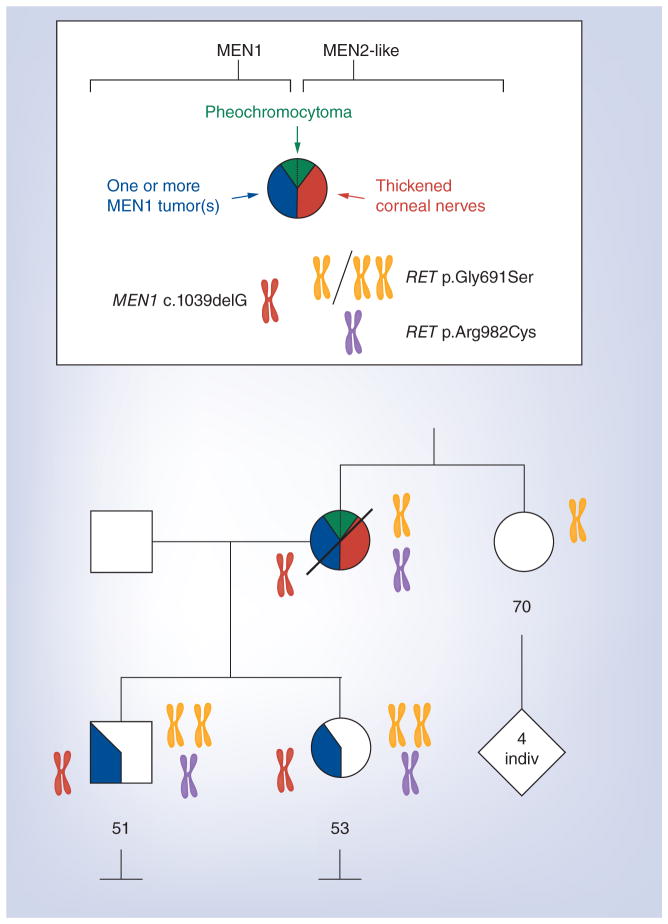
The patient’s son and daughter also tested positive for the MEN1 mutation 1132delG, and full RET sequencing showed that they carry the same both RET polymorphisms (Gly691Ser and Arg982Cys) as their mother. Both are homozygous for Gly691Ser rather than heterozygous (Figure 1).
Figure 1. Pie chart shows tumors of MEN1 or of MEN2-like type.
The pie is divided from 12:00 to 6:00 o’clock. A chromosome indicates a mutation or polymorphism of a gene. The color denotes the specific locus. Two identical chromosomes show homozygosity for a polymorphism.
Note: index case’s unaffected sister does not carry the MEN1 mutation.
The patient’s daughter was diagnosed at age 15 with hyperparathyroidism and subsequently underwent three neck surgeries. She was diagnosed with pituitary macroadenoma at age 18 (hormonal secretion profile unknown); she underwent trans-sphenoidal resection with postsurgical central adrenal insufficiency and central hypothyroidism. She was found to have an elevated prolactin level years later, which necessitated medical therapy consisting of bromocriptine; she developed ZES that was also treated medically. She underwent thoracotomy at age 40 for a bronchial carcinoid tumor. She underwent multiple surgeries for metastatic gastrinoma. Her features of MEN1 also included cutaneous lipomas. At age 50 she presented with a 4-cm left inguinal mass found to be benign fibromyxoid neoplasm on excisional biopsy. As this tumor was initially misdiagnosed as a neurofibroma, loss of heterozygosity (LOH) analysis at the site of the frameshift mutation was attempted but unsuccessful. Testing of her germline p27 CDKI gene both by sequencing and deletion/duplication methods was negative.
The patient’s son was found to have hypercalcemia in his teens and underwent surgical management for hyperparathyroidism at age 27. At age 24 he underwent distal pancreatectomy for a nonfunctioning pancreatic neuroendocrine tumor. Other MEN1 features in this patient included lipomas and angiofibromas. A summary of findings in all three family members is shown in Table 1 & Figure 1.
Table 1.
Clinical and genetic description of our patient and two other relevant families.
| Family member | Mutated gene | Clinical manifestations (age at diagnosis) | |
|---|---|---|---|
| MEN1 | MEN2-like or MEN2 | ||
| Current report: MEN1= 1132delG frameshift mutation in MEN1 | |||
| Index case (F)† | MEN1 | HPT (23), CS (29), PNET (29), ZES (35), PR (39), CA (54), LI (55), AF (55), FI (55) | PH (29), Thickened corneal nerves (50) |
| Daughter of index case | MEN1 | HPT (15), PI/PR (18), ZES (31), CA (40), LI (40) | |
| Son of index case | MEN1 | HPT (?15), PNET (24), AF (24), LI (24) | |
| Other reported families in the literature | |||
|
Mastroianno et al.[22] MEN1= IVS5 + 1G > A RET = K666M in exon 11 of RET |
|||
| Index case (M) | MEN1 and RET | PI (38), HPT (45), GA (47), CA (47) | MTC (46) |
| – Son of index case | MEN1 and RET | ||
| – Son of index case | RET | ||
| Brother 1 of index case | MEN1 | ZES (46), HPT (46) | |
| – Son of brother 1 | MEN1 | HPT (22), IN (23), GL (23), LI (26) | |
| – Son of brother 1 | -- | ||
| – Son of brother 1 | MEN1 | HPT (18) | |
| – Daughter of brother 1 | -- | ||
| Brother 2 of index case | RET | CCH (45) | |
| – Daughter of brother 2 | RET | CCH (16) | |
| Brother 3 of index case | MEN1 and RET | HPT (40), CS (40), CA (40), LI (40), AF (40), GA (41) | MTC (40) |
| – Daughter of brother 3 | -- | ||
| – Daughter of brother 3 | MEN1 and RET | HPT (13), PI (15) | |
| Niece 4 of index case | MEN1 | IN (17), HPT (18) | |
| Nephew 5 of index case | -- | ||
| Nephew 6 of index case | RET | CCH (41) | |
| Niece 5 of index case | MEN1 | IN (17), HPT (39), AF (41) | |
| – Son of niece 5 | -- | ||
| – Son of niece 5 | MEN1 | ||
| – Daughter of niece 5 | MEN1 | HPT (20), PI (20) | |
| – Daughter of niece 5 | MEN1 | ||
|
Frank-Raue et al. [23] MEN1 = IVS5+1G>A RET = Y791F in exon 13 of RET (polymorphism)‡ |
|||
| Mother of index case | MEN1 | HPT, PI, FI | |
| Maternal uncle of index case | MEN1 | PNET, FI, AD | |
| Maternal cousin (M) of index case | MEN1 | HPT, PI, PNET | |
| Maternal cousin (F) of index case | MEN1 | HPT, PNET | |
| Maternal cousin (M) of index case | MEN1 | PI, PNET, FI, AD | |
| Daughter of index case | MEN1 | ||
| Father of index case | RET | ||
| Index case (M) | MEN1 and RET | HPT, PNET, FI | |
| Brother of index case | MEN1 and RET | HPT, PI, PNET, FI | CCH |
| Daughter of index case | MEN1 and RET | ||
When not shown in parentheses, age at diagnosis was not reported.
Other features included thyroid follicular adenoma.
First characterized as pathogenic variant and, more recently, defined as a rare benign polymorphism and as potential phenotypic modifying factor [26].
--: No mutation; AD: Adrenal tumor; AF: Angiofibroma; CA: Carcinoid; CCH: C-cell hyperplasia; CS: Cushing’s syndrome; F: Female; FI: Fibroma; GA: Gastrinoma; GL: Glucagonoma; HPT: Primary hyperparathyroidism; IN: Insulinoma; LI: Lipoma; M: Male; MTC: Medullary thyroid cancer; PH: Phechromocytoma; PI: Pituitary adenoma; PNET: Pancreatic neuroendocrine tumor; PR: Prolactinoma; ZES: Zollinger–Ellison syndrome.
Discussion
We report here MEN1 and MEN2-like manifestations together in one patient. Although the patient carries a germline MEN1 mutation, yet she manifests MEN2-like features, including pheochromocytoma and thickened corneal nerves [8–11]. Pheochromocytoma has also been a rare (<1%) feature of MEN1, reported in fewer than ten cases in the literature [12–18]. LOH at 11q13 was found in two tumors, thus reinforcing the likelihood that most of these rare tumors result from biallelic MEN1 gene inactivation [13]. In mouse models of MEN1, the prevalence of pheochomocytoma was much higher at 7%, further supporting this notion [19].
The finding of thickened corneal nerves is rare, and has been described in several diseases including nonendocrinological and endocrinological diseases such as neurofibromatosis 1 (NF1) and MEN2 [8,20,21]. Although typically characteristic of MEN2B [8,10], thickened corneal nerves have been described also in MEN2A [9,11]. There is however no previous report of MEN1 cases associated with thickened corneal nerves. The presence of thickened corneal nerves along with pheochromocytoma but no neurofibromas in the index case was suggestive of, but not confirmative, of a MEN-2 like picture rather than neurofibromatosis 1, however DNA sequence testing for NF1 was not performed.
To our knowledge, this is the first report in the literature of a patient showing classical MEN1 features harboring a germline MEN1 mutation, in addition to MEN2-like features with negative RET mutation analysis. Only one previously reported family had manifestations of both MEN1 and MEN2 [22]; however in this family, germline mutation in the RET proto-oncogene, in addition to germline MEN1 mutation, was detected (Table 1). Testing revealed widespread expression of the RET mutation, Lys666Met; and clinical manifestations included medullary thyroid cancer (MTC) in two members and CCH in three members, out of the eight total members carrying RET mutation [22]. Twelve members of this family also carried a mutation in IVS4 + 1G > A of the MEN1 gene, with a total of four patients carrying both MEN1 and RET mutations. In this family, the co-existence of MEN1 and RET mutations did not seem to alter the onset or course of any one tumor type, tumor behavior, or typical phenotype as compared with patients with the same MEN1 or RET mutations separately. In contrast to the previously reported family [22], full RET sequencing was negative for mutation in our case.
In another reported case with MEN1 manifestations, an index case expressed recurrent hyperparathyroidism [23]. In addition to a MEN1 mutation IVS5 + 1G > A, RET analysis revealed the polymorphism Tyr791Phe (Table 1). Subsequently, C-cell hyperplasia (CCH), but no other feature of MEN2, was detected in the brother of the index case, although two other family members carried the RET polymorphism without tumors (to date). The potential pathogenicity of Tyr791Phe acting alone, or as a phenotypic modifying variant has been debated since 2010 [24,25]. More recently, a wide study indicated this variant as a rare benign polymorphism [26]. Therefore, it is possible, but not proven, that CCH in this family occurred as a result of phenotypic modifying effect of Tyr791Phe in combination to chronic hypercalcemia.
The prevalence of the RET polymorphism Gly691Ser is common, occurring in about 40% of Europeans and 37% in Latino populations, whereas Arg982Cys is present in about 2.4% of Europeans and about 5% of Asian populations [27]. It is notable that the RET polymorphism Gly691Ser, while not believed to be a pathogenic mutation on its own, has been suggested to modulate the effect of RET mutations [28–31]. Even when present along with the RET polymorphism Arg982Cys as in our patient, it may modulate the effect of a mutation in a separate gene [32]. The allelic frequency of Gly691Ser has been found to be significantly higher in patients with sporadic MTC as compared with normal controls (28 vs 19%) [31]. While our patient displayed some features of MEN2, neither the patient nor any family member showed MTC or CCH.
In a case series report of four unrelated patients having neurofibromas and thickened corneal nerves, testing in one patient was negative for NF1 and RET analysis, however, revealed the presence of RET Gly691Ser polymorphism [33]. Our patients did not display other features seen in that patient such as Marfanoid habitus or multiple neurofibromas. A study researching congenital anomalies of the kidney or urinary tract found that when the Gly691Ser polymorphism is combined with the rarer Arg982Cys RET polymorphism in vitro, this resulted in almost complete loss of MAPK phosphorylation that is critical for kidney formation, which neither polymorphism individually had any effect on [32]. This raises the question of whether in our patient the RET polymorphisms Gly691Ser and Arg982Cys, either individually or in combination, are working in synergy with the MEN1 mutation 1132delG or with another gene to produce features of MEN2.
This current 1132delG MEN1 mutation, which represents a frameshift mutation at codon 341 leading to premature truncation of menin, has not been previously reported in germline or sporadic tumors. The presence of the RET polymorphisms Gly691Ser or Arg982Cys could in part explain some of the MEN2-like features of this patient. Other possible explanations of the MEN2-like features include a previously unrecognized phenotype–genotype association with the 1132delG frameshift mutation in MEN1, the presence of an occult RET mutation in the patient undetected by full gene sequencing, association with an unknown MEN2-like syndrome, or the influence of potential phenotypic modifying RET variants.
Although we were unable to perform LOH analysis or demonstrate the expression of menin protein in the patient’s pheochromocytoma, neither would confirm the hypothesis that this tumor was not part of MEN1. Other limitations of this study include the small size of the case report (one patient).
Conclusion
This is the first case report of a combination of typical clinical findings of MEN1 harboring a germline MEN1 mutation and the MEN2-like phenotype with negative full RET gene analysis of pathogenic variants. Germline MEN1 mutation can rarely be associated with some of the classic expressions of both MEN1 and MEN2-like features together. The combination of classical MEN1 and MEN2-like features in one patient is rare. This may represent a previously unrecognized phenotype–genotype association with this previously unreported mutation. The presence of potential phenotypic modifying RET variants, could, at least in part, explain the MEN2-like phenotype in this MEN1 patient. The combination observed in this patient may point to a single molecular pathway, and supports the possibility of as yet unrecognized connections between the molecular pathways for MEN1 (menin protein) and MEN2 (RET protein). These explanations are speculative in the absence of more clinical data or more fundamental knowledge. This report could promote future recognition of similar cases. The detection of future such cases, the establishment of a larger cohort with international collaborations, in addition to next-generation sequencing would all be important to achieve the goal of better understanding of this disorder.
Future perspective
The multiple endocrine neoplasias (MEN1, MEN2) are rare disorders involving germline inactivation/mutation of different genes (MEN1, RET). It is even more rare to find features of MEN1 and MEN2 both in one patient, with genetic mutation of only one, but not both, genes involved. Although some tumors are manifested in both syndromes, it is not known if there is interaction between the molecular pathways involving the products of MEN1 and RET genes. Furthermore, the presence of certain polymorphisms, may influence the disease course, or result in the manifestation of a new phenotype. This study is important to raise awareness among clinicians taking care of patients with rare diseases about unexpected manifestations which subsequently lead to the detection of cases similar to ours. In order to better understand the underlying mechanisms, a larger cohort of such cases is needed, which, when combined with next-generation sequencing, will allow for better understanding of the underlying molecular pathways and clinical course of these disorders.
Practice points.
MEN1 and MEN2 are rare autosomal dominant disorders, that result from germline mutations in the MEN1 and RET genes, respectively.
Mutations in other genes, like CDKI, result in a MEN1-like syndrome in man, but in mice result in manifestation of tumors of MEN1 and MEN2.
The patient presented with typical MEN1 features, in addition MEN2-like features, namely pheochromocytoma and thickened corneal nerves.
The patient had a germline 1132delG frameshift mutation in MEN1, no mutation in CDKN1B (p27) and no RET mutation. She had both RET polymorphisms Gly691Ser and Arg982Cys.
Pheochromocytoma has been a rare presentation of MEN1, however loss of heterozygosity testing was unavailable for further testing.
Thickened corneal nerves is typically a feature of MEN2B, or NF1, and has been reported in MEN2A as well, but not in MEN1.
The current 1132delG MEN1 mutation, has not been previously reported in germline or sporadic tumors, and the combination of features may represent a previously unrecognized phenotype–genotype association with this mutation.
The presence of the RET polymorphisms Gly691Ser or Arg982Cys may account for the MEN2-like features of this patient.
Future detection of such cases, in addition to next-generation sequencing, are needed to better understand the underlying mechanisms.
Acknowledgments
We thank Dr. Martha Quezado for her expertise in review of pathology, and Mr. Craig Cochran for his help in clinical patient care coordination.
Footnotes
Informed consent disclosure
The authors state that they have obtained verbal and written informed consent from the patient/patients for the inclusion of their medical and treatment history within this case report.
Financial & competing interests disclosure
This research was funded by the Intramural Research Program of the NIH, and the National Institutes of Diabetes and Digestive and Kidney Diseases (NIDDK)/NIH. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest;
•• of considerable interest
- 1••.Marx SJ. Molecular genetics of multiple endocrine neoplasia types 1 and 2. Nat Rev Cancer. 2005;5(5):367–375. doi: 10.1038/nrc1610. Discusses the molecular genetics underlying the two syndromes. [DOI] [PubMed] [Google Scholar]
- 2.Wells SA, Jr, Asa SL, Dralle H, et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015;25(6):567–610. doi: 10.1089/thy.2014.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agarwal SK, Mateo CM, Marx SJ. Rare germline mutations in cyclin-dependent kinase inhibitor genes in multiple endocrine neoplasia type 1 and related states. J Clin Endocrinol Metab. 2009;94(5):1826–1834. doi: 10.1210/jc.2008-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franklin DS, Godfrey VL, O’brien DA, Deng C, Xiong Y. Functional collaboration between different cyclin-dependent kinase inhibitors suppresses tumor growth with distinct tissue specificity. Mol Cell Biol. 2000;20(16):6147–6158. doi: 10.1128/mcb.20.16.6147-6158.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piotrowska K, Pellegata NS, Rosemann M, Fritz A, Graw J, Atkinson MJ. Mapping of a novel MEN-like syndrome locus to rat chromosome 4. Mamm Genome. 2004;15(2):135–141. doi: 10.1007/s00335-003-3027-8. [DOI] [PubMed] [Google Scholar]
- 6.Pellegata NS, Quintanilla-Martinez L, Siggelkow H, et al. Germ-line mutations in p27Kip1 cause a multiple endocrine neoplasia syndrome in rats and humans. Proc Natl Acad Sci USA. 2006;103(42):15558–15563. doi: 10.1073/pnas.0603877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alberts WM, Mcmeekin JO, George JM. Mixed multiple endocrine neoplasia syndromes. JAMA. 1980;244(11):1236–1237. [PubMed] [Google Scholar]
- 8.Eter N, Klingmuller D, Hoppner W, Spitznas M. Typical ocular findings in a patient with multiple endocrine neoplasia type 2b syndrome. Graefes Arch Clin Exp Ophthalmol. 2001;239(5):391–394. doi: 10.1007/s004170000245. [DOI] [PubMed] [Google Scholar]
- 9.Kinoshita S, Tanaka F, Ohashi Y, Ikeda M, Takai S. Incidence of prominent corneal nerves in multiple endocrine neoplasia type 2A. Am J Ophthalmol. 1991;111(3):307–311. doi: 10.1016/s0002-9394(14)72314-1. [DOI] [PubMed] [Google Scholar]
- 10.Spector B, Klintworth GK, Wells SA., Jr Histologic study of the ocular lesions in multiple endocrine neoplasia syndrome type IIb. Am J Ophthalmol. 1981;91(2):204–215. doi: 10.1016/0002-9394(81)90175-6. [DOI] [PubMed] [Google Scholar]
- 11.Takai S, Kinoshita S, Tanaka F, Ikeda M, Tanaka N, Kobayashi T. Prominent corneal nerves in patients with multiple endocrine neoplasia type 2A: diagnostic implications. World J Surg. 1992;16(4):620–623. doi: 10.1007/BF02067337. discussion 624. [DOI] [PubMed] [Google Scholar]
- 12.Carty SE, Helm AK, Amico JA, et al. The variable penetrance and spectrum of manifestations of multiple endocrine neoplasia type 1. Surgery. 1998;124(6):1106–1113. doi: 10.1067/msy.1998.93107. discussion 1113–1104. [DOI] [PubMed] [Google Scholar]
- 13•.Cote GjLJ, Evans Db. The spectrum of mutations in MEN-1 variant syndromes. Presented at: 80th Annual Meeting of the Endocrine Society; New Orleans, LA, USA. 24–27 June 1998; Demonstrates LOH in 2 pheochromocytomas presenting in MEN1. [Google Scholar]
- 14.Dackiw AP, Cote GJ, Fleming JB, et al. Screening for MEN1 mutations in patients with atypical endocrine neoplasia. Surgery. 1999;126(6):1097–1103. doi: 10.1067/msy.2099.101376. discussion 1103–1094. [DOI] [PubMed] [Google Scholar]
- 15.Denes J, Swords F, Rattenberry E, et al. Heterogeneous genetic background of the association of pheochromocytoma/paraganglioma and pituitary adenoma: results from a large patient cohort. J Clin Endocrinol Metab. 2015;100(3):e531–e541. doi: 10.1210/jc.2014-3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Langer P, Cupisti K, Bartsch DK, et al. Adrenal involvement in multiple endocrine neoplasia type 1. World J Surg. 2002;26(8):891–896. doi: 10.1007/s00268-002-6492-4. [DOI] [PubMed] [Google Scholar]
- 17.Trump D, Farren B, Wooding C, et al. Clinical studies of multiple endocrine neoplasia type 1 (MEN1) QJM. 1996;89(9):653–669. doi: 10.1093/qjmed/89.9.653. [DOI] [PubMed] [Google Scholar]
- 18.Waldmann J, Bartsch DK, Kann PH, Fendrich V, Rothmund M, Langer P. Adrenal involvement in multiple endocrine neoplasia type 1: results of 7 years prospective screening. Langenbecks Arch Surg. 2007;392(4):437–443. doi: 10.1007/s00423-006-0124-7. [DOI] [PubMed] [Google Scholar]
- 19••.Crabtree JS, Scacheri PC, Ward JM, et al. A mouse model of multiple endocrine neoplasia, type 1, develops multiple endocrine tumors. Proc Natl Acad Sci USA. 2001;98(3):1118–1123. doi: 10.1073/pnas.98.3.1118. Shows two rodent models suggesting MEN1 and MEN2 features could be hereditary also in humans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim SK, Dohlman CH. Causes of enlarged corneal nerves. Int Ophthalmol Clin. 2001;41(1):13–23. doi: 10.1097/00004397-200101000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Lewis RA, Riccardi VM. Von Recklinghausen neurofibromatosis. Incidence of iris hamartomata. Ophthalmology. 1981;88(4):348–354. doi: 10.1016/s0161-6420(81)35034-9. [DOI] [PubMed] [Google Scholar]
- 22•.Mastroianno S, Torlontano M, Scillitani A, et al. Coexistence of multiple endocrine neoplasia type 1 and type 2 in a large Italian family. Endocrine. 2011;40(3):481–485. doi: 10.1007/s12020-011-9501-2. Presents the only family reported with MEN1 and MEN2 syndromes. [DOI] [PubMed] [Google Scholar]
- 23.Frank-Raue K, Rondot S, Hoeppner W, Goretzki P, Raue F, Meng W. Coincidence of multiple endocrine neoplasia types 1 and 2: mutations in the RET protooncogene and MEN1 tumor suppressor gene in a family presenting with recurrent primary hyperparathyroidism. J Clin Endocrinol Metab. 2005;90(7):4063–4067. doi: 10.1210/jc.2004-1759. [DOI] [PubMed] [Google Scholar]
- 24.Erlic Z, Hoffmann MM, Sullivan M, et al. Pathogenicity of DNA variants and double mutations in multiple endocrine neoplasia type 2 and von Hippel-Lindau syndrome. J Clin Endocrinol Metab. 2010;95(1):308–313. doi: 10.1210/jc.2009-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toledo RA, Wagner SM, Coutinho FL, et al. High penetrance of pheochromocytoma associated with the novel C634Y/Y791F double germline mutation in the RET protooncogene. J Clin Endocrinol Metab. 2010;95(3):1318–1327. doi: 10.1210/jc.2009-1355. [DOI] [PubMed] [Google Scholar]
- 26.Toledo RA, Hatakana R, Lourenco DM., Jr Comprehensive assessment of the disputed RET Y791F variant shows no association with medullary thyroid carcinoma susceptibility. Endocr Relat Cancer. 2015;22(1):65–76. doi: 10.1530/ERC-14-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.ExAC Browser (Beta) – Exome Aggregation Consortium. Cambridge, MA, USA: 2015. http://exac.broadinstitute.org. [Google Scholar]
- 28.Borrello MG, Aiello A, Peissel B, et al. Functional characterization of the MTC-associated germline RET-K666E mutation: evidence of oncogenic potential enhanced by the G691S polymorphism. Endocr Relat Cancer. 2011;18(4):519–527. doi: 10.1530/ERC-10-0306. [DOI] [PubMed] [Google Scholar]
- 29.Cardot-Bauters C, Leteurtre E, Leclerc L, et al. Does the RET variant G691S influence the features of sporadic medullary thyroid carcinoma? Clin Endocrinol (Oxf) 2008;69(3):506–510. doi: 10.1111/j.1365-2265.2008.03230.x. [DOI] [PubMed] [Google Scholar]
- 30.Colombo C, Minna E, Rizzetti MG, et al. The modifier role of RET-G691S polymorphism in hereditary medullary thyroid carcinoma: functional characterization and expression/penetrance studies. Orphanet J Rare Dis. 2015;10(1):25. doi: 10.1186/s13023-015-0231-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elisei R, Cosci B, Romei C, et al. RET exon 11 (G691S) polymorphism is significantly more frequent in sporadic medullary thyroid carcinoma than in the general population. J Clin Endocrinol Metab. 2004;89(7):3579–3584. doi: 10.1210/jc.2003-031898. [DOI] [PubMed] [Google Scholar]
- 32.Chatterjee R, Ramos E, Hoffman M, et al. Traditional and targeted exome sequencing reveals common, rare and novel functional deleterious variants in RET-signaling complex in a cohort of living US patients with urinary tract malformations. Hum Genet. 2012;131(11):1725–1738. doi: 10.1007/s00439-012-1181-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Babovic-Vuksanovic D, Messiaen L, Nagel C, et al. Multiple orbital neurofibromas, painful peripheral nerve tumors, distinctive face and marfanoid habitus: a new syndrome. Eur J Hum Genet. 2012;20(6):618–625. doi: 10.1038/ejhg.2011.275. [DOI] [PMC free article] [PubMed] [Google Scholar]