Abstract
Background
Cognitive behavioral therapy (CBT) is an evidence-based treatment for alcohol use disorders, yet is rarely implemented with high fidelity in clinical practice. Computer-based delivery of CBT offers the potential to address dissemination challenges, but to date there have been no evaluations of a web-based CBT program for alcohol use within a clinical sample.
Methods
This study randomized treatment-seeking individuals with a current alcohol use disorder to one of three treatments at a community outpatient facility: (1) standard treatment-as-usual (TAU); (2) TAU plus on-site access to a computerized CBT targeting alcohol use (TAU+CBT4CBT); or (3) CBT4CBT plus brief weekly clinical monitoring (CBT4CBT+monitoring). Participant alcohol use was assessed weekly during an 8-week treatment period, as well as 1, 3, and 6 months after treatment.
Results
Sixty-eight individuals (65% male; 54% African American) were randomized (TAU = 22; TAU+CBT4CBT = 22; CBT4CBT+monitoring = 24). There were significantly higher rates of treatment completion among participants assigned to one of the CBT4CBT conditions compared to TAU (Wald = 6.86, p < .01). Significant reductions in alcohol use were found across all conditions within treatment, with participants assigned to TAU+CBT4CBT demonstrating greater increases in percentage of days abstinent (PDA) compared to TAU, t(536.4) = 2.68, p < .01, d = 0.71, 95% CI [0.60, 3.91], for the full sample. Preliminary findings suggest the estimated costs of all self-reported AUD-related services utilized by participants were considerably lower for those assigned to CBT4CBT conditions compared to TAU, both within treatment and during follow-up.
Conclusions
This trial demonstrated the safety, feasibility, and preliminary efficacy of web-based CBT4CBT targeting alcohol use. CBT4CBT was superior to TAU at increasing PDA when delivered as an add-on, and it was not significantly different from TAU or TAU+CBT4CBT when delivered with clinical monitoring only.
Keywords: CBT4CBT, Alcohol Use Disorders, Computer-Delivered Treatment
INTRODUCTION
Cognitive behavioral therapy (CBT) has demonstrated effectiveness at treating a wide range of substance use disorders, including alcohol. Despite the support from efficacy trials, it has proven challenging to disseminate CBT to the clinical community. Clinician surveys typically report that CBT is one of the most common approaches used to treat substance use disorders in clinical practice (Ball et al., 2002, McCarty et al., 2007), but objective evidence indicates this may not be the case (Hoffman and McCarty, 2013, Humphreys and McLellan, 2011, McLellan et al., 2003). For example, independent ratings of recorded counseling sessions as practiced in 11 substance use treatment facilities across the US found CBT interventions were strikingly infrequent (Santa Ana et al., 2008). Furthermore, the level of training and supervision needed to achieve clinician competence in CBT (Sholomskas et al., 2005) is likely to be too costly and time-intensive for most substance use treatment settings (McLellan et al., 2003).
The emergence of computer-delivered interventions offers the potential to address many of these challenges (Carroll and Rounsaville, 2010, Marsch and Dallery, 2012). There are multiple potential advantages of computer delivery, including broad availability and access, standardization and consistent quality, and reduction of cost and clinician time (Wright et al., 2005, Postel et al., 2008, Olmstead et al., 2010, Marks and Cavanagh, 2009, McCrone et al., 2004). Recent years have seen tremendous growth in the development of computer-delivered interventions for alcohol use (e.g., Hester et al., 2012, Hester et al., 2011, Riper et al., 2008, Saitz et al., 2007, Kypri et al., 2014). However, most have been designed for or evaluated in undergraduate samples that may not generalize to clinical samples (Khadjesari et al., 2011), or have been designed as screening and brief interventions (Bewick et al., 2008, Dedert et al., 2015). There is as yet no comprehensive web-based CBT program designed specifically for individuals with alcohol problems at a level of severity for which specialty treatment has been sought or recommended.
We developed a computer-based version of CBT (CBT4CBT; Carroll et al., 2008) that would provide consistent and high quality delivery of key CBT concepts for avoiding or reducing substance use. The CBT4CBT program is user-friendly, requires no prior experience with computers, and includes minimal text-based material (i.e., no reading is required). Content is based closely on a CBT manual published by NIDA (Carroll, 1998), with material presented via graphic illustrations, videotaped examples, verbal instructions, audio voiceovers, interactive assessments, and practice exercises (Carroll et al., 2008). The program was evaluated initially as an adjunct to standard addiction treatment versus standard treatment alone in an 8-week trial with a broad range of individuals seeking treatment at an outpatient substance use treatment facility. Participants assigned to the CBT4CBT condition had longer periods of abstinence during treatment (Carroll et al., 2008), with effects maintained through a 6-month follow-up (Carroll et al., 2009). These findings were recently replicated in a larger trial with cocaine-dependent individuals maintained on methadone (Carroll et al., 2014).
We also developed a web-based version of CBT4CBT specifically for individuals with alcohol use disorders (AUDs). This version retained the structure, features, and basic content of the original version, but included additional alcohol-specific content and new video-based examples featuring individuals confronting a range of alcohol-related risks drawn from the NIAAA CBT manual (Kadden et al., 1992). In this article, we describe results of a randomized Stage I pilot trial (Rounsaville et al., 2001) that evaluated the feasibility, safety, preliminary efficacy and marginal costs of the program in an 8-week trial with a 6-month follow-up. This trial evaluated CBT4CBT in two forms compared to standard treatment: (1) as an add-on to standard treatment, and (2) as a virtual ‘stand-alone’ delivered with minimal clinical monitoring. The delivery of CBT4CBT as an add-on to standard treatment parallels the prior approach (Carroll et al., 2008, Carroll et al., 2014), whereas the ‘stand-alone’ CBT4CBT condition is novel and has not yet been evaluated in a clinical population. The primary hypothesis was that either delivery method of CBT4CBT would be more effective than standard treatment at reducing rates of alcohol use, as indicated by the percentage of days abstinent (PDA). We also explored safety and feasibility of the ‘stand-alone’ implementation of CBT4CBT in a treatment-seeking sample as well as the estimated marginal costs of delivering the three models of treatment in an outpatient setting.
MATERIALS AND METHODS
Participants
Participants were recruited from the Substance Abuse Treatment Unit (SATU), an outpatient substance abuse treatment facility in New Haven, CT between March 2012 and December 2014 (based on funding period). Eligibility criteria included: (1) 18 years of age or older, (2) fluent in English with at least a 6th grade reading level, (3) seeking outpatient treatment for alcohol use and meet current (past 30 days) Diagnostic and Statistical Manual – 4th edition (DSM-IV; American Psychiatric Association, 1994) criteria for alcohol abuse or dependence, and (4) psychiatrically stable such that outpatient treatment was appropriate. Exclusions were: (1) an untreated bipolar or psychotic disorder, (2) a current legal case pending such that incarceration was likely during the 8-week trial, (3) seeking alcohol pharmacotherapy, or (4) DSM-IV criteria for current dependence on a drug other than alcohol. Individuals reporting drug use other than alcohol in the past 30 days were eligible provided they reported alcohol as their primary drug of choice, and the severity of other drug use did not meet DSM-IV criteria for current dependence.
As depicted in the CONSORT diagram (Figure 1), 87 individuals provided written informed consent approved by the Yale University School of Medicine Human Investigations Committee and were screened for eligibility. Sixty-eight were deemed eligible, completed the pre-treatment assessment battery, and were randomly assigned to treatment in equal numbers using a computerized urn randomization program (Stout et al., 1994) which concealed the sequence until treatment conditions were assigned. The urn program was designed to balance groups with respect to gender, ethnicity (minority versus non-minority), education level (less than high school/high school grad or higher), probation status (yes/no), and severity of alcohol use as assessed by the Alcohol Use Disorders Identification Test (Saunders et al., 1993) (AUDIT score of 15 or below versus 16 or above).
Figure 1.
CONSORT diagram of participant flow
Treatments
Eligible participants were randomly assigned to one of the following three treatment conditions for an 8-week period: (1) standard treatment as usual (TAU), which consisted of weekly group or individual psychotherapy delivered by masters-level counselors at the outpatient facility. Participants assigned to this (or any other condition) were also offered standard ancillary services provided by the treatment facility, which included psychiatric, pharmacologic, and emergency services; (2) TAU plus CBT4CBT, which consisted of standard treatment in addition to access to the web-based CBT4CBT program (described in more detail below); or (3) CBT4CBT with brief clinical monitoring (CBT4CBT+monitoring). In this condition, CBT4CBT was implemented as a virtual stand-alone treatment, wherein participants were asked to complete one CBT4CBT module each week on-site as their principal form of treatment, in conjunction with brief (10 minute) weekly clinical monitoring provided in-person by a doctoral level psychologist. Monitoring sessions were manual guided (Carroll et al., 1998); sessions followed guidelines for low-intensity interventions used in previous placebo-controlled trials (Volpicelli et al., 2001, Pettinati et al., 2004, Pettinati et al., 2005) and trials of internet-delivered treatment (Kenwright et al., 2005). The monitoring sessions were intended as a means of evaluating each participant’s current functional status and safety, address participants’ questions or concerns, and review the participants’ use of the CBT4CBT program.
Participants assigned to either of the CBT4CBT conditions were provided access to the web-based program on a dedicated computer in a private room within the clinic, using a unique username and password. The program consists of 7 modules (each covering one basic CBT concept, such as drink refusal skills, coping with craving, etc.), each taking approximately 45 minutes to complete. Modules were structured to parallel clinician-delivered CBT sessions, which included introduction to a skill topic, didactic instruction, and opportunity for practice. The key concepts were conveyed through a series of brief videos using actors and realistic settings depicting high-risk situations for alcohol use, with characters demonstrating a targeted skill for avoiding alcohol. Multiple interactive exercises and game-like tasks followed each video to reinforce the skill being taught and how it could be applied to other problems. Each module concluded with a demonstration of how to complete the practice assignment (i.e., homework). Participants were asked to complete one module per week on-site during the 8-week trial.
Assessments
Assessments were administered before randomization, weekly during the treatment phase, at the 8-week treatment termination point, and 1-, 3-, and 6-months following treatment termination. Treatment completion was defined a priori as completing at least 5 sessions within 8 weeks. The Structured Clinical Interview for DSM-IV (SCID; First et al., 1995) was used to determine eligibility with respect to alcohol use and psychiatric diagnoses. The AUDIT (Saunders et al., 1993, Babor et al., 2001) was administered before randomization to measure hazardous drinking. The Substance Use Calendar, similar to the Timeline Follow Back (Sobell and Sobell, 1992), was administered weekly during treatment to collect day-by-day self-reports of frequency and quantity of alcohol and other drug use throughout the 8-week treatment period, as well as for the 28 days prior to randomization. It was also administered at each follow-up interview to cover the six months following treatment termination. Breathalyzer samples were collected at each visit to assess recent alcohol intake (99% concordance between BAC and self-report); urine toxicology screens for illicit drugs were also obtained at every assessment visit (97% concordance with self-reported recent drug use). Use of medical, psychiatric, and substance use services accessed outside of protocol treatments (including Emergency Department visits, hospitalizations, outpatient care, utilization of self-help) as well as involvement with the criminal justice system were assessed before treatment, at termination, and through follow-up with the Program And Client Costs – Substance Abuse Treatment (PACC-SAT; Jofre-Bonet et al., 2004, Olmstead et al., 2007), adapted from the Treatment Services Review (French et al., 2000, McLellan et al., 1992). The therapeutic alliance was evaluated at weeks 2 and 6 using the Working Alliance Inventory (WAI; Horvath and Greenberg, 1986) for all participants (completed by both clients and clinicians), and a version of the WAI for technology-based interventions (WAI-Tech; Kiluk et al., 2014) for those assigned to CBT4CBT. Participants assigned to one of the CBT4CBT conditions also completed a 17-item satisfaction survey (Carroll et al., 2008) that evaluated various aspects of the program using a Likert-type scale ranging from 1 (indicating low satisfaction) to 5 (indicating high satisfaction).
Data Analyses
Demographic and baseline descriptive variables, as well as treatment adherence indicators, were evaluated across treatment conditions with Analysis of Variance (ANOVA) or Chi-square. Usability data regarding the CBT4CBT program included the mean number of CBT4CBT modules completed (for those assigned to one of the CBT4CBT conditions), the average amount of time to complete each module (which was recorded as part of the program’s administrative database), and the number of homework assignments completed.
The primary outcome to evaluate treatment effects was a change in self-reported alcohol use over time, indicated by PDA by week, with the percentage of heavy drinking days (PHDD) (HDD = 5 or more standard drinks for men, and 4 or more standard drinks for women) as a secondary indicator. The principal analytic strategy was random effects regression analysis (maximum likelihood approach for handling missing data), with time modeled by week during the 8-week treatment period and by month through the 6-month follow-up. Two contrasts were evaluated: (1) TAU versus TAU+CBT4CBT and (2) TAU versus CBT4CBT+monitoring.
The percentage of subjects with no heavy drinking days (PSNHDDs) in the final four weeks of treatment (Falk et al., 2010) was evaluated post hoc as an endpoint indicator of treatment efficacy using Chi-square analysis. All analyses were conducted for the full intention to treat (ITT) sample (N=68) using data collected both before and after withdrawal/last clinical contact, but due to the differences in treatment exposure and retention by condition we also conducted exploratory analyses for the ITT sample up to the point of withdrawal/last clinical contact (i.e., data collected only while each participant was still enrolled in the treatment arm of the protocol). Secondary outcomes also included treatment utilization and cost, as well as treatment satisfaction. Estimates for marginal costs of treatment sessions and other AUD-related services received outside of protocol treatment were calculated using the most recently available State of Connecticut reimbursement rates (downloaded from www.CTDSSMAP/CTportal and www.vera.org/price of prisons).
RESULTS
Participants
Table 1 displays demographic and baseline characteristics across treatment conditions for the 68 participants randomized. ANOVA and chi square tests indicated no differences across treatment conditions on any of these baseline variables. The majority of the sample were male (65%), African American (54%) and had a mean age of 42.7 (SD=11.9). Most were unemployed (74%), not married (91%), and had completed high school (79%). A quarter of the sample indicated they had been referred to treatment by the criminal justice system. In terms of alcohol use during the 28-day period prior to randomization, participants reported drinking any alcohol on approximately 13 days, heavy drinking on nearly 8 days, and averaged 7 drinks per drinking day. Their mean AUDIT score at baseline was 18.4 (SD=8.4).
Table 1.
Sample characteristics at baseline by treatment assignment, N=68
| TAU N = 22 |
TAU + CBT4CBT N = 22 |
CBT4CBT + monitoring N = 24 |
Total N = 68 |
Χ2 | p | |
|---|---|---|---|---|---|---|
|
| ||||||
| Categorical variables | n (%) | n (%) | n (%) | n (%) | ||
|
| ||||||
| Female | 8 (36.4) | 7 (31.8) | 9 (37.5) | 24 (35.3) | 0.18 | 0.92 |
| Hispanic ethnicity | 2 (9.1) | 2 (9.1) | 4 (16.7) | 8 (11.8) | 0.86 | 0.65 |
| Race | ||||||
| Caucasian | 6 (27.3) | 8 (36.4) | 9 (37.5) | 23 (33.8) | 8.20 | 0.22 |
| African-American | 12 (54.4) | 13 (59.1) | 12 (50) | 37 (54.4) | ||
| Responded Hispanic only | 1 (4.5) | 1 (4.5) | 3 (12.5) | 5 (7.4) | ||
| Multiracial/other | 3 (13.6) | 0 | 0 | 3 (4.4) | ||
| Completed high school | 20 (90.9) | 17 (77.3) | 17 (70.8) | 54 (79.4) | 2.92 | 0.23 |
| Never married/living alone | 21 (95.9) | 19 (86.4) | 22 (91.7) | 62 (91.2) | 1.14 | 0.57 |
| Unemployed | 17 (77.3) | 14 (63.6) | 19 (79.2) | 50 (73.5) | 1.66 | 0.44 |
| Referred by criminal justice system | 6 (27.3) | 5 (22.7) | 6 (25) | 17 (25) | 0.12 | 0.94 |
| Previous inpatient psychiatric treatment | 5 (22.7) | 3 (13.6) | 6 (25.0) | 14 (20.6) | 1.00 | 0.61 |
| Previous substance use treatment | 8 (36.4) | 9 (40.9) | 8 (33.3) | 25 (36.8) | 0.29 | 0.87 |
|
| ||||||
| Continuous variables (mean, SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | F | p |
|
| ||||||
| Age, mean (SD) | 42.3 (11.6) | 41.9 (11.2) | 43.9 (13.1) | 42.7 (11.9) | 0.19 | 0.83 |
| Days of alcohol use past 28 | 10.9 (7.8) | 15.0 (10.6) | 12.2 (7.2) | 12.7 (8.7) | 1.27 | 0.29 |
| Days of heavy drinking past 28 | 6.6 (6.7) | 7.8 (8.4) | 8.8 (7.0) | 7.8 (7.3) | 0.53 | 0.59 |
| Days of binge drinking past 28 | 5.6 (6.6) | 2.6 (4.8) | 4.5 (4.4) | 4.3 (5.4) | 1.75 | 0.18 |
| Drinks per drinking day past 28 | 8.7 (6.4) | 6.1 (4.1) | 7.4 (3.9) | 7.4 (4.9) | 1.56 | 0.22 |
| Days of cigarette use past 28 | 19.4 (11.8) | 15.9 (13.1) | 16.0 (13.0) | 17.1 (12.6) | 0.55 | 0.58 |
| Days of marijuana use past 28 | 2.7 (6.8) | 2.3 (5.4) | 0.4 (1.7) | 1.8 (5.1) | 1.37 | 0.26 |
| Days of cocaine use past 28 | 0.6 (1.6) | 0.2 (0.6) | 1.0 (2.7) | 0.6 (1.8) | 0.90 | 0.41 |
| Age of first alcohol use | 14.4 (3.0) | 16.1 (4.0) | 15.0 (4.0) | 15.2 (3.7) | 1.30 | 0.28 |
| Number prior alcohol treatments | 1.5 (1.8) | 1.1 (1.6) | 2.1 (3.9) | 1.6 (2.7) | 0.81 | 0.45 |
| Number of arrests in lifetime | 3.5 (4.6) | 2.5 (3.4) | 4.0 (5.8) | 3.4 (4.7) | 0.59 | 0.56 |
| Months incarcerated lifetime | 10.3 (19.4) | 17.4 (44.3) | 23.1 (51.3) | 17.1 (40.8) | 0.55 | 0.58 |
| AUDIT Score | 19.5 (9.0) | 17.8 (8.9) | 18.0 (7.4) | 18.4 (8.4) | 0.29 | 0.75 |
| Shipley estimated IQ | 94.7 (15.7) | 94.6 (13.1) | 92.9 (11.4) | 94.0 (13.2) | 0.13 | 0.89 |
Note: AUDIT indicates Alcohol Use Disorders Identification Test, higher scores indicate higher risk of hazardous drinking.
Treatment Engagement, Retention, and Completion
As seen in Figure 1 (CONSORT diagram), of the 63 individuals who initiated treatment (i.e., attended at least one session), 33 completed the treatment protocol (52%). Of those who initiated, participants assigned to either of the CBT4CBT conditions were more likely to complete treatment than those assigned to TAU (TAU = 26%, TAU+CBT4CBT = 65%, CBT4CBT+monitoring = 63%; Wald = 6.86, p < .01). Seven participants were withdrawn from the treatment arm and referred to a higher level of care by their clinician; of these, 4 were in TAU (1 person enrolled in an inpatient detoxification program followed by a 28-day inpatient program; 1 completed 13 inpatient days and 8 IOP days, and another completed a 4-day inpatient detoxification program); 2 were in TAU+CBT4CBT (none connected with outside services); 1 was in CBT4CBT+monitoring (completed a 4-day inpatient detoxification program). During the follow-up period, five participants reported they had received inpatient treatment for alcohol use; four of those had been assigned to TAU and one had been assigned to TAU+CBT4CBT. Two participants reported they had been incarcerated during the follow-up period (TAU, 148 days; CBT4CBT+monitoring, 93 days).
There were marked differences across conditions with respect to exposure to protocol treatments. Of those who initiated treatment (N = 63), participants assigned to TAU attended a mean of 2.8 (SD = 2.5) group sessions and 1.4 (SD = 2.0) individual sessions (4.3 total sessions; SD = 2.2). Participants assigned to TAU+CBT4CBT attended a mean of 3.1 (SD = 2.4) group sessions, 2.0 (SD = 2.5) individual sessions, and completed a mean of 5.6 (SD = 1.9) of the 7 CBT4CBT modules (10.8 total sessions; SD = 3.7). Those in the CBT4CBT+monitoring condition completed an average of 5.4 (SD=1.9) of the 7 modules offered and attended 5.0 (SD = 1.9) sessions of brief clinical monitoring (mean number of minutes = 11.3, SD = 1.4).
Despite the disproportionate levels of treatment exposure and retention across groups, data were collected on 66 of the 68 randomized participants at treatment termination (97% of the ITT sample), and 62 participants (91%) completed the final follow-up interview 6-months following treatment termination. Thus, data were fairly complete and data availability was comparable across treatment conditions.
The percentage of participants in each condition completing all 7 CBT4CBT modules was comparable across conditions and consistent with our previous work: TAU+CBT4CBT = 50.0%; CBT4CBT+monitoring = 41.7% (p = ns). Participants spent an average of 192.6 (SD = 85.6) minutes working on the CBT4CBT program, with an average of 35 minutes per module (SD = 7.6), with no significant differences between the two CBT4CBT conditions. Most participants (88.4%) reported completing at least one of the six homework assignments, as indicated by their responses within the CBT4CBT program; participants reported completing an average of 3.7 homework assignments (SD=2.1), with no significant differences between the two CBT4CBT conditions.
Effect of treatment on change in drinking within treatment
Results of random effects regression analyses evaluating change in drinking are presented in Table 2. For the ITT sample using all data collected, there was a main effect of time on PDA by week [F(1, 535.93) = 10.28, p < .01] indicating an overall increase in abstinence during the 8-week period for the sample as a whole. There was also a significant time by condition effect for the contrast comparing TAU to TAU plus CBT4CBT, indicating those assigned to TAU plus CBT4CBT group made more rapid reductions in drinking across time [t (536.36) = 2.68, p < .01]. The contrast comparing TAU to CBT4CBT+monitoring was not significant. Results were similar for PDA when only data collected up to the point of withdrawal or last treatment contact were used.
Table 2.
Change in drinking over time, weeks 0 – 8, intention to treat sample (N = 68)
| Treatment condition ndf = 2 |
Week by condition ndf = 2 |
Treatment week ndf = 1 |
Contrast 1 by Week ndf = 1 |
Contrast 2 by Week ndf = 1 |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | ddf | p | F | ddf | p | F | ddf | p | t | ddf | p | d* | t | ddf | p | d* | |
|
All data collected (observations = 606)
| |||||||||||||||||
| PDA | 2.14 | 87.62 | .12 | 5.93 | 535.93 | <.01 | 10.28 | 535.95 | <.01 | −0.50 | 535.08 | .62 | .13 | 2.68 | 536.36 | .01 | .71 |
| PHDD | 0.28 | 91.52 | .75 | 2.36 | 536.21 | .10 | 0.24 | 536.24 | .62 | 1.25 | 535.23 | .21 | .01 | −0.89 | 536.72 | .37 | .64 |
|
| |||||||||||||||||
|
Excluding data collected after withdrawal or last treatment contact (observations = 464)
| |||||||||||||||||
| PDA | 2.14 | 76.23 | .12 | 8.46 | 404.04 | <.01 | 38.32 | 404.52 | <.01 | −0.10 | 405.27 | .92 | .03 | 3.28 | 406.90 | .00 | .94 |
| PHDD | 0.47 | 78.18 | .62 | 3.24 | 405.38 | .04 | 11.58 | 405.94 | <.01 | 0.05 | 406.85 | .96 | .01 | −2.04 | 408.59 | .04 | .64 |
Note: PDA = Percentage of Days Abstinent; PHDD = Percentage of Heavy Drinking Days; Contrast 1 indicates effect for TAU versus CBT4CBT+monitoring; Contrast 2 indicates effect for TAU versus TAU+CBT4CBT;
Effect size for growth model analysis (Feingold, 2009)
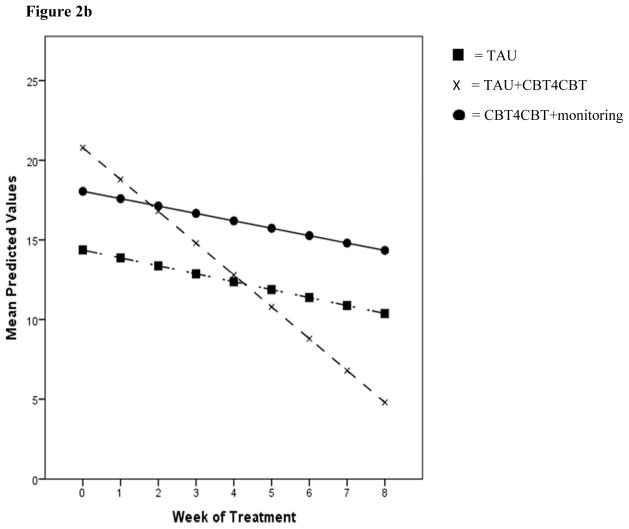
In terms of PHDD by week, there were no main or interaction effects using all data collected, however when restricted to data collected prior to withdrawal/last contact, there was a main effect of time [F(1, 405.94) = 11.58, p < .01] indicating a decrease in heavy drinking, as well as a significant effect for the contrast of TAU versus TAU+CBT4CBT by time [t (408.59) = −2.04, p < .05], indicating a more rapid decrease in heavy drinking for the TAU+CBT4CBT group. Contrasts evaluating TAU versus CBT4CBT+monitoring were non-significant for PHDD using all data collected or data collected prior to withdrawal/last contact. Results for changes in PDA and PHDD over time are illustrated in Figures 2a and 2b.
Figure 2.
Figure 2a. Percentage of Days Abstinent (PDA) by treatment week
Figure 2b. Percentage of Heavy Drinking Days (PHDD) by treatment week
For PSNHDDs in the last 4 weeks of treatment, only 2 participants assigned to TAU (9.1%) met this criterion versus 8 for both TAU+CBT4CBT and CBT4CBT+monitoring (36.5% and 33.3%, respectively), (χ2 = 5.10, p = .08, Cramer’s V = .27). Contrasts indicated a significantly higher rate of PSNHDDs in the TAU+CBT4CBT condition versus TAU (Wald = 4.07, p < .05), and a trend for the contrast of the CBT4CBT+monitoring condition versus TAU (Wald = 3.51, p = .06). Means and standard deviations for PDA and PHDD across treatment conditions at each time point, including follow-up assessments, are presented in Supplemental Table 1.
Change in drinking through the 6-month follow-up
Results of random effects regression analyses evaluating primary drinking outcomes by month from treatment termination to the 6-month follow-up interview revealed a significant effect of time for PHDD [F(1,377.98) = 6.88, p < .01], indicating an overall decrease in heavy drinking during the follow-up period. The contrast of TAU versus CBT4CBT+monitoring by time was significant for PDA [t(377.85) = −2.15, p < .05], indicating a greater increase in PDA by month for those assigned to TAU compared to CBT4CBT+monitoring, although the lack of a significant main effect for time [F(1, 376.22) = 0.4, p = ns] suggests these differences are relatively small. Also, when drinking data for those who reported time in a controlled environment during the follow-up period were excluded (inpatient treatment or incarceration; n = 7), the contrast of TAU versus CBT4CBT+monitoring by time was no longer significant for PDA [t(388.55) = −1.58, p = ns]. Contrasts evaluating TAU versus TAU+CBT4CBT as well as TAU versus CBT4CBT+monitoring over time were non-significant for PHDD during the follow-up period.
Relative costs of treatments
We estimated marginal costs of delivering each protocol treatment in 3 ways: First, an ‘intent to treat’ analysis, estimating marginal costs as if each participant had completed all sessions offered in the protocol (TAU = 1 individual + 8 group sessions; TAU+CBT4CBT = 1 individual + 8 group sessions + CBT4CBT; CBT4CBT+monitoring = 8 sessions brief monitoring + CBT4CBT) (see Supplemental Table 2). Second, we calculated ‘as treated’ marginal costs, based on utilization of protocol treatments (i.e., actual session attendance). Finally, we estimated costs of all AUD-related services utilized by participants (i.e., all outpatient and inpatient services reported both within and outside of protocol treatment). Based on the 2015 Current Procedural Terminology (CPT) medical billing codes to determine reimbursement rates for the state of Connecticut, we assumed costs of $34.77 per group session, $66.17 per individual session, and $21.64 for each monitoring visit. Including estimates from larger national studies regarding use of other services (e.g., French and McGeary, 1997), we estimated $572 per day of inpatient detoxification, $511 per inpatient substance use hospitalization day, and $200 per day of intensive outpatient treatment. As we focused on marginal costs, we did not include costs that were constant across the three treatment conditions (e.g., standard program costs such as rent and utilities, costs of urine monitoring). Currently, as CBT4CBT is provided at fixed cost of $100 per patient (rather than by module); we used a fixed cost of $100 per participant regardless of how many modules were completed.
Results are displayed in Figure 3 (calculations shown in Supplemental Table 1). For the ‘intent to treat analysis’, per participant estimates were $310.83 for TAU, $410.83 for TAU+CBT4CBT, and $273.12 for CBT4CBT+monitoring for the 8-week treatment period. Means for protocol treatments ‘as treated’ were $164.48, $318.85 and $219.07 per participant, respectively. Average marginal costs including all services received during the 8-week treatment period (e.g., inpatient detoxification, inpatient rehabilitation, and intensive outpatient treatment) were estimated as $1345.14, $318.85, and $296.10 per participant, respectively. Including estimates of costs for inpatient treatment or time in jail during the six-month follow-up (not displayed in Figure 3) further magnifies the cost differences incurred during the study (total costs of $8,886.55, $760.17 and $826.98 per participant, for TAU, TAU+CBT4CBT and CBT4CBT+monitoring, respectively).
Figure 3.
Marginal costs of treatment per participant by condition
Note: Intention to treat = expected attendance at all sessions offered in protocol
As treated = actual session attendance
All services included = all services utilized both within and outside of protocol treatments
Treatment satisfaction and working alliance
Indicators of treatment satisfaction were high and comparable across conditions. For example, percentages of participants indicating they were “highly satisfied with treatment” were 70% for TAU, 65% for TAU+CBT4CBT, and 82% for CBT4CBT+monitoring (n = 62, p = ns). Ratings of satisfaction with different aspects of the program were very positive (mean ratings of 4.0 on a 5-point scale for the 13 satisfaction items assessed) and did not differ across the two CBT4CBT conditions; for example, most indicated that the program made them think about their alcohol use in a new way (90% of those in CBT4CBT+monitoring, 85% of those in TAU+CBT4CBT, p = ns).
The Working Alliance Inventory - Client version (WAI-C) and Therapist version (WAIT) were completed by participants and clinicians, respectively, after sessions 2 and 6. At session 2, WAI-C ratings indicated high positive alliances but did not differ by condition, with mean scores for the Task, Bond, Goal and Total scales of 4.1, 4.8, 5.8, and 4.9 on the 7-point scale (p = ns). Alliance ratings also did not differ after Session 6, but only 5 participants in TAU attended that many sessions. Ratings from each participant’s clinician assessed at Session 2 and 6 also did not differ by condition, with mean scores for the Task, Bond, Goal and Total scales of 3.9, 4.7, 3.8, and 4.1 across conditions at Session 2 (similar mean scores at Session 6). For the WAI-Tech version, measuring participants’ rated alliance with the CBT4CBT program, scores were also high and did not differ across the two CBT4CBT conditions, with mean scores of 5.9, 5.5, 5.6, and 5.7 across the Task, Bond, Goal, and Total scores, respectively.
DISCUSSION
In this preliminary trial of CBT4CBT for AUDs, our main findings were as follows: First, there were marked effects on treatment retention favoring both forms of CBT4CBT over TAU, whether delivered in addition to TAU or with minimal clinical monitoring. Second, in terms of the primary outcome of reduced alcohol use, there was an overall increase in PDA (primary indicator) and decrease in PHDD (secondary indicator) across conditions during the 8-week treatment period, with significant effects favoring the TAU+CBT4CBT condition over TAU in terms of change over time. Third, for primary and secondary indicators of drinking during the 6-month follow-up period, PDA increased and PHDD decreased across conditions. Finally, for the secondary outcome of marginal costs, the three protocol treatments would have been comparable if participants utilized protocol sessions offered, but when the costs of non-protocol AUD-related treatments received were included, costs of TAU were substantially (about 4 times) higher, than costs of the two CBT4CBT conditions.
Retention was the most striking difference between protocol treatments in this study, with very few participants completing treatment in TAU (n = 5; 26%) and using on average about 4 sessions (individual and group) over 8 weeks. Participants assigned to either CBT4CBT condition were more likely to complete treatment and completed the majority of CBT4CBT sessions offered. While the use of a TAU condition provided a ‘real-world’ comparison condition for this initial evaluation of computerized CBT for AUDs, this complicated interpretation of outcome data because a relatively high number of participants in this condition either failed to attend a sufficient number of sessions or were referred to a higher level of care by a clinician during the 8-week period. It is not clear why levels of treatment non-completion or withdrawal were relatively high in the TAU condition; this differed from our experience evaluating CBT4CBT as an add-on to TAU in other outpatient settings, where levels of retention and treatment engagement were higher and comparable across conditions (Carroll et al., 2014, Carroll et al., 2008). The higher rates of treatment non-completion or withdrawal in TAU do not appear to be due to a particular clinician or group therapy orientation assigned by the clinic (e.g., motivational, skills-based, or disease model), as non-completers/withdrawals were relatively evenly distributed across clinicians/groups within TAU. Further, the same clinicians and group therapy orientation assignments were utilized in both TAU and TAU+CBT4CBT, which suggests the addition of on-site access to CBT4CBT may have contributed to greater treatment retention at this facility. Nevertheless, extensive efforts to follow and obtain data from all randomized participants were successful and prevented differential data availability across conditions. Thus, efficacy analyses and estimates of cost were based on the intention to treat sample, rather than only on those retained in treatment or carrying forward their ‘last value’, which may have further disadvantaged the TAU condition.
This study was a first evaluation of the feasibility and safety of CBT4CBT when delivered as a virtual stand-alone condition, rather than as an add-on to treatment in a treatment-seeking population. The brief weekly sessions were intended to provide clinical monitoring to assess safety and address potential ethical concerns with offering only a web-based intervention on-site to a treatment-seeking sample of individuals with AUD. This approach appeared feasible and safe in this sample; one participant was withdrawn and referred to a higher level of care and there were no other serious adverse events reported within this condition. Participants assigned to either CBT4CBT condition completed a similar number of modules and homework assignments and reported similar levels of treatment satisfaction. While equivalence was not demonstrated, the CBT4CBT+monitoring condition did not do significantly worse than either the TAU or the TAU+CBT4CBT condition.
In terms of alcohol use, participants across all conditions demonstrated a significant increase in PDA during the 8-week treatment period, however those assigned to the combination TAU+CBT4CBT consistently demonstrated greater increases in alcohol abstinence compared to TAU regardless of the manner in which data were handled (e.g., all data collected as well as that collected prior to withdrawal/last contact). When analyses were restricted to within-treatment data only, participants assigned to TAU+CBT4CBT also showed greater reductions in heavy drinking compared to TAU. These findings are consistent with our hypothesis and parallel those from our prior trials with drug users, which demonstrated greater rates of drug abstinence for the combination TAU+CBT4CBT compared to TAU only (Carroll et al., 2008; Carroll et al., 2014). Although our hypothesis that CBT4CBT+monitoring would be more effective than TAU at reducing alcohol use was not supported, we did find a trend-level effect indicating participants assigned to CBT4CBT+monitoring were more likely to complete treatment and report no heavy drinking during the final month compared to TAU. During the 6-month period following treatment, there were significant decreases in heavy drinking for the sample as a whole, yet those assigned to TAU showed a greater increase in PDA compared to CBT4CBT+monitoring. While this may suggest ‘stand-alone’ delivery of CBT4CBT provided with brief monitoring may not be sufficient to sustain abstinence outcomes following treatment, it should be noted that this effect was no longer significant after excluding drinking data for those who reported time in a controlled environment during the follow-up period (the majority of which had been assigned to TAU).
One of the major differences across conditions was in estimates of marginal costs of AUD-related treatments associated with achieving these outcomes. When costs for the protocol treatments were calculated as delivered in the trial, the per-participant cost for TAU+CBT4CBT condition was, as anticipated, higher than the other two conditions. Yet when costs for AUD-related treatment services delivered outside of the protocol were included, there were marked differences in costs. This is notable because cost saving associated with a computer-delivered treatment is often presented as a means to reduce clinician time (Marks et al., 2004). In this study, the cost difference associated with the computer-delivered treatment was largely the result of greater retention in outpatient treatment and less use of more intensive and expensive interventions while achieving comparable outcomes. While preliminary, this may be an additional aspect of cost savings associated with a computer-delivered intervention.
Limitations of this preliminary study include a small sample size, with lower alcohol severity overall compared to large trials (e.g., COMBINE; Anton et al., 2006), as well as an unequal time commitment across treatment conditions. As designed, participants assigned to TAU+CBT4CBT had potential for greater exposure to treatment (total sessions) than TAU, which may have contributed to more favorable alcohol use outcomes. However, participants across conditions spent equivalent amounts of time per week meeting with research staff, thereby limiting any effect of bias from interactions with research staff for those assigned to CBT4CBT.
In sum, these results support the safety, feasibility, and preliminary efficacy of a web-based version of CBT4CBT specifically targeting alcohol use. It appeared efficacious in reducing alcohol use when delivered as an add-on to standard outpatient treatment; it also demonstrated potential as a virtual stand-alone treatment in conjunction with minimal clinical monitoring, particularly with respect to treatment retention, satisfaction, and cost savings.
Supplementary Material
Acknowledgments
Sources of Support:
This research was supported by grant R21 AA021405 from the National Institute on Alcohol Abuse and Alcoholism and P50 DA09241 from the National Institute on Drug Abuse.
This randomized trial was registered at: Clinical trials.gov - NCT 01615497.
We are grateful to the staff of SATU and the individuals who participated in this trial. We thank Drs. Elise DeVito for her helpful comments on the project.
Footnotes
Disclosure: Kathleen M. Carroll, PhD, is a member of CBT4CBT LLC, which makes some versions of CBT4CBT available to qualified clinicians. The alcohol version of CBT4CBT is not yet released for clinical use.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. APA Press; Washington DC: 1994. [Google Scholar]
- Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift RM, Weiss RD, Williams LD, Zweben A for the COMBINE Study Research Group. Combined pharmacotherapies and behavioral interventions for alcohol dependence: The COMBINE Study. JAMA. 2006;2006:2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG. Series AUDIT: The Alcohol Use Disorders Identification Test: Guidelines for use in primary health care. World Health Organization; Geneva, Switzerland: 2001. AUDIT: The Alcohol Use Disorders Identification Test: Guidelines for use in primary health care. [Google Scholar]
- Ball SA, Bachrach K, DeCarlo J, Farentinos C, Keen M, McSherry T, Polcin D, Snead N, Sockriter R, Wrigley P, Zammarelli L, Carroll KM. Characteristics of community clinicians trained to provide manual-guided therapy for substance abusers. J Subst Abuse Treat. 2002;23:309–318. doi: 10.1016/s0740-5472(02)00281-7. [DOI] [PubMed] [Google Scholar]
- Bewick BM, Trusler K, Barkham M, Hill AJ, Cahill J, Mulhern B. The effectiveness of web-based interventions designed to decrease alcohol consumption--a systematic review. Prev Med. 2008;47:17–26. doi: 10.1016/j.ypmed.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Carroll KM. A Cognitive-Behavioral Approach: Treating Cocaine Addiction. NIDA; Rockville, Maryland: 1998. [Google Scholar]
- Carroll KM, Ball SA, Martino S, Nich C, Babuscio T, Gordon MA, Portnoy GA, Rounsaville BJ. Computer-assisted cognitive-behavioral therapy for addiction. A randomized clinical trial of ‘CBT4CBT’. Am J Psychiatry. 2008;165:881–888. doi: 10.1176/appi.ajp.2008.07111835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Ball SA, Martino S, Nich C, Babuscio TA, Rounsaville BJ. Enduring effects of a computer-assisted training program for cognitive behavioral therapy: A 6-month follow-up of CBT4CBT. Drug Alcohol Depend. 2009;100:178–181. doi: 10.1016/j.drugalcdep.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Kiluk BD, Nich C, Gordon MA, Portnoy G, Marino D, Ball SA. Computer-assisted delivery of cognitive-behavioral therapy: Efficacy and durability of CBT4CBT among cocaine-dependent individuals maintained on methadone. Am J Psychiatry. 2014;171:436–444. doi: 10.1176/appi.ajp.2013.13070987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Nuro KF, Rounsaville BJ, Petrakis I. Compliance Enhancement: A Clinician’s Manual for Pharmacotherapy Trials in the Addictions, Unpublished manual. Yale University Psychotherapy Development Center; 1998. [Google Scholar]
- Carroll KM, Rounsaville BJ. Computer-assisted therapy in psychiatry: Be brave - it’s a new world. Current Psychiatry Reports. 2010;12:426–432. doi: 10.1007/s11920-010-0146-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedert EA, McDuffie JR, Stein R, McNiel JM, Kosinski AS, Freiermuth CE, Hemminger A, Williams JW., Jr Electronic interventions for alcohol misuse and alcohol use disorders: A systematic review. Ann Intern Med. 2015;163:205–214. doi: 10.7326/M15-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk D, Wang XQ, Liu L, Fertig J, Mattson M, Ryan M, Johnson B, Stout R, Litten RZ. Percentage of subjects with no heavy drinking days: Evaluation as an efficacy endpoint for alcoholclinical trials. Alcoholism: Clinical and Experimental Research. 2010;34:2022–2034. doi: 10.1111/j.1530-0277.2010.01290.x. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV, Patient Edition. American Psychiatric Press; Washington, D.C: 1995. [Google Scholar]
- French MT, McGeary KA. Estimating the economic cost of substance abuse treatment. Health Economics. 1997;6:539–544. doi: 10.1002/(sici)1099-1050(199709)6:5<539::aid-hec295>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- French MT, Roebuck MC, McLellan AT, Sindelar JL. Can the Treatment Services Review (TSR) be used to estimate the costs of addiction and ancillary services? J Subst Abuse. 2000;12:341–361. doi: 10.1016/s0899-3289(01)00058-x. [DOI] [PubMed] [Google Scholar]
- Hester RK, Delaney HD, Campbell W. ModerateDrinking.Com and moderation management: Outcomes of a randomized clinical trial with non-dependent problem drinkers. J Consult Clin Psychol. 2011;79:215–224. doi: 10.1037/a0022487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester RK, Delaney HD, Campbell W. The college drinker’s check-up: outcomes of two randomized clinical trials of a computer-delivered intervention. Psychol Addict Behav. 2012;26:1–12. doi: 10.1037/a0024753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman KA, McCarty D. Improving the quality of addiction treatment. In: MILLER PM, editor. Interventions for Addiction: Comprehensive Addictive Behaviors and Disorders,, Vol. 3, Interventions for Addiction: Comprehensive Addictive Behaviors and Disorders. Academic Press; San Diego, CA: 2013. pp. 579–588. [Google Scholar]
- Horvath AO, Greenberg LS. The development of the Working Alliance Inventory. In: GREENBERG LS, PINSOF WM, editors. The Psychotherapeutic Process: A Research Handbook, The Psychotherapeutic Process: A Research Handbook. Guilford; New York: 1986. pp. 529–556. [Google Scholar]
- Humphreys K, McLellan AT. A policy-oriented review of strategies for improving the outcomes of services for substance use disorder patients. Addiction. 2011;106:2058–2066. doi: 10.1111/j.1360-0443.2011.03464.x. [DOI] [PubMed] [Google Scholar]
- Jofre-Bonet M, Sindelar JL, Petrakis IL, Nich C, Frankforter T, Rounsaville BJ, Carroll KM. Cost effectiveness of disulfiram: treating cocaine use in methadone-maintained patients. Journal of substance abuse treatment. 2004;26:225–232. doi: 10.1016/S0740-5472(04)00004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadden R, Carroll KM, Donovan D, Cooney JL, Monti P, Abrams D, Litt M, Hester RK. Cognitive-behavioral Coping Skills Therapy Manual: A Clinical Research Guide for Therapists Treating Individuals with Alcohol Abuse and Dependence. NIAAA; Rockville, MD: 1992. [Google Scholar]
- Kenwright M, Marks IM, Graham C, Franses A, Mataix-Cols D. Brief scheduled phone support from a clinician to enhance computer-aided self-help for obsessive-compulsive disorder: Randomized clinical trial. Journal of clinical psychology. 2005;61:1499–1508. doi: 10.1002/jclp.20204. [DOI] [PubMed] [Google Scholar]
- Khadjesari Z, Murray E, Hewitt C, Hartley S, Godfrey C. Can stand-alone computer-based interventions reduce alcohol consumption? A systematic review. Addiction. 2011;106:267–282. doi: 10.1111/j.1360-0443.2010.03214.x. [DOI] [PubMed] [Google Scholar]
- Kiluk BD, Serafini K, Frankforter T, Nich C, Carroll KM. Only connect: The working alliance in computer-based cognitive behavioral therapy. Behaviour research and therapy. 2014;63C:139–146. doi: 10.1016/j.brat.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kypri K, Vater T, Bowe SJ, Saunders JB, Cunningham JA, Horton NJ, McCambridge J. Web-based alcohol screening and brief intervention for university students: a randomized trial. JAMA. 2014;311:1218–1224. doi: 10.1001/jama.2014.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks I, Cavanagh K. Computer-aided psychological treatments: Evolving issues. Annual Review of Clinical Psychology. 2009;5:121–141. doi: 10.1146/annurev.clinpsy.032408.153538. [DOI] [PubMed] [Google Scholar]
- Marks IM, Kenwright M, McDonough M, Whittaker M, Mataix-Cols D. Saving clinicians’ time by delegating routine aspects of therapy to a computer: A randomized controlled trial in phobia/panic disorder. Psychological Medicine: A Journal of Research in Psychiatry and the Allied Sciences. 2004;34:9–17. doi: 10.1017/s003329170300878x. [DOI] [PubMed] [Google Scholar]
- Marsch LA, Dallery J. Advances in the psychosocial treatment of addiction: the role of technology in the delivery of evidence-based psychosocial treatment. Psychiatr Clin North Am. 2012;35:481–493. doi: 10.1016/j.psc.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty D, Fuller BE, Arfken C, Miller M, Nunes EV, Edmundson E, Copersino M, Floyd A. Direct care workers in the National Drug Abuse Treatment Clinical Trials Network: Characteristics, opinions, and beliefs. Psychiatric Services. 2007;58:181–190. doi: 10.1176/appi.ps.58.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrone P, Knapp M, Proudfoot J, Ryden C, Cavanagh K, Shapiro DA, Ilson S, Gray JA, Goldberg D, Mann A, Marks I, Everitt B, Tylee A. Cost-effectiveness of computerised cognitive-behavioural therapy for anxiety and depression in primary care: randomised controlled trial. Br J Psychiatry. 2004;185:55–62. doi: 10.1192/bjp.185.1.55. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Alterman AI, Cacciola J, Metzger D, O’Brien CP. A new measure of substance abuse treatment. Initial studies of the treatment services review. The Journal of nervous and mental disease. 1992;180:101–110. doi: 10.1097/00005053-199202000-00007. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Carise D, Kleber HD. Can the national addiction treatment infrastructure support the public’s demand for quality care? J Subst Abuse Treat. 2003;25:117–121. [PubMed] [Google Scholar]
- Olmstead TA, Ostrow CD, Carroll KM. Cost-effectiveness of computer-assisted training in cognitive-behavioral therapy as an adjunct to standard care for addiction. Drug Alcohol Depend. 2010;110:200–207. doi: 10.1016/j.drugalcdep.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmstead TA, Sindelar JL, Easton CJ, Carroll KM. The cost-effectiveness of four treatments for marijuana dependence. Addiction. 2007;102:1443–1453. doi: 10.1111/j.1360-0443.2007.01909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettinati HM, Weiss RD, Dundon W, Miller WR, Donovan D, Ernst DB, Rounsaville BJ. A structured approach to medical management: a psychosocial intervention to support pharmacotherapy in the treatment of alcohol dependence. Journal of studies on alcohol Supplement. 2005:170–178. doi: 10.15288/jsas.2005.s15.170. discussion 168–179. [DOI] [PubMed] [Google Scholar]
- Pettinati HM, Weiss RD, Miller WR, Donovan DM, Rounsaville BJ. Medical Management (MM) Treatment Manual: A Guide for Medically-Trained Clinicians Providing Pharmacotherapy as Part of the Treatment of Alcohol Dependence. NIAAA; Bethesda, MD: 2004. [Google Scholar]
- Postel MG, de Haan HA, DeJong CAJ. E-therapy for mental health problems: A systematic review. Telemedicine and E-Health. 2008;14:707–714. doi: 10.1089/tmj.2007.0111. [DOI] [PubMed] [Google Scholar]
- Riper H, Kramer J, Smit F, Conijn B, Schippers G, Cuijpers P. Web-based self-help for problem drinkers: A pragmatic randomized trial. Addiction. 2008;103:218–227. doi: 10.1111/j.1360-0443.2007.02063.x. [DOI] [PubMed] [Google Scholar]
- Rounsaville BJ, Carroll KM, Onken LS. A stage model of behavioral therapies research: Getting started and moving on from Stage I. Clinical Psychology: Science and Practice. 2001;8:133–142. [Google Scholar]
- Saitz R, Palfai TP, Freedner N, Winter MR, MacDonald A, Lu J, Ozonoff A, Rosenbloom DL, DeJong W. Screening and brief intervention online for college students: The iHealth study. Alcohol and Alcoholism. 2007;42:28–36. doi: 10.1093/alcalc/agl092. [DOI] [PubMed] [Google Scholar]
- Santa Ana EJ, Martino S, Ball SA, Nich C, Frankforter TL, Carroll KM. What is usual about ‘treatment-as-usual’? Data from two multisite effectiveness trials. J Subst Abuse Treat. 2008;35:369–379. doi: 10.1016/j.jsat.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Sholomskas DE, Syracuse-Siewert G, Rounsaville BJ, Ball SA, Nuro KF, Carroll KM. We don’t train in vain: a dissemination trial of three strategies of training clinicians in cognitive-behavioral therapy. J Consult Clin Psychol. 2005;73:106–115. doi: 10.1037/0022-006X.73.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline followback: A technique for assessing self-reported alcohol consumption. In: LITTEN RZ, ALLEN J, editors. Measuring alcohol consumption: Psychosocial and biological methods, Measuring alcohol consumption: Psychosocial and biological methods. Humana Press; New Jersey: 1992. pp. 41–72. [Google Scholar]
- Stout RL, Wirtz PW, Carbonari JP, DelBoca FK. Ensuring balanced distribution of prognostic factors in treatment outcome research. J Stud Alcohol Supplement. 1994;12:70–75. doi: 10.15288/jsas.1994.s12.70. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR, Pettinati HM, McLellan AT, O’Brien CP. Combining medication and psychosocial treatments for addictions: The BRENDA approach. Guilford; New York: 2001. [Google Scholar]
- Wright JH, Wright AS, Albano AM, Basco MR, Goldsmith LJ, Raffield T, Otto MW. Computer-assisted cognitive therapy for depression: maintaining efficacy while reducing therapist time. Am J Psychiatry. 2005;162:1158–1164. doi: 10.1176/appi.ajp.162.6.1158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.