Abstract
Regulation of hyphal morphogenesis in Candida albicans can occur through quorum sensing (QS). A QS signal, farnesol, is produced during high-density growth and inhibits morphogenesis. However, the signal transduction pathway that regulates QS is unknown. Here, we show that a C. albicans mutant lacking Chk1p but not either the Sln1p or the Nik1p histidine kinase is refractory to the inhibitory effect of farnesol both in cell suspension and during the formation of a biofilm. This study is the first to demonstrate a role for a two-component signal transduction protein in QS by a eukaryotic organism.
Cell density is a critical factor in the regulation of Candida albicans hyphal morphogenesis. At a density of >106 cells/ml, yeast cells do not shift (germinate) to hyphae or do so at low frequencies, while at a density of <106 cells/ml, germination occurs (3). The relationship between cell density and new gene transcription (hyphal morphogenesis) resembles quorum sensing (QS) in some bacteria (14). Recent observations indicate that a QS system operates in C. albicans and that the isoprenoid farnesol is the QS autoinducer signal (12). Cells exposed to farnesol do not germinate, even at low cell densities. However, the regulatory and signal transduction events that direct QS are unknown, not only for C. albicans but for other fungi and eukaryotes in general. In some bacteria, two-component signaling regulates QS. Since C. albicans has several two-component signal proteins that are critical to a number of processes, including cell wall biosynthesis, adaptation to stress conditions, and virulence, our rationale was that farnesol sensing could be mediated through two-component proteins. C. albicans has three hybrid-histidine kinases, two of which have orthologues in Saccharomyces cerevisiae (Sln1p) and Neurospora crassa (Nik1p) that are presumed to play a role in an osmotic stress response (1, 15, 19, 20). The third histidine kinase, Chk1p, has some similarity to two Schizosaccharomyces pombe proteins, Mak2p and Mak3p, which are known to function as sensors for oxidative stress (2, 5). In addition to the histidine kinases, C. albicans has two response regulator proteins, Ssk1p and Skn7p, whose S. cerevisiae homologs act downstream of the Sln1p histidine kinase (11). In C. albicans, Ssk1p and Skn7p function in the adaptation of cells to oxidant stress, while Ssk1p, in addition, regulates the expression of structural cell wall proteins and negatively regulates Chk1p expression (7, 18).
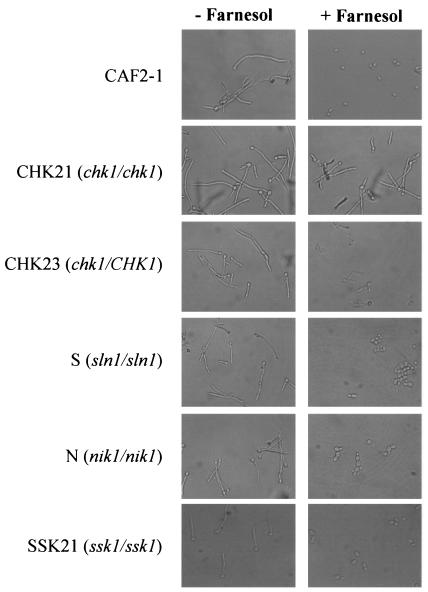
The C. albicans strains used for this study have been described previously (4, 6, 9, 20). Unless noted, cells were routinely cultured in YPD (1% yeast extract, 2% dextrose, 2% peptone) or YNB (0.67% yeast nitrogen base [pH 7.0], 50 mM glucose) at 30°C. To assess whether the two-component signal transduction proteins of C. albicans play a role in QS, all strains (see Table 1) were first cultured overnight at 30°C in YPD. Subsequently, the cells were washed twice and then inoculated into 10 ml of prewarmed medium 199 (pH 7.5) with or without 250 μM trans,trans-farnesol (Sigma-Aldrich, St. Louis, Mo.) at a concentration of 5 × 105 cells/ml. Cells were incubated at 37°C for 4 h, in order to allow germination to occur. Identical experiments were performed with 10% serum at 37°C as the inducing condition. The percentages of yeast cells and hyphae were then determined by light microscopy. Photographs were taken using a Canon digital camera, and figures were prepared with Adobe Photoshop 6.0. The C. albicans sln1, nik1, and chk1 histidine kinase mutants and the ssk1 response regulator mutant were compared to strain CAF2-1 (wild type) in hypha-inducing medium (10% serum or medium 199 [pH 7.5] with or without 250 μM farnesol). In medium 199 (pH 7.5) lacking farnesol, germination proceeded normally (89 to 96%) for all strains (Fig. 1, left column; Table 1). In the presence of farnesol, the percentages of germination for CAF2-1 and for strains S (sln1/sln1), N (nik1/nik1), and SSK21 (ssk1/ssk1) decreased significantly to values ranging from 15 to 30%, while germination of the chk1 mutant (CHK21) was 84% of that of CAF2-1 (Fig. 1, right column; Table 1). The germination of a strain reconstituted with a single copy of CHK1 (CHK23) was intermediate to that of CAF2-1 and the null counterpart (Fig. 1, right column, panel for CHK23; Table 1), indicating that the phenotype observed may be a result of the gene dosage. Similar results were seen when strains were grown in 10% serum (data not shown), indicating that the farnesol response is not medium dependent.
TABLE 1.
Germination of C. albicans in the presence or absence of 250 μM farnesola
| Strain | Without farnesol
|
With farnesol
|
||
|---|---|---|---|---|
| % Yeast cells | % Hyphae | % Yeast cells | % Hyphae | |
| CAF2-1 (wild type) | 4.5 ± 1.8 | 95.5 ± 1.8 | 70.1 ± 10 | 29.9 ± 10 |
| CHK21 (chk1Δ) | 5.7 ± 4.2 | 94.3 ± 4 | 8.2 ± 8 | 91.8 ± 8 |
| CHK23 (chk1/CHK1) | 3 ± 2.7 | 97 ± 2.7 | 40.4 ± 20 | 59.6 ± 20 |
| S (sln1Δ) | 10.4 ± 7 | 89.6 ± 7 | 69.4 ± 19 | 30.6 ± 19 |
| N (nik1Δ) | 12.8 ± 8 | 87 ± 8 | 72 ± 29 | 28 ± 29 |
| SSK21 (ssk1Δ) | 6.7 ± 1.8 | 93.3 ± 1.8 | 84.3 ± 12 | 15.7 ± 12 |
All strains were grown in medium 199 (pH 7.5) at 37°C. The values presented are the means of results from three experiments; standard deviations are indicated.
FIG. 1.
Representative photomicrographs of C. albicans grown without (left) and with (right) 250 μM farnesol. Strains were grown for 4 h at 37°C in medium 199 (pH 7.5) at a density of 5 × 105 cells/ml.
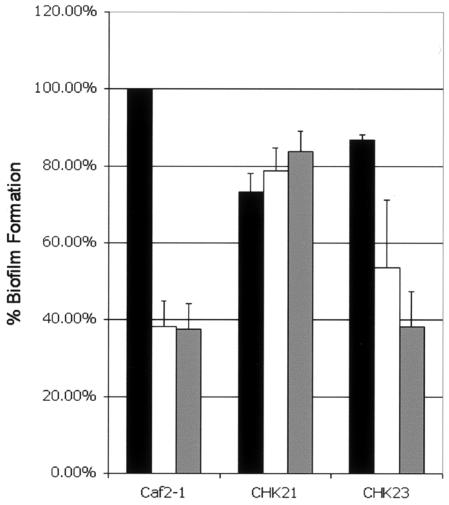
An important aspect of the effect of farnesol on C. albicans is its influence on biofilm formation (8, 16). C. albicans forms biofilms on a variety of substrates both in vitro and in clinical settings, such as indwelling intravenous catheters of patients (8). In the clinical setting, biofilm formation also represents a problem for therapeutic management of patients due to the resistance of the biofilm cells to antifungal therapy. In vitro studies indicate that farnesol inhibits biofilm formation, possibly by inhibiting the ability of the organism to shift to a filamentous morphology (16). Since our data indicate that the chk1 null mutant is not morphologically responsive to farnesol compared to parental and other mutants, the effect of farnesol on biofilm formation by this mutant was determined. C. albicans strains were grown overnight in YNB (pH 7) containing 50 mM glucose at 30°C, harvested, and washed twice in phosphate-buffered saline (PBS). The cell density was standardized to 107 CFU/ml, and cells (100 μl of cell suspension) were allowed to adhere to the bottoms of 96-well microtiter plates. After 90 min of incubation at 37°C, the nonadhered cells were removed by washing twice with 200 μl of PBS. Then, 200 μl of YNB (pH 7) containing 50 mM glucose with or without farnesol (25 or 250 μM) was added, and biofilms were allowed to develop for 48 h at 37°C while being shaken at 150 rpm. The biofilms were washed twice with 200 μl of PBS and incubated in a solution containing 150 μl of PBS with 50 mM glucose, 2.5 mg of XTT [2,3-bis(2-methoxy-4-nitro-sulfophenyl)-5-[(phenylamine)carbonyl]-2H-tetrazolium hydroxide]/ml, and 2 μM menadione (Sigma-Aldrich) (16). After 2 h of incubation at 37°C, 100 μl of each sample was transferred to a fresh plate, and the reduction in XTT was determined by measuring the optical density of the sample at 492 nm. In the absence of farnesol, biofilm formation occurred for all strains (Fig. 2). However, in the presence of subinhibitory concentrations of farnesol (25 μM), CAF2-1 biofilm formation was reduced by 60%, while strain CHK21 (chk1/chk1) formed a biofilm that was approximately twofold greater than that of CAF2-1 (Fig. 2). At a 250 μM concentration of farnesol, CAF2-1 again produced a biofilm that was approximately 40% of the size of that produced in the absence of farnesol by that strain. In contrast, the biofilm formation of the chk1 mutant exceeded that of CAF2-1. At a 25 μM concentration of farnesol, strain CHK23, containing one copy of CHK1, formed a biofilm somewhat intermediate in size to those formed by strain CAF2-1 and the CHK21 null mutant (averaging 55% of the sizes of those formed by CAF2-1 and CHK21) (Fig. 2). With 250 μM farnesol, biofilm formation by CHK23 was equal to that of CAF2-1. The ssk1, sln1, and nik1 null mutants resembled CAF2-1 and formed biofilms only in the absence of farnesol (data not shown). The data on biofilm formation by these strains support the observed effects of farnesol on germination (Fig. 1; Table 1).
FIG. 2.
Influence of farnesol on biofilm formation. CHK21 (chk1/chk1Δ) is nonresponsive to farnesol. Biofilm formation was assessed using the XTT metabolic assay. The percentages of CHK21 and CHK23 cells that formed a biofilm are indicated relative to that of CAF2-1 without farnesol (black bars), with 25 μM farnesol (white bars), and with 250 μM farnesol (grey bars). The data represent averages of the results from four experiments.
Our observation of the role of the two-component signal transduction protein Chk1p in QS is the first for a eukaryotic organism. However, it is unclear how new gene transcription is initiated upon perception of the farnesol signal. Since Chk1p is a cytoplasmic protein, it is possible that another protein, which is upstream of Chk1p, perceives the farnesol signal and then activates a pathway that includes Chk1p. This hypothesis may be quite likely since no apparent motifs are found in Chk1p that would suggest a binding site for farnesol. The histidine box domain and the receiver domain of Chk1p contain residues of histidine and aspartate, respectively, that may be sequentially phosphorylated during signal transfer. In addition, a partial mitogen-activated protein kinase domain that may also be critical to signal transfer is located in the N-terminal half of Chk1p (5). The phenotype of the chk1 mutant in the presence of farnesol suggests that this kinase participates in a signal pathway. If so, this pathway would be unique among organisms that utilize two-component signal transduction.
Our observation that QS is mediated through a two-component pathway may help in developing new strategies to inhibit hyphal morphogenic shifting in yeast cells. Furthermore, QS inhibitors in bacteria have been shown to be successful as antibiotics against biofilm formation (10). Since two-component signaling genes are not found in humans, it is likely that the development of two-component inhibitors may be useful in the treatment of candidiasis and candidal biofilms as well as in the dissection of the QS pathway. Such studies have recently been reported (13, 17).
Acknowledgments
This work was supported in part by Public Health Service grants NIAID-47047 and NIAID-43465 to R.A.C. and CA88456 and DE13478 to R.L.C. M.K. was supported in part by NIH training grant T32AI37251. B.P.K. was supported in part by a grant from The Netherlands Organization for Scientific Research (NWO).
B.P.K. thanks Jesse Cohen for his technical assistance and Julia Douglas and her laboratory personnel for their valuable discussions. M.K. thanks Katie Kierpiec for assistance with the morphogenesis assays of strains grown in medium 199. Strains S and N (sln and nik mutants) were kindly provided by Mikio Arisawa, Nippon Roche, Kamakura, Japan.
REFERENCES
- 1.Alex, L. A., C. Korch, C. P. Selitrennikoff, and M. I. Simon. 1998. COS1, a two-component histidine kinase that is involved in hyphal development in the opportunistic pathogen Candida albicans. Proc. Natl. Acad. Sci. USA 95:7069-7073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buck, V., J. Quinn, T. Soto Pino, H. Martin, J. Saldanha, K. Makino, B. A. Morgan, and J. B. Millar. 2001. Peroxide sensors for the fission yeast stress-activated mitogen-activated protein kinase pathway. Mol. Biol. Cell 12:407-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calderone, R. A. 2002. Candida and candidiasis. ASM Press, Washington, D.C.
- 4.Calera, J. A., and R. Calderone. 1999. Flocculation of hyphae is associated with a deletion in the putative CaHK1 two-component histidine kinase gene from Candida albicans. Microbiology 145:1431-1442. [DOI] [PubMed] [Google Scholar]
- 5.Calera, J. A., G. H. Choi, and R. A. Calderone. 1998. Identification of a putative histidine kinase two-component phosphorelay gene (CaHK1) in Candida albicans. Yeast 14:665-674. [DOI] [PubMed] [Google Scholar]
- 6.Calera, J. A., X.-J. Zhao, and R. Calderone. 2000. Defective hyphal development and avirulence caused by a deletion of the SSK1 response regulator gene in Candida albicans. Infect. Immun. 68:518-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chauhan, N., D. Inglis, E. Roman, J. Pla, D. Li, J. A. Calera, and R. Calderone. 2003. Candida albicans response regulator gene SSK1 regulates a subset of genes whose functions are associated with cell wall biosynthesis and adaptation to oxidative stress. Eukaryot. Cell 2:1018-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Douglas, L. J. 2003. Candida biofilms and their role in infection. Trends Microbiol. 11:30-36. [DOI] [PubMed] [Google Scholar]
- 9.Fonzi, W. A., and M. Y. Irwin. 1993. Isogenic strain construction and gene mapping in Candida albicans. Genetics 134:717-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hentzer, M., and M. Givskov. 2003. Pharmacological inhibition of quorum sensing for the treatment of chronic bacterial infections. J. Clin. Investig. 112:1300-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hohmann, S. 2002. Osmotic stress signaling and osmoadaptation in yeasts. Microbiol. Mol. Biol. Rev. 66:300-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hornby, J. M., E. C. Jensen, A. D. Lisec, J. J. Tasto, B. Jahnke, R. Shoemaker, P. Dussault, and K. W. Nickerson. 2001. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl. Environ. Microbiol. 67:2982-2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim, S., E. Kim, D. S. Shin, H. Kang, and K. B. Oh. 2002. Evaluation of morphogenic regulatory activity of farnesoic acid and its derivatives against Candida albicans dimorphism. Bioorg. Med. Chem. Lett. 12:895-898. [DOI] [PubMed] [Google Scholar]
- 14.Miller, M. B., and B. L. Bassler. 2001. Quorum sensing in bacteria. Annu. Rev. Microbiol. 55:165-199. [DOI] [PubMed] [Google Scholar]
- 15.Nagahashi, S., T. Mio, N. Ono, T. Yamada-Okabe, M. Arisawa, H. Bussey, and H. Yamada-Okabe. 1998. Isolation of CaSLN1 and CaNIK1, the genes for osmosensing histidine kinase homologues, from the pathogenic fungus Candida albicans. Microbiology 144:425-432. [DOI] [PubMed] [Google Scholar]
- 16.Ramage, G., S. P. Saville, B. L. Wickes, and J. L. López-Ribot. 2002. Inhibition of Candida albicans biofilm formation by farnesol, a quorum-sensing molecule. Appl. Environ. Microbiol. 68:5459-5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shchepin, R., J. M. Hornby, E. Burger, T. Niessen, P. Dussault, and K. W. Nickerson. 2003. Quorum sensing in Candida albicans: probing farnesol's mode of action with 40 natural and synthetic farnesol analogs. Chem. Biol. 10:743-750. [DOI] [PubMed] [Google Scholar]
- 18.Singh, P., N. Chauhan, A. Ghosh, F. Dixon, and R. Calderone. 2004. SKN7 of Candida albicans: mutant construction and phenotype analysis. Infect. Immun. 72:2390-2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Srikantha, T., L. Tsai, K. Daniels, L. Enger, K. Highley, and D. R. Soll. 1998. The two-component hybrid kinase regulator CaNIK1 of Candida albicans. Microbiology 144:2715-2729. [DOI] [PubMed] [Google Scholar]
- 20.Yamada-Okabe, T., T. Mio, N. Ono, Y. Kashima, M. Matsui, M. Arisawa, and H. Yamada-Okabe. 1999. Roles of three histidine kinase genes in hyphal development and virulence of the pathogenic fungus Candida albicans. J. Bacteriol. 181:7243-7247. [DOI] [PMC free article] [PubMed] [Google Scholar]