Abstract
Objective
To determine the overall use of extracorporeal membranous oxygenation (ECMO) for influenza-associated illness and describe risk factors associated with mortality in these patients.
Design
Retrospective multicenter cohort analysis
Setting
The international Extracorporeal Life Support Organization (ELSO) database was queried for patients with influenza-associated illness on ECMO from 1992–2014.
Patients
1654 patients with influenza associated illness on ECMO
Measurements and Main Results
Demographic and clinical data collected included: age, type of support, duration of support, type of microbial co-detection, complications, and survival status at discharge. The primary outcome of interest was survival to hospital discharge. From 1992–2014, 1688 (3%) of the 61,336 ECMO runs were due to influenza-associated illness reflecting 1654 unique patients: 30 (2%) were neonates, 521 (31%) were pediatric patients, and 1,103 (67%) were adults. ECMO use for influenza-associated illness increased from 1992–2014, with a marked increase in use after the 2009 H1N1 pandemic. Survival to hospital discharge of patients with influenza-associated illness on ECMO was 63% and was not affected by bacterial co-detection. However, when patients with S. aureus co-detection were compared to those with another bacterial co-detection, their survival to hospital discharge was significantly lower (52% vs. 67%, p<0.01). In a logistic regression model, the effect of S. aureus on in-hospital mortality varied by age group, with younger patients with S. aureus having increased in-hospital mortality.
Conclusions
ECMO use for individuals with influenza increased over time, particularly after the 2009 H1N1 pandemic, most notably among older adults. Survival to hospital discharge for patients with influenza on ECMO was slightly higher than survival to hospital discharge for respiratory illness due to any cause. Bacterial co-detection was common among patients with influenza on ECMO and was associated with increased days on ECMO but not increased mortality. Only S. aureus co-detection in children was associated with increased in-hospital mortality.
Keywords: influenza, S. aureus, extracorporeal membranous oxygenation, ELSO
Introduction
Influenza infection is associated with a significant amount of morbidity and mortality globally. Although influenza impacts all ages, infants and the elderly have increased severity of illness and risk for mortality compared to the other age groups.1, 2 In the United States, during the 2013–2014 influenza season, there were an estimated 35.6 influenza-associated hospitalizations per 100,000 persons, accounting for 5–9% of adult deaths during this study period and contributing to 96 pediatric deaths.3 Bacterial co-detection, more commonly with S. aureus and S. pneumoniae, occurs in 0.5–2.5% of influenza cases, and may increase severity of illness.4, 5 Severe viral lower respiratory tract infection can progress to the acute respiratory distress syndrome (ARDS).6 ARDS is a result of apoptosis of respiratory epithelial cells.6 The process of influenza associated apoptosis is thought to be multifactorial. First, influenza enters epithelial cells and replicates, leading to T-cell induced apoptosis and altered cellular function. Additionally, cytokine production of respiratory epithelial cells activates neutrophils and macrophages, resulting in further inflammation, pulmonary edema and respiratory failure, which can make infected individuals more susceptible to bacterial co-infection. ARDS is routinely managed using lung protective ventilator strategies to maximize mean airway pressure and lung recruitment, while avoiding lung injury from excessive tidal volumes and barotrauma from high peak pressures.7 If lung protective mechanical ventilation strategies are insufficient to provide adequate oxygenation, individuals may require extracorporeal membrane oxygenation (ECMO).8, 9
ECMO reduces the requirement for high positive pressure ventilation by allowing gas exchange to occur outside the pulmonary vasculature, reducing the risk of compounding the primary injury with ventilator induced lung injury.10 Although ECMO has been most frequently used in pediatric patients, there is a growing use in adults with ARDS.8, 9 During the 2009 H1N1 pandemic, there was a spike in case reports in the literature related to ECMO use for influenza-associated illness (IAI) in both adults and children.9, 11–14 However, the overall trend of ECMO use for all IAI before and after 2009 pandemic has not been reported. Therefore, we sought to query a large, international ECMO database to describe the characteristics, epidemiology, and predictors of survival of IAI patients on ECMO. We hypothesized that ECMO for IAI use over time would increase, especially in adults, and that young children would have the highest in-hospital survival.
Materials and Methods
Extracorporeal Life Support Organization (ELSO) houses an international, voluntary registry that was established in 1986 that compiles reports of patients placed on ECMO in order to evaluate treatments and outcomes.15 Over 200 institutions affiliated with ELSO prospectively report de-identified information on the use, complications and outcomes of patients on ECMO and this information combined into one large database and maintained by ELSO.
The ELSO database was queried for patients on ECMO from 1992–2014 with either a primary or secondary ICD-9 code for “influenza” (487 or 488) or an organism code for influenza. Data analysis included all subjects from 1992–2014; however, since ECMO use for IAI increased significantly after 1995 our graphs do not depict data prior to 1996. Data extraction included demographic information (age, race, gender), microbial co-detection (bacterial, fungal and viral), neurologic, renal, hemorrhagic, cardiovascular and pulmonary complications, and survival to hospital discharge. To identity if co-detections with IAI were present, preselected organism codes were used and were further classified into three categories: bacteria, viruses, or fungi. Additionally, S. aureus co-detection, which was identified by secondary ICD-9 code, was separately analyzed because of its high frequency. ECMO support mode was classified as either venoarterial (VA) if there was any arterial component listed or venovenous (VV).
Statistical Analysis
Demographic and clinical characteristics of individuals with and without co-detection were evaluated for balance using the Student’s t-test with unequal variances for continuous variables and the Pearson’s Chi-squared for categorical variables with a type I error rate of α=0.05. Pearson’s Chi squared and an unadjusted logistic regression were performed to compare survivors to non-survivors. The odds ratios and 95% confidence intervals were calculated. A Bayesian multivariable logistic regression was also performed stratifying by age group, using in hospital mortality as a primary outcome. Covariates included: pre-ECMO pH, age, type of ECMO support (VA vs VV), and staphylococcal co-detection. Pre-ECMO pH was centered at 7.4 and missing values were imputed by treating them as random variables in the Bayesian modeling framework. Age was treated as non-linear using a 5-knot smoothing spline. Odds ratios (OR) were calculated and 95% posterior credible intervals were calculated. All descriptive calculations were done using Stata 13 (Stata Corp, College Station, TX), the logistic regression was done using R, and the multivariate survival model was fit using PyMC 3.16
This study contained only de-identified information and was approved by the Vanderbilt University Institutional Review Board.
Results
From 1992–2014, out of the 61,336 total ECMO runs listed in the ELSO registry, 1688 (3%) were associated with IAI. A total of 1654 unique patients contributed to these ECMO runs. Of these patients, 30 (2%) were neonates, 521 (32%) were pediatric patients, and 1,103 (67%) were adults. The demographic and clinical characteristics are reported in Table 1, with majority of patients classified as white race (69%).
Table 1.
Characteristics of Patients with IAI on ECMO and Comparison of Individuals with Documented Bacterial Co-Detection Compared to those without Bacterial Co-Detection from 1992–2014.
| All Influenza N=1654 |
Influenza without Bacterial
Co-infection N=1033 |
Influenza with Bacterial
Co-infection N=621 |
p-value* | |
|---|---|---|---|---|
|
| ||||
| Mean age in years (range) | 30.7 (0–81) | 30.4 (0–81) | 31.1 (0–76) | 0.51 |
|
| ||||
| Race/Ethnicitya | 0.62 | |||
| White | 66% | 65% | 66% | |
| Black | 8% | 9% | 7% | |
| Asian | 12% | 11% | 13% | |
| Hispanic | 8% | 9% | 8% | |
| Other | 6% | 6% | 6% | |
|
| ||||
| Gender b (% female) | 46% | 46% | 47% | 0.72 |
|
| ||||
| Survival (% survival) | 63% | 65% | 61% | 0.11 |
|
| ||||
| Mean days on ECMOc | 13 | 11 | 17* | <0.01 |
|
| ||||
| Mean days of mechanical ventilationd | 26 | 23 | 31* | <0.01 |
|
| ||||
| Any complication | 1450 (87%) | 883 (86%) | 567(91%)* | <0.01 |
|
| ||||
| Complications | ||||
| Neurologic | 212(13%) | 103(13%) | 82(13%) | 0.72 |
| Renal | 857(52%) | 501(49%) | 356(57%)* | <0.01 |
| Hemorrhagic | 647(39%) | 372(36%) | 275(44%)* | <0.01 |
| Cardiovascular | 946(57%) | 570(55%) | 376(61%)* | 0.03 |
| Pulmonary | 336(20%) | 166(16%) | 170(27%)* | <0.01 |
Comparison of influenza with and without bacterial co-infection
n=1600;
n=1628;
n=1615;
n=803
Trends over time
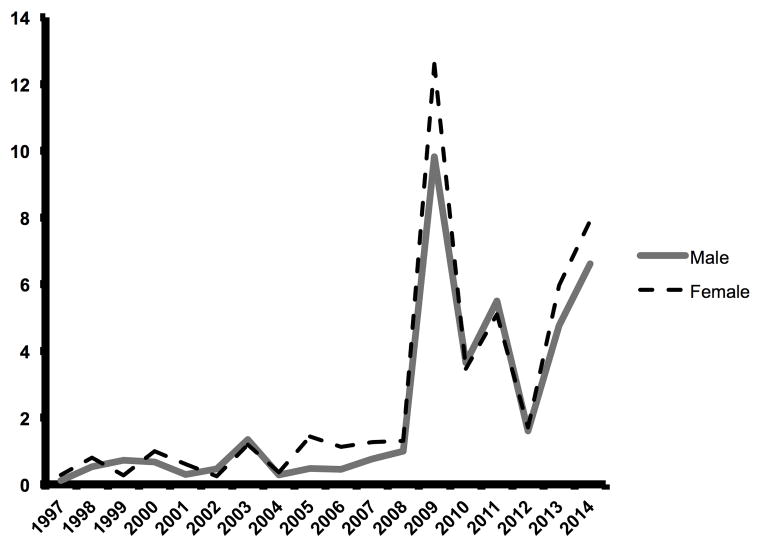
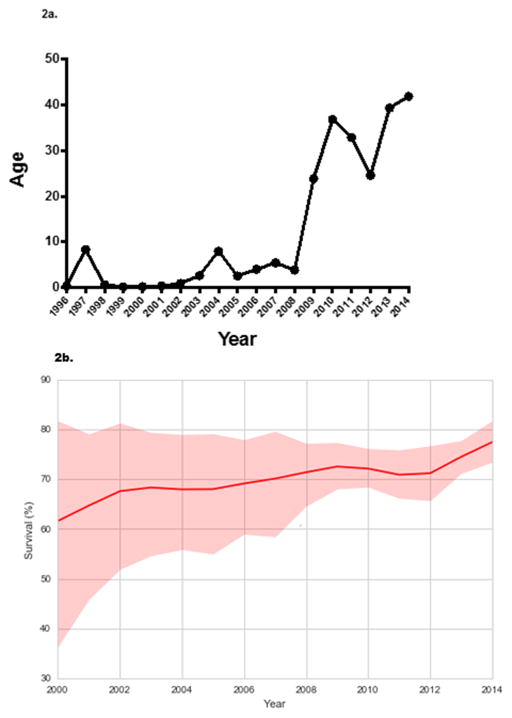
The proportion of ECMO use for IAI increased over time with peak use in 2009 (Figure 1). We fit a robust linear regression to the logarithm of ECMO proportion that yielded an estimated slope of 0.22, and whose posterior 95% credible interval [0.19, 0.26] excluded zero. The median age of patients with IAI on ECMO also increased in a log-linear fashion (Figure 2a), with a notable increase after 2008. A log-linear robust regression model fit to the annual log-median age values had an estimated slope of 0.31 (95% credible interval [0.23, 0.39]). The survival of patients on ECMO for IAI increased over time, ranging from 21–78%, with a median survival of 63% (Figure 2b).
Figure 1.
The percent of patients with IAI on ECMO in the ELSO database from 1992–2014, stratified by gender.
Figure 2.
Figure 2a. The median age of patients with influenza on ECMO over time from 1996–2014.
Figure 2b. The percent of individuals with IAI on ECMO who survived hospital discharge over time from 1996–2014. The shaded area represents the 95% posterior credible intervals for survival of patients with IAI on ECMO over time.
Co-detection with Other Organisms
Among patients with IAI, 621(38%) had a recorded co-detection with bacteria, 215 (13%) with fungus, and 90 (5%) with viral infection. The most frequent bacterial co-detection reported was S. aureus, which was identified in 244/621 (39%) patients. Other pathogens identified in this group included: Pseudomonas (14%), Enterococcus (9%), S. pneumoniae (8%), E. coli (6%), and Klebsiella sp. (7%). Of those with bacterial co-detection, 35% of bacterial cultures were obtained from an unknown site, with the remaining bacterial cultures being obtained from: a respiratory site (45%), blood (25%), wound site (3%), and other sites (e.g. stool and urine) (12%). Among those patients without bacterial co-detection, the most frequent co-detection was Candida sp./yeast (19%).
The average age of subjects with and without bacterial co-detection was similar (30 vs. 31 years, respectively) (Table 1). Median duration of ECMO and duration of mechanical ventilation were longer in patients with bacterial co-detection, (17 vs.11 days, p<0.01 and 23 vs. 31 days, p<0.01, respectively). Renal, hemorrhagic and pulmonary complications were more common among patients with bacterial co-detection than patients with influenza alone (Table 1). In contrast, the overall survival to hospital discharge was similar in patients with and without bacterial co-detection.
Staphylococcal Co-detection
Patients with S. aureus co-detection were on average 7 years younger than patients with another bacterial co-detection (27 vs. 34 years, p=0.001) and had decreased survival to hospital discharge (Table 2). Although days on ECMO did not differ between the two groups, the number of days on mechanical ventilation was shorter among patients with S. aureus co-detection (27 vs. 34 days, p=0.04). However, only about 50% of patients had complete information about duration of mechanical ventilation. Patients with S. aureus co-detection had more frequent neurologic and renal complications than those with other bacterial co-detections (17% vs 11% and 63% vs 54%, respectively, p<0.05). Additionally, patients with S. aureus co-detection were more likely to be on VA ECMO (43% vs 28% p<0.01).
Table 2.
Demographics of Patients with S. aureus Co-detection Compared to those with Bacterial Co-detection without S. aureus from 1992–2014.
| Influenza with S. aureus
Co-detection N=244 |
Influenza with Bacterial Co-detection
without S. aureus N=377 |
p-value | |
|---|---|---|---|
|
| |||
| Mean age in years (range) | 27 (0–68) | 34 (0–76) | <0.01 |
|
| |||
| Race/Ethnicitya | 0.07 | ||
| White | 166(69%) | 236(64%) | |
| Black | 21(9%) | 22(6%) | |
| Asian | 22(9%) | 59(16%) | |
| Hispanic | 16(7%) | 34(9%) | |
| Other | 15(6%) | 15(5%) | |
|
| |||
| Gender (% female)b | 112(46%) | 175(47%) | 0.93 |
|
| |||
| Survival (% survival) | 126(52%) | 253(67%)* | <0.01 |
|
| |||
| Mean days on ECMOc | 16 | 17 | 0.69 |
|
| |||
| Mean days of mechanical ventilationd | 27 | 34* | 0.04 |
|
| |||
| Any complication | 223(91%) | 344(91%) | 0.94 |
|
| |||
| Complications | |||
| Neurologic | 42(17%) | 40(11%)* | 0.02 |
| Renal | 153(63%) | 203(54%)* | 0.03 |
| Hemorrhagic | 114(47%) | 161(43%) | 0.33 |
| Cardiovascular | 151(62%) | 225(60%) | 0.58 |
| Pulmonary | 276(61%) | 101(59%) | 0.69 |
|
| |||
| Percent on VA ECMO | 43% | 28%* | <0.01 |
n=610;
n=617;
n=606;
n=320
Survivors vs. Non-survivors
The median age of non-survivors vs. survivors with IAI on ECMO did not differ (Table 3). However, neonates and adolescents were more likely to die in the hospital compared to other patients (OR 2.7 and 2.2 respectively; p<0.01) (Table 3). Older adults also had an increased risk of dying in the hospital compared to other age groups (OR 1.5; p<0.01) while young adults age 18–49 years were more likely to survive (OR 0.51; p<0.01) (Table 3). Subjects with S. aureus co-detection were more likely to die in the hospital compared to subjects with no S. aureus co-detection (OR 2.2; p<0.01) (Table 3). The non-survivors did have a lower pH, higher PCO2, and lower bicarbonate before ECMO, and were more likely to be on VA ECMO than the survivors (Table 3).
Table 3.
Comparison of Survivors with IAI on ECMO to Non-Survivors from 1992–2014.
| Survivors N=1050 |
Non-Survivors N=604 |
p-value or Odds Ratio (95% CI) | |
|---|---|---|---|
|
| |||
| Median Age (years) | 31 | 30 | p=0.29 |
|
| |||
| Gendera (% female) | 47% | 44% | p=0.27 |
|
| |||
| Neonate (<30 days) | 12 (1.1%) | 18 (3.0%) | 2.7 (1.2–6.1) |
| Infant (30 days to <1 year) | 63 (6.0%) | 51 (8.4%) | 1.4 (0.96–2.2) |
| Child (1 year to 12 years) | 174 (17%) | 90 (15%) | 0.88 (0.66–1.2) |
| Adolescent (13 years to 17 years) | 58 (5.5%) | 68 (11%) | 2.2 (1.5–3.2) |
| Early adulthood (18 years to 49 years) | 535 (51%) | 210 (35%) | 0.51 (0.41–0.59) |
| Late adulthood (≥50 years) | 201 (19%) | 157 (26%) | 1.5 (1.2–1.9) |
|
| |||
| Type of co-infection | |||
| Bacterial | 36% | 40% | 1.2 (0.96–1.5) |
| Fungal | 13% | 13% | 1.0 (0.76–1.4) |
| Viral | 5% | 6% | 1.2 (0.78–1.9) |
|
| |||
| Percent with S. aureus co-infection | 12% | 20%* | 1.8 (1.3–2.4) |
|
| |||
| Mean blood gas values prior to ECMO (95% CI) | |||
| pHb | 7.26 (7.25–7.27) | 7.21 (7.20–7.23)* | <0.01 |
| PCO2c | 56 (55–58) | 62 (59–64)* | <0.01 |
| HCO3d | 26 (25–26) | 24 (24–25)* | 0.01 |
| PaO2e | 63 (59–67) | 60 (56–65) | 0.4 |
|
| |||
| Percent on VA ECMO | 24% | 45%* | 2.6 (2.1–3.2) |
n=1628,
n=1476;
n=1404;
n=1355;
n=1502
Model
A multivariate logistic regression was developed to identify patient characteristics associated with increased in-hospital mortality when stratified by age group (Table 4). In infants, pre-ECMO pH, PO2, VA ECMO and co-detection did not appear to have a significant impact on mortality (Table 4). However, in children 1–17 years of age, co-detection with S. aureus was associated with a significant increase in in-hospital mortality, (OR 2.5, 95% CI 1.17–4.10). In adults, VA ECMO was the only factor associated with an increased risk of death in the hospital, (OR 2.83, 95% CI 1.80–4.00). (Table 4)
Table 4.
Multivariate Model for Predictors of In-Hospital Mortality of Patients with IAI on ECMO from 1992–2014.
| Adjusted Odds Ratio | 95% Credible Interval | |
|---|---|---|
|
| ||
| Infant (<1 year) | ||
| pH | 1.34 | 0.67–1.62 |
| PO2 | 1.13 | 0.67–1.62 |
| VA | 3.31 | 0.83–6.44 |
| Co-infection with S. aureus | 5.41 | 0.51–14.4 |
|
| ||
| Child (1–17 years) | ||
| pH | 0.55 | 0.07–1.24 |
| PO2 | 0.86 | 0.65–1.08 |
| VA | 1.50 | 0.89–2.14 |
| Co-infection with S. aureus | 2.50 | 1.17–4.10 |
|
| ||
| Adult (≥18 years) | ||
| pH | 0.09 | 0.03–0.16 |
| PO2 | 0.93 | 0.80–1.07 |
| VA | 2.83 | 1.80–4.00 |
| Co-infection with S. aureus | 0.22 | 0.63–1.47 |
Discussion
ECMO use for life threatening IAI has been described in several retrospective studies, case-reports, and some randomized controlled trials, with the majority of these reports during and after the 2009 H1N1 pandemic. 9, 11, 14, 17, 18 However, our study is unique because it represents a comprehensive report of the overall trends for IAI and ECMO use and associated risks for mortality over a 23-year time period using the ELSO international database. We report both an increase in the use of ECMO for IAI and an overall increase in the median age of these individuals over the study period, most notably during and after the 2009 H1N1 pandemic. Yet despite the increase in age and use of ECMO overtime, the average proportion of patients with IAI who survived ECMO was slightly higher than that of patients over 1 month of age on ECMO for any cause (63% versus 55–56% ).19 Survival during the 2009 pandemic in our cohort was 60%, which is slightly lower than two other reports, 79% in an Australian/New Zealand study and 72.5% in a UK study.9, 18 However, the Australia and New Zealand study, excluded neonates, transplant recipients, and those on ECMO for primary cardiac failure,18 while our study was all-inclusive, thus potentially explaining the higher survivor rate observed in those studies. Our results suggest that individuals with IAI and respiratory failure could benefit from ECMO use, including older adults.
Even though there was not a difference in overall survival of IAI patients compared to other individuals with respiratory illness on ECMO, we noted in-hospital mortality differences by age group. For example, when stratified by age group, overall survival was significantly lower in three distinct populations, with the lowest survival recorded in neonates (40%), then adolescents (46%), and then adults ≥50 years (56%). Increased in-hospital mortality due to IAI in neonates and older adults is well described.20–22 However, increased in-hospital mortality among adolescents with IAI may be under-recognized.14 Therefore, our findings suggest prevention strategies such as influenza immunization are particularly important in this age group. For example, a study conducted by the Pediatric Acute Lung Injury and Sepsis Investigators reported that during the 2010–2011 and 2011–2012 US, influenza vaccination was associated with a three-quarters reduction in the risk of life-threatening influenza illness in children,23 which may lead to reduction in the need for ECMO.
Co-detection with another organism was commonly documented in our IAI cohort, with bacterial co-detection in over a third of the cases. Although patients with bacterial co-detection had more frequent complications and longer duration of ECMO and ventilator time, their overall in-hospital mortality was similar to that of patients without co-detection. Therefore, our study highlights the importance recognizing the frequency of bacterial co-detection in patients with IAI on ECMO without a difference in mortality. Thus, suggesting it is reasonable to continue ECMO in patients with IAI, despite bacterial co-detection, because the overall mortality is not different despite the longer duration on ECMO.
Although patients with bacterial co-detection did not appear to have increased in hospital mortality in general, co-detection with S. aureus had a significant effect on severity of illness and mortality in patients with IAI, with only 52% of these patients surviving to hospital discharge. The association of S. aureus with severe influenza disease has been reported previously.4, 24–27 When adjusting for other co-variates including type of ECMO and pre-ECMO blood gas values, S. aureus co-detection was associated with increased mortality in children 1–17 years of age. These data should be interpreted with caution because the diagnosis of S. aureus is not laboratory or site-confirmed. Prospective trials with ECMO and IAI use are needed to establish if our findings are reproducible.
The strength of our study is the comprehensive overview of individuals of all ages and co-morbidities with IAI on ECMO using a database over two decades. However, there are some limitations associated with the ELSO database. The data are self-reported by designated individuals each institution and not validated by other investigators Some data were missing or incomplete, including time on mechanical ventilation, details regarding site of infection, and in some instances, the specific type of infection. Also, the clinical database does not have a severity of illness score, however disease complications and duration of mechanical ventilation and ECMO were used as indicators of disease severity. The diagnosis of influenza was not laboratory confirmed and some cases of influenza disease may not have been included in our cohort; but our definition was consistent throughout the study. Additionally, S. aureus sensitivities were not recorded in most cases, so we could not distinguish between methicillin-sensitive versus methicillin-resistant species. Additional therapies such as antibiotics were not included in the registry; therefore we were unable to account for differences in management. We also were not able to verify if individuals received influenza vaccination.
Conclusions
ECMO use for IAI has increased over the past two decades, especially during and after the 2009 pandemic. The overall mortality in this cohort decreased over time and is comparable to patients on ECMO for other causes. In addition, despite the fact IAI patients with bacterial co-detection had longer duration of ECMO and mechanical ventilation, their overall survival was similar to those without co-detection. However, we did note that children with S. aureus co-detection appeared to have more severe disease and increased mortality and the mortality rates were higher in neonates, older adults, and adolescents. Therefore, ECMO for IAI can be beneficial in certain populations. Our study highlights the importance of identifying bacterial co-detection in patients with influenza, particularly those with S. aureus infection. More importantly, given the severity of disease associated with IAI, our study emphasizes the need to promote prevention strategies such as influenza vaccination, which is recommended yearly to all individuals ≥6 months of age.28
Acknowledgments
Funding Support: NIH T32 AI095202-04
References
- 1.Clark NM, Lynch JP. Influenza: Epidemiology, Clinical Features, Therapy, and Prevention. Semin Respir Crit Care Med. 2011;32(04):373–392. doi: 10.1055/s-0031-1283278. [DOI] [PubMed] [Google Scholar]
- 2.Louie JK, Acosta M, Winter K, et al. Factors associated with death or hospitalization due to pandemic 2009 influenza a(H1N1) infection in california. JAMA. 2009;302(17):1896–1902. doi: 10.1001/jama.2009.1583. [DOI] [PubMed] [Google Scholar]
- 3.Epperson S, Blanton L, Kniss K, Mustaquim D, Steffens C, Wallis T, et al. Influenza Activity - United States, 2013–14 Season and Composition of the 2014–15 Influenza Vaccines. MMWR: Morbidity and Mortality Weekly Report. 2014;63(22):483–490. [PMC free article] [PubMed] [Google Scholar]
- 4.Chertow DS, Memoli MJ. Bacterial coinfection in influenza: a grand rounds review. JAMA. 2013;309(3):275–282. doi: 10.1001/jama.2012.194139. [DOI] [PubMed] [Google Scholar]
- 5.Metersky ML, Masterton RG, Lode H, File TM, Jr, Babinchak T. Epidemiology, microbiology, and treatment considerations for bacterial pneumonia complicating influenza. IJID. 2012;16(5):e321–331. doi: 10.1016/j.ijid.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Short KR, Kroeze EJ, Fouchier RA, Kuiken T. Pathogenesis of influenza-induced acute respiratory distress syndrome. The Lancet Infectious Diseases. 2014;14(1):57–69. doi: 10.1016/S1473-3099(13)70286-X. [DOI] [PubMed] [Google Scholar]
- 7.Ventilation with Lower Tidal Volumes as Compared with Traditional Tidal Volumes for Acute Lung Injury and the Acute Respiratory Distress Syndrome. NEJM. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 8.Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374(9698):1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 9.Noah MA, Peek GJ, Finney SJ, Griffiths MJ, Harrison DA, Grieve R, et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1) JAMA. 2011;306(15):1659–1668. doi: 10.1001/jama.2011.1471. [DOI] [PubMed] [Google Scholar]
- 10.Brodie D, Bacchetta M. Extracorporeal membrane oxygenation for ARDS in adults. NEJM. 2011;365(20):1905–1914. doi: 10.1056/NEJMct1103720. [DOI] [PubMed] [Google Scholar]
- 11.Zangrillo A, Biondi-Zoccai G, Landoni G, Frati G, Patroniti N, Pesenti A, et al. Extracorporeal membrane oxygenation (ECMO) in patients with H1N1 influenza infection: a systematic review and meta-analysis including 8 studies and 266 patients receiving ECMO. Critical Care. 2013;17(1):R30. doi: 10.1186/cc12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roch A, Lepaul-Ercole R, Grisoli D, Bessereau J, Brissy O, Castanier M, et al. Extracorporeal membrane oxygenation for severe influenza A (H1N1) acute respiratory distress syndrome: a prospective observational comparative study. Intensive Care Medicine. 2010;36(11):1899–1905. doi: 10.1007/s00134-010-2021-3. [DOI] [PubMed] [Google Scholar]
- 13.Pham T, Combes A, Roze H, Chevret S, Mercat A, Roch A, et al. Extracorporeal membrane oxygenation for pandemic influenza A(H1N1)-induced acute respiratory distress syndrome: a cohort study and propensity-matched analysis. Am J Respir Crit Care Med. 2013;187(3):276–285. doi: 10.1164/rccm.201205-0815OC. [DOI] [PubMed] [Google Scholar]
- 14.Kumar A, Zarychanski R, Pinto R, Cook DJ, Marshall J, Lacroix J, et al. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA. 2009;302(17):1872–1879. doi: 10.1001/jama.2009.1496. [DOI] [PubMed] [Google Scholar]
- 15.Organizationn ELS. http://www.elsonet.org/index.php?option=com_content&view=article&id=11&Itemid=485. Published 2014.
- 16.Patil AHD, Fonnesbeck C. PyMC: Bayesian Stochastic Modelling in Python. Journal of Statistical Software. 2010;35(4):1–80. [PMC free article] [PubMed] [Google Scholar]
- 17.Papadopoulos N, Ahmad Ael S, Marinos S, Moritz A, Zierer A. Extracorporeal membrane oxygenation for influenza-associated acute respiratory distress syndrome. Thorac Cardiovasc Surg. 2013;61(6):516–521. doi: 10.1055/s-0032-1330923. [DOI] [PubMed] [Google Scholar]
- 18.Davies A, Jones D, Bailey M, Beca J, Bellomo R, Blackwell N, et al. Extracorporeal Membrane Oxygenation for 2009 Influenza A(H1N1) Acute Respiratory Distress Syndrome. JAMA. 2009;302(17):1888–1895. doi: 10.1001/jama.2009.1535. [DOI] [PubMed] [Google Scholar]
- 19.Paden ML, Conrad SA, Rycus PT, Thiagarajan RR, Registry E. Extracorporeal Life Support Organization Registry Report 2012. ASAIO Journal. 2013;59(3):202–210. doi: 10.1097/MAT.0b013e3182904a52. [DOI] [PubMed] [Google Scholar]
- 20.Bhat N, Wright JG, Broder KR, Murray EL, Greenberg ME, Glover MJ, et al. Influenza-associated deaths among children in the United States, 2003–2004. NEJM. 2005;353(24):2559–2567. doi: 10.1056/NEJMoa051721. [DOI] [PubMed] [Google Scholar]
- 21.Wong KK, Jain S, Blanton L, Dhara R, Brammer L, Fry AM, et al. Influenza-Associated Pediatric Deaths in the United States, 2004–2012. Pediatrics. 2013;132(5):796–804. doi: 10.1542/peds.2013-1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Epperson S, Blanton L, Kniss K, Mustaquim D, Steffens C, Wallis T, et al. Influenza activity - United States, 2013–14 season and composition of the 2014–15 influenza vaccines. MMWR. 2014;63(22):483–490. [PMC free article] [PubMed] [Google Scholar]
- 23.Ferdinands JM, Olsho LE, Agan AA, Bhat N, Sullivan RM, Hall M, et al. Effectiveness of influenza vaccine against life-threatening RT-PCR-confirmed influenza illness in US children, 2010–2012. The Journal of Infectious Diseases. 2014;210(5):674–683. doi: 10.1093/infdis/jiu185. [DOI] [PubMed] [Google Scholar]
- 24.Tasher D, Stein M, Simoes EA, Shohat T, Bromberg M, Somekh E. Invasive bacterial infections in relation to influenza outbreaks, 2006–2010. Clin Infect Dis. 2011;53(12):1199–1207. doi: 10.1093/cid/cir726. [DOI] [PubMed] [Google Scholar]
- 25.Reed C, Kallen AJ, Patton M, Arnold KE, Farley MM, Hageman J, et al. Infection with community-onset Staphylococcus aureus and influenza virus in hospitalized children. The Pediatric Infectious Disease Journal. 2009;28(7):572–576. doi: 10.1097/INF.0b013e31819d8b71. [DOI] [PubMed] [Google Scholar]
- 26.Finelli L, Fiore A, Dhara R, Brammer L, Shay DK, Kamimoto L, et al. Influenza-associated pediatric mortality in the United States: increase of Staphylococcus aureus coinfection. Pediatrics. 2008;122(4):805–811. doi: 10.1542/peds.2008-1336. [DOI] [PubMed] [Google Scholar]
- 27.Muscedere J, Ofner M, Kumar A, Long J, Lamontagne F, Cook D, et al. The occurrence and impact of bacterial organisms complicating critical care illness associated with 2009 influenza A(H1N1) infection. Chest. 2013;144(1):39–47. doi: 10.1378/chest.12-1861. [DOI] [PubMed] [Google Scholar]
- 28.Grohskopf LA, Olsen SJ, Sokolow LZ, Bresee JS, Cox NJ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP) -- United States, 2014–15 influenza season. MMWR. 2014;63(32):691–697. [PMC free article] [PubMed] [Google Scholar]