Abstract
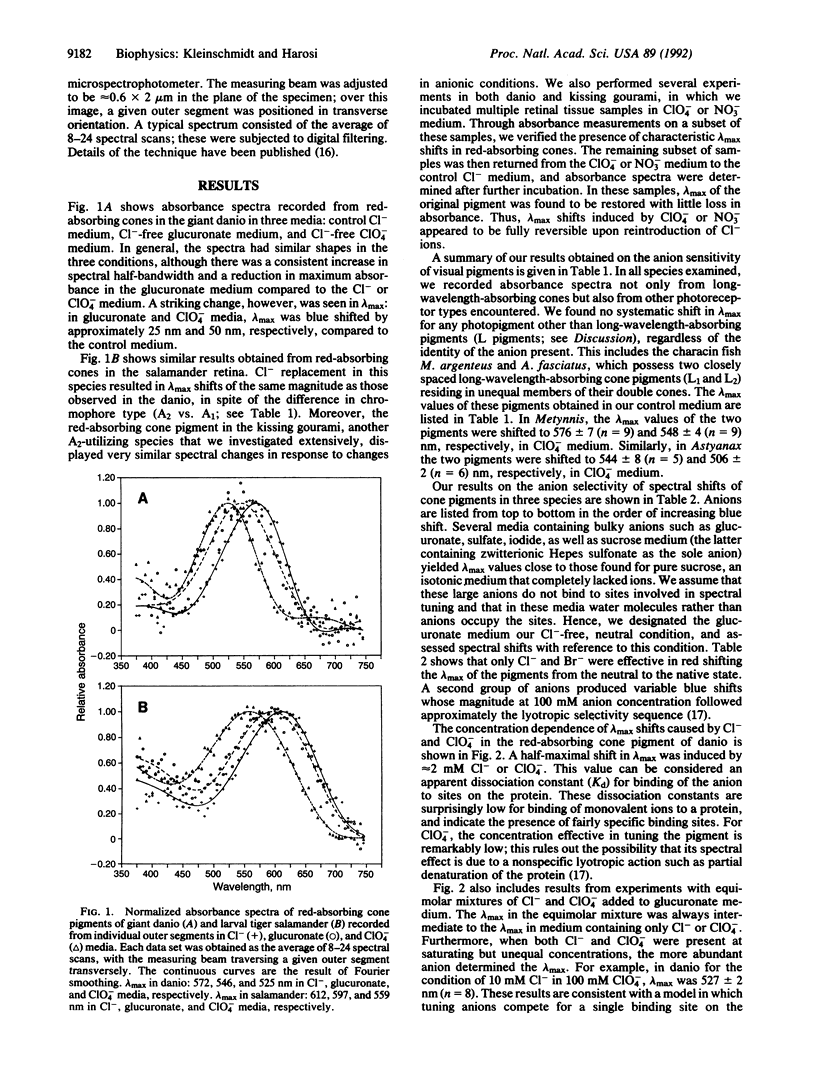
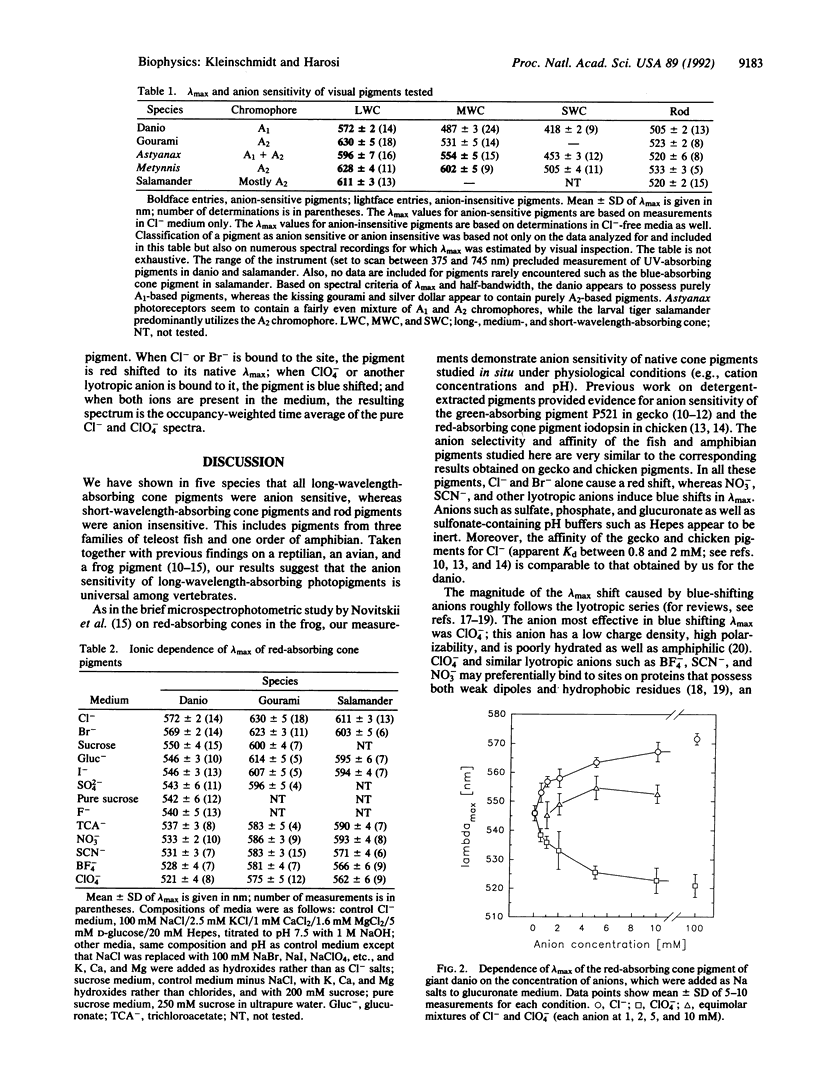
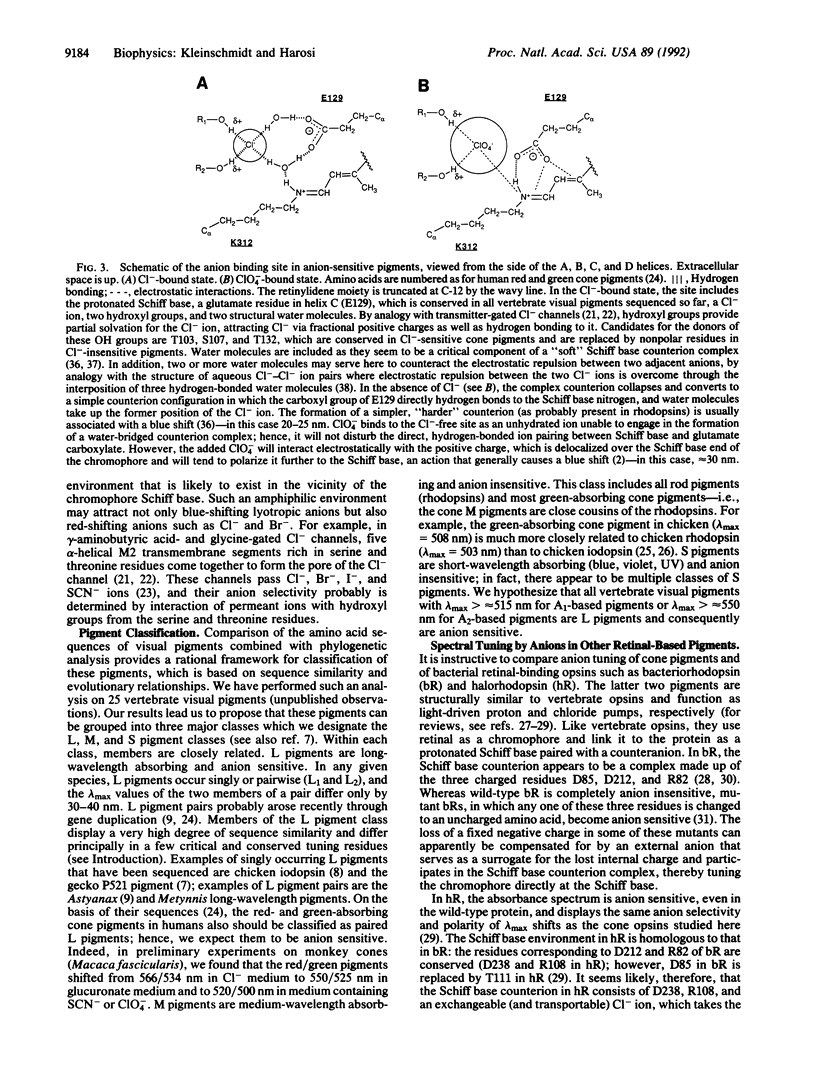
We tested the effect of anions on the absorbance spectrum of native visual pigments as measured by microspectrophotometry in individual cone outer segments of four species of fish and one species of amphibian. In all species tested, the long-wavelength-absorbing cone pigments were anion sensitive, and their lambda max could be tuned over a range of 55 nm depending on the identity of the anion present. Cl- and Br- were the only anions that produced native pigment spectra by red shifting lambda max from its value under anion-free conditions. Lyotropic anions such as NO3-, SCN-, BF4-, and ClO4- caused substantial and graded blue shifts of lambda max. The apparent Kd of binding sites on the pigment for Cl- and for ClO4- was approximately 2 mM. Taken together with previous findings on three visual pigments from the reptilian, avian, and amphibian classes, our results support the hypothesis that all long-wavelength-absorbing vertebrate visual pigments are spectrally tuned in part through the binding of a chloride ion. We propose that the site of anion tuning is near the protonated Schiff base of the chromophore, whose counterion may be complex and include Cl- as an exchangeable anion. This counterion configuration may resemble the one present in the light-driven Cl- pump halorhodopsin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birge R. R. Nature of the primary photochemical events in rhodopsin and bacteriorhodopsin. Biochim Biophys Acta. 1990 Apr 26;1016(3):293–327. doi: 10.1016/0005-2728(90)90163-x. [DOI] [PubMed] [Google Scholar]
- Blatz P. E., Mohler J. H., Navangul H. V. Anion-induced wavelength regulation of absorption maxima of Schiff bases of retinal. Biochemistry. 1972 Feb 29;11(5):848–855. doi: 10.1021/bi00755a026. [DOI] [PubMed] [Google Scholar]
- Bormann J., Hamill O. P., Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J Physiol. 1987 Apr;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins K. D., Washabaugh M. W. The Hofmeister effect and the behaviour of water at interfaces. Q Rev Biophys. 1985 Nov;18(4):323–422. doi: 10.1017/s0033583500005369. [DOI] [PubMed] [Google Scholar]
- Crescitelli F. Ionochromic behavior of Grecko visual pigments. Science. 1977 Jan 14;195(4274):187–188. doi: 10.1126/science.831267. [DOI] [PubMed] [Google Scholar]
- Crescitelli F., Karvaly B. The gecko visual pigment: the anion hypsochromic effect. Vision Res. 1991;31(6):945–950. doi: 10.1016/0042-6989(91)90202-g. [DOI] [PubMed] [Google Scholar]
- Crescitelli F. The gecko visual pigment: a pH indicator with a salt effect. J Physiol. 1981 Dec;321:385–399. doi: 10.1113/jphysiol.1981.sp013991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crescitelli F. The gecko visual pigment: the chromophore dark exchange reaction. Exp Eye Res. 1988 Feb;46(2):239–248. doi: 10.1016/s0014-4835(88)80081-2. [DOI] [PubMed] [Google Scholar]
- Dani J. A., Sanchez J. A., Hille B. Lyotropic anions. Na channel gating and Ca electrode response. J Gen Physiol. 1983 Feb;81(2):255–281. doi: 10.1085/jgp.81.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dér A., Száraz S., Tóth-Boconádi R., Tokaji Z., Keszthelyi L., Stoeckenius W. Alternative translocation of protons and halide ions by bacteriorhodopsin. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4751–4755. doi: 10.1073/pnas.88.11.4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fager L. Y., Fager R. S. Halide control of color of the chicken cone pigment iodopsin. Exp Eye Res. 1979 Oct;29(4):401–408. doi: 10.1016/0014-4835(79)90056-3. [DOI] [PubMed] [Google Scholar]
- Henderson R., Baldwin J. M., Ceska T. A., Zemlin F., Beckmann E., Downing K. H. Model for the structure of bacteriorhodopsin based on high-resolution electron cryo-microscopy. J Mol Biol. 1990 Jun 20;213(4):899–929. doi: 10.1016/S0022-2836(05)80271-2. [DOI] [PubMed] [Google Scholar]
- Hárosi F. I. Cynomolgus and rhesus monkey visual pigments. Application of Fourier transform smoothing and statistical techniques to the determination of spectral parameters. J Gen Physiol. 1987 May;89(5):717–743. doi: 10.1085/jgp.89.5.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakitani H., Kakitani T., Rodman H., Honig B. On the mechanism of wavelength regulation in visual pigments. Photochem Photobiol. 1985 Apr;41(4):471–479. doi: 10.1111/j.1751-1097.1985.tb03514.x. [DOI] [PubMed] [Google Scholar]
- Khorana H. G. Bacteriorhodopsin, a membrane protein that uses light to translocate protons. J Biol Chem. 1988 Jun 5;263(16):7439–7442. [PubMed] [Google Scholar]
- Khorana H. G. Rhodopsin, photoreceptor of the rod cell. An emerging pattern for structure and function. J Biol Chem. 1992 Jan 5;267(1):1–4. [PubMed] [Google Scholar]
- Kojima D., Okano T., Fukada Y., Shichida Y., Yoshizawa T., Ebrey T. G. Cone visual pigments are present in gecko rod cells. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6841–6845. doi: 10.1073/pnas.89.15.6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwata O., Imamoto Y., Okano T., Kokame K., Kojima D., Matsumoto H., Morodome A., Fukada Y., Shichida Y., Yasuda K. The primary structure of iodopsin, a chicken red-sensitive cone pigment. FEBS Lett. 1990 Oct 15;272(1-2):128–132. doi: 10.1016/0014-5793(90)80465-u. [DOI] [PubMed] [Google Scholar]
- Langosch D., Becker C. M., Betz H. The inhibitory glycine receptor: a ligand-gated chloride channel of the central nervous system. Eur J Biochem. 1990 Nov 26;194(1):1–8. doi: 10.1111/j.1432-1033.1990.tb19419.x. [DOI] [PubMed] [Google Scholar]
- Lanyi J. K. Halorhodopsin, a light-driven electrogenic chloride-transport system. Physiol Rev. 1990 Apr;70(2):319–330. doi: 10.1152/physrev.1990.70.2.319. [DOI] [PubMed] [Google Scholar]
- Loppnow G. R., Barry B. A., Mathies R. A. Why are blue visual pigments blue? A resonance Raman microprobe study. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1515–1518. doi: 10.1073/pnas.86.5.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti T., Rösselet S. J., Otto H., Heyn M. P., Khorana H. G. The retinylidene Schiff base counterion in bacteriorhodopsin. J Biol Chem. 1991 Oct 5;266(28):18674–18683. [PubMed] [Google Scholar]
- Mathies R. A., Lin S. W., Ames J. B., Pollard W. T. From femtoseconds to biology: mechanism of bacteriorhodopsin's light-driven proton pump. Annu Rev Biophys Biophys Chem. 1991;20:491–518. doi: 10.1146/annurev.bb.20.060191.002423. [DOI] [PubMed] [Google Scholar]
- Nathans J. Determinants of visual pigment absorbance: identification of the retinylidene Schiff's base counterion in bovine rhodopsin. Biochemistry. 1990 Oct 16;29(41):9746–9752. doi: 10.1021/bi00493a034. [DOI] [PubMed] [Google Scholar]
- Nathans J., Thomas D., Hogness D. S. Molecular genetics of human color vision: the genes encoding blue, green, and red pigments. Science. 1986 Apr 11;232(4747):193–202. doi: 10.1126/science.2937147. [DOI] [PubMed] [Google Scholar]
- Neitz M., Neitz J., Jacobs G. H. Spectral tuning of pigments underlying red-green color vision. Science. 1991 May 17;252(5008):971–974. doi: 10.1126/science.1903559. [DOI] [PubMed] [Google Scholar]
- Novitskii I. Iu, Zak P. P., Ostrovskii M. A. Vliianie anionov na spektral'nye svoistva iodopsin v nativnykh kolobochkakh setchatki liagushki (mikrospektrofotometricheskoe issledovanie). Bioorg Khim. 1989 Aug;15(8):1037–1043. [PubMed] [Google Scholar]
- Okano T., Kojima D., Fukada Y., Shichida Y., Yoshizawa T. Primary structures of chicken cone visual pigments: vertebrate rhodopsins have evolved out of cone visual pigments. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5932–5936. doi: 10.1073/pnas.89.13.5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen R. W., Tobin A. J. Molecular biology of GABAA receptors. FASEB J. 1990 Mar;4(5):1469–1480. doi: 10.1096/fasebj.4.5.2155149. [DOI] [PubMed] [Google Scholar]
- Sakmar T. P., Franke R. R., Khorana H. G. Glutamic acid-113 serves as the retinylidene Schiff base counterion in bovine rhodopsin. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8309–8313. doi: 10.1073/pnas.86.21.8309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schobert B., Lanyi J. K., Oesterhelt D. Effects of anion binding on the deprotonation reactions of halorhodopsin. J Biol Chem. 1986 Feb 25;261(6):2690–2696. [PubMed] [Google Scholar]
- Shichida Y., Kato T., Sasayama S., Fukada Y., Yoshizawa T. Effects of chloride on chicken iodopsin and the chromophore transfer reactions from iodopsin to scotopsin and B-photopsin. Biochemistry. 1990 Jun 19;29(24):5843–5848. doi: 10.1021/bi00476a028. [DOI] [PubMed] [Google Scholar]
- Wang S. Z., Adler R., Nathans J. A visual pigment from chicken that resembles rhodopsin: amino acid sequence, gene structure, and functional expression. Biochemistry. 1992 Apr 7;31(13):3309–3315. doi: 10.1021/bi00128a002. [DOI] [PubMed] [Google Scholar]
- Wright E. M., Diamond J. M. Anion selectivity in biological systems. Physiol Rev. 1977 Jan;57(1):109–156. doi: 10.1152/physrev.1977.57.1.109. [DOI] [PubMed] [Google Scholar]
- Yokoyama R., Yokoyama S. Convergent evolution of the red- and green-like visual pigment genes in fish, Astyanax fasciatus, and human. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9315–9318. doi: 10.1073/pnas.87.23.9315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhukovsky E. A., Oprian D. D. Effect of carboxylic acid side chains on the absorption maximum of visual pigments. Science. 1989 Nov 17;246(4932):928–930. doi: 10.1126/science.2573154. [DOI] [PubMed] [Google Scholar]
- de Groot H. J., Smith S. O., Courtin J., van den Berg E., Winkel C., Lugtenburg J., Griffin R. G., Herzfeld J. Solid-state 13C and 15N NMR study of the low pH forms of bacteriorhodopsin. Biochemistry. 1990 Jul 24;29(29):6873–6883. doi: 10.1021/bi00481a017. [DOI] [PubMed] [Google Scholar]



