Abstract
Dietary restriction (DR) without malnutrition is associated with longevity in various organisms. However, it has also been shown that reduced calorie intake is often ineffective in extending lifespan. Selecting optimal dietary regimens for DR studies is complicated, as the same regimen may lead to different outcomes depending on genotype and environmental factors. Recent studies suggested that interventions such as moderate protein restriction with/without adequate nutrition (e.g. particular amino acids or carbohydrates) may have additional beneficial effects mediated by certain metabolic and hormonal factors implicated in the biology of aging, regardless of total calorie intake. In particular, it was shown that restriction of a single amino acid, methionine, can mimic the effects of DR and extend lifespan in various model organisms. We discuss beneficial effects of methionine-restricted (MR) diet, the molecular pathways involved, and the use of this regimen in longevity interventions.
Keywords: Dietary restriction, protein restriction, methionine restriction, lifespan extension
Introduction
Dietary restriction (DR) is an evolutionarily conserved strategy that was reported to extend lifespan in a broad range of organisms.1 It is generally believed that reduced food intake (a term often used interchangeably with calorie restriction, CR) is the basis for the DR effect on lifespan2. While the beneficial effects of DR are known for all major model organisms of aging, including yeast, fruit flies, worms and mice, a word of caution is needed regarding the diets used in these experiments. Dietary composition necessarily differs for different experimental models and among research labs, which may lead to different or even opposite lifespan effects.3 In addition, the duration and severity of the DR regimen that achieves maximal longevity may not be feasible outside of lab settings.4 In humans, DR may be associated with severe side effects and elevated risk of malnutrition, especially with regard to protein and micronutrient requirements.5 Although analyses of those practicing CR showed that humans exhibit some of the same molecular and metabolic signatures observed in long-lived CR rodents6, currently it is impractical to directly apply CR to increase longevity and reduce the risk of age-associated diseases in humans.
In order to alleviate the detrimental consequences of DR, while preserving its beneficial effects, several studies attempted to develop modified DR regimens. In particular, a combination of optimized diet with meal frequency and timing (prolonged fasting) was suggested to achieve the DR-associated lifespan extension.7 Another promising DR regimen involves the decrease in the levels of protein and/or individual essential amino acids, such as methionine and tryptophan. It was suggested that these diets may support longevity without decreasing daily calorie intake.8 It may also reduce possible undesirable effects of DR diet in humans and avoid the risk of malnutrition. In this review, we focus on the importance of the composition of DR diets involving methionine restriction (MR), discuss mechanisms involved, and provide examples that illustrate the relationship between MR and longevity through the balance of protein-based dietary regimens.
Dietary restriction: the royal road to longevity?
Since CR was first shown to be a reliable intervention for extending the lifespan of laboratory rats,9 there have been numerous studies that applied this dietary regimen to a broad range of species.10–15 It has been demonstrated that the incidence of many age-associated disorders could be decreased by reduced calorie intake.16,17 However, the benefits of this powerful regimen were also questioned by the research community because of its ineffectiveness in extending lifespan under many conditions, challenging researchers to both understand the basis of these contrasting effects and develop better dietary approaches and a stronger theory.18–21 For instance, the effect of decreased dietary toxicity associated with DR could be easily confused with the effect of lifespan extension upon reduced food intake. For example, this phenomenon is observed when highly concentrated sugar-yeast or yeast diets are used in Drosophila.18–19 To distinguish the toxic effects of diets on lifespan, fecundity may also be assessed in tested organisms. For example, egg production in cohorts of mated female flies may be measured, because it is decreased by toxicity associated with excessive food intake, whereas it is elevated with the increase in food intake when the diet is truly limiting and approaches malnutrition.19 However, such analyses are still insufficient and lead to further questions. What is the underlying mechanism of food toxicity? Why the toxic effect is not observed under conditions of reduced dietary intake? Is it too small to be observed by assessing egg production? In addition, DR in Drosophila may be achieved by simply diluting sugar-yeast levels, thereby resulting in differences in water intake. As in the case of water stress, it was reported that lifespan extension by DR could be abolished by providing ad libitum water without altering food intake in Drosophila, i.e., water consumption could be a factor in assessing lifespan extension by DR. Nevertheless, it is of interest that lifespan extension in Canton-S flies upon reduction of dietary yeast alone was not affected by water availability.22 Another challenge to DR is that we do not know the exact ingested amounts of food as well as its distribution in the organism following consumption. Compensatory feeding under DR conditions was reported in many studies and is considered as another variable for the lifespan effects of DR.23–24 Current methods to quantify the eating behavior in Drosophila have limitations. Though specific dyes such as erioglaucine can be used to assess this behavior in Drosophila,25 such analyses assess the amounts of food consumed in a short period of time and, in addition, the absorption rates of individual nutrients, their distribution in the body and their contribution to various metabolic processes may be different and may be further influenced by DR.
Importance of reduced protein intake in dietary restriction
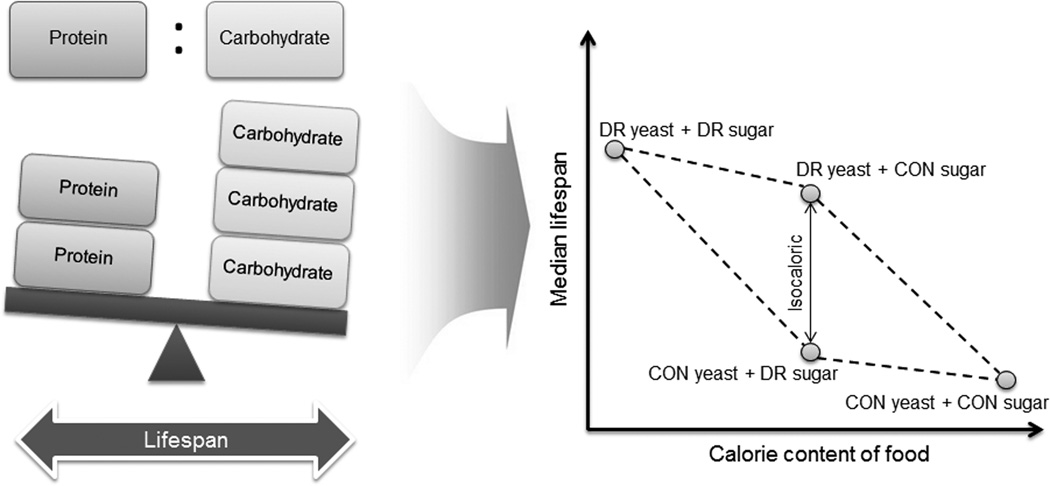
Recent evidence suggests that reduced calorie intake does not necessarily extend lifespan in Drosophila, whereas it was found that reduction of specific nutritional components such as dietary yeast is important in supporting lifespan extension. Specifically, sugar reduction had little or no influence on lifespan, even though calorie intake was reduced by this regimen, whereas yeast reduction was consistently very effective in lifespan extension (Figure 1).18,26–27 In mammals, including humans, protein restriction (PR) diets without malnutrition also show beneficial effects: PR contributes to reduced mortality and incidence of age-associated diseases, ultimately supporting longevity.28–30 Interestingly, low protein intake leads to a reduction in serum IGF-1 levels in respondents aged 50–65 and is associated with reduced cancer incidence and overall reduced mortality.29 Importantly, two long-term DR studies (1 and 6 years) without malnutrition did not find reduced IGF-1 levels and the IGF:IGFBP-3 ratio in humans subjected to severe CR (CR diet group: 1800 kcal day−1 with 24% calories from protein and 28% calories from fat; Western diet group: approximately 2500 kcal day−1 with 16% calories from protein and 33.6% calories from fat), whereas IGF-1 levels could be reduced by moderately restricted protein diet (Low-protein diet: 0.76 g kg−1 per day; ~10% of energy intake from protein; High-protein diet: 1.73 g kg−1 per day; ~24% of energy intake from protein). 30 This finding shows that PR may be an effective regimen in humans and that it may delay aging by influencing the insulin/IGF-1 signaling pathway, an evolutionarily conserved mechanism that regulates longevity.30–31 For example, Ames dwarf mice, which exhibit low levels of growth hormone (GH)/IGF-1, are characterized by small body size and long lifespan.32 It was also reported that oxidative stress may be reduced by the PR diet. Decreased protein ingestion (by 40%) in Wistar rats leads to lower levels of reactive oxygen species (ROS), reduced oxidative damage to DNA and proteins, and decreased expression of respiratory complex I in the liver.28 Overall, growing evidence regarding reduced protein intake strongly supports the idea that among the three major nutritional components (proteins, carbohydrates, lipids), proteins are in the unique position to regulate lifespan and mimic the effect of DR on aging.
Figure 1.
Protein and carbohydrate components of diet differentially influence lifespan of Drosophila. Low protein diet (DR yeast) is more effective in extending lifespan than low carbohydrate diet (DR sugar). In the figure, DR refers to dietary restriction, and CON to control diet. Modified from [27].
With respect to the effect of PR diet on aging, it was also reported that the protein/carbohydrate ratio needs to be considered if one wants to maximize the beneficial effects of reduced protein levels on aging. It was shown that Drosophila lifespan is the longest on the diet with the 1:16 ratio of protein to carbohydrate and that this lifespan gradually decreases with the increase in the ratio. This finding supports a general theme that not only low protein levels, but the specific balance between proteins and carbohydrates are critical determinants of maximal lifespan of Drosophila.33 Consistent with this notion, an analysis of dietary geometry in mice showed that energy restriction achieved by food dilution in ad libitum-fed mice did not extend lifespan, probably because of the compensatory consumption of protein, but a low protein, high carbohydrate diet could limit such compensatory protein consumption. Here, energy requirement was met by adding more carbohydrates and reducing protein intake, suppressing hepatic mammalian target of rapamycin (mTOR) and mitochondrial function due to reduced circulating branched-chain amino acids.34 Therefore, though PR is the main mechanism that supports the benefits of DR, compensatory food consumption due to diluted food can be a potential problem. It may lead to spurious results on lifespan due to compensatory protein intake, up to a normal level. However, balanced macronutrient composition can help avoid compensatory food consumption, thereby optimizing the effect of diet on lifespan.
Methionine restriction and aging
With growing evidence on the importance of protein and amino acid restriction in regulating lifespan, several studies focused on dissecting the roles of dietary amino acids in order to identify nutrients that regulate longevity.35–37 In this regard, the most successful case has been the use of methionine restriction (MR) diet. Its beneficial effect on lifespan was described in rat, mouse, and fruit fly models, although it was also questioned for being inconsistent, similarly to the effect of DR. For example, 2–4 week old rats were examined on two diets that contained 0.86% or 0.17% methionine. 23 Compensatory food consumption occurred on the diet with the lower level of methionine that led to reduced body weight, showing the same pattern of eating behavior as in the case of DR.23 Nevertheless, lifespan of rats on the lower methionine diet was extended against increased dietary intake.38 As a supportive evidence, it was reported that both maximum and median lifespan of mice could be extended when the low methionine diet was provided starting at 12 months of age.39 It was also shown that metformin altered E. coli methionine metabolism, leading to methionine restriction in E. coli, which in turn led to lifespan extension of the host C. elegans.40 In human diploid fibroblasts, MR could extend replicative lifespan and inhibited senescence by downregulating mitochondrial protein synthesis and respiratory chain assembly41. As such, many studies strongly support the idea that MR can be effective in extending lifespan in various species, and that it mimics the effect of DR. On the other hand, ineffectiveness of MR has been noted in Drosophila, similar to the cases of DR and PR. A chemically defined diet was developed for Drosophila, which allowed regulating the levels of individual dietary components, including methionine. Unexpectedly, initial studies did not observe a positive regulation of lifespan on the low methionine diet, either when 5% or 15% glucose levels were used.42
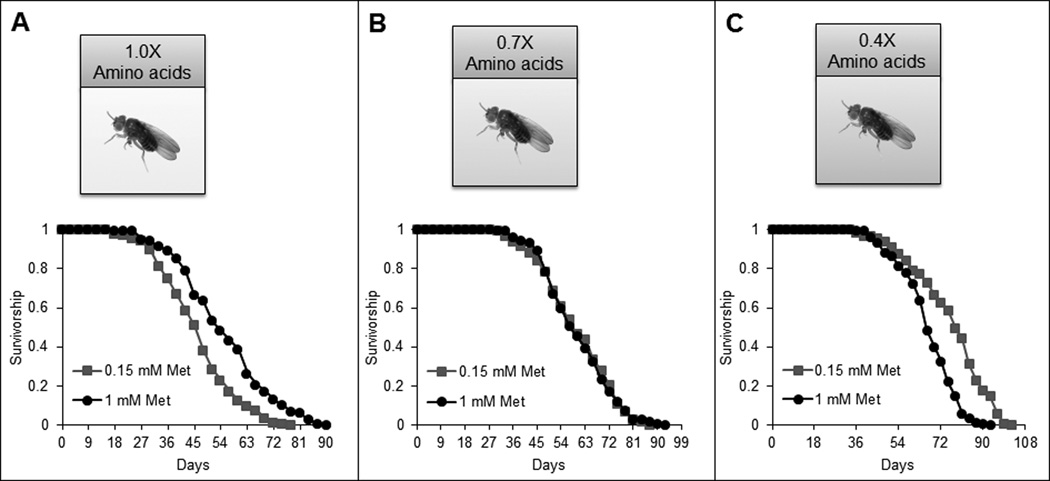
As discussed above, many of the MR, PR and DR studies face the same challenges. For example, CR did not extend lifespan in rhesus macaques in a study carried out at the National Institute on Aging, whereas a study at the Wisconsin National Primate Research Center reported a positive effect of CR on lifespan.10,17 Leaving aside potential differences, including the study design, dietary regimes of control groups, housing environment, timing of food consumption and diet composition, which may have influenced the results, a clear fact is that CR is not always successful in achieving lifespan extension and may show opposing results even in very similar and well controlled studies. These considerations must be taken into account when one considers the use of DR regimens and interprets the results. A recent approach that employs nutrient geometry can help gain deeper insights into the DR effects on aging. Like in DR studies, the ineffectiveness of methionine restriction in extending lifespan could reflect the fact that not only methionine, but its balance with other nutrients is important in achieving the desired effect. A recent study tested the effect of amino acid balance on lifespan in Drosophila, examining various combinations and proportions of methionine and other amino acids. Interestingly, lifespan of Drosophila was extended by MR under conditions of low amino acid status, while MR did not work under conditions of high amino acid status (Figure 2).43 In conclusion, as in the case of DR, methionine is not a magic bullet that regulates lifespan, as certain conditions must be met for its beneficial effect. For that reason, future studies may benefit from the use of diverse combinations of methionine and other nutrients.
Figure 2.
Methionine restriction extends Drosophila lifespan under conditions of low amino acid status. Comparison of Drosophila lifespan on 1 mM (black curves) and 0.15 mM (red curves) methionine when the levels of other amino acids are (A) 1.0X, (B) 0.7X, (C) 0.4X. Modified from [43].
Benefits of methionine restriction in age-related health
Aging is accompanied by organ and tissue dysfunction and elevated incidence of chronic diseases. Thus, it is not surprising that nutritional interventions involving DR and PR may also offer different benefits in preventing age-associated diseases and supporting longevity. Likewise, there have been many reports regarding the benefits of MR in health and longevity.44 For example, significant reduction in the development of prostatic intraepithelial neoplastic lesions in transgenic mice was observed on the 0.12% methionine diet, when compared with the 0.86% methionine diet.45 Also, ten-week old ob/ob mice fed 0.12% (MR) or 0.86% (Control) methionine diets for 14 weeks were analyzed. Interestingly, mice raised on the 0.12% methionine diet were rescued from severe steatosis and significantly reduced triglyceride, serum alanine aminotransferase and aspartate aminotransferase, and plasma insulin levels.46 It was also demonstrated that MR in mice induced secretion of cardioprotective hormones such as adiponectin and FGF21, thus leading to protection against hyperhomocysteinemia.47
How to reduce oxidative stress-induced damage is also critical for pursuing healthy aging. In this regard, some studies examined how MR diet affects ROS generation. Wistar rats were maintained on isocaloric 40% MR diet for 7 weeks and then analyzed for parameters of oxidative stress. This diet decreased heart mitochondrial ROS generation, particularly from complex I, as well as damage to mitochondrial DNA, proteins, and lipids.48 It was also shown that mitochondrial ROS generation and subsequent damage to mitochondrial DNA and protein were reduced in heart, liver, brain, and kidney mitochondria in animals fed the 40% MR diet.49–51 In addition, an increase in glutathione levels and peroxidase activity were observed in blood and kidney of animals on the 80% MR diet. 52 Moreover, biomarkers of oxidative stress including plasma 8-hydoxydeoxyguanosine, 8-isoprostane, and erythrocyte protein-bound glutathione, were maintained at low levels in animals fed 80% MR diet for 6 months.52 Overall, lower ROS levels may contribute to maintaining healthy lifespan in rodent models fed MR diets, thereby leading to lifespan extension.
Mechanisms of lifespan extension by methionine restriction
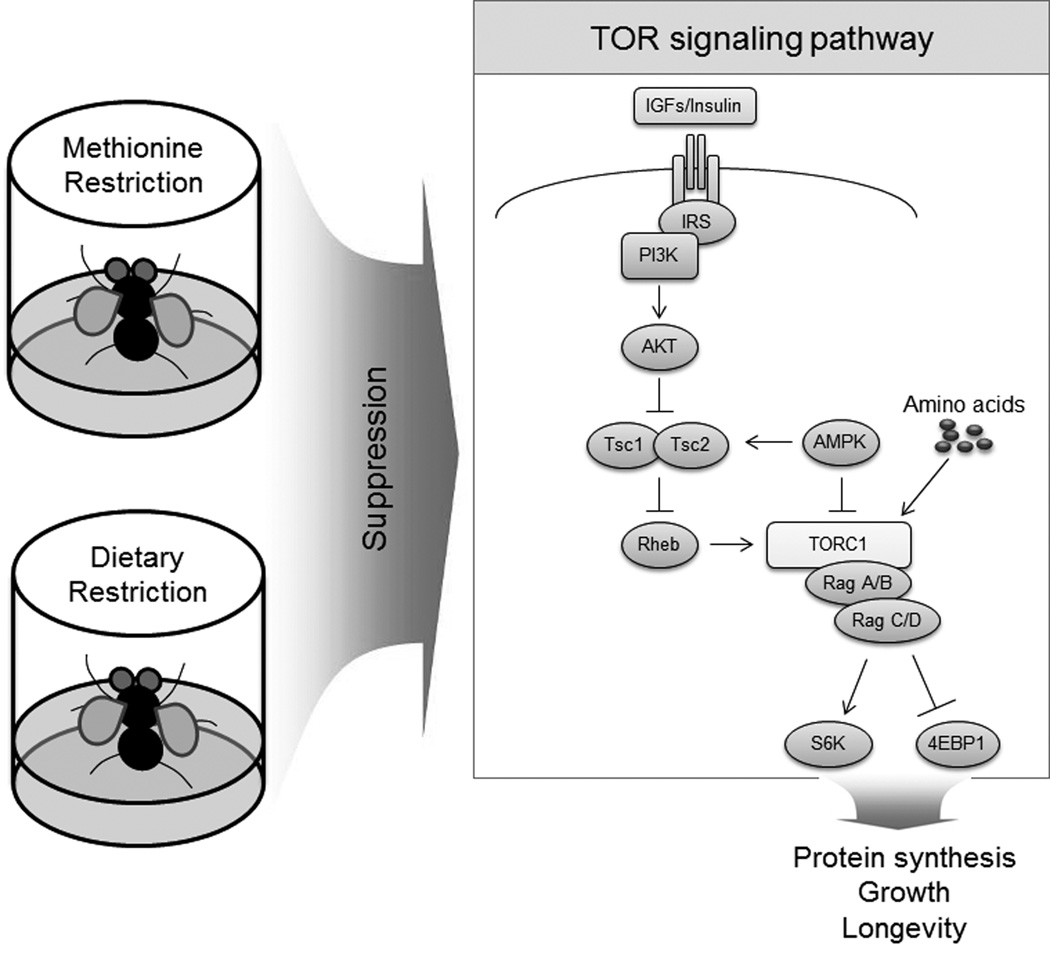
Despite many studies, the mechanisms of lifespan regulation by DR and PR remain incompletely understood. It was proposed that insulin/IGF-1 and mTOR signaling represent a conserved upstream nutrition signaling pathway, which regulates longevity from yeast to mammals and mediates the effects of DR and PR53–55 (Figure 3). Consistent with this idea, overexpression of dTsc1, dTsc2, the dominant-negative forms of dTOR, or dS6K, all of which are involved in TOR signaling, extended Drosophila lifespan.43 Moreover, lifespan extension upon overexpression of dTsc2 depended on the levels of dietary yeast, supporting the idea that lifespan extension by DR or PR is due to suppression of TOR signaling.13 This longevity strategy is also conserved in other organisms. Deletion of ribosomal S6 protein kinase 1 (S6K1), which acts downstream of mTOR, extended lifespan of mice, and the corresponding gene expression pattern mimicked that observed in response to DR.56 It was also found that rats subjected to DR and treated with 1-methyl-1-nitrosourea to induce breast cancer showed decreased levels of phosphorylated mTOR, thereby down-regulating mTOR activity and changing the phosphorylation status of p70S6K and 4E-BP1.57 In the case of yeast, knockout of SCH9, a homolog of mammalian AKT and S6K and a major target of yeast TORC1, extended both chronological and replicative lifespans.58–59 Decreased yeast TOR activity itself was also shown to extend chronological and replicative lifespan.60 Finally, it was reported that CR of SCH9Δ or TOR1Δ failed to further extend yeast replicative lifespan, suggesting that the lifespan extension by CR in yeast involves suppression of TOR signaling.61
Figure 3.
Dietary restriction, methionine restriction, and mTOR signaling43,53–55. Lifespan extension by both DR and MR depends on suppression of mTOR signaling. Insulin receptor and TSC2 were examined for their roles in lifespan extension by MR under conditions of low amino acid status in Drosophila.
The MR diet mimics both DR or PR with regard to phenotypes associated with lifespan extension and induces similar gene expression and physiological changes.11,38,43,49,62–63 Thus, the underlying mechanism of lifespan extension by MR may indeed involve the same pathways utilized by DR. Overexpression of dTsc2 or a dominant negative form of the insulin receptor (dInRDN) failed to further extend the lifespan of Drosophila subjected to MR under conditions of low amino acid status,43 suggesting the role of mTOR signaling. However, whether this mechanism applies to MR under conditions of high amino acid levels remains unknown. Based on the analysis of lifespan in response to MR and involving graded amino acid levels, MR did not extend Drosophila lifespan under conditions of high amino acid status.
A recent study reported that sulfur amino acid restriction increased the expression of cystathionine γ-lyase (CGL), which in turn elevated hydrogen sulfide production. 64 This effect was observed in yeast, worms, fruit flies, and mice subjected to DR media/diets, suggesting its importance for the DR-mediated longevity benefits. Consistent with this idea, MR-mediated lifespan extension in fruit flies was accompanied by increased hydrogen sulfide production. Thus, the data support the notion that the lifespan extension induced by MR shares the same underlying mechanisms with the lifespan extension by DR.
One of the conclusions from these studies is that decreased calorie intake from proteins, specifically their methionine, may be a factor directly responsible for the lifespan extension effect of DR.37–38,65 In fact, neither carbohydrate nor lipid restriction had a consistent effect on lifespan in rodents.66–67 Based on these findings, it is possible that these nutritional pathways also play a role in longevity regulation under MR conditions. In addition, many physiological changes in mice under MR conditions overlap with the effects of DR.65 The involvement of mTOR together with GCN2 in amino acid sensing mechanisms is well known.68 Furthermore, requirement of GCN2 for longevity extension by MR in yeast was shown. A decline in IGF-1, serum glucose and insulin levels were also implicated in the MR effect in mice, suggesting a possible role for the GH/IGF signaling pathway in longevity control by methionine levels.65 Moreover, additional studies support the hypothesis that Ames dwarf and GH receptor knockout mice do not respond to 0.16% methionine diet, whereas the respective control animals and mice overexpressing GH respond with lifespan extension.69 Overall, when consumption of the essential amino acid methionine is limited, lifespan extension in different organisms is observed, similar to the interventions that reduce calorie intake. However, further studies are required to better understand the molecular mechanisms involved and apply these principles to human diet to achieve potential beneficial effects with regard to the aging process and/or age-associated chronic diseases.64–71
Acknowledgments
This work is supported by NIH AG021518 to V.N.G. and an intramural grant (K1505311) from Korea University to B.C.L.
Footnotes
Conflict of interest
The authors declare no conflicts of interest.
References
- 1.Fontana L, Partridge L, Longo VD. Extending healthy life span—from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bordone L, Guarente L. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat. Rev. Mol. Cell Biol. 2005;6:298–305. doi: 10.1038/nrm1616. [DOI] [PubMed] [Google Scholar]
- 3.Grandison RC, Wong R, Bass TM, Patridge L, Piper MD. Effect of a standardised dietary restriction protocol on multiple laboratory strains of Drosophila melanogaster. Plos One. 2009;4:e4067. doi: 10.1371/journal.pone.0004067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harper JM, Leathers CW, Austad SN. Does caloric restriction extend life in wild mice? Aging Cell. 2006;5:441–449. doi: 10.1111/j.1474-9726.2006.00236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Speakman JR, Hambly C. Starving for life: what animal studies can and cannot tell us about the use of caloric restriction to prolong human lifespan. J. Nutr. 2007;137:1078–1086. doi: 10.1093/jn/137.4.1078. [DOI] [PubMed] [Google Scholar]
- 6.Cava E, Fontana L. What are the roles of calorie restriction and diet quality in promoting healthy longevity? Aging (Albany NY) 2013;13:38–45. doi: 10.1016/j.arr.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Brandhorst S, Choi IY, Wei M, et al. A priodic diet that mimics fasting promotes multi-system regeneration, enhanced cognitive performance, and health span. Cell Metabolism. 2015;22:86–99. doi: 10.1016/j.cmet.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spindler SR. Caloric restriction: from soup to nuts. Ageing Res. Rev. 2009;9:324–353. doi: 10.1016/j.arr.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 9.McCay CM, Crowel MF, Maynard LA. The effect of retarded growth upon the length of the life span and upon the ultimate body size. J. Nutr. 1935;10:63–79. [PubMed] [Google Scholar]
- 10.Mattison JA, Wright C, Bronson RT, et al. Studies of aging in ames dwarf mice: Effects of caloric restriction. J. Am. Aging Assoc. 2000;23:9–16. doi: 10.1007/s11357-000-0002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin SJ, Kaeberlein M, Andalis AA, et al. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature. 2002;418:344–348. doi: 10.1038/nature00829. [DOI] [PubMed] [Google Scholar]
- 12.Barger JL, Walford RL, Weindruch R. The retardation of aging by caloric restriction: Its significance in the transgenic era. Exp. Gerontol. 2003;38:1343–1345. doi: 10.1016/j.exger.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 13.Kapahi P, Zid BM, Harper T, et al. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr. Biol. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wood JG, Rogina B, Lavu S, et al. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- 15.Partridge L, Piper MD, Mair W. W. Dietary restriction in Drosophila. Mech. Ageing Dev. 2005;126:938–950. doi: 10.1016/j.mad.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 16.Rizza W, Veronese N, Fontana L. What are the roles of calorie restriction and diet quality in promoting healthy longevity? Ageing Res. Rev. 2014;13:38–45. doi: 10.1016/j.arr.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Colman RJ, Anderson RM, Johnson SC, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piper MD, Partridge L. Dietary restriction in Drosophila: Delayed aging or experimental artefact? PLoS Genet. 2007;3:461–466. doi: 10.1371/journal.pgen.0030057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Partridge L, Gems D, Withers DJ. Sex and death: What is the connection? Cell. 2005;120:461–472. doi: 10.1016/j.cell.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 20.Turturro A, Duffy PH, Hart RW. Modulation of toxicity by diet and dietary macronutrient restriction. Mutat. Res. 1993;295:151–164. doi: 10.1016/0921-8734(93)90017-w. [DOI] [PubMed] [Google Scholar]
- 21.Morley JE, Chahla E, Alkaade S. Antiaging, longevity and calorie restriction. Curr. Opin. Clin. Nutr. Metab. Care. 2010;13:40–45. doi: 10.1097/MCO.0b013e3283331384. [DOI] [PubMed] [Google Scholar]
- 22.Ja WW, Carvalho GB, Zid BM, et al. Water- and nutrient-dependent effects of dietary restriction on Drosophila lifespan. Proc. Natl. Acad. Sci. U. S. A. 2009;106:18633–18637. doi: 10.1073/pnas.0908016106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carvalho GB, Kapahi P, Benzer S. Compensatory ingestion upon dietary restriction in Drosophila melanogaster. Nat. Methods. 2005;2:813–815. doi: 10.1038/nmeth798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tatar M, Post S, Yu K. Nutrient control of Drosophila longevity. Trends Endocrinol. Metab. 2014;25:509–517. doi: 10.1016/j.tem.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong R, Piper MD, Wertheim B, Partridge L. Quantification of Food Intake in Drosophila. Plos One. 2009;4:e6063. doi: 10.1371/journal.pone.0006063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cooper T, Mockett RJ, Sohal B, Sohal R, Orr W. Effect of caloric restriction on life span of the housefly, Musca domestica. FASEB J. 2004;13:1–13. doi: 10.1096/fj.03-1464fje. [DOI] [PubMed] [Google Scholar]
- 27.Mair W, Piper MD, Partridge L. Calories do not explain extension of life span by dietary restriction in Drosophila. PLoS Biol. 2005;3:1305–1311. doi: 10.1371/journal.pbio.0030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ayala V, Naudi A, Sanz A, et al. Dietary protein restriction decreases oxidative protein damage, peroxidizability index, and mitochondrial complex I content in rat liver. J. Gerontol. A. Biol. Sci. Med. Sci. 2007;62:352–360. doi: 10.1093/gerona/62.4.352. [DOI] [PubMed] [Google Scholar]
- 29.Levine ME, Suarez JA, Brandhorst S, et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 2014;19:407–417. doi: 10.1016/j.cmet.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fontana L, Weiss EP, Villareal DT, Klein S, Holloszy JO. Long-term effects of calorie or protein restriction on serum IGF-1 and IGFBP-3 concentration in humans. Aging Cell. 2008;7:681–687. doi: 10.1111/j.1474-9726.2008.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barbieri M, Bonafè M, Franceschi C, Paolisso G. Insulin/IGF-I-signaling pathway: an evolutionarily conserved mechanism of longevity from yeast to humans. Am. J. Physiol. Endocrinol. Metab. 2003;285:E1064–E1071. doi: 10.1152/ajpendo.00296.2003. [DOI] [PubMed] [Google Scholar]
- 32.Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384:33–33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- 33.Lee KP, Simpson SJ, Clissold FJ, et al. Lifespan and reproduction in Drosophila: New insights from nutritional geometry. Proc. Natl. Acad. Sci. U. S. A. 2008;105:2498–2503. doi: 10.1073/pnas.0710787105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Solon-Biet SM, McMahon AC, Ballard JW, et al. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab. 2014;19:418–430. doi: 10.1016/j.cmet.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Min KJ, Tatar M. Restriction of amino acids extends lifespan in Drosophila melanogaster. Mech. Ageing Dev. 2006;127:643–646. doi: 10.1016/j.mad.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 36.Flurkey K, Astle CM, Harrison DE. Life extension by diet restriction and N-acetyl-L-cysteine in genetically heterogeneous mice. Journals Gerontol. - Ser. A Biol. Sci. Med. Sci. 2010;65:1275–1284. doi: 10.1093/gerona/glq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grandison RC, Piper MD, Partridge L. Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature. 2009;462:1061–1064. doi: 10.1038/nature08619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orentreich N, Matias JR, DeFelice A, Zimmerman JA. Low methionine ingestion by rats extends life span. J. Nutr. 1993;123:269–274. doi: 10.1093/jn/123.2.269. [DOI] [PubMed] [Google Scholar]
- 39.Sun L, Sadighi Akha AA, Miller RA, Harper JM. Life-span extension in mice by preweaning food restriction and by methionine restriction in middle age. Journals Gerontol. - Ser. A Biol. Sci. Med. Sci. 2009;64:711–722. doi: 10.1093/gerona/glp051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cabreiro F, Au C, Leung KY, et al. Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell. 2013;153:228–239. doi: 10.1016/j.cell.2013.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koziel R, Ruckenstuhl C, Albertini E, et al. Methionine restriction slows down senescence in human diploid fibroblasts. Aging Cell. 2014;13:1038–1048. doi: 10.1111/acel.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Troen AM, Emily EF, Jessica F, et al. Lifespan modification by glucose and methionine in Drosophila melanogaster fed a chemically defined diet. Age. 2007;29:29–39. doi: 10.1007/s11357-006-9018-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee BC, Kaya A, Ma S, et al. Methionine restriction extends lifespan of Drosophila melanogaster under conditions of low amino-acid status. Nat. Commun. 2014;5:3592. doi: 10.1038/ncomms4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wanders D, Ghosh S, Stone KP, Van NT, Gettys TW. Transcriptional impact of dietery methionine restriction on systemic inflammation: relevance to biomarkers of metabolic disease during aging. Biofactors. 2014;40:13–26. doi: 10.1002/biof.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sinha R, Cooper TK, Rogers CJ, et al. Dietary methionine restriction inhibits prostatic intraepithelial neoplasia in TRAMP mice. Prostate. 2014;74:1663–1673. doi: 10.1002/pros.22884. [DOI] [PubMed] [Google Scholar]
- 46.Malloy VL, Perrone CE, Dwight AL, et al. Methionine restriction prevents the progression of hepatic steatosis in leptin-deficient obese mice. Metabolism. 2013;62:1651–1661. doi: 10.1016/j.metabol.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 47.Ables GP, Ouattara A, Hampton TG, et al. Dietary Methionine Restriction in Mice Elicits an Adaptive Cardiovascular Response to Hyperhomocysteinemia. Sci. Rep. 2015;5:8886. doi: 10.1038/srep08886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanchez-Roman I, Gomez A, Gomez J, et al. Forty percent methionine restriction lowers DNAmethylation, complex i ROS generation, and oxidative damage to mtDNA and mitochondrial proteins in rat heart. J. Bioenerg. Biomembr. 2011;43:699–708. doi: 10.1007/s10863-011-9389-9. [DOI] [PubMed] [Google Scholar]
- 49.Sanz A, Caro P, Ayala V, et al. Methionine restriction decreases mitochondrial oxygen radical generation and leak as well as oxidative damage to mitochondrial DNA and proteins. FASEB J. 2006;20:1064–1073. doi: 10.1096/fj.05-5568com. [DOI] [PubMed] [Google Scholar]
- 50.Caro P, Gomez J, Lopez-Torres M, et al. Forty percent and eighty percent methionine restriction decrease mitochondrial ROS generation and oxidative stress in rat liver. Biogerontology. 2008;9:183–196. doi: 10.1007/s10522-008-9130-1. [DOI] [PubMed] [Google Scholar]
- 51.Caro P, Gomez J, Sanchez I, et al. Forty percent methionine restriction decreases mitochondrial oxygen radical production and leak at complex I during forward electron flow and lowers oxidative damage to proteins and mitochondrial DNA in rat kidney and brain mitochondria. Rejuvenation Res. 2009;12:421–434. doi: 10.1089/rej.2009.0902. [DOI] [PubMed] [Google Scholar]
- 52.Maddineni S, Nichenametla S, Sinha R, Wilson RP, Richie JP. Methionine restriction affects oxidative stress and glutathione-related redox pathways in the rat. Exp. Biol. Med. 2013;238:392–399. doi: 10.1177/1535370213477988. [DOI] [PubMed] [Google Scholar]
- 53.Sanchez-Roman I, Barja G. Regulation of longevity and oxidative stress by nutritional interventions: Role of methionine restriction. Exp. Gerontol. 2013;48:1030–1042. doi: 10.1016/j.exger.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 54.Kaeberlein M, Kapahi P Cell signaling. Aging is RSKy business. Science. 2009;326:55–56. doi: 10.1126/science.1181034. [DOI] [PubMed] [Google Scholar]
- 55.Kapahi P, Chen D, Rogers AN, et al. With TOR, less is more: A key role for the conserved nutrient-sensing TOR pathway in aging. Cell Metab. 2010;11:453–465. doi: 10.1016/j.cmet.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Selman C, Tullet JM, Wieser D, et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science. 2009;326:140–144. doi: 10.1126/science.1177221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiang W, Zhu Z, Thompson HJ. Dietary energy restriction modulates the activity of AMP-activated protein kinase, Akt, and mammalian target of rapamycin in mammary carcinomas, mammary gland, and liver. Cancer Res. 2008;68:5492–5499. doi: 10.1158/0008-5472.CAN-07-6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fabrizio P, Pozza F, Pletcher SD, Gendron CM, Longo VD. Regulation of longevity and stress resistance by Sch9 in yeast. Science. 2001;292:288–290. doi: 10.1126/science.1059497. [DOI] [PubMed] [Google Scholar]
- 59.Urban J, Soulard A, Huber A, et al. Sch9 Is a Major Target of TORC1 in Saccharomyces cerevisiae. Mol. Cell. 2007;26:663–674. doi: 10.1016/j.molcel.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 60.Powers RW, Kaeberlein M, Caldwell SD, Kennedy BK, Fields S. Extension of chronological life span in yeast by decreased TOR pathway signaling. Genes Dev. 2006;20:174–184. doi: 10.1101/gad.1381406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kaeberlein M, Powers RW, Steffen KK, et al. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science. 2005;310:1193–1196. doi: 10.1126/science.1115535. [DOI] [PubMed] [Google Scholar]
- 62.Wei M, Fabrizio P, Hu J, et al. Life span extension by calorie restriction depends on Rim15 and transcription factors downstream of Ras/PKA, Tor, and Sch9. PLoS Genet. 2008;4:0139–0149. doi: 10.1371/journal.pgen.0040013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Petti AA, Crutchfield CA, Rabinowitz JD, Botstein D. Survival of starving yeast is correlated with oxidative stress response and nonrespiratory mitochondrial function. Proc. Natl. Acad. Sci. U. S.A. 2011;108:1089–1098. doi: 10.1073/pnas.1101494108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hine C, Harputlugil E, Zhang Y, et al. Endogenous hydrogen sulfide production is essential for dietary restriction benefits. Cell. 2014;15:132–144. doi: 10.1016/j.cell.2014.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miller RA, Buehner G, Chang Y, et al. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell. 2005;4:119–125. doi: 10.1111/j.1474-9726.2005.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shimokawa I, Higami Y, Yu BP, Masoro EJ, Ikeda T. Influence of dietary components on occurrence of and mortality due to neoplasms in male F344 rats. Aging Clin. Exp. Res. 1996;8:254–262. doi: 10.1007/BF03339576. [DOI] [PubMed] [Google Scholar]
- 67.Khorakova M, Deil Z, Khausman D, Matsek K. Effect of carbohydrate-enriched diet and subsequent food restriction on life prolongation in Fischer 344 male rats. Fiziol. Zh. 1990;36:16–21. [PubMed] [Google Scholar]
- 68.Li W, Li X, Miller RA. ATF4 activity: a common feature shared by many kinds of slow-aging mice. Aging Cell. 2014;13:1012–1018. doi: 10.1111/acel.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brown-Borg HM, Rakoczy SG, Wonderlich JA, et al. Growth hormone signaling is necessary for lifespan extension by dietary methionine. Aging Cell. 2014;13:1019–1027. doi: 10.1111/acel.12269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cavuoto P, Fenech MF. A review of methionine dependency and the role of methionine restriction in cancer growth control and life-span extension. Cancer Treat. Rev. 2012;38:726–736. doi: 10.1016/j.ctrv.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 71.Mirzaei H, Suarez JA, Longo VD. Protein and amino acid restriction, aging and disease: from yeast to humans. Trends Endocrinol. Metab. 2014;25:558–566. doi: 10.1016/j.tem.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]