Abstract
Background
Impaired inhibitory control is considered a behavioural phenotype in patients with bulimia nervosa. However, the underlying neural correlates of impaired general and food-specific behavioural inhibition are largely unknown. Therefore, we investigated brain activation during the performance of behavioural inhibition to general and food-related stimuli in adults with bulimia nervosa.
Methods
Women with bulimia and healthy control women underwent event-related fMRI while performing a general and a food-specific no-go task.
Results
We included 28 women with bulimia nervosa and 29 healthy control women in our study. On a neuronal level, we observed significant group differences in response to general no-go stimuli in women with bulimia nervosa with high symptom severity; compared with healthy controls, the patients showed reduced activation in the right sensorimotor area (postcentral gyrus, precentral gyrus) and right dorsal striatum (caudate nucleus, putamen).
Limitations
The present results are limited to adult women with bulimia nervosa. Furthermore, it remains unclear whether impaired behavioural inhibition in patients with this disorder are a cause or consequence of chronic illness.
Conclusion
Our findings suggest that diminished frontostriatal brain activation in patients with bulimia nervosa contribute to the severity of binge eating symptoms. Gaining further insight into the neural mechanisms of behavioural inhibition problems in individuals with this disorder may inform brain-directed treatment approaches and the development of response inhibition training approaches to improve inhibitory control in patients with bulimia nervosa. The present study does not support greater behavioural and neural impairments to food-specific behavioural inhibition in these patients.
Introduction
Bulimia nervosa is a common mental disorder with heightened morbidity and all-cause mortality.1 It is characterized by recurrent episodes of binge eating accompanied by a feeling of lack of control over eating. The binge eating episodes are followed by inappropriate compensatory behaviours, such as self-induced vomiting or laxative misuse to prevent weight gain.2 The frequency of binging/purging behaviour in patients with bulimia nervosa is considered an important predictor of therapeutic outcome. Less frequent binging/purging before the beginning of treatment is associated with better therapeutic outcome and lower dropout rate in treatment trials.3–5 Given the limited treatment response of patients with a high frequency of binging/purging, a better understanding of the neurobiological mechanisms of the behaviour as well as the neural markers contributing to the severity of these core symptoms are of great interest.
Numerous neuropsychological studies have investigated the role of inhibitory control in bulimic-type eating disorders.6–9 Inhibitory control refers to the ability to suppress inappropriate and unwanted actions.10 Impaired response inhibition in individuals with bulimic-type eating disorders is not limited to binge eating, but frequently includes several impulsive behaviours (e.g., excessive drinking, substance abuse),11 suggesting a more general dysregulation.12 A meta-analysis by our group13 examined data from neuropsychological studies on response inhibition to general and disease-salient stimuli in individuals with bulimic-type eating disorders. In those with bulimia nervosa, we found impairments in response inhibition to general stimuli with a small effect size and to disease-related stimuli with a medium effect size, indicating a larger impairment in response inhibition in patients when confronted with eating disorder–salient stimuli. A basic difference of inhibitory controls is in mechanisms that relate to overt behaviour, such as the control of activated motor responses (i.e., behavioural inhibition), and those that refer to mental processes, such as the control of cognitive contents and attentional processes (i.e., cognitive inhibition).14 In neuropsychological studies of bulimia nervosa, inhibitory control has been most frequently investigated with cognitive inhibition tasks (e.g., Stroop task, Simon task). These tasks measure the expression of inhibitory control involving the cognitive and attentional suppression of task-irrelevant information (i.e., interference control).15 The stop signal task (SST) and the no-go task (as part of the go/no-go task) are able to more specifically measure behavioural inhibition. While the SST measures the inhibition of an already started action (action cancellation), the no-go task measures the inhibition of a planned response (action restraint).16 In the present study, we applied a classic no-go paradigm to assess differences in the level of impairment to suppress motor responses to both general and food-related stimuli.
The functional neuroanatomy of response inhibition has been studied extensively in nonclinical samples. Functional MRI studies consistently reveal frontal lobe activation during these tasks, especially in the right lateral prefrontal cortex (PFC), dorsomedial frontal cortex (presupplementary motor area [pre-SMA]), right middle/inferior frontal and right inferior parietal regions and subcortical brain regions.17–22 The PFC and pre-SMA may work together in sending the stop signal to the basal ganglia, which inhibits the behavioural output of the primary motor cortex.23 Medial frontal areas, particularly the pre-SMA, have been implicated not only in response inhibition, but also in response selection.20,22 Frontoparietal activation, which is typically elicited by no-go signals, indicates additional attentional or working memory resources in addition to inhibitory processes.18 However, the localization of brain activation within the frontal cortex during response inhibition tasks varies across studies, and this variation appears to be task dependent.24
Preliminary evidence from food-specific no-go tasks suggests an association between body mass index (BMI) and food-related behavioural inhibition.25 The authors demonstrated that BMI was negatively correlated with brain activation in the PFC, with more overweight adolescents showing lower dorsolateral PFC, ventrolateral PFC, medial PFC and lateral orbitofrontal cortex (OFC) activation. Impairments in behavioural inhibition were associated with increased activation in food reward regions (e.g., insula, temporal operculum), reflecting a failure to deactivate food reward regions if necessary.25 These preliminary findings suggest that a food-specific response inhibition paradigm is effective in assessing response inhibition in patients with overeating, including bulimic-type eating disorders.2 However, the specificity of this finding remains unclear, as no nonfood control condition was included.
The underlying neural correlates of impaired inhibitory control in patients with bulimia nervosa are not well understood. So far, 1 fMRI study26 investigated neural mechanisms of behavioural inhibition in a sample of adolescents with binge eating/purging behaviour and showed increased activation relative to healthy controls in the right dorsolateral PFC, bilateral hypothalamus, right anterior cingulate cortex (ACC), right middle temporal cortex (TC) and bilateral precentral gyri in an event-related no-go task with nonfood stimuli. However, a limitation of this study was that the authors grouped anorexia nervosa from the binge eating/purging subtype together with bulimia nervosa in a single category of binge eating/purging behaviour, limiting the specificity of the results to either the binge eating/purging subtype of anorexia nervosa or bulimia nervosa. Furthermore, neural correlates of cognitive inhibition (i.e., interference control) have been assessed in adolescents27 and adults28 with bulimia nervosa. During correct responding on incongruent nonfood trials, adolescent and adult patients showed diminished activation in frontostriatal circuits, including the right lateral PFC and striatum. Diminished activation in frontostriatal circuits was closely associated with symptom severity. Adolescent patients with bulimia nervosa with the most objective bulimic and vomiting episodes deactivated the inferior frontal gyrus and precuneus.27 Adults with the disorder with the most objective episodes of binge eating/purging engaged cortical areas (medial PFC, TC, inferior parietal cortex) and the head of the caudate nucleus the least.28 These results suggest that the association of cortical–subcortical activation with symptom severity is dose-dependent. In sum, preliminary findings of impaired behavioural and cognitive inhibition in patients with bulimia nervosa suggest frontostriatal dysfunction, although the findings regarding increased or deficient activation are controversial.
Given the existing literature, there is a dearth of studies addressing behavioural inhibition in adults with bulimia nervosa and including food-specific inhibitory control. Therefore, the aim of the present study was to investigate whether adults with bulimia nervosa show dysfunctional activation in the brain inhibitory control network and to examine whether these differences are specific to food-related stimuli or whether they relate to a generalized impairment in behavioural inhibition.
Consistent with previous studies, we expected to find impaired behavioural inhibition in patients with bulimia nervosa associated with decreased activation within frontostriatal networks to general no-go and food-specific no-go stimuli and increased activation in limbic reward regions (amygdala, striatum, OFC) in the food-specific no-go paradigm, reflecting a failure of top–down control to appetizing food stimuli. Given the relevance of the symptom severity (i.e., frequency of binge eating) for therapeutic outcome, we additionally expected that neural markers of impaired response inhibition would be most pronounced in a subgroup of patients with a high frequency of binge eating.3–5,29
Methods
Participants
We recruited women who met the diagnostic criteria for bulimia nervosa according to DSM-5 from our outpatient department at the Medical University Hospital in Heidelberg, Germany, and through advertisements in the local media. Additionally, we recruited age-, education- and BMI-matched healthy controls through announcements at the hospital and other public institutions as well as through advertisements in the local media. All participants underwent a Structured Clinical Interview for DSM-5 Axis I disorders (SCID).2 Objective binge eating episodes were thereby defined according to the DSM-5 criteria. Participants were excluded from the healthy control group if they had a lifetime history of an eating disorder. Further general exclusion criteria were claustrophobia; metallic implants; bipolar disorder; psychosis; a history of head injury; hearing impairment; neurologic disorders; diabetes mellitus; nicotine, drug or alcohol abuse; and current psychotropic medication in controls or medication other than antidepressants in patients with bulimia nervosa. Participants reporting a lifetime diagnosis of a borderline personality disorder were also excluded, as this disorder is known to be associated with impairments in inhibitory control and decision making.30 We obtained oral and written informed consent from all participants before including them in the study. The study was conducted in accordance with the Declaration of Helsinki and was approved by the local ethics committee of the Medical School of the University of Heidelberg.
Procedure
The participants were tested individually in a single session between 9 am and 2 pm. They were asked to come to the clinic without having breakfast and to refrain from consuming alcoholic drinks for 24 hours before the experiment. After arriving in the clinic, all participants received a light, standardized breakfast containing approximately 550 kcal. The breakfast was followed by the SCID, after which participants were asked to complete self-report questionnaires and a battery of neuropsychological tasks. We assessed depressive symptoms using the Beck Depression Inventory-II (BDI-II).31 Eating behaviour was assessed using the Dutch Eating Behaviour Questionnaire (DEBQ),32 consisting of the restrained eating, emotional eating and external eating subscales, and different aspects of impulsiveness as a personality trait were assessed using the Barratt Impulsiveness Scale (BIS-11), consisting of the attentional, motor and nonplanning impulsiveness subscales.33 At 12 pm, the MRI scanning was performed for all participants, as this time corresponded to the lunchtime of most of the participants. We explained the experimental procedure to the participants and trained them in the no-go paradigm before the fMRI measurement (described in the section that follows). Additionally, all participants performed a reward paradigm (monetary and food incentive delay tasks) during the same scanning session. The findings of the latter are reported elsewhere.34 Furthermore, hunger and mood ratings were obtained from participants before the fMRI measurement.
Experimental paradigm
The no-go component of the go/no-go paradigm is an established task to examine brain activation during response inhibition. The general and disorder-specific no-go paradigms were conducted in accordance with previous studies by our group and cooperating groups.35–37 The event-related design was presented in 2 runs, each containing 8 blocks. The block sequence of the different modalities in both runs was either GFFGGFFG or FGGFFGGF (G = general block, F = food block) and was counterbalanced across the participants. One no-go block lasted 75 000 ms, 1 imaging run lasted 10.1 min or 303 scans. Participants were instructed to respond as quickly and accurately as possible to the frequent target stimulus (go stimulus: square in the general no-go task, household item in the disorder-specific no-go task) but to inhibit any reaction to the rare nontarget stimulus (no-go stimulus: circle in the general no-go task, food picture in the disorder-specific no-go task). Before entering the scanner, all participants selected 8 pictures of their most favourite foods from a set of 85 custom-made pictures showing high-caloric sweet and savoury foods.38,39 The 8 pictures of household items were the same for all participants. After leaving the scanner, participants were shown the 8 individually selected food pictures used in the paradigm again on a computer screen and were asked to rate their arousal, valence, incentive salience (i.e., wanting), hedonic value (i.e., liking) and urge to binge eat during the initial viewing. The participants were also asked to rate their arousal, valence, incentive salience and hedonic value of the 8 household items. Further details related to the task are given in Appendix 1, Fig. S1, available at jpn.ca.
Behavioural data analysis
For each participant, we calculated the percentage of right reactions to both go stimuli and no-go stimuli for the general no-go task (correct(%)_go_square, correct(%)_no-go_circle) as well as the food-specific no-go task (correct(%)_go_nonfood, correct(%)_no-go_food). Additionally, we calculated the mean reaction times (RT) to go stimuli and no-go stimuli (that were incorrectly responded to) for the general no-go task (mean_RT(ms)_go_square, mean_RT(ms)_no-go_circle) as well as the food-specific no-go task (mean_RT(ms)_go_nonfood, mean_RT(ms)_no-go_food). We used Bonferroni corrections for the accuracy as well as for the RT, resulting in a statistical significance level of p = 0.013 for the accuracy as well as p = 0.013 for the RT in the no-go task. A correlation analysis between number of binge eating episodes per week in the bulimia nervosa group and the behavioural data of the no-go task were performed using a Spearman correlation analysis. We compared the valence and arousal ratings for the food pictures and household items between patients and healthy controls using repeated-measures analysis of variance (ANOVA) with rating as the within-subjects factor and group as the between-subjects factor.
Image acquisition and analysis
Imaging was performed using a 3 T Siemens Trio MRI scanner (Siemens Medical Solutions). We used a standard 32-channel head coil to acquire data from the entire brain. The parameters used for image collection are described in Appendix 1.
Data were preprocessed and analyzed using SPM8 software (Wellcome Department of Neurology) based on MATLAB version 7.13 (Mathworks, Inc.). Preprocessing of functional scans included slice time correction; realignment; coregistration; normalization of functional and anatomic images to a standard Montreal Neurological Institute (MNI) template brain (ICBM152), resulting in a voxel size of 3 × 3 × 4 mm3 for functional images and a voxel size of 1 × 1 × 1 mm3 for high-resolution structural images; and smoothing. We used a 256-s high-pass filter to remove low-frequency noise and signal drift. First-level statistical analyses were performed within a general linear model (GLM) approach.40 Details on the preprocessing and first-level analysis are described in Appendix 1.
On the second level of analysis, the individual contrast estimates from the first-level analysis for all participants were entered into a random-effects model, allowing population inference.41 Within-group activation was analyzed using a 1-sample t test (no-go_circle–go_square, or no-go_food–go_nonfood, respectively) and between-groups activation using a 2-sample t test (no-go_circle–go_square of first group minus no-go_circle–go_square of second group, or no-go_circle–go_square of second group minus no-go_circle–go_square of first group, respectively, as well as no-go_food–go_nonfood of first group minus no-go_food–go_nonfood of second group, or no-go_food–go_nonfood of second group minus no-go_food–go_nonfood of first group, respectively). Concerning our third hypothesis, we separated the bulimia nervosa group into subgroups of low and high binge eating frequency using a median split. We used a median split to address the observed high variance of objective binge eating episodes per week within the bulimia nervosa group in the best way. Furthermore, a median split was also used to obtain 2 bulimia nervosa groups of the same but still sufficient sample size. We report results of the 1-sample t test that were significant at p < 0.05, cluster-level uncorrected with a cluster-defining threshold of p < 0.05, family-wise error (FWE)–corrected, cluster size k > 10. The FWE rate was used to correct for multiple comparison and implemented in SPM. We report results of the 2-sample t test that were significant at p < 0.05, cluster-level FWE-corrected with a cluster-defining threshold of p < 0.001, uncorrected, cluster size k > 10. To analyze activation in smaller volumes (i.e., the right dorsal striatum), we performed a small volume correction42 using the cluster of activation in the right dorsal striatum significant at p < 0.05, cluster-level uncorrected with a cluster-defining threshold of p < 0.001 uncorrected, minimal voxel size of 10. The location of the peak activity associated with each cluster of activation is reported in MNI coordinates.
Additionally, brain activation was extracted from regions found in the whole brain analysis. For each cluster, the mean percent signal change was extracted using MarsBar.43 Correlation analysis between the number of binge eating episodes per week and the mean percent signal changes extracted from the significant clusters were performed using a Pearson correlation analysis. We used SPSS version 20 (IBM Corp.) to analyze the data.
Results
We recruited 30 women with bulimia nervosa and 31 healthy controls for participation in our study. Data from 4 people had to be excluded due to technical problems, resulting in a final sample of 28 women with bulimia nervosa (mean age 27.54 ± 10.52 yr) and 29 controls (mean age 27.25 ± 6.68 yr). All participants were right-handed, older than 18 years and had normal or corrected-to-normal vision. The mean BMI was 21.24 ± 2.99 in the bulimia group and 21.83 ± 1.82 in the control group. The mean years of education was 12.68 ± 1.42 in the bulimia group and 12.72 ± 0.84 in the control group.
The clinical characteristics of the participants are summarized in Table 1. The mean number of objective binge eating episodes per week was 3.71 ± 2.53, with a range of 1–7.5 objective binge eating episodes per week within the 3 months preceding the study. One participant with bulimia nervosa reported no binges during the 28 days preceding the study (beginning of hospital treatment), but 1–2 binges per week before the beginning of hospital treatment. Women with bulimia nervosa scored significantly higher on the BDI-II and on the restraint, emotional and external eating scales of the DEBQ (Table 1). For the BIS-11, patients with bulimia nervosa had significantly higher attentional impulsiveness scores and a lower motor impulsiveness scores than healthy controls. No group differences were found for the total score and the nonplanning impulsiveness score on the BIS-11 (Table 1). To address our third hypothesis, we divided the bulimia nervosa group into 2 subgroups based on the median number of binge eating episodes per week over the 3 months preceding the study (median 2.75 episodes); patients with fewer than 3 binge eating episodes per week were assigned to the low-frequency group (n = 14) and those with 3 or more binge eating episodes per week were assigned to the high-frequency group (n = 14). The low- and high-frequency binge eating groups did not differ in age (26.43 ± 10.35 yr v. 28.66 ± 10.95 yr, t = −0.552, p = 0.59), BMI (21.71 ± 3.24 v. 20.76 ± 2.76, t = 0.834, p = 0.41) or years of education (12.36 ± 1.55 yr v. 13.00 ± 1.24 yr, t = −1.212, p = 0.24). These 2 bulimia nervosa subgroups also did not differ in depression scores on the BDI-II, restraint eating (DEBQ) total score, BIS-11 total and subscale scores, duration of the disorder, antidepressant use or current psychotherapy (Appendix 1, Table S1). Women in the high-frequency binge eating group scored significantly higher in emotional and external eating than women in the low-frequency binge eating group (Appendix 1, Table S1).
Table 1.
Clinical characteristics of study participants
| Group; mean ± SD or % | ||||
|---|---|---|---|---|
|
|
||||
| Characteristic | Control, n = 29 | Bulimia, n = 28 | t55 | p value |
| BDI-II total | 3.59 ± 3.13 | 25.63 ± 12.43 | 9.248 | < 0.001 |
| DEBQ | ||||
| Restrained eating | 11.79 ± 8.13 | 28.89 ± 7.76 | 8.119 | < 0.001 |
| Emotional eating | 7.28 ± 4.74 | 28.45 ± 8.05 | 12.148 | < 0.001 |
| External eating | 18.38 ± 6.68 | 25.14 ± 7.31 | 3.649 | < 0.001 |
| BIS-11 | ||||
| Total score | 58.48 ± 6.44 | 61.81 ± 8.96 | 1.606 | 0.11 |
| Attentional impulsiveness | 14.52 ± 2.08 | 17.89 ± 3.71 | 4.229 | < 0.001 |
| Motor impulsiveness | 21.28 ± 3.02 | 19.00 ± 3.68 | −2.537 | 0.014 |
| Nonplanning impulsiveness | 22.69 ± 3.53 | 24.93 ± 5.12 | 1.915 | 0.06 |
| Objective binge eating episodes/wk | — | 3.71 ± 2.53 | — | — |
| Purging-type (self-induced vomiting, laxative misuse, diuretics) | — | 85.71 | — | — |
| Disorder duration, yr | — | 10.19 ± 11.35 | — | — |
| Antidepressants | — | 25.00 | — | — |
| Current psychotherapy | — | 53.57 | — | — |
| Subclinical bulimia* | — | 3.57 | — | — |
BDI = Beck Depression Inventory-II; BIS-11 = Barratt Impulsiveness Scale; DEBQ = Dutch Eating Behaviour Questionnaire; SD = standard deviation.
Patient with subclinical bulimia nervosa with no binge eating episodes during the 28 d before participation.
Behavioural data
Table 2 summarizes the behavioural data of the no-go task. A 2-sample t test revealed a trendwise group difference in accuracy in the general no-go task (p = 0.040) that failed to reach statistical significance after correcting for multiple testing. Healthy controls performed better than women with bulimia nervosa in inhibiting the motor response to general no-go stimuli. For the food-specific no-go task, no group differences in accuracy were observed. Regarding the RT, no group differences were observed for the general or the food-specific no-go task.
Table 2.
Group differences regarding behavioural performance in the no-go task
| Group; mean ± SD | ||||
|---|---|---|---|---|
|
|
||||
| Measure | Control, n = 29 | Bulimia, n = 28 | t55 | p value |
| Correct% | ||||
| Go_square | 98.73 ± 3.25 | 99.04 ± 1.94 | 0.426 | 0.67 |
| No-go_circle | 83.46 ± 10.41 | 77.12 ± 12.32 | −2.101 | 0.040 |
| Go_nonfood | 98.67 ± 3.68 | 98.47 ± 3.41 | −0.214 | 0.83 |
| No-go_food | 81.25 ± 9.21 | 78.91 ± 8.58 | −0.993 | 0.33 |
| RT, ms | ||||
| Go_square | 357.88 ± 38.06 | 358.86 ± 41.62 | 0.093 | 0.93 |
| No-go_circle | 322.28 ± 42.70 | 326.02 ± 49.58 | 0.306 | 0.76 |
| Go_nonfood | 415.28 ± 38.83 | 412.80 ± 40.67 | −0.236 | 0.81 |
| No-go_food | 377.32 ± 48.72 | 389.76 ± 57.98 | 0.878 | 0.38 |
RT = reaction time; SD = standard deviation.
Appendix 1, Table S2, summarizes the behavioural data of the no-go task of women in the low- and high-frequency binge eating groups. We observed no group differences in accuracy or RT between these subgroups in the general or in the food-specific no-go task.
The Spearman correlation analysis between the number of binge eating episodes per week in the bulimia nervosa group and the behavioural data of the no-go task resulted in no significant correlations between number of binge eating episodes per week and accuracy (all p > 0.59) and RT (all p > 0.76) for the general and the food-specific no-go tasks.
Valence and arousal ratings for food pictures relative to pictures of household items differed between women with bulimia nervosa and healthy controls. For valence, we observed no main effect of condition (F1,55 = 0.224, p = 0.64), but there was a significant group (bulimia v. control) × condition (food pictures v. household items) interaction in valence (F1,55 = 17.701, p < 0.001), in that patients with bulimia nervosa rated the food pictures more positively. For arousal, we observed a significant main effect of condition (F1,54 = 43.463, p < 0.001), indicating less arousal to food pictures as well as a significant group (bulimia v. control) × condition (food pictures v. household items) interaction (F1,55 = 16.656, p < 0.001), in that patients with bulimia nervosa showed less arousal in response to food pictures.
Imaging data
Whole brain activation within the control group
Whole brain activation within the control group during the no-go_circle–go_square and no-go_food–go_nonfood contrasts are shown in Appendix 1, Table S3 and Table S4.
During general behavioural inhibition, we observed activation in the bilateral frontoparietal network, bilateral precentral cortex, right postcentral cortex, right supplementary motor area (SMA), bilateral insula extending into the putamen, bilateral occipital cortex, right inferior temporal cortex and right thalamus.
During food-specific inhibition, we observed activation in the inferior parietal cortex, right pre-SMA/SMA, bilateral insula extending into the putamen, right hippocampus, bilateral occipital cortex, left inferior temporal cortex, bilateral midcingulate cortex and bilateral thalamus.
Whole brain activation within the bulimia nervosa group
Whole brain activation within the bulimia nervosa group during the no-go_circle–go_square and no-go_food–go_nonfood contrasts are shown in Appendix 1, Table S5 and Table S6.
During general behavioural inhibition, we observed activation in the bilateral frontoparietal network, left precentral cortex, left SMA, right insula extending into the putamen, left putamen, bilateral occipital cortex and right temporal cortex.
During food-specific inhibition, we observed activation in the bilateral frontoparietal network, right precentral cortex, bilateral SMA, bilateral insula, right putamen and bilateral midcingulate cortex.
Whole brain comparison between the control group and the bulimia nervosa group
During the no-go_circle–go_square and no-go_food–go_nonfood contrasts, the whole brain analysis between healthy controls and patients with bulimia nervosa revealed no significant differences in brain activation.
Bulimia nervosa subgroups
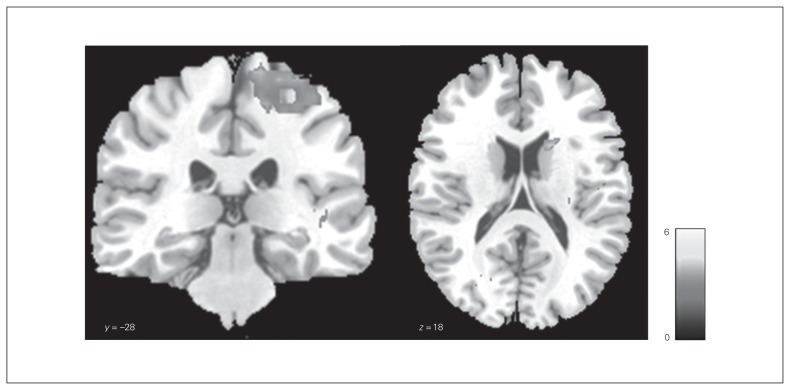
We observed significant differences in brain activation using a 2-sample t test during the no-go_circle–go_square contrast between healthy controls and women with bulimia nervosa in the high-frequency binge eating subgroup (Table 3, Fig. 1). The control group showed stronger activation in the right postcentral gyrus, right precentral gyrus, right caudate nucleus and right putamen than the high-frequency binge eating patient subgroup. The right dorsal striatum (caudate nucleus, putamen) was significant after small volume correction (pFWE < 0.05). Owing to the unequal sample size of the healthy control group and the high-frequency binge eating bulimia nervosa subgroup, we additionally performed a nonparametrical approach using the statistical nonparametric mapping (SnPM13) software44 based on SPM8. Nonparametric testing was conducted with 5000 random permutations. This approach confirmed our findings of stronger activation in the right postcentral gyrus, right precentral gyrus, right caudate nucleus and right putamen in the control group relative to the high-frequency binge eating bulimia nervosa subgroup (p < 0.05, cluster-level uncorrected; cluster-defining threshold p < 0.001, uncorrected; cluster size k > 10). Controls did not differ from the low-frequency binge eating subgroup, and women in the low- and high-frequency binge eating subgroups did not differ from each other in brain activation during the no-go_circle–go_square contrast.
Table 3.
Whole brain results of the comparison between healthy controls (n = 29) and patients with bulimia nervosa with severe symptoms (n = 14) for the general no-go task
| MNI coordinates | |||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Region | Laterality | t value | x | y | z | BA | Cluster size |
| Controls > bulimia nervosa | |||||||
| Postcentral gyrus (parietal lobe)* | R | 5.05 | 33 | −25 | 38 | 3 | 141 |
| Precentral gyrus (frontal lobe)* | R | 4.04 | 15 | −28 | 70 | 4 | |
| Postcentral gyrus (parietal lobe)* | R | 3.99 | 33 | −28 | 66 | 4/6 | |
| Caudate nucleus† | R | 4.55 | 18 | 11 | 18 | 36 | |
| Putamen† | R | 4.01 | 27 | 17 | 10 | ||
BA = Brodmann area; FWE = family-wise error; MNI = Montreal Neurological Institute; R = right.
Results significant at p < 0.05 cluster-level, FWE-corrected; cluster-defining threshold p < 0.001, uncorrected. Cluster size k > 10 voxels.
Results significant at p < 0.05 cluster-level uncorrected, cluster-defining threshold p < 0.001 uncorrected. Cluster size k > 10 voxels.
Fig. 1.
Group comparison of brain activation during the general no-go task. Differences in group activation between healthy controls and patients with bulimia nervosa with a high frequency of binge eating (controls > high-BN) during the contrast no-go_circle–go_square. The significance threshold was set at p < 0.05, cluster-level uncorrected. The SPM t-map was rendered on a T1-weighted template image supplied with mricron (Colin brain). The colour scale represents the t-scores.
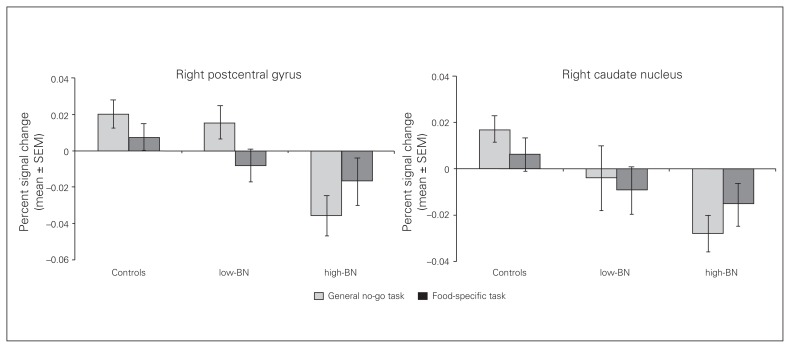
For the no-go_food–go_nonfood contrast, we did not observe any significant differences in brain activation during the whole brain analysis between healthy controls and the 2 bulimia nervosa subgroups or between the low- and high-frequency binge eating bulimia nervosa subgroups. Levels of brain activation in healthy controls and in the low- and high-frequency binge eating bulimia nervosa subgroups extracted from the right sensorimotor area (postcentral gyrus, precentral gyrus) and right dorsal striatum (caudate nucleus, putamen) for the general and food-specific no-go tasks are shown in Figure 2.
Fig. 2.
Brain activation extracted from the significant clusters of the whole brain analysis (regions of interest) for the general and food-specific no-go task in healthy controls and bulimia nervosa subgroups. Bar charts depicting percent signal change from the significant clusters (right postcentral gyrus, right caudate nucleus) for the controls and patients during the no-go_circle–go_square (light colour) and the no-go_food–go_nonfood (dark colour) contrasts. Error bars depict standard errors of the mean (SEM). High-BN = patients with bulimia nervosa with a high frequency of binge eating; low-BN = patients with bulimia nervosa with a low frequency of binge eating.
Within the bulimia nervosa group we found a negative correlation for the general no-go task between percent signal change extracted from the right sensorimotor area (postcentral gyrus, precentral gyrus) during the no-go_circle–go_square contrast and the number of binge eating episodes per week (r = −0.454, p = 0.015). Controlling for hunger and mood of patients with bulimia nervosa before the fMRI scanning showed that these factors did not influence the negative correlation between percent signal change extracted from the right sensorimotor area (postcentral gyrus, precentral gyrus) during the no-go_circle–go_square contrast and the number of binge eating episodes per week (hunger: r = −0.458, p = 0.016; mood: r = −0.449, p = 0.019).
Discussion
The purpose of this study was to identify the neural mechanisms underlying behavioural inhibition in patients with bulimia nervosa. To the best of our knowledge, this is the first neuroimaging study investigating behavioural inhibition in adults with this disorder using both disorder-specific and disorder-unspecific no-go paradigms.
Contrary to our first hypothesis, we observed no significant brain differences during behavioural inhibition to general and food-specific stimuli in the bulimia nervosa group compared with the control group. Our second hypothesis was also not supported, as there were no greater engagements of the limbic system during food-specific behavioural inhibition in patients with bulimia nervosa than in healhty controls. In accordance with our third hypothesis, we observed diminished frontostriatal activity in patients with bulimia nervosa with a high symptom severity compared with healthy controls. More specifically, we observed diminished brain activation in sensorimotor brain regions and the dorsal striatum in those with high symptom severity. This finding was specific to the general no-go paradigm as we observed no group differences in the food-specific no-go paradigm, suggesting a more generalized impairment of behavioural inhibition rather than a disorder-specific impairment. As there were no significant differences between the high– and low–symptom severity groups in duration of the disorder, depression, medication use or psychotherapy, it is rather unlikely that the observed differences were influenced to a greater extent by these factors.
In accordance with neuroimaging data, the behavioural findings showed a trendwise impaired behavioural inhibition in response to general but not to food-specific no-go stimuli in patients with bulimia nervosa. This result is in contrast to previous neuropsychological findings, suggesting pronounced impairments of inhibitory control specifically in response to food stimuli.13 However, these findings are largely based on food-specific cognitive inhibition, suggesting that in patients with bulimia nervosa, the need to suppress distracting food stimuli is a greater challenge than the control of prepotent motor responses to food stimuli. On the other hand, longer RTs in the food-specific than in the general no-go paradigm also indicate that inherent paradigm factors, such as visual complexity of the stimuli, may have contributed to the unexpected findings in the present study. However, our food pictures were effective in eliciting greater positive valence associated with lower arousal in patients with bulimia nervosa. Notably, the same task design with alcohol-related stimuli was successfully used to differentiate between alcohol-dependent patients and controls.34,35 The authors of these studies found impairments of inhibition in response to alcoholic stimuli but not to general stimuli for binge drinkers.34 However, the authors also observed that both alcohol-dependent patients and healthy controls showed a higher number of commission errors in response to alcohol-associated stimuli in an alcohol go/no-go task and concluded that the difference in picture complexity between alcohol-associated stimuli and geometrical stimuli might be the reason for this finding.35
The neural findings of diminished frontostriatal recruitment during general behavioural inhibition are in contrast to those of a previous study in adolescents with bulimic-type binge eating behaviour that reported increased activity in this network.26 However, 2 previous studies addressing general cognitive inhibition in adolescents and adults support diminished activity in the frontostriatal network during inhibitory control.27,28
In the present study, significant differences within fronto-striatal networks were observed only in patients with bulimia nervosa with 3 or more binge eating/purging episodes per week. Patients with less severe symptoms showed no significant neural alterations compared with healthy controls and patients with high symptom severity. This finding extends previous research that focused exclusively on patients with high symptom severity (mean binge eating frequency was > 4 per week in all 3 previous studies). Furthermore, in the present study as well as in the study by Marsh and colleagues,28 diminished activation in frontostriatal networks was closely related to symptom severity (i.e., number of objective bulimic episodes). Future research is necessary to investigate whether diminished frontostriatal activity during inhibitory control in patients with bulimia nervosa may serve as a neural predictor (in extension to clinical markers) of therapeutic responsiveness and outcome.
Diminished control over motor responses in the present study was associated with diminished cortical activation in the motor cortex, including the primary motor, premotor and primary somatosensory cortices. The motor cortex is involved in the planning, preparation and control of motor responses, including behavioural inhibition.45
The present concept of frontostriatal loops is based on the assumption of a somatotopic organization of the striatum, suggesting a functional subdivision of the basal ganglia into limbic (ventral striatum), cognitive (caudate nucleus) and sensorimotor networks (putamen).46,47 In the present study, we observed deficient activation in the dorsal striatum with activation peaks in the caudate nucleus and in the putamen. The caudate receives input from frontal and parietal association areas involved in goal-directed behaviour, whereas the putamen receives projections from primary sensory and motor cortices forming the sensorimotor network.46,47 Thus, the neural findings predominantly support diminished inhibitory motor control in patients with bulimia nervosa with high symptom severity.
Previous studies investigating cognitive inhibition27,28 have identified diminished activation of the right lateral PFC and striatum as core regions of impaired inhibitory control in patients with bulimia nervosa. A meta-analysis48 has identified clusters of activation in the PFC, pre-SMA and insula during behavioural inhibition. Thus, different inhibitory control tasks engage overlapping but distinct neural circuits, suggesting that the observed regional differences within the PFC are task dependent.49
Thus, in addition to the cognitive frontostriatal network, the present study may indicate that the sensorimotor frontostriatal network is also involved in diminished inhibitory control in patients with bulimia nervosa.
Limitations
Our study has the following limitations. We included only adult women, limiting the generalizability to men or adolescents with bulimia nervosa. Furthermore, we were not able to control for potentially confounding variables, such as depression, medication use, patient treatment status and duration of the disorder. However, the low symptom severity group served as a clinical control group, as there were no significant differences between the low and high symtpom severity groups concerning these variables. In addition, we did not account for menstrual status, which can modulate neural function.50 However, menstrual status differed systematically across groups, so we don’t think that this confounded our findings. Furthermore, it remains unclear whether impaired behavioural inhibition should be considered as a cause or a consequence of chronic illness in patients with bulimia nervosa. Thus, future studies should evaluate the functioning of behavioural inhibition processes using a longitudinal approach and investigate patients after the remission of symptoms.
Clinical implications
Gaining further insight into the neural mechanisms of behavioural inhibition problems in patients with bulimia nervosa may inform brain-directed treatment approaches. Previous studies provide evidence of positive effects of neuromodulation techniques (i.e., repetitive transcranial magnetic stimulation [rTMS]) on eating disorder symptomatology in individuals with bulimic-type eating disorders.51–53 Repetitive transcranial magnetic stimulation of the dorsolateral PFC in individuals with bulimic-type eating disorders resulted in lower cue-induced food cravings and could therefore reduce binge eating. A recently published study reported rTMS-induced decreases in cerebral oxygenation of the left dorsolateral PFC in patients with bulimia nervosa using near-infrared spectroscopy.54 In another study, rTMS of the dorsomedial PFC in a sample of healthy adults resulted in enhanced impulsivity via striatal mechanisms on the dopamine D2 receptor using positron emission tomography, indicating the importance of the dorsomedial PFC and striatum in decision making and dopamine modulation.55 Furthermore, there is evidence from previous studies with mostly nonclinical samples indicating that response inhibition training reduces problematic and impulsive behaviours and enhances prefrontal cortical and striatal activation.56,57 Evidence of treatment effects should be extended to patients with eating disorders to see whether response inhibition training could improve inhibitory control, especially regarding binge eating episodes.
Conclusion
Our findings provide novel insight into the neural correlates of behavioural inhibition in adults with bulimia nervosa. Altered neurotransmission in the sensorimotor frontostriatal network may contribute to behavioural dysfunction in thesepatients, especially by moderating the severity of binge eating symptoms. However, it remains unclear whether these alterations are a cause or consequence of bulimia nervosa.
Acknowledgements
The authors thank the individuals who participated in the study for their time and effort. This study was supported by the German Research Foundation (FR 2626/3-1).
Footnotes
Competing interests: M. Bendszus has received grants from DFG, Guerbet, Novartis, Hopp Foundation, Stryker, Medtronic and Siemens and personal fees from Guerbet, Novartis, Roche, Bayer, Teva and Vascular Dynamics. No other competing interests declared.
Contributors: M. Wu, M. Bendszus and H.-C. Friederich designed the study. M. Skunde, M. Wu and W. Herzog acquired the data, which M. Skunde, S. Walther, J. Simon and H.-C. Friederich analyzed. M. Skunde and H.-C. Friederich write the article, which all authors reviewed and approved for publication.
References
- 1.Suokas JT, Suvisaari JM, Gissler M, et al. Mortality in eating disorders: a follow-up study of adult eating disorder patients treated in tertiary care, 1995–2010. Psychiatry Res. 2013;210:1101–6. doi: 10.1016/j.psychres.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 2.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. Washington, DC: APA; 2013. [Google Scholar]
- 3.Olmsted MP, MacDonald DE, McFarlane T, et al. Predictors of rapid relapse in bulimia nervosa. Int J Eat Disord. 2015;48:337–40. doi: 10.1002/eat.22380. [DOI] [PubMed] [Google Scholar]
- 4.Turnbull SJ, Schmidt U, Troop NA, et al. Predictors of outcome for two treatments for bulimia nervosa: short and long-term. Int J Eat Disord. 1997;21:17–22. doi: 10.1002/(sici)1098-108x(199701)21:1<17::aid-eat2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 5.Fairburn CG, Agras WS, Walsh BT, et al. Prediction of outcome in bulimia nervosa by early change in treatment. Am J Psychiatry. 2004;161:2322–4. doi: 10.1176/appi.ajp.161.12.2322. [DOI] [PubMed] [Google Scholar]
- 6.Galimberti E, Martoni RM, Cavallini MC, et al. Motor inhibition and cognitive flexibility in eating disorder subtypes. Prog Neuropsychopharmacol Biol Psychiatry. 2012;36:307–12. doi: 10.1016/j.pnpbp.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 7.Rosval L, Steiger H, Bruce K, et al. Impulsivity in women with eating disorders: Problem of response inhibition, planning, or attention? Int J Eat Disord. 2006;39:590–3. doi: 10.1002/eat.20296. [DOI] [PubMed] [Google Scholar]
- 8.Kemps E, Wilsdon A. Preliminary evidence for a role for impulsivity in cognitive disinhibition in bulimia nervosa. J Clin Exp Neuropsychol. 2010;32:515–21. doi: 10.1080/13803390903264122. [DOI] [PubMed] [Google Scholar]
- 9.Mobbs O, Van der Linden M, d’Acremont M, et al. Cognitive deficits and biases for food and body in bulimia: investigation using an affective shifting task. Eat Behav. 2008;9:455–61. doi: 10.1016/j.eatbeh.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–7. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Holderness CC, Brooks-Gunn J, Warren MP. Co-morbidity of eating disorders and substance abuse review of the literature. Int J Eat Disord. 1994;16:1–34. doi: 10.1002/1098-108x(199407)16:1<1::aid-eat2260160102>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 12.Dansky BS, Brewerton TD, Kilpatrick DG. Comorbidity of bulimia nervosa and alcohol use disorders: results from the National Women’s Study. Int J Eat Disord. 2000;27:180–90. doi: 10.1002/(sici)1098-108x(200003)27:2<180::aid-eat6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 13.Wu M, Hartmann M, Skunde M, et al. Inhibitory control in bulimic-type eating disorders: a systematic review and meta-analysis. PLoS One. 2013;8:e83412. doi: 10.1371/journal.pone.0083412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bari A, Robbins TW. Inhibition and impulsivity: behavioral and neural basis of response control. Prog Neurobiol. 2013;108:44–79. doi: 10.1016/j.pneurobio.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Nigg JT. On inhibition/disinhibition in developmental psychopathology: views from cognitive and personality psychology and a working inhibition taxonomy. Psychol Bull. 2000;126:220–46. doi: 10.1037/0033-2909.126.2.220. [DOI] [PubMed] [Google Scholar]
- 16.Eagle DM, Bari A, Robbins TW. The neuropsychopharmacology of action inhibition: cross-species translation of the stop-signal and go/no-go tasks. Psychopharmacology (Berl) 2008;199:439–56. doi: 10.1007/s00213-008-1127-6. [DOI] [PubMed] [Google Scholar]
- 17.Buchsbaum BR, Greer S, Chang WL, et al. Meta-analysis of neuroimaging studies of the Wisconsin card-sorting task and component processes. Hum Brain Mapp. 2005;25:35–45. doi: 10.1002/hbm.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Criaud M, Boulinguez P. Have we been asking the right questions when assessing response inhibition in go/no-go tasks with fMRI? A meta-analysis and critical review. Neurosci Biobehav Rev. 2013;37:11–23. doi: 10.1016/j.neubiorev.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Rubia K, Russell T, Overmeyer S, et al. Mapping motor inhibition: conjunctive brain activations across different versions of go/no-go and stop tasks. Neuroimage. 2001;13:250–61. doi: 10.1006/nimg.2000.0685. [DOI] [PubMed] [Google Scholar]
- 20.Simmonds DJ, Pekar JJ, Mostofsky SH. Meta-analysis of go/no-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia. 2008;46:224–32. doi: 10.1016/j.neuropsychologia.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steele VR, Aharoni E, Munro GE, et al. A large scale (N=102) functional neuroimaging study of response inhibition in a go/nogo task. Behav Brain Res. 2013;256:529–36. doi: 10.1016/j.bbr.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watanabe J, Sugiura M, Sato K, et al. The human prefrontal and parietal association cortices are involved in no-go performances: an event-related fMRI study. Neuroimage. 2002;17:1207–16. doi: 10.1006/nimg.2002.1198. [DOI] [PubMed] [Google Scholar]
- 23.Aron AR. From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biol Psychiatry. 2011;69:e55–68. doi: 10.1016/j.biopsych.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mostofsky SH, Schafer JG, Abrams MT, et al. fMRI evidence that the neural basis of response inhibition is task-dependent. Brain Res Cogn Brain Res. 2003;17:419–30. doi: 10.1016/s0926-6410(03)00144-7. [DOI] [PubMed] [Google Scholar]
- 25.Batterink L, Yokum S, Stice E. Body mass correlates inversely with inhibitory control in response to food among adolescent girls: an fMRI study. Neuroimage. 2010;52:1696–703. doi: 10.1016/j.neuroimage.2010.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lock J, Garrett A, Beenhakker J, et al. Aberrant brain activation during a response inhibition task in adolescent eating disorder subtypes. Am J Psychiatry. 2011;168:55–64. doi: 10.1176/appi.ajp.2010.10010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marsh R, Horga G, Wang Z, et al. An fMRI study of self-regulatory control and conflict resolution in adolescents with bulimia nervosa. Am J Psychiatry. 2011;168:1210–20. doi: 10.1176/appi.ajp.2011.11010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marsh R, Steinglass JE, Gerber AJ, et al. Deficient activity in the neural systems that mediate self-regulatory control in bulimia nervosa. Arch Gen Psychiatry. 2009;66:51–63. doi: 10.1001/archgenpsychiatry.2008.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keel PK, Mitchell JE, Miller KB, et al. Long-term outcome of bulimia nervosa. Arch Gen Psychiatry. 1999;56:63–9. doi: 10.1001/archpsyc.56.1.63. [DOI] [PubMed] [Google Scholar]
- 30.Sebastian A, Jung P, Krause-Utz A, et al. Frontal dysfunctions of impulse control — a systematic review in borderline personality disorder and attention-deficit/hyperactivity disorder. Front Hum Neurosci. 2014;8:698. doi: 10.3389/fnhum.2014.00698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hautzinger M, Keller F, Kühner C. Beck Depressions-Inventar: BDI II. Harcourt Test Services Frankfurt/Main; 2006. Revision. [Google Scholar]
- 32.Grunert SC. Ein inventar zur erfassung von selbstaussagen zum ernährungsverhalten. Diagnostica. 1989;35:167–79. [Google Scholar]
- 33.Preuss UW, Rujescu D, Giegling I, et al. Psychometrische evaluation der deutschsprachigen version der Barratt-Impulsiveness-Skala. Nervenarzt. 2008;79:305–19. doi: 10.1007/s00115-007-2360-7. [DOI] [PubMed] [Google Scholar]
- 34.Simon JJ, Skunde M, Walther S, et al. Neural signature of food reward processing in bulimic-type eating disorders. Soc Cogn Affect Neurosci. 2016 doi: 10.1093/scan/nsw049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Czapla M, Simon JJ, Friederich HC, et al. Is binge drinking in young adults associated with an alcohol-specific impairment of response inhibition? Eur Addict Res. 2015;21:105–13. doi: 10.1159/000367939. [DOI] [PubMed] [Google Scholar]
- 36.Czapla M, Simon JJ, Richter B, et al. The impact of cognitive impairment and impulsivity on relapse of alcohol-dependent patients: implications for psychotherapeutic treatment. Addict Biol. 2015 doi: 10.1111/adb.12229. 10.1111. [DOI] [PubMed] [Google Scholar]
- 37.Walther S, Goya-Maldonado R, Stippich C, et al. A supramodal network for response inhibition. Neuroreport. 2010;21:191–5. doi: 10.1097/WNR.0b013e328335640f. [DOI] [PubMed] [Google Scholar]
- 38.Brooks SJ, O’Daly OG, Uher R, et al. Differential neural responses to food images in women with bulimia versus anorexia nervosa. PLoS One. 2011;6:e22259. doi: 10.1371/journal.pone.0022259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Friederich HC, Kumari V, Uher R, et al. Differential motivational responses to food and pleasurable cues in anorexia and bulimia nervosa: a startle reflex paradigm. Psychol Med. 2006;36:1327–35. doi: 10.1017/S0033291706008129. [DOI] [PubMed] [Google Scholar]
- 40.Friston KJ, Holmes AP, Worsley KJ, et al. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1994;2:189–210. [Google Scholar]
- 41.Holmes A, Friston K. Generalisability, random E ects & population inference. Neuroimage. 1998;7:S754. [Google Scholar]
- 42.Worsley KJ, Marrett S, Neelin P, et al. A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 43.Brett M, Anton J-L, Valabregue R, et al. Region of interest analysis using the MarsBaR toolbox for SPM 99. Neuroimage. 2002;16:S497. [Google Scholar]
- 44.Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haggard P. Human volition: towards a neuroscience of will. Nat Rev Neurosci. 2008;9:934–46. doi: 10.1038/nrn2497. [DOI] [PubMed] [Google Scholar]
- 46.Redgrave P, Rodriguez M, Smith Y, et al. Goal-directed and habitual control in the basal ganglia: implications for Parkinson’s disease. Nat Rev Neurosci. 2010;11:760–72. doi: 10.1038/nrn2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7:464–76. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- 48.Swick D, Ashley V, Turken U. Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. Neuroimage. 2011;56:1655–65. doi: 10.1016/j.neuroimage.2011.02.070. [DOI] [PubMed] [Google Scholar]
- 49.Nee DE, Wager TD, Jonides J. Interference resolution: insights from a meta-analysis of neuroimaging tasks. Cogn Affect Behav Neurosci. 2007;7:1–17. doi: 10.3758/cabn.7.1.1. [DOI] [PubMed] [Google Scholar]
- 50.Dreher JC, Schmidt PJ, Kohn P, et al. Menstrual cycle phase modulates reward-related neural function in women. Proc Natl Acad Sci U S A. 2007;104:2465–70. doi: 10.1073/pnas.0605569104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McClelland J, Bozhilova N, Campbell I, et al. A systematic review of the effects of neuromodulation on eating and body weight: evidence from human and animal studies. Eur Eat Disord Rev. 2013;21:436–55. doi: 10.1002/erv.2256. [DOI] [PubMed] [Google Scholar]
- 52.Van den Eynde F, Claudino AM, Mogg A, et al. Repetitive transcranial magnetic stimulation reduces cue-induced food craving in bulimic disorders. Biol Psychiatry. 2010;67:793–5. doi: 10.1016/j.biopsych.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 53.Dunlop K, Woodside B, Lam E, et al. Increases in frontostriatal connectivity are associated with response to dorsomedial repetitive transcranial magnetic stimulation in refractory binge/purge behaviors. Neuroimage Clin. 2015;8:611–8. doi: 10.1016/j.nicl.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sutoh C, Koga Y, Kimura H, et al. Repetitive transcranial magnetic stimulation changes cerebral oxygenation on the left dorsolateral prefrontal cortex in bulimia nervosa: a near-infrared spectroscopy pilot study. Eur Eat Disord Rev. 2016;24:83–8. doi: 10.1002/erv.2413. [DOI] [PubMed] [Google Scholar]
- 55.Cho SS, Koshimori Y, Aminian K, et al. Investing in the future: stimulation of the medial prefrontal cortex reduces discounting of delayed rewards. Neuropsychopharmacology. 2015;40:546–53. doi: 10.1038/npp.2014.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Houben K. Overcoming the urge to splurge: influencing eating behavior by manipulating inhibitory control. J Behav Ther Exp Psychiatry. 2011;42:384–8. doi: 10.1016/j.jbtep.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 57.Houben K, Jansen A. Training inhibitory control. A recipe for resisting sweet temptations. Appetite. 2011;56:345–9. doi: 10.1016/j.appet.2010.12.017. [DOI] [PubMed] [Google Scholar]