Abstract
This study has explored the feasibility of using spent coffee grounds as a good supporting material for the Paenibacillus chitinolyticus CKS1 cellulase immobilization. An optimal operational conditions in a batch-adsorption system were found to be: carrier mass of 12 g/L, under the temperature of 45 °C and no pH adjustments. The immobilization yield reached about 71%. An equilibrium establishment between the cellulase and the carrier surface occurred within 45 min, whereas the process kinetics may be predicted by the pseudo-second-order model. An immobilized cellulase preparation expressed very good avicelase activity, this reached up to 2.67 U/g, and revealed an improved storage stability property, compared to free enzyme sample counterpart. The addition of metal ions, such as K+ and Mg2+ did not affect positively immobilization yield results, but on the contrary, contributed to an improved bio-activities of the immobilized cellulase, thus may be employed before each enzyme application. The method developed in this study offers a cheap and effective alternative for immediate enzyme isolation from the production medium and its stabilization, compared to other carriers used for the immobilization.
Keywords: Biotechnology, Food science, Materials science, Bioengineering, Ecology
1. Introduction
The use of cellulase has been increased during the last decades, due to the significant role which enzyme plays in various industries for human and animal purposes [1, 2, 3]. This is a key biocatalyst enzyme, which hydrolyzes β-1,4-glycosidic bonds of cellulose to produce oligomeric or soluble sugar [4, 5, 6]. The practical applicability of free cellulase has been, unfortunately, greatly limited by the stability and reusability of the enzyme [5]. During a typical hydrolysis reaction, the multimeric cellulase structure may tend to dissociate, resulting in the enzyme rapid inactivation, which becomes difficult to reuse or recover [3, 4, 5]. Therefore, the prevention of the enzyme disruption and its physiological destabilization are of great concern. Accordingly, an enzyme immobilization on solid supports has been suggested as a viable alternative. The immobilization process may help to improve the overall operational cellulases stability by increasing their resilience to changes in external factors [1, 3, 4]. Moreover, the immobilization provides a facile route to recycle the enzyme over multiple cycles, allows possible modulation of the catalytic properties, such as higher activity or selectivity [3, 5]. Selection of a suitable immobilization strategy greatly influences the properties of the resulting biocatalyst, including the operational costs [1]. The matrices for enzyme immobilization should provide a biocompatible and inert environment, by not interfering with the native structure of enzymes [4].
In that manner, several immobilization protocols have been proposed in the literature in order to increase cellulase binding capacity, stability and enzymolysis efficiency, wherein the immobilized enzyme preparations exhibited better performances compared to the free enzyme [1, 4, 5, 7, 8]. The supports, which were employed, have originated from either synthetic or biological materials. Although synthetic materials are efficient at recognizing and binding of molecular targets, they are difficult to obtain and require more additional costs than bio-macromolecules [9]. Therefore, this investigation has been focused on a production of a suitable enzyme immobilization carrier, derived from an agroindustrial waste material, named spent coffee grounds, in order to develop cost-effective biocatalyst preparations for industrial use. Many efforts have been devoted to retrieve spent coffee grounds, discarded as environmental pollutants, for a number of viable purposes, such as fertilizers, animal feed supplements, as a source for obtaining a fuel, or bioactive compound isolation [10, 11, 12]. In particular, spent coffee grounds have been proved to possess a high adsorption capacity, owing to their adjustable pore size, large surface area, pore volume and opened structures, thus, have been exploited for adsorbents production [1, 13, 14, 15, 16]. It is a well-known that porous materials have favored features for immobilizing bioactive proteins, such as enzymes, compared to non-porous materials.
The aim of this study was to explore the feasibility of using pre-activated spent coffee grounds as the solid carrier for immediate immobilization of the enzyme cellulase from the production medium. The optimal immobilization conditions were evaluated, whereby the effectiveness of the resultant immobilized cellulase was determined on the avicel hydrolysis as a substrate model. The activity and stability of the immobilized enzyme preparations were investigated and compared with those of the free enzyme counterpart.
2. Materials and methods
2.1. Material and chemicals
Spent coffee grounds, used for the cellulase immobilization, are the waste material, discarded after espresso coffee beverage preparation (using Italian Frog Espresso Coffee Pod Machine, power: 450W, voltage: 230/120 V, under the following conditions: 7 g of ground coffee were extracted under the pressure within 25 s at about 90 °C) in the local cafe. The coffee was produced from “Doncafé - Espresso Aromatico - cialde”, Strauss Adriatic d.o.o., Šimanovci, Belgrade, Serbia and contained a mix of about 40% of Arabica, originated from Santos, Guatemala, Columbia, India and Honduras and around 60% of Robusta originated from India and Vietnam (depending on the current offer on the market, while taking into account similarity in composition). The working 1% Avicel solution (w/v), purchased from Merck, Germany, has been prepared fresh in tri-sodium citrate buffer (Na3C6H5O7), pH 4.8. The chlorine dioxide (ClO2) solution has been prepared by using 27% (w/v) sodium chlorite (NaClO2) and 10% (w/v) citric acid (C6H8O7), purchased from ACRŌS ORGANIC, New Jersey, USA and Lachema, Czech Republic, respectively, in ratio NaClO2: C6H8O7: dH2O = 1:5:14 (30% ClO2 solution). The potassium chloride (KCl) and magnesium chloride (MgCl2) salts were procured by Lachema, Czech Republic.
2.2. Enzyme production
Cellulase enzyme has been produced by Paenibacillus chitinolyticus CKS1, cellulolytic bacterium isolated from forest land. One liter of growing medium for cellulase production was prepared by using of 5 g of the casein hydrolysate, 3 g of the yeast extract powder and 5 g of the D (+)-cellobiose, these have been dissolved in the 0.1 M phosphate buffer pH 7. After 48 h of the bacterial culture growing period at 30 °C on a translatory shaker (IKA − KS 4000i control, Staufen, Germany) under 150 rpm, the medium was centrifuged to remove the cells. The collected supernatant was stored in the fridge at 4 °C and represents a cellulase enzyme working solution. Each group of the experiments has been done with such a freshly produced enzyme solution.
2.3. Determination of cellulase activity
Determination of the cellulase activities have been done by DNS (3,5-dinitrosalicylic acid reagent) method, based on the sugar content, that originated from the enzymatic hydrolysis at 80 °C, during 30 min [6, 17, 18]. Avicel has been used as a substrate medium and glucose as a standard. The reaction was stopped by adding of DNS reagent, afterward the samples were boiled, cooled, diluted and filtrated before measuring of the activity. One unit of enzyme activity was defined as the amount of cellulase producing 1 μmol of glucose equivalents per minute [5]. Avicelase activity was measured in triplicate using UV/VIS spectrophotometer (Ultrospec 3300 pro, Amersham Biosciences, USA) at 540 nm.
2.4. Preparation of the carrier
After coffee beverage preparation, spent coffee grounds have been collected, dried and subjected to the process of polyphenols compounds isolation, on the basis of the previously researching [12]. The remaining solid material has been dried for overnight at 105 °C and further treated with the 30% chlorine dioxide aqueous solution in the ratio of 6:1 (v/w), during 40 min, at room temperature. The solid phase was separated from the mixture by vacuum pump, washed with distilled water and dried at 105 °C, over 24 h. The material prepared in this way has served as the carrier for the further cellulase immobilization experiments.
2.5. Immobilization experiments
The cellulase enzyme immobilization process has been performed in two manners, by mixing the samples in a batch system, as well as in a dynamic column, loaded with the coffee carrier. Batch experiments were carried out in a 100 mL round-bottomed flask on a translatory shaker (150 rpm) with 30 mL of the cellulase supernatant solution and predetermined mass of the coffee carrier, for the duration of 30 min. The continuous mode of immobilization experiments was carried out in columns height of 15 cm, loaded up to 1 cm, 2 cm and 3 cm of the coffee carrier, with the addition of 40 mL of the enzyme solution. The experiments were performed at room temperature (27 °C). After certain period of the immobilization process duration, the solid phase (containing of immobilized enzyme preparation) was separated by filtration, washed with distilled water and then dried to a total loss of moisture content at room temperature. The residual liquid phase from the reaction mixture, together with the immobilized specimen has been subjected to avicelase activity determination, based on spectro-photometrical analysis.
The immobilization yield (%) was the term used to determine the success of the cellulase immobilization process onto the coffee carrier, and was calculated by the following equation [19]:
| (1) |
where the immobilized activity has been calculated by subtracting the starting activity of free cellulase supernatant and the unbound cellulase activity, remained in the liquid phase of the reaction mixture (units, i.e. μmol/min). The amount of free protein in residual solution was determined using the BCA method with standard protocol by BSA as the protein standard [20].
2.6. Kinetic study
The kinetic experiments were performed at different temperatures (30, 40, 45 and 50 °C) in 250 mL Erlenmeyer flasks. The coffee carrier and cellulase supernatant solution were mixed in the ratio 1:60 (w/v). After the per-determined reaction time on a translatory shaker (150 rpm), the solid phase was filtered, washed with distilled water and then dried at room temperature. The immobilized and residual specimens have been subjected to avicelase activities determination.
The equilibrium adsorption capacity (qe (U/g)) of cellulase on the coffee carrier was calculated according to the following equation:
| (2) |
where C0 (U/mL) and Ce (U/mL) are the initial and the equilibrium concentration of cellulase solution, respectively, V (mL) is the volume of cellulase solution, and m (g) is the mass of the coffee carrier [1].
2.7. FT-IR study
The binding of cellulase onto coffee carrier was analyzed by FT-IR spectrometry (BOMEM MB100, Canada). The dried samples were prepared in a form of KBr pellets (0.6 mg of the sample and 200 mg of KBr). The measurements were carried out with a 4 cm−1 resolution in the 400–4000 cm−1 wave number range.
2.8. Storage stability
The stability analysis of the immobilized and free cellulase enzyme, storaged at 4 °C, was conducted by periodically determination of the remaining avicelase activities, as stated above. The tests were monitored during 10 days using 1-day intervals.
3. Results and discussion
3.1. Cellulase immobilization onto coffee carrier
3.1.1. Effect of immobilization performance with regard to the carrier mass
The results of the batch immobilization method with regard to coffee carrier mass have been presented in Fig. 1(a). The immobilization yields were ranged between 49% and 62%. An increase of the coffee carrier mass up to 12 g/L affected the improved immobilization yield, providing greater surface area and availability of more binding sites. Further increase of the carrier amount resulted in decreased cellulase immobilization success. There is a possibility that formation of coffee particles layers, even in a completely mixed batch reactor system, induced the reduction of the effective surface area. As a consequence, the percent of cellulase immobilized per unit of mass of the carrier had been diminished. In addition, it may be speculated that coffee material porosity affected the non-uniformly an enzyme binding onto it. However, under the optimal coffee carrier dose (12 g/L), the immobilized enzyme activity resulted in its maximal value of 1.97 U/g of the coffee carrier (Fig. 1).
Fig. 1.
Effect of the carrier mass (a), pH (b) and contact time and temperature (c) on the cellulase immobilization and avicelase activity in a batch.
On the other hand, the results of the immobilization yield obtained during the continuous immobilization experiments (Table 1) were found to be several fold lower compared to batch immobilization method and reached up to 15%.
Table 1.
The success of the continuous immobilization process.
| Column height (cm) | Immobilization yield (%) | Avicelase activity (U/g) |
|---|---|---|
| 1 | 11.86 ± 2.14 | 0.43 ± 2.77 |
| 2 | 14.75 ± 2.21 | 0.54 ± 2.53 |
| 3 | 15.41 ± 2.33 | 0.57 ± 2.17 |
A careful analysis of the current performance revealed that the raw cellulase solution flow through the densely crammed coffee carrier layer in the column had been labored and slow. There is the possibility that channels and the narrow passages formation occurred through the column length, creating some kind of screening effect by hindering the attachment of cellulase on carrier binding sites. As a consequence, it affected that a large part of the carrier remained unavailable for enzyme bindings. In addition, it has been observed that the increase of the column height affected an achievement of the improved results; however, these remarks did not contribute importantly to the overall process accomplishment.
Anyhow, the findings reached in this study have been quite satisfactory compared to literature reports. Yu et al. (2013) have noted that cellulase was loaded onto the soluble–insoluble reversible polymer Eudragit S-100 with the success of 40% [21]. Furthermore, about 50% of loaded commercial cellulase onto biomass solids have been achieved by Kumar and Wyman (2008) [22].
In the following investigations, the effects of pH, additives (metal ions), reaction time and temperature on cellulase immobilization yield in a batch system have been further examined. The single factor batch experiments were carried out and the conditions were shown in Table 2.
Table 2.
Factors and levels of single factor experiment.
| Factors | Level | ||||||
|---|---|---|---|---|---|---|---|
| Mass of carrier (g/L) | 8 | 12* | 16 | 20 | |||
| pH | 3 | 4 | 5 | 6 | 7* | 8 | 9 |
| Metal ions concentration (mmol) | 0* | 5 | 10 | 15 | 20 | ||
| Reaction time (min) | 5 | 10 | 15 | 30* | 45 | 60 | 90 |
| Temperature (°C) | 27* | 40 | 45 | 50 | |||
the levels kept constant when other factors were investigated.
3.1.2. Effect of pH
Bearing in mind that enzyme activity is highly dependent on the ionization state of the amino acids in the active site, the pH influence on the process has been monitored, as well. More precisely, the cellulase immobilization onto coffee carrier was studied by varying the pH from 3.0 to 9.0 (Fig. 1(b)).
It was observed that the immobilization yields reached the highest values between the pH from 5 to 7. However, the avicelase activity of immobilized cellulase was slightly lower when the immobilization process was occurring at pH 5, and reached maximum at pH near to 7. At the endpoints, pH 3 and 9, the immobilization yield was drastically decreased, as well as avicelase activities of the immobilized enzyme. Jianqin Zhou observed that optimal pH for the immobilization of cellulase from Trichoderma viride onto chitosan carrier, that resulted at pH 5, was quite similar to our results [23]. Anyhow, it should be also taken under consideration that the pH of the starting cellulase solution without additional adjustments was equaled to 7.21 and remained almost unchanged until the end of the immobilization process. Thus, it may be suggested that there was no the essential need to modify and adjust the pH values of the initial enzyme solution for achieving of the successful immobilization onto the coffee carrier.
3.1.3. Effect of the immobilization time and reaction temperature
Cellulase immobilization onto coffee carrier with relation to the contact time has been observed depending on reaction temperature (Fig. 1(c)). At the beginning of the process, a relatively fast exponential phase up to 45 min occurred, suggesting the involvement of a passive process of enzyme binding on the coffee surface. In addition, a large number of surface sites have been available for enzyme adsorption. The initial phase was rapidly followed by a second stage of equilibrium attainment. The equilibrium stage considers the state where no change has been observed between the amount of enzymes being adsorbed onto the carrier and the amount of enzymes desorbed from the carrier [9].
It was clear that temperature variation highly affected the degree of the immobilization yield, that had reached about 71%. Cellulase immobilization onto coffee carrier was found to be optimal under the temperature of 45 °C. Lv et al. (2013) noted that the amount of enzyme amylase adsorbed onto the adsorbents originated from corn stalks core material has been temperature affected too, and reached a maximum at 36 °C [9]. On contrary, Prieto et al. (2014) found that the amount of enzyme β- galactosidase adsorbed by mass unit of the commercial activated carbon decreased with the increase of temperature from 25 to 50 °C [24]. This group of researchers noted that the negative effect of elevated temperature on the enzyme adsorption may be due to some alteration in the adsorbed enzyme conformation on the active sites of the support. The immobilized cellulase activity onto coffee carrier, observed in this study, was found to be the highest at 45 °C whereas it resulted in 2.67 U/g and decreased in the following order: 50 °C > 40 °C > 30 °C. To make these results clearer, it was determined that free cellulase activity was higher at 50 °C compared to 40 °C, which indicates that the celulase produced by Paenibacillus chitinolyticus CKS1 tolerates well the higher reaction temperatures.
3.1.4. Effect of metal ions
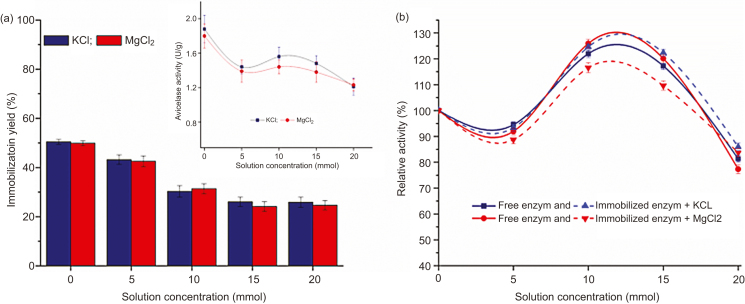
The influence of additives, such as metal ions (K+ and Mg2+) on the cellulase immobilization onto coffee carrier was studied by varying KCl and MgCl2 concentrations from 5 to 20 mmol in supernatant working solution (Fig. 2(a)). Initial work solutions were prepared from 1 mol stock solution in ratio 1:10 (v:v).
Fig. 2.
Effect of metal ions on the cellulase immobilization and avicelase activity (a) and relative activity (b).
It was found that with the increasing of KCl and MgCl2 concentration up to 15 mmol, the yield of cellulase adsorption onto coffee carrier was decreased, whereupon it remained unchanged. The possible reason may be the phenomenon of the carrier surface saturation, so that metal ions compete against the cellulase for binding sites on the coffee surface. In addition, the salt presence may cause the change of the cellulase enzyme configuration, thus resulting in a decrease in hydrophobic interactions between the enzyme and adsorption surface [25, 26]. But, on contrary, it was observed an increased immobilized cellulase activity. Because of that, the effect of metal ions has been further considered according to the activities of the free and immobilized cellulase.
The effects of KCl and MgCl2 on both free cellulase supernatant activity and immobilized cellulase were performed by using of different salt solution concentrations, prepared from 1 mol stock solution. 30 μL of the determined salt solution (5, 10, 15 and 20 mmol) was mixed with 3 mL of the total reaction mixture, which has been further used for determination of avicelase activity. The relative activity of the free and the immobilized cellulase was calculated by using the avicelase activity before and after addition of the salt content in the reaction mixture. As shown in Fig. 2(b), both free and immobilized avicelase relative activities have been increased up to 25% with addition of 10 mmol concentration of the salt solution. More precisely, the free enzyme has expressed a slightly higher avicelase activity by employment of MgCl2 than KCl, while for the immobilized cellulase KCl had more significant influence on avicelase activities.
These positive effects of the magnesium and potassium ions on the cellulase enzyme activity have been perceived to improve the functional properties of the immobilized enzyme. Furthermore, it should be noted that coffee material also contains potassium, magnesium and calcium in a certain amounts [27], thus it probably contributed partly to the enzyme stabilization.
3.2. Cellulase binding conformation
The cellulase immobilization onto coffee carrier was revealed by evaluation of the FT-IR spectra of coffee surface before and after process completion (Fig. 3). The most significant changes on the cellulase immobilized sample have occurred in region from 1650 cm−1 to 1540 cm−1 (I), which is specific for proteins molecules [5]. A characteristic NH2 band for cellulase has been observed at 1633.5 cm−1 (I). The NH2 group may be associated with NH stretch at 3436.9 cm−1 (II), too [5, 28]. The stretching pattern near 1550.6 cm−1 (I) also suggests the presence of the enzyme carboxyl group [29]. Other changes on the coffee carrier after cellulase immobilization were less pronounced and recorded slight intensifying intensity bands at 1147.5 cm−1 and 995.12 cm−1 (III). These absorption bands may be associated to cellobiose, indicating its presence in traces in a supernatant after enzyme production [30].
Fig. 3.
FTIR spectra of coffee carrier before and after cellulase immobilization.
3.3. Kinetic analysis
The kinetic analysis has been conducted on the basis of experimental data fitting with the most common pseudo-first and pseudo-second order models. The linearized form of pseudo-first and pseudo-second order kinetic model are presented as follows:
| (3) |
| (4) |
where k1 (1/min) and k2 (g/Umin) are the rate constants of the pseudo-first-order and pseudo-second-order adsorption kinetics, respectively, qe is the ratio of enzyme adsorbed on the surface of the adsorbent at equilibrium (U/g) and qt, is the ratio of enzyme adsorbed any time (U/g) [1].
The corresponding kinetic parameters from the pseudo-first and pseudo-second-order linear plots (Fig. 4) were obtained, with regard to four different reaction temperatures and were reported in Table 3.
Fig. 4.
Pseudo-second-order kinetic plot for the adsorption of cellulase onto spent coffee grounds.
Table 3.
Kinetic model parameters for cellulase immobilization onto coffee carrier at different temperatures (adsorbent mass 20 g/L, 150 rpm).
| Parameters |
Pseudo-first order kinetic model |
Pseudo-second order kinetic model |
|||||
|---|---|---|---|---|---|---|---|
| Temperature (° C) | qe,exp (U/g) | k1 (1/min) |
qe,cal (U/g) |
R2 | k2 (g/U min) |
qe,cal (U/g) |
R2 |
| 30 | 2.8501 | 0.0059 | 0.7789 | 0.0653 | 0.0581 | 2.7785 | 0.9742 |
| 40 | 3.3246 | 0.0672 | 1.5018 | 0.4679 | 0.0191 | 3.9404 | 0.9834 |
| 45 | 5.4813 | 0.0116 | 1.0481 | 0.0783 | 0.0123 | 5.5788 | 0.9872 |
| 50 | 4.1725 | 0.0233 | 3.1737 | 0.9429 | 0.0148 | 5.0123 | 0.9881 |
The applicability of these kinetic models was determined by measuring the coefficients of correlation, R2, and predicted qe values. Based on these criteria, it was found that the immobilization process of cellulase onto the coffee carrier has been followed by the pseudo-second-order kinetic model. The correlation coefficients were high enough, in the range of 0.97–0.99, to render this model as an appropriate process approximation. In addition, the calculated qe values were in a good agreement with the experimental qe values. The kinetic constant (k2) has been increased with the temperature increase up to 45 °C, which provides that higher temperatures improved the adsorption rate of cellulases, whereas the highest estimated temperature of 50 °C affected delay of the process rate. Du et al. (2012), in a similar manner, also found that immobilization rate of cellulases onto pretreated corncob increased with the increase of the temperature from 4 to 50 °C [31]. These results implied that low temperatures (especially 4 °C) decreased the movement abilities of cellulases along the reaction medium and consequently decreased the immobilization rate of cellulases.
Based on a principle of pseudo-second order kinetics, the rate limiting step of the immobilization process may be chemical connection. Compared to the results obtained in this study, the literature reports have been opposed. For instance, the immobilization of cellulase from Aspergillus niger on a commercial activated carbon was found to be followed by the kinetic of pseudo-second order model [1], whereas the cellulase immobilization kinetics onto pretreated lignocelluloses had been characterized by pseudo-first order kinetics [31].
3.4. Storage stability
The enzyme storage stability is of significant importance for the enzyme future applications. Both free and immobilized enzyme samples were stored under the same conditions (4 °C), for 10 days, whereas the residual activity has been determined as a function of the storage time.
The results of free and immobilized cellulase storage stability are presented on Fig. 5. It was observed that the free and immobilized enzyme lost about 33% of their activities after the postponement of 9 days. In the first four days, activities of the immobilized cellulase were even increased and thereafter the progressively activity losses have been recorded.
Fig. 5.
Storage stability of the free and the immobilized cellulase recorded from 1 to 10 days.
An increasing of the enzyme activities may be due to a conformational stabilization of cellulase molecules after attachment to coffee surface. In addition, the potassium and magnesium ions, existing on a coffee surface, probably contributed to the manifestation of an improved immobilized enzyme stability. But also, it should be taken into consideration that the enzyme was concentrated on a relatively small amount of coffee carrier with respect to the initial solution. Anyhow, the cellulase immobilized onto coffee carrier provided better storage properties within the 9 days, compared to the free enzyme, offering the protection from being directly affected by the environment and thus giving the advantages of using such an immobilized cellulase preparations.
4. Conclusions
This study investigates the effects of main process parameters, that influence cellulase (produced from the new isolate Paenibacillus chitinolyticus CKS1) immobilization onto spent coffee grounds. The results indicate that such a waste material may be exploited as an appropriate solid carrier for performing an effective enzyme immobilization under the mild experimental conditions, offering economically viable perspective, particularly in relation to the use of the commercially available activated carbons. The immobilized enzyme preparation revealed an improved storage stability and avicelase activity, compared to the free enzyme sample. The developed method offers a great possibility of immediate enzyme isolation from the production medium and its stabilization before further potential application.
Declarations
Author contribution statement
Aneta V. Buntić, Marija D. Pavlović, Dušan G. Antonović, Slavica S. Šiler-Marinković, Suzana I. Dimitrijević-Branković: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by Ministry of Science and Education of the Republic of Serbia (TR 31035).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Daoud F.B.-O., Kaddour S., Sadoun T. Adsorption of cellulase Aspergillus niger on a commercial activated carbon: kinetics and equilibrium studies. Colloid. Surface. B. 2010;75(1):93–99. doi: 10.1016/j.colsurfb.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 2.Dinçer A., Telefoncu A. Improving the stability of cellulase by immobilization on modified polyvinyl alcohol coated chitosan beads. J. Mol. Catal. B-Enzym. 2007;45(1):10–14. [Google Scholar]
- 3.Gokhale A.A., Lu J., Lee I. Immobilization of cellulase on magnetoresponsive graphene nano-supports. J. Mol. Catal. B-Enzym. 2013;90(0):76–86. [Google Scholar]
- 4.Wang S., Su P., Ding F., Yang Y. Immobilization of cellulase on polyamidoamine dendrimer-grafted silica. J. Mol. Catal. B-Enzym. 2013;89(0):35–40. [Google Scholar]
- 5.Khoshnevisan K., Bordbar A.-K., Zare D., Davoodi D., Noruzi M., Barkhi M., Tabatabaei M. Immobilization of cellulase enzyme on superparamagnetic nanoparticles and determination of its activity and stability. Chem. Eng. J. 2011;171(2):669–673. [Google Scholar]
- 6.Mihajlovski K.R., Carević M.B., Dević M.L., Šiler-Marinković S., Rajilić-Stojanović M.D., Dimitrijević-Branković S. Lignocellulosic waste material as substrate for Avicelase production by a new strain of Paenibacillus chitinolyticus CKS1. Int. Biodeter. Biodegr. 2015;104:426–434. [Google Scholar]
- 7.Bayramoglu G., Senkal B.F., Arica M.Y. Preparation of clay?poly(glycidyl methacrylate) composite support for immobilization of cellulase. Appl. Clay. Sci. 2013;85(0):88–95. [Google Scholar]
- 8.Zhou J. Immobilization of Cellulase on a Reversibly Soluble-Insoluble Support: Properties and Application. J. Agr. Food. Chem. 2010;58(11):6741–6746. doi: 10.1021/jf100759c. [DOI] [PubMed] [Google Scholar]
- 9.Lv J.-S., Liu X.-Y., Xu J.-X., Deng Y.-F., Wu Z., Wang Y.-M., Fan M.-Y., Xu H. Preparation and properties of adsorption material from corn stalks core when used for enzyme immobilization and the subsequent activities of the adsorbed enzymes. Ind. Crop. Prod. 2013;50:787–796. [Google Scholar]
- 10.Zuorro A., Lavecchia R. Spent coffee grounds as a valuable source of phenolic compounds and bioenergy. J. Clean. Prod. 2012;34(0):49–56. [Google Scholar]
- 11.Chen K.-I., Lo Y.-C., Liu C.-W., Yu R.-C., Chou C.-C., Cheng K.-C. Enrichment of two isoflavone aglycones in black soymilk by using spent coffee grounds as an immobiliser for β-glucosidase. Food Chem. 2013;139(1–4):79–85. doi: 10.1016/j.foodchem.2013.01.093. [DOI] [PubMed] [Google Scholar]
- 12.Pavlović M.D., Buntić A.V., Šiler-Marinković S.S., Dimitrijević-Branković S.I. Ethanol influenced fast microwave-assisted extraction for natural antioxidants obtaining from spent filter coffee. Sep. Purif. Technol. 2013;118(0):503–510. [Google Scholar]
- 13.Kyzas G.Z., Lazaridis N.K., Mitropoulos A.C. Removal of dyes from aqueous solutions with untreated coffee residues as potential low-cost adsorbents: Equilibrium, reuse and thermodynamic approach. Chem. Eng. J. 2012;189-190(0):148–159. [Google Scholar]
- 14.Roh J., Umh H., Yoo C., Rengaraj S., Lee B., Kim Y. Waste coffee-grounds as potential biosorbents for removal of acid dye 44 from aqueous solution. Korean. J. Chem. Eng. 2012;29(7):903–907. [Google Scholar]
- 15.Reffas A., Bernardet V., David B., Reinert L., Lehocine M.B., Dubois M., Batisse N., Duclaux L. Carbons prepared from coffee grounds by H3PO4 activation: Characterization and adsorption of methylene blue and Nylosan Red N-2RBL. J. Hazard. Mater. 2010;175(1–3):779–788. doi: 10.1016/j.jhazmat.2009.10.076. [DOI] [PubMed] [Google Scholar]
- 16.Ahmaruzzaman M. Industrial wastes as low-cost potential adsorbents for the treatment of wastewater laden with heavy metals. Adv. Colloid. Interfac. 2011;166(1):36–59. doi: 10.1016/j.cis.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Qi B., Chen X., Su Y., Wan Y. Enzyme adsorption and recycling during hydrolysis of wheat straw lignocellulose. Bioresource Technol. 2011;102(3):2881–2889. doi: 10.1016/j.biortech.2010.10.092. [DOI] [PubMed] [Google Scholar]
- 18.Miller G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959;31(3):426–428. [Google Scholar]
- 19.Matte C.R., Bussamara R., Dupont J., Rodrigues R.C., Hertz P.F., Ayub M.A.Z. Immobilization of Thermomyces lanuginosus Lipase by Different Techniques on Immobead 150 Support: Characterization and Applications. Appl. Biochem. Biotech. 2014;172(5):2507–2520. doi: 10.1007/s12010-013-0702-4. [DOI] [PubMed] [Google Scholar]
- 20.Kumar R., Wyman C.E. Cellulase adsorption and relationship to features of corn stover solids produced by leading pretreatments. Biotechnol. Bioeng. 2009;103(2):252–267. doi: 10.1002/bit.22258. [DOI] [PubMed] [Google Scholar]
- 21.Yu Y., Yuan J., Wang Q., Fan X., Wang P., Sun X. Immobilization of cellulases on the reversibly soluble polymer Eudragit S‐100 for cotton treatment. Eng. Life. Sci. 2013;13(2):194–200. [Google Scholar]
- 22.Kumar R., Wyman C.E. An improved method to directly estimate cellulase adsorption on biomass solids. Enzyme. Microb. Tech. 2008;42(5):426–433. [Google Scholar]
- 23.Zhou J. Immobilization of cellulase on a reversibly soluble- insoluble support: properties and application. J. Agr. Food. Chem. 2010;58(11):6741–6746. doi: 10.1021/jf100759c. [DOI] [PubMed] [Google Scholar]
- 24.Prieto L.M., Ricordi R.G., Kuhn R.C., Foletto E.L., Mazutti M.A., Burkert C.A.V. Evaluation of β-galactosidase adsorption into pre-treated carbon. Biocatalysis and Agricultural Biotechnology. 2014;3(3):26–29. [Google Scholar]
- 25.Ko J.K., Ximenes E., Kim Y., Ladisch M.R. Adsorption of enzyme onto lignins of liquid hot water pretreated hardwoods. Biotechnol. Bioeng. 2014;112(3):447–456. doi: 10.1002/bit.25359. [DOI] [PubMed] [Google Scholar]
- 26.Tu M., Pan X., Saddler J.N. Adsorption of Cellulase on Cellulolytic Enzyme Lignin from Lodgepole Pine. J. Agr. Food. Chem. 2009;57(17):7771–7778. doi: 10.1021/jf901031m. [DOI] [PubMed] [Google Scholar]
- 27.Mussatto S.I., Carneiro L.M., Silva J.P., Roberto I.C., Teixeira J.A. A study on chemical constituents and sugars extraction from spent coffee grounds. Carbohyd. Polym. 2011;83(2):368–374. [Google Scholar]
- 28.Pavlović M.D., Buntić A.V., Mihajlovski K.R., Šiler-Marinković S.S., Antonović D.G., Radovanović Ž., Dimitrijević-Branković S.I. Rapid cationic dye adsorption on polyphenol-extracted coffee groundsćA response surface methodology approach. J. Taiwan. Inst. Chem. E. 2014;45(4):1691–1699. [Google Scholar]
- 29.Abraham R.E., Verma M.L., Barrow C.J., Puri M. Suitability of magnetic nanoparticle immobilised cellulases in enhancing enzymatic saccharification of pretreated hemp biomass. Biotechnol. Biofuel. 2014;7(1):90. doi: 10.1186/1754-6834-7-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nikonenko N.A., Buslov D.K., Sushko N.I., Zhbankov R.G. Investigation of stretching vibrations of glycosidic linkages in disaccharides and polysaccarides with use of IR spectra deconvolution. Biopolymers. 2000;57(4):257–262. doi: 10.1002/1097-0282(2000)57:4<257::AID-BIP7>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 31.Du R., Su R., Li X., Tantai X., Liu Z., Yang J., Qi W., He Z. Controlled adsorption of cellulase onto pretreated corncob by pH adjustment. Cellulose. 2012;19(2):371–380. [Google Scholar]