Abstract
Valence is a principal dimension by which we understand emotional experiences, but oftentimes events are not easily classified as strictly positive or negative. Inevitably, individuals vary in how they tend to interpret the valence of ambiguous situations. Surprised facial expressions are one example of a well-defined, ambiguous affective event that induces trait-like differences in the propensity to form a positive or negative interpretation. To investigate the nature of this affective bias, we asked participants to organize emotional facial expressions (surprised, happy, sad) into positive/negative categories while recording their hand-movement trajectories en route to each response choice. We found that positivity-negativity bias resulted in differential hand movements for modal versus non-modal response trajectories, such that when an individual categorized a surprised face according to his or her non-modal interpretation (e.g., a negatively biased individual selecting a positive interpretation), the hand showed an enhanced spatial attraction to the alternative, modal response option (e.g., negative) in the opposite corner of the computer screen (Experiment 1). Critically, we also demonstrate that this asymmetry between modal versus non-modal response trajectories is mitigated when the valence interpretations are made under a cognitive load, although the frequency of modal interpretations is unaffected by the load (Experiment 2). These data inform a body of seemingly disparate findings regarding the effect of cognitive effort on affective responses, by showing within a single paradigm that varying cognitive load selectively alters the dynamic motor movements involved in indicating affective interpretations, whereas the interpretations themselves remain consistent across variable cognitive loads.
Keywords: affect, ambiguity, cognitive load, mouse-tracking, valence
People often make judgments concerning what is positive and what is negative, but sometimes we are faced with circumstances that are not easily classified as one or the other. Under such ambiguous conditions, personality or other trait-like factors can influence an individual to assume a positive or negative interpretation. The tendency to ‘see’ negativity versus positivity in one’s experiences can co-vary with various health outcomes and symptoms. For example, an extreme negative bias is observed in major depression, whereas an extreme positive bias is observed in mania (Beck, 1967). In addition, for some individuals, a negative interpretation bias can have similar consequences to experiencing actual danger (Mathews & Mackintosh, 2000) and may play a causal role in producing high levels of state anxiety (Mathews & MacLeod, 2002). We investigated this type of affective bias in a psychologically healthy population using surprised facial expressions as a means of evoking trait-like individual differences in the tendency to make a positive or negative interpretation (positivity-negativity bias; Neta, Norris, & Whalen, 2009). Whereas most facial expressions displaying discrete emotions have a strongly stereotyped valence, surprised facial expressions are uniquely ambiguous, because they occur in response to positive or negative events (Kim, Somerville, Johnstone, Alexander, & Whalen, 2003).
Prior research shows that an individual’s propensity to interpret surprised expressions as either positive or negative appears to be trait-like as it is consistent over the course of a year and can be predicted by short-latency corrugator supercilii responses preceding the ultimate categorization (Neta et al., 2009). Furthermore, interpretations can be manipulated to be more negative by presenting low-spatial-frequency images of surprised expressions (Neta & Whalen, 2010). These converging data are consistent with the notion that this interpretive bias results from relatively low-level, automatic processes that are fairly stable over time. However, reaction time data show that categorizing the valence of surprised expressions takes longer compared to less ambiguous facial expressions (e.g., anger, happy), suggesting a more effortful process may be involved in resolving the valence of surprise (Neta et al., 2009). Still, reaction times offer limited information about the process by which individuals resolve ambiguous valence and can be difficult to interpret when comparing facial expression categories (e.g., Lindquist, Barrett, Bliss-Moreau, & Russell, 2006).
In order to better characterize the process of interpreting ambiguous valence, we used a computer mouse-tracking method to continuously record the trajectory of participants’ hand movements as they selected a valence category for surprised expressions. Computer mouse-tracking can capture an individual’s attraction to competing response options during a forced choice task, by recording the real-time spatial attraction of a participant’s hand to the competing option when en route to the ultimate interpretation (Freeman & Ambady, 2010a; Figure 1). This method has been applied to investigations of ambiguity resolution with respect to race (Freeman, Pauker, Apfelbaum, & Ambady, 2010b) and gender (Hehman, Carpinella, Johnson, Leitner, & Freeman, 2014). Here, we apply this tool to investigate ambiguity resolution in the affective domain.
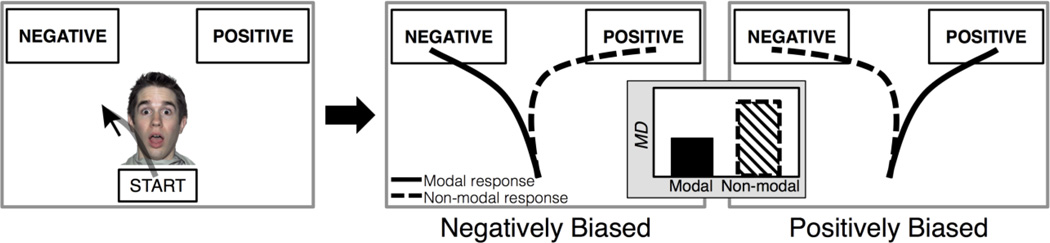
Figure 1.
Schematic illustration of a mouse-tracking trial: after clicking the start button, a face would be displayed and participants moved the mouse to select an interpretation of the emotional expression. One prediction (Hypothesis 1) is that maximum deviation (MD) from an idealized straight-line trajectory will be higher during positive versus negative interpretations for negatively biased participants and higher during negative versus positive interpretations for positively biased participants (i.e., MD is greater during non-modal versus modal response choices).
One clear hypothesis is that whenever individuals choose their non-modal response (e.g., a negatively biased individual interpreting a surprised expression as positive), they will exhibit a spatial attraction towards the alternative modal option (e.g., negative), (Hypothesis 1; Figure 1). After confirming that this relationship between positivity-negativity bias and hand movements indeed occurs (Experiment 1), we subsequently adapted this method to investigate the effect of cognitive load on these biased response patterns (Experiment 2). Given the evidence that positivity-negativity biases arise from lower-level, automatic processing (e.g., Neta & Whalen, 2010), possibly requiring the engagement of a higher-level, regulatory mechanism when choosing the alternative option (Kim et al., 2003), one might predict that adding a cognitive load would exaggerate biased responding (Hypothesis 2a: the response asymmetry found in Exp. 1 will be more pronounced under cognitive load). Alternatively, since participants take longer to categorize surprised expressions compared to clearly valenced expressions (Neta et al., 2009), perhaps the biased behavior requires more effortful processing. Thus, it is possible that adding a cognitive load might mitigate biased responding, particularly if performing the cognitive load task co-opts the neural machinery necessary to organize the biased response patterns (Hypothesis 2b: the response asymmetry found in Exp. 1 will be mitigated under cognitive load).
Experiment 1
Method
Participants
Forty Dartmouth College undergraduates participated for course credit (32 female), a sample size consistent with previous work (Neta et al., 2009). One female subject was excluded because she reported that she was feeling ill such that her condition interfered with her ability to perform the task (resulting N = 39, 31 female). All participants gave written and informed consent in accordance with the Dartmouth Committee for the Protection of Human Subjects.
Stimuli
Stimuli consisted of images of faces taken from the NimStim set of facial expressions (Tottenham, Tanaka, Leon, McCarry, Nurse, et al., 2009). Thirty identities were used (14 female), and each identity had 1 happy, 1 sad, and 1 surprised facial expression, resulting in 90 total images used in the experiment. Happy and sad faces were included in the design as clearly valenced anchors, forcing participants to be ready to choose either response option throughout the experiment regardless of how biased they were with respect to the surprised face condition. Consequently, including these clearly valenced trials reduces the possibility that biased hand movements could result from the mere repetition of a particular movement. These trials also allowed us to ensure that participants were not categorizing faces at random.
Procedure
Prior to the experiment, participants watched an instructional video to introduce them to the experiment presentation software (MouseTracker; Freeman & Ambady, 2010a). This ensured that all participants knew how to correctly respond during trials. Next, participants were instructed that they would be evaluating facial expressions, and that on each trial they should use the computer mouse to choose whether or not the person in the image had just experienced something positive or negative, and that they should respond as quickly as possible. The positive and negative response options were located at the top left and right corners of the computer screen. Trials were self-paced, and participants began each trial by clicking a start button at the bottom center of the screen. Immediately after pressing the start button, a face appeared centered in the screen (with a happy, sad, or surprised expression), and participants moved the mouse to click on “positive” or “negative” response options located in the top corners of the screen (spatial positioning of response options was counterbalanced across participants). Immediately following each response, participants rated on a 5-point Likert scale how confident they were in their response choice for that trial (1 = not at all confident, 5 = extremely confident). If a participant did not respond to the face in 5 seconds, the experiment would move to the next trial. Consistent with previous research, if participants did not begin initiating mouse movement in under 400 milliseconds, after that trial they would see a message instructing them to start moving sooner, even if they had not finalized their decision yet (Freeman & Ambady, 2009; Freeman et al., 2010b). Participants completed 12 practice trials before starting the task (practice trials consisted of face identities different from those in the task trials). The task consisted of 90 self-paced trials (30 trials per emotion category).
Preprocessing and dependent measure
All trajectory data were preprocessed in accordance with previous work (Freeman et al., 2009, 2010a, 2010b). Trajectories were rescaled into a standardized coordinate space (top-left: “−1, 1.5”; bottom-right: “1, 0”) and remapped rightward for comparison. Trajectories were converted into a normalized time space of 101 time bins. Maximum deviation (MD) from an idealized straight-line trajectory (i.e., a straight line from the observed trajectory’s start and endpoints) was computed for each trial as a measure of spatial attraction towards the alternative response option. MD is a commonly used index of the degree to which a competing alternative is activated when a participant is deciding between two response options (Freeman & Ambady, 2010a).
Results
Our report focuses on assessing the relationship between positivity-negativity bias and the MD of mouse movements in response to surprised expressions. Sad and happy faces served as clearly valenced anchors and ensured that participants had to use both response options across the entire experiment and were not responding at random. Thus, the results for these clearly valenced trials are analyzed separately.
Positivity-negativity bias
Participants varied in their tendency to rate surprised faces as positive or negative (% negative ratings: M = 62.48%, SE = 2.87%, Range = 20 – 90%). The percentage of surprised faces rated as negative was used as an index for positivity-negativity bias, consistent with previous research (Neta et al., 2009, 2010).
Maximum Deviation
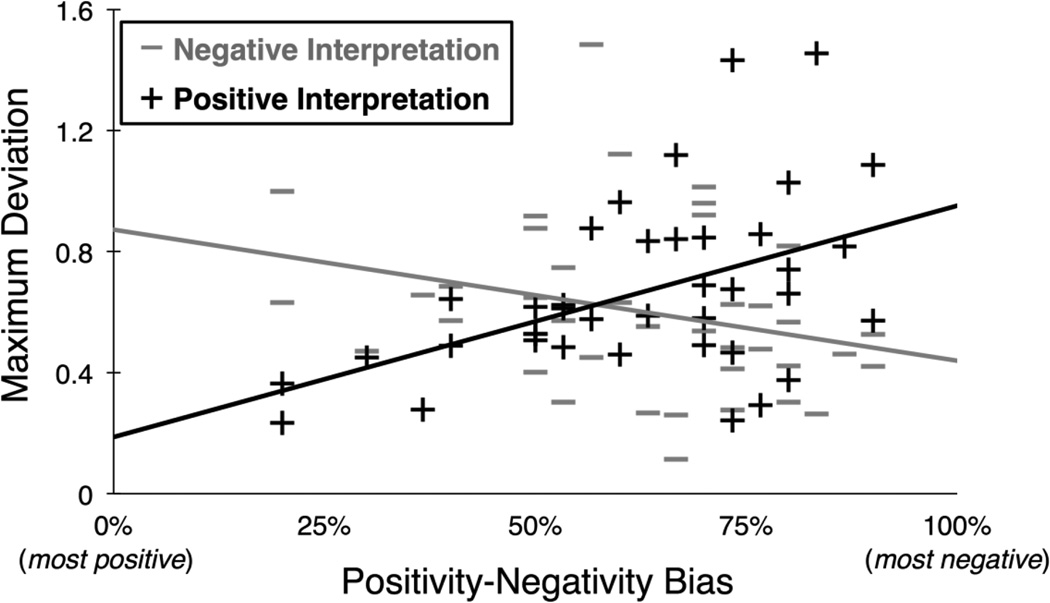
We conducted a repeated measures ANOVA to assess the effect of interpretation outcome (positive or negative) and positivity-negativity bias (which varied between 0 and 100%) on maximum deviation (MD) for surprised face trials. The effect of positivity-negativity bias on MD was not significant, F(1,37) = 0.954, p = 0.335, η2 = 0.025. There was a significant main effect of interpretation outcome, such that MD was larger on trials where the surprised face was rated positive compared to trials where it was rated negative, F(1,37) = 9.620, p = 0.004, η2 = 0.163. Critically, this main effect was qualified by a significant interaction of interpretation outcome × positivity-negativity bias, F(1,37) = 12.387, p = 0.001, η2 = 0.210. As seen in Figure 2, negatively biased participants exhibited a stronger spatial attraction to the negative response option (as indexed by MD) in cases where their ultimate interpretation was positive (correlation between positivity-negativity bias and MD during negative interpretations was marginally significant; r(37) = −0.277, p = 0.088). Additionally, positively biased participants exhibited a stronger spatial attraction to the positive option in cases where their ultimate interpretation was negative (correlation between positivity-negativity bias and MD during positive interpretations was significant; r(37) = 0.474, p = 0.002). This interaction shows that the amount of spatial attraction toward the alternate valence category can be predicted by a combination of the participant’s expected interpretation (i.e., positivity-negativity bias) and the actual interpretation (i.e., positive or negative) on any given trial. Specifically, when a participant did not interpret a surprised face in a manner consistent with his or her positivity-negativity bias, the hand was nevertheless partially attracted to the response option consistent with that bias.
Figure 2.
Positivity-negativity bias influences hand movements when using a computer mouse to categorize surprised facial expressions, such that participants exhibit a spatial attraction to their modal response option when choosing their non-modal response option (consistent with the prediction schematic in Figure 1). Here, MDs for all participants are plotted as a function of positivity-negativity bias, separately for each interpretation outcome (i.e., each participant has one data point for mean MD during positive interpretations and one data point for mean MD during negative interpretations), showing the significant bias × interpretation interaction (p = 0.001, η2 = 0.210).
As a supplementary analysis, we tested whether or not spatial ordering of the positive and negative options could account for biased responses. An independent-samples t-test showed there was no difference between the bias of participants with one spatial ordering versus the other spatial ordering (t(37) = −1.254, p = 0.205). Furthermore, adding spatial ordering as a between subjects covariate to the above analysis on MD only made the reported effects slightly more significant (main effect of response outcome: F(1,36) = 10.025, p = 0.003; response outcome × positivity-negativity bias interaction: F(1,36) = 12.766, p = 0.001). In this supplementary analysis, the effect of bias on MD remained non-significant (F(1,36) = 1.115, p = 0.298), and the effect of spatial ordering and the spatial ordering × response outcome interaction were both not significant (F(1,36) = 0.535, p = 0.469; F(1,36) = 0.288, p = 0.595). This suggests that the observed effects of bias are not simply due to more general leftward or rightward biases.
Happy and sad trials
For completeness, we note that surprised face trials were significantly different than happy and sad face trials for the dependent measures investigated in this study. Predictably, paired-samples t-tests showed that MD was considerably larger on surprised face trials (M = 0.581; SE = 0.033) compared to happy face trials (M = 0.328; SE = 0.027) and sad face trials (M = 0.433; SE = 0.038): happy vs. surprise (t(38) = −6.955, p < 0.001) and sad vs. surprise (t(38) = −4.297, p < 0.001). Additionally, participants were significantly less confident in their categorizations of surprised faces (M = 3.201; SE = 0.089) compared to their categorizations of happy faces (M = 4.863; SE = 0.046) and sad faces (M = 4.618; SE = 0.066): happy vs. surprise (t(38) = 17.874, p < 0.001) and sad vs. surprise (t(38) = 18.541, p < 0.001), confirming that surprise represents an ambiguous valence condition compared to happy and sad. Only correctly categorized trials were included in the calculation of these statistics, as there were not enough incorrect trials to allow for interpretation outcome to be analyzed as a factor with respect to the unambiguous face trials (the vast majority of subjects had 0 incorrect trials for happy and sad conditions).
Reaction Time and Initiation Time
When the above analysis of MD was applied using reaction time (RT) as a dependent measure instead, the results were similar (main effect of positivity-negativity bias was not significant: F(1,37) = 0.356, p = 0.554, η2 = 0.009; main effect of response outcome was significant: F(1,37) = 12.762, p = 0.001, η2 = 0.183; positivity-negativity bias × interpretation outcome interaction was significant: F(1, 37) = 17.674, p < 0.001, η2 = 0.262). However, separate analyses of the positive and negative response options show a non-significant correlation between bias and RT for negative responses (r(37)= −0.104, p = 0.530), and the correlation between bias and RT for positive responses was marginally significant (r(37) = 0.302, p = 0.062). These follow up analyses suggest that MD may be more sensitive to bias than RT in this paradigm. An analysis using initiation time as a dependent measure showed that there was no effect of bias (F(1,37) = 0.403, p = 0.529, η2 = 0.0208) or interpretation outcome (F(1,37) = 1.513, p = 0.226, η2 = 0.0378) on initiation time, nor was there a significant bias × interpretation outcome interaction (F(1,37) = 1.501, p = 0.228, η2 = 0.0375).
Discussion
These results confirm Hypothesis 1, demonstrating that mouse-tracking is sensitive to positivity-negativity bias in the context of ambiguous valence categorization. Specifically, when a participant makes an interpretation counter to his or her bias, that participant is partially attracted to his or her expected (modal) response option located at the opposite corner of the computer screen. In other words, the asymmetry in an individual’s distribution of responses (% surprised faces rated as negative versus positive) is reflected in the asymmetry of their hand trajectories when en route to a modal versus non-modal response choice (deviation to the alternative option was greater during non-modal responses).
One possible mechanism that might explain these asymmetrical hand trajectories is that some form of motor learning occurred as a consequence of practicing a frequent behavior. However, because this task did not include feedback or reinforcement, it is difficult to know why an individual would learn to respond in one direction versus another. Furthermore, the inclusion of clearly valenced conditions ensured that an individual had to be ready to respond in either direction throughout the experiment. For example, although a negatively biased individual would show an increased spatial attraction to the negative option when choosing the positive interpretation for a surprised expression, this attraction did not occur when choosing the positive interpretation for a happy expression. Taken together, it seems unlikely that the effects reported here could be explained by the frequency with which an individual made a particular, directional hand movement.
In Experiment 2, we use this additional measure of biased behavior (i.e., asymmetrical hand trajectories) to investigate the influence of cognitive load on positivity-negativity bias. Previous findings have suggested that positivity-negativity bias depends on automatic, low-level processing that would require a regulatory mechanism when choosing the alternative, unbiased option (Neta et al., 2010; Kim et al., 2003). Given this previous line of work, one might expect bias to be enhanced under cognitive load, particularly if regulating this biased behavior indeed requires effortful processing (Hypothesis 2a). However, participants take longer to categorize surprised faces compared to clearly valenced expressions (Neta et al., 2009), suggesting that positivity-negativity bias follows effortful (not automatic) processing. In the latter case, one might predict that a cognitive load could influence participants to behave in a less biased manner (Hypothesis 2b).
Hypotheses 2a & 2b assume that our two measures of positivity-negativity bias (% negative ratings; asymmetry between modal vs. non-modal hand trajectories) will co-vary, which is expected given that the processing dynamics of any choice task should be inherently linked to the response outcome. However, with respect to this particular task, the distribution of responses (% negative ratings) is thought to represent a trait-like construct that is stable over time (Neta et al., 2009, 2010), whereas the spatial attraction revealed in computer mouse trajectories is thought to reflect a transitory dynamic process of integrating perceptual input with prior knowledge over the course of arriving at a categorization (Freeman & Ambady, 2011). Furthermore, affective motor responses measured by facial electromyography (EMG) reveal that short latency corrugator responses tend to reflect one’s overall distribution of responses, rather than the interpretation on any given trial (Neta et al., 2009). This suggests that on any given trial, the dynamic motor activations are somewhat independent of the particular interpretation outcome (i.e., positive or negative). Therefore, Experiment 2 also allowed us to test if one’s distribution of interpretations (% negative ratings) would be differentially susceptible to changes in cognitive demands compared to the dynamic motor process by which those outcomes are reported (i.e., asymmetrical hand trajectories). That is, Experiment 2 allowed us to investigate two additional hypotheses: whether the asymmetry in hand trajectories is always a direct function of the asymmetry in the distribution of interpretations (Hypothesis 3a), or if a cognitive load differentially affects hand movements compared to the distribution of interpretations (Hypothesis 3b).
Experiment 2
Method
Participants
One hundred and six participants (63 female) took part in this study online via Amazon Mechanical Turk (MTurk) using an in-house, Javascript-based implementation of MouseTracker software. This implementation has been used in tandem with MTurk in previous work (e.g., Hehman et al., 2014). MTurk is a website that coordinates data collection by allowing its users to participate in tasks that are posted by researchers, and in turn are able to receive compensation from the researcher (www.MTurk.com). The quality of MTurk data has been shown to meet or exceed the standards of other published research in the social sciences (Buhrmester, Kwang, & Gosling, 2011). All participation in this study was voluntary.
Procedure
The face stimuli for Experiment 2 were the same as for Experiment 1, and the procedure was generally the same but with an added working memory task that varied between high and low cognitive load. First, participants viewed a brief description of the task and were given the opportunity to consent to their participation. Next, a page with instructions appeared. Participants could take as much time as necessary reading the instructions before continuing with the 12 practice trials and 90 experiment trials. Like Experiment 1, trials were self-paced, and the subject would select a start button at the bottom of the screen to begin a trial. First, a number would appear centered on the screen that the participant was instructed to hold in his or her memory. The number was only on the screen for 4 seconds, then a face would appear centered on the screen (happy, sad, or surprised), and the participant would choose whether the person in the image just experienced something positive or negative by moving the mouse from the bottom center start position to one of the response options at the top left or top right corners of the screen (i.e., positive or negative; spatial ordering counterbalanced across participants). Participants were instructed to begin moving the mouse immediately after a face appeared, even if they had not yet finalized their choice. After a response was selected, a single digit probe was presented, and participants had to choose whether or not the probe was in the number presented at the beginning of the trial. If the participant did not respond to the probe in 5 seconds the experiment would move to the next trial. Trials were divided into blocks with high cognitive load and low cognitive load. For high cognitive load blocks, the number at the beginning always contained 7 digits, whereas for low cognitive load blocks, the number was just a single digit. Ordering of the cognitive load levels was counter balanced across participants: either the first half of trials were high cognitive load and the second half was low cognitive load, or vice versa.
Preprocessing
Trajectories were preprocessed in the same fashion as in Experiment 1. Trials on which participants either took too long to initiate mouse movements (initiation cutoff = 750 milliseconds, mean initiation time = 481.96 milliseconds) or started moving the mouse before the face appeared were considered invalid trials and were excluded from the analyses. The initiation time cut-off was longer for this experiment compared to Experiment 1, because here the presentation of a face prompted the subjects to initiate mouse movement, whereas in the previous experiment the subjects clicked a start button to initiate the face presentation (and in turn initiate mouse movement). The exact value of 750 ms was chosen based on informal pilot experiments conducted in the lab, with an aim to choose a value that was feasible for participants but still required them to initiate a response quickly. Participants received error messages on invalid trials informing them that they should begin initiating movement immediately after the face appeared, even if they had not finalized their choice, but that they should not initiate movement until the face appeared.
Results
Excluded Participants
Sixteen participants that did not score above chance on the working memory task were excluded from the analyses. Eleven additional participants were excluded because they did not have any trials in one or more cells of the design matrix (2 interpretation outcomes × 2 cognitive loads; for example, if a subject rated all surprised faces in high cognitive load as negative they would have no values in one cell of the design). The remaining number of participants was seventy-nine (47 female).
Positivity-Negativity Bias
As in Experiment 1, the percentage of surprised faces rated as negative was used as an index for positivity-negativity bias (% negative: M = 59.26%, SE = 2.29%, Range = 11.54 – 92%). A paired samples t-test showed that positivity-negativity bias was not different for low versus high cognitive load trials (t(78) = 0.822, p = 0.413). We also specifically tested if the load caused individuals to become more or less biased in how they distributed their responses (Hypotheses 2a & 2b). Positively biased participants (a bias score < 50%) on average made more frequent positive interpretations in the low load versus high load condition, and this change in bias score was marginally different from zero (single-sample t-test for change in bias score (high load minus low load) against 0 for the 26 participants with a bias score < 50%: t(25) = 1.789, p = 0.086; Shapiro-Wilks statistic: W = 0.9588, p = 0.368). Similarly, negatively biased participants (bias score > 50%) on average were made more frequent negative interpretations in the low versus high load condition, but the change in bias score was not different from zero (single-sample t-test for change in bias score versus 0, for the 50 participants with a bias score > 50%: t(49) = −0.161, p = 0.872; Shapiro-Wilks statistic: W = 0.9918, p = 0.979). Comparing these two groups directly, an independent samples t-test showed that the difference in the change in bias score (from high to low load) for positively biased individuals versus negatively biased individuals was not significant (t(44.115) = 1.610, p = 0.114). Although Levene’s test was not significant for these two groups (p = 0.352), equal variances were not assumed for this test due to the highly unequal N across conditions (for completeness, assuming equal variances results in marginal significance: t(74) = 1.696, p = 0.094). This finding gives some support for Hypothesis 2a, as positively bias participants made more frequent positive interpretations under high versus low load. However, this effect was only marginally significant, and the frequency of negative interpretations did not change with load for negatively biased participants.
Maximum Deviation
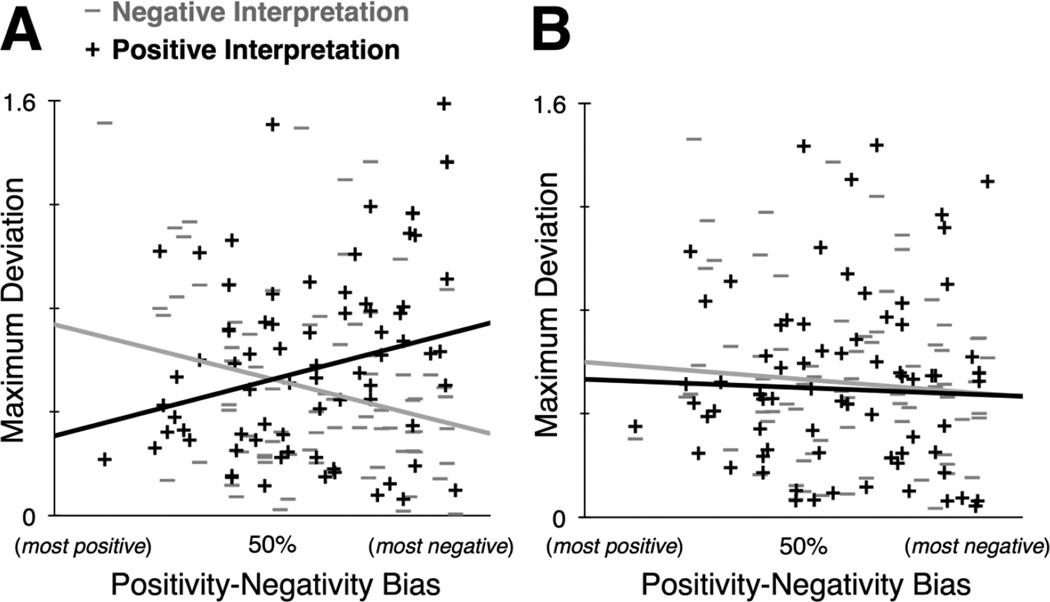
As in Experiment 1, MD was used as a dependent measure quantifying the degree to which the competing alternative interpretation was activated when deciding whether a surprised face was positive or negative. A repeated measures ANOVA was used to determine the effect of the experimental predictors on MD, including two within-subject factors (high/low cognitive load; positive/negative interpretation outcome) and two continuous between-subject predictors (positivity-negativity bias and overall accuracy on the working memory task). Replicating the finding in Experiment 1, we observed a significant positivity-negativity bias × interpretation outcome interaction (F(1,76) = 4.401, p = 0.039, η2 = 0.053). However, this effect was qualified by a 3-way positivity-negativity bias × interpretation outcome × cognitive load interaction (F(1,76) = 7.757, p = 0.007, η2 = 0.091), such that the positivity-negativity bias × interpretation outcome interaction essentially goes away under high cognitive load (Figure 3). This finding is in support of Hypothesis 2b, showing that the asymmetry between modal and non-modal response trajectories is mitigated with high cognitive load. There was also a main effect of working memory accuracy on MD (F(1,76) = 9.232, p = 0.003, η2 = 0.108), such that low accuracy on the working memory task predicted greater MD on all surprised face trials. No other main effects or interactions in the model were significant: (main effect of positivity-negativity bias: F(1,76) = 0.402, p = 0.528, η2 = 0.005; main effect of interpretation outcome: F(1,76) = 1.580, p = 0.213, η2 = 0.019; main effect of load condition: F(1,76) = 0.007, p = 0.935, η2 = 0.0002; interpretation outcome × working-memory accuracy interaction: F(1,76) = 0.383, p = 0.539, η2 = 0.005; load × positivity-negativity bias interaction: F(1,76) = 0.475, p = 0.493, η2 = 0.006; load × working-memory accuracy interaction: F(1,76) = 0.008, p = 0.927, η2 = 0.0002; interpretation outcome × load interaction: F(1,76) = 0.171, p = 0.681, η2 = 0.002; interpretation outcome × load × working-memory accuracy interaction: F(1,76) = 1.508, p = 0.223, η2 = 0.018).
Figure 3.
MD is greater for modal versus non-modal response trajectories only when valence interpretations are made under low (and not high) cognitive load. (A) Under low cognitive load, we find a replication of the bias × interpretation outcome interaction found in Experiment 1 (compare to Figure 2). (B) Under high cognitive load, the bias × interpretation outcome interaction is mitigated. Together these scatterplots illustrate the significant 3-way bias × interpretation outcome × cognitive load interaction on MD (p = 0.007, η2 = 0.091).
Similar to the analyses of Experiment 1, adding spatial ordering as a covariate did not change the significance levels of the effects listed above (bias × interpretation outcome interaction: F(1,75) = 4.145, p = 0.045; 3-way bias × interpretation outcome × load interaction: F(1,75) = 7.243, p = 0.009; main effect of working-memory accuracy: F(1,75) = 8.739, p = 0.004; all other main effects and interactions: p’s > 0.2), and the effect of spatial ordering on MD was not significant (F(1,75) = 0.265, p = 0.608).
Reaction Time and Initiation Time
A repeated measures ANOVA using RT as the dependent measure rather than MD showed that the experimental predictors had no significant effects on RT (main effect of positivity-negativity bias: F(1,76) = 0.881, p = 0.351; main effect of working-memory accuracy: F(1,76) = 0.323, p = 0.571; main effect of interpretation outcome: F(1,76) = 1.708, p = 0.195; main effect of load condition: F(1,76) = 0.000, p = 0.997; bias × interpretation outcome interaction: F(1,76) = 0.041, p = 0.840; interpretation outcome × working-memory accuracy interaction: F(1,76) = 1.974, p = 0.164; load × bias interaction: F(1,76) = 0.317, p = 0.575; load × working-memory accuracy interaction: F(1,76) = 0.006, p = 0.937; interpretation outcome × load interaction: F(1,76) = 1.252, p = 0.267; interpretation outcome × load × working-memory accuracy interaction: F(1,76) = 2.006, p = 0.161; bias × load × interpretation outcome interaction: F(1,76) = 0.630 p = 0.430). Positivity-negativity bias was a significant predictor of initiation time, such that a more negative bias predicted longer initiation times (F(1,76) = 8.300, p = 0.005, η2 = 0.062), which is an effect that was not found in Experiment 1. Overall accuracy on the working memory task was also a significant predictor of initiation time (F(1,76) = 15.642, p < 0.001, η2 = 0.117), such that participants with lower accuracy scores had longer initiation times. There were no other significant effects on initiation time (main effect of interpretation outcome: F(1,76) = 0.003, p = 0.959; main effect of load condition: F(1,76) = 1.146, p = 0.288; bias × interpretation outcome interaction: F(1,76) = 3.877, p = 0.053; interpretation outcome × accuracy interaction: F(1,76) = 0.613, p = 0.436; load × bias interaction: F(1,76) = 0.055, p = 0.814; load × working-memory accuracy interaction: F(1,76) = 1.881, p = 0.174; interpretation outcome × load interaction: F(1,76) = 0.377, p = 0.541; bias × load × interpretation outcome interaction: F(1,76) = 0.319 p = 0.574; interpretation outcome × load × working-memory accuracy interaction: F(1,76) = 0.165, p = 0.686).
General Discussion
Experiment 1 established that positivity-negativity bias is reflected in hand movements that are made during the resolution of ambiguous valence. Specifically, participants showed an enhanced spatial attraction to their modal interpretation whenever selecting their non-modal interpretation. In Experiment 2 we replicated this effect, but further showed that the effect is contingent upon cognitive resources being readily available (supporting Hypothesis 2b). Critically, even though increasing cognitive load mitigated the difference between modal and non-modal trajectories in biased participants, the distribution of valence interpretations themselves remained consistent across different loads (supporting Hypothesis 3b). This finding is in line with previous data showing that these valence interpretations are trait-like (Neta et al., 2009). Taken together, these data suggest that one’s ultimate affective interpretations (as measured via subjective report), are more resistant to an increase in cognitive demands compared to the dynamic motor processes used to express those interpretations.
Generally speaking, a response bias can be towards or against any given category, affective or otherwise. Therefore, it is possible that the effects observed in the current report would hold for biases in other types of non-affective categorization tasks. As a specific example (provided by a reviewer), ambiguous visual illusions can be interpreted in more than one way, and individuals may show consistent biases with respect to such perceptual interpretations. In support of this broader generalization, a similarly structured paradigm was performed with rhesus monkeys, who were trained to move their forearm in two directions in order to move a visual cue up or down (Crutcher & Alexander, 1990). Comparable to the present study, Crutcher and Alexander used a variable physical load to separate neurons that code for the goal of a movement (i.e., up or down were the two possible goals in their paradigm) from those that code for the actual muscle activations required to accomplish that goal (which varied with the weight of the physical load). Similarly, we showed that a variable cognitive load selectively interfered with the dynamic motor process required to indicate an affective interpretation, whereas the ultimate interpretations (i.e., positive or negative interpretations were the two possible goals in the current paradigm) were independent of the load condition. Therefore, the differentiation of a response goal (in this case the affective interpretation) from the response itself may be the result of more domain-general processes. The extension of the current effects to other domains might speak to the overlap between affective and non-affective processes, which is one key objectives of the “conceptual act theory” (Barrett, 2014). However, here we focus our discussion on the implications of these findings for affective biases in particular, as well as affective responses more generally.
Affective responses are theorized to include subjective interpretations as well as motor activations (e.g., James, 1884). A primary output channel for such motor activations is facial movements (e.g., Ekman, 1992; Cacioppo & Gardner, 1999), but arm and hand movements also play a large role in the expression of affect (Pollick, Paterson, Bruderlin, & Sanford, 2001). The current data show that increasing cognitive load has a selective effect on the transitory motor expression of an affective bias, whereas the trait-like subjective report measure of that bias (% negative ratings) was unaffected by the load. This is consistent with the idea that measures of affect obtained via subjective report are less influenced by concurrent cognitive demands compared to more “objective” measures of observable, dynamic motor responses that are used to infer affective interpretations (e.g., facial electromyography; overt approach/avoid behavior, such as deviation in the hand’s response trajectories used in the current report). The possibility that the observable motor component of an affective response may be more malleable than a concomitant subjective report measure is consistent with what has been repeatedly noted by over a century of emotion theorists—that the presence of a particular feeling or emotion cannot be sufficiently classified according to any single, objective behavioral index (Cannon, 1927; Dashiell, 1928; Schachter & Singer, 1962; Griffiths, 1997). For example, although activation of the corrugator supercilli can be correctly taken to indicate the perception of negativity (Dimberg, 1997), this muscle is also activated in response to increases in visual brightness (Plainis, Murray, & Carden, 2006) or increases in the difficulty of a task (Cohen, Davidson, Senulis, Saron, & Weisman, 1992). Consequently, subjective report data (in addition to overt motor behavior) are critical for understanding how a given individual interprets an affective event. Therefore, the current data may speak to the general limitation of characterizing particular affective interpretations with specific motor responses.
The present data also provide insight into the complex relationship between cognitive effort and concurrent emotional responding (see Vuilleumier, 2005; Phelps, 2006; Pessoa, 2008 for reviews). Several studies have found that increasing cognitive effort mitigates emotional responses (e.g., Pessoa, McKenna, Gutierrez, & Ungerleider, 2002; Van Dillen, Heslenfeld, & Koole, 2009). However, other findings suggest that cognitive effort occupies attentional resources required for regulating emotion, ultimately causing a disinhibition that increases emotional responding (e.g., Ward & Mann, 2000; Dolcos & McCarthy, 2006). The present results offer additional information regarding this complex relationship, by demonstrating a mitigating effect of concurrent cognitive effort on the process, but not the outcome, of affective interpretations. Specifically, our results support the relative automaticity of affective biases, and show more generally that it is possible to predict which aspects of affective responses are more likely to be influenced by cognitive effort. Further characterization of this distinction could contribute to our understanding of emotional reactions that are resistant to cognitive emotion-regulation methods (e.g., Ochsner & Gross, 2005; Johnstone, van Reekum, Urry, Kalin, & Davidson, 2007; Raio, Orederu, Palazzol, Shurick, & Phelps, 2013). The proposed dissociation between subjective interpretations and motor expressions may be a useful framework for exploring such distinctions.
Acknowledgments
Special thanks to: George Wolford for statistical advice; an anonymous reviewer for helpful comments; Zach Ingbretsen and Samantha Jacobs for additional assistance.
Funding
This work was supported by National Institute of Mental Health Grant 080716.
Footnotes
Declaration of Conflicting Interests
The authors declare no conflicts of interest with respect to the authorship or publication of this article.
References
- Amin N, Foa EB, Coles ME. Negative interpretation bias in social phobia. Behaviour Research and Therapy. 1998;36(10):945–957. doi: 10.1016/s0005-7967(98)00060-6. [DOI] [PubMed] [Google Scholar]
- Barrett LF. The conceptual act theory: A précis. Emotion Review. 2014;6(4):292–297. [Google Scholar]
- Beck AT. Depression: Clinical, experimental, and theoretical aspects. New York: Harper & Row; 1967. [Google Scholar]
- Buhrmester M, Kwang T, Gosling SD. Amazon's Mechanical Turk a new source of inexpensive, yet high-quality, data? Perspectives on Psychological Science. 2011;6(1):3–5. doi: 10.1177/1745691610393980. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Gardner WL. Emotion. Annual Review of Psychology. 1999;50(1):191–214. doi: 10.1146/annurev.psych.50.1.191. [DOI] [PubMed] [Google Scholar]
- Cannon WB. The James-Lange theory of emotions: A critical examination and an alternative theory. The American Journal of Psychology. 1927;39(1/4):106–124. [PubMed] [Google Scholar]
- Cohen BH, Davidson RJ, Senulis JA, Saron CD, Weisman DR. Muscle tension patterns during auditory attention. Biological Psychology. 1992;33(2):133–156. doi: 10.1016/0301-0511(92)90028-s. [DOI] [PubMed] [Google Scholar]
- Crutcher MD, Alexander GE. Movement-related neuronal activity selectively coding either direction or muscle pattern in three motor areas of the monkey. Journal of Neurophysiology. 1990;64(1):151–163. doi: 10.1152/jn.1990.64.1.151. [DOI] [PubMed] [Google Scholar]
- Dashiell JF. Are there any native emotions? Psychological Review. 1928;35(4):319–327. [Google Scholar]
- Dimberg U. Facial reactions: Rapidly evoked emotional responses. Journal of Psychophysiology. 1997;11(2):115–123. [Google Scholar]
- Dolcos F, McCarthy G. Brain systems mediating cognitive interference by emotional distraction. The Journal of Neuroscience. 2006;26(7):2072–2079. doi: 10.1523/JNEUROSCI.5042-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P. An argument for basic emotions. Cognition & Emotion. 1992;6(3/4):169–200. [Google Scholar]
- Freeman JB, Ambady N. Motions of the hand expose the partial and parallel activation of stereotypes. Psychological Science. 2009;20(10):1183–1188. doi: 10.1111/j.1467-9280.2009.02422.x. [DOI] [PubMed] [Google Scholar]
- Freeman JB, Ambady N. MouseTracker: Software for studying real-time mental processing using a computer mouse-tracking method. Behavior Research Methods. 2010a;42(1):226–241. doi: 10.3758/BRM.42.1.226. [DOI] [PubMed] [Google Scholar]
- Freeman JB, Pauker K, Apfelbaum EP, Ambady N. Continuous dynamics in the real-time perception of race. Journal of Experimental Social Psychology. 2010b;46(1):179–185. [Google Scholar]
- Freeman JB, Ambady N. A dynamic interactive theory of person construal. Psychological Review. 2011;118(2):247. doi: 10.1037/a0022327. [DOI] [PubMed] [Google Scholar]
- Griffiths PE. What emotions really are: The problem of psychological categories. Chicago: University of Chicago Press; 1997. [Google Scholar]
- Hehman E, Carpinella CM, Johnson KL, Leitner JB, Freeman JB. Early processing of gendered facial cues predicts the electoral success of female politicians. Social Psychological and Personality Science. 2014;5(7):815–824. [Google Scholar]
- James W. What is an emotion? Mind. 1884;34:188–205. [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. The Journal of Neuroscience. 2007;27(33):8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Somerville LH, Johnstone T, Alexander AL, Whalen PJ. Inverse amygdala and medial prefrontal cortex responses to surprised faces. Neuroreport. 2003;14(18):2317–2322. doi: 10.1097/00001756-200312190-00006. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. NIMH, Center for the Study of Emotion & Attention; 2005. [Google Scholar]
- Lindquist KA, Barrett LF, Bliss-Moreau E, Russell JA. Language and the perception of emotion. Emotion. 2006;6(1):125–138. doi: 10.1037/1528-3542.6.1.125. [DOI] [PubMed] [Google Scholar]
- Mathews A, Mackintosh B. Induced emotional interpretation bias and anxiety. Journal of Abnormal Psychology. 2000;109(4):602–615. [PubMed] [Google Scholar]
- Mathews A, MacLeod C. Induced processing biases have causal effects on anxiety. Cognition & Emotion. 2002;16(3):331–354. [Google Scholar]
- Neta M, Norris CJ, Whalen PJ. Corrugator muscle responses to surprised facial expressions are associated with individual differences in positivity-negativity bias. Emotion. 2009;9(5):640–648. doi: 10.1037/a0016819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neta M, Whalen PJ. The primacy of negative interpretations when resolving the valence of ambiguous facial expressions. Psychological Science. 2010;21(7):901–907. doi: 10.1177/0956797610373934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Pessoa L, McKenna M, Gutierrez E, Ungerleider LG. Neural processing of emotional faces requires attention. Proceedings of the National Academy of Sciences. 2002;99(17):11458–11463. doi: 10.1073/pnas.172403899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. On the relationship between emotion and cognition. Nature Reviews Neuroscience. 2008;9(2):148–158. doi: 10.1038/nrn2317. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annual Reviews of Psychology. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- Plainis S, Murray IJ, Carden D. The dazzle reflex: electrophysiological signals from ocular muscles reveal strong binocular summation effects. Ophthalmic and Physiological Optics. 2006;26(3):318–325. doi: 10.1111/j.1475-1313.2006.00350.x. [DOI] [PubMed] [Google Scholar]
- Pollick FE, Paterson HM, Bruderlin A, Sanford AJ. Perceiving affect from arm movement. Cognition. 2001;82(2):B51–B61. doi: 10.1016/s0010-0277(01)00147-0. [DOI] [PubMed] [Google Scholar]
- Raio CM, Orederu TA, Palazzolo L, Shurick AA, Phelps EA. Cognitive emotion regulation fails the stress test. Proceedings of the National Academy of Sciences. 2013;110(37):15139–15144. doi: 10.1073/pnas.1305706110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter S, Singer J. Cognitive, social, and physiological determinants of emotional state. Psychological Review. 1962;69(5):379–399. doi: 10.1037/h0046234. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Nelson C. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Research. 2009;168(3):242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dillen LF, Heslenfeld DJ, Koole SL. Tuning down the emotional brain: an fMRI study of the effects of cognitive load on the processing of affective images. Neuroimage. 2009;45(4):1212–1219. doi: 10.1016/j.neuroimage.2009.01.016. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P. How brains beware: neural mechanisms of emotional attention. Trends in Cognitive Sciences. 2005;9(12):585–594. doi: 10.1016/j.tics.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Ward A, Mann T. Don't mind if I do: disinhibited eating under cognitive load. Journal of Personality and Social Psychology. 2000;78(4):753. doi: 10.1037//0022-3514.78.4.753. [DOI] [PubMed] [Google Scholar]