Abstract
Palmitoylethanolamide (PEA) and oleoylethanolamide (OEA) are endogenous lipid mediators that suppress inflammation. Their actions are terminated by the intracellular cysteine amidase, N-acylethanolamine acid amidase (NAAA). Even though NAAA may offer a new target for anti-inflammatory therapy, the lipid-like structures and reactive warheads of current NAAA inhibitors limit the use of these agents as oral drugs. Here, we describe a series of novel benzothiazole-piperazine derivatives that inhibit NAAA in a potent and selective manner via a non-covalent mechanism. A prototype member of this class (8) displays high oral bioavailability, access to the central nervous system (CNS), and strong activity in mouse model of multiple sclerosis (MS). This compound exemplifies a second generation of non-covalent NAAA inhibitors that may be useful in the treatment of MS and other chronic CNS disorders.
Keywords: N-acylethanolamine acid amidase, Cysteine hydrolase, Palmitoylethanolamide, Oleoylethanolamide, Neuroinflammation, Multiple sclerosis
Graphical abstract
The cysteine amidase N-acyl ethanolamine acid amidase (NAAA) promotes inflammation by degrading endogenous anti-inflammatory lipid amides such as palmitoylethanolamide (PEA). A new class of non-covalent orally available NAAA inhibitors (e.g., 8) interrupt NAAA-mediated lipid amide degradation in the brain and are strongly protective in a mouse model of neuroinflammation.
NAAA is cysteine hydrolase that catalyses the biodegradation of PEA and OEA (Figure 1),[1] two lipids that suppress inflammation by activating the ligand-operated transcription factor, peroxisome proliferator-activated receptor-α (PPAR-α).[2] Macrophages and other host-defense cells constitutively generate PEA and OEA in amounts that are sufficient to fully engage PPAR-α.[3] This process is halted during inflammation leading to decrease in PPAR-α signaling and acceleration of the inflammatory response.[3] Accordingly, small-molecule NAAA inhibitors restore normal PEA and OEA levels in inflamed tissues and exert marked anti-inflammatory effects in animal models, pointing to NAAA as a potential target for therapy.[4]
Figure 1.

NAAA hydrolyzes saturated and monounsaturated fatty acid ethanolamides (e.g., PEA, 1) into fatty acid (e.g., palmitic acid, 2) and ethanolamine.
Figure 2 illustrates the three most potent classes of NAAA inhibitors described so far. Each class is defined by the presence of a chemical warhead – β-lactone, β-lactam or isothiocyanate – that can react covalently with NAAA's catalytic cysteine to form a thioester bond.[5] While potent and, in some cases, systemically active, these molecules share two features that limit their use as oral drugs: first, the presence of a reactive warhead lowers their metabolic stability (e.g., for β-lactone 3)[6] or increases risk of allergic reactions (e.g., for β-lactam 4 and isothiocyanate 6);[7] and, second, the hydrophobic fragment that ensures target recognition by these agents negatively impacts drug-likeness.
Figure 2.

Structures of published NAAA inhibitors (3,[6] 4,[5b] 6[8]), activity-based NAAA probe (5)[9] (top), and compounds reported here (bottom).
Here, we describe a series of benzothiazole-piperazine derivatives that non-covalently inhibit NAAA (Figure 2). Our experiments indicate that compound 8, a representative member of this class, is potent, selective for NAAA and orally available. Moreover, 8 crosses the blood-brain barrier, elevates PEA and OEA levels in the CNS, and is strongly active in a mouse model of MS.
A screening campaign to discover new scaffolds for NAAA inhibition yielded the low-potency hit 7 (Table 1). To improve potency, we launched a structure-activity relationship (SAR) study starting with the benzamide portion of 7. Removal of the o-methyl group (9) or replacement of such group with a halogen (10, 11) caused complete loss of activity. By contrast, substitutions with a methoxy (12), methylsulfonyl (13) or ethylsulfonyl (14) group yielded compounds of progressively greater potency. Since moving the ethylsulfonyl substituent to the meta or para position of the phenyl ring strongly reduced activity (15, 16), we focused our exploration on o-sulfonyl derivatives containing linear, branched or cyclic alkyl groups (Table 2). We found that potency was highly sensitive to length and size of the alkyl fragment, with bulkier substituents producing weaker inhibition (e.g., 18, 23).
Table 1.
Inhibitory potencies (IC50 in μM) of compounds 7 and 9–16 on the activity of hNAAA expressed in HEK-293 cells.

| Compound | X | hNAAA IC50 (μM)[a] |
|---|---|---|
| 7 | o-CH3 | 88.9 |
| 9 | H | NA[b] |
| 10 | o-Cl | NA |
| 11 | o-F | NA |
| 12 | O-OCH3 | 20.5 |
| 13 | O-SO2CH3 | 5.69 ± 2.54 |
| 14 | O-SO2CH2CH3 | 0.45 ± 0.11 |
| 15 | m-SO2CH2CH3 | NA |
| 16 | p-SO2CH2CH3 | NA |
Values are the mean ± SEM of three or more determinations, or the mean of triplicate determinations in a single experiment.
Not Active, <30% inhibition at 100 μM.
Table 2.
Potencies of compounds 17–23 on hNAAA.
| Compound | R | hNAAA IC50 (μM)[a] |
|---|---|---|
| 17 | (CH2)2CH3 | 0.39 ± 0.06 |
| 18 | (CH2)3CH3 | 8.80 ± 2.40 |
| 19 | i-propyl | 0.50 ± 0.09 |
| 20 | c-propyl | 0.19 ± 0.03 |
| 21 | c-butyl | 0.32 ± 0.00 |
| 22 | c-pentyl | 1.10 ± 0.09 |
| 23 | c-hexyl | 38.11 ± 5.49 |
See Footnote for Table 1.
We then turned to the benzothiazole system, using 14 as an entry point (Table 3). Removal of the 6-F phenyl substituent did not affect potency (24), whereas insertion of electron-withdrawing (25, 26) or -donating (27, 28) groups was detrimental. Moving a halogen at various positions of the ring caused either minor effects or decrease in potency (29–32).
Table 3.
Potencies of compounds 24–32 on hNAAA.
| Compound | Y | hNAAA IC50 (μM)[a] |
|---|---|---|
| 24 | H | 0.43 ± 0.03 |
| 25 | 6-Cl | 1.55 ± 0.29 |
| 26 | 6-CF3 | 7.2 |
| 27 | 6-CH3 | 6.87 |
| 28 | 6-OCH3 | 5.73 |
| 29 | 4-F | 0.55 ± 0.20 |
| 30 | 5-F | 2.95 ± 0.84 |
| 31 | 7-F | 0.30 ± 0.02 |
| 32 | 7-Cl | 0.71 ± 0.21 |
See Footnote for Table 1.
Next, we probed the role of the piperazine by replacing it with alternatively oriented piperidines (Table 4). The compounds obtained were either weakly (33) or moderately (34) active, with 33 being ≈60 times less potent than 14. Loss of potency was also observed with 35, in which a methylene bridge replaced the benzamide carbonyl of 14. To examine the effect of conformational changes in the piperazine ring, we introduced one or two methyl groups at various positions of this moiety (36–39). These attempts did not lead to improvements in potency, but confirmed the role for a mono-substituted piperazine in this region, as shown by the drop in activity caused by 2,2-dimethyl substitution (38). Confirming this, we noted that potency was highly sensitive to the configuration of the methyl group, with 40, the (R)-enantiomer of 36, being 15 times less potent than the (S)-enantiomer 8. Alkyl chain modifications on the sulfonyl substituent of 8 yielded no further improvement (41, 42).
Table 4.
Potencies of compounds 8 and 33–42 on hNAAA.
| Compound | Structure | hNAAA IC50 (μM)[a] |
|---|---|---|
| 33 |

|
26.17 ± 7.15 |
| 34 |

|
1.77 ± 0.18 |
| 35 |

|
1.82 ± 0.32 |
| 36 |

|
0.50 ± 0.075 |
| 37 |

|
0.72 ±0.12 |
| 38 |

|
NA[b] |
| 39 |

|
1.44 ± 0.14 |
| 40 |

|
3.43 ± 0.50 |
| 8 |

|
0.23 ± 0.04 |
| 41 |
|
0.20 ± 0.05 |
| 42 |

|
0.18 ± 0.04 |
See Footnote for Table 1.
We focused subsequent mechanistic work on 8 because of its greater water solubility, compared to other similarly potent compounds (Table 5; Table S1, Supporting Info). To support this work, an easily scalable synthesis was developed (Scheme 1).
Table 5.
In vitro metabolism and pharmacokinetic properties of 8 in mice.[a]
| Solubility in PBS (μM) | 139 ± 1 |
| Plasma t1/2 (min) | >120[b] |
| MLM (NADPH) t1/2 (min) | >60[c] |
| MLM (UDPG) t1/2 (min) | >60[d] |
| 3 mg/kg, i.v | 3 mg/kg, p.o. | |
|---|---|---|
| Cmax (ng/mL) | 1660±166 | 613±68 |
| Tmax (min) | (5.0) | 30 |
| Cl (mL/min/Kg) | 33.2±1.6 | 49±8 |
| t1/2 (min) | 73.9±3.7 | 104±16 |
| Vd (L/Kg) | 3.5±0.2 | 7.4±1.1 |
| AUCplasma (h × ng/mL) | 1366.8±68.3 | 988±157 |
| AUCbrain (h × ng/mL) | 404.3±109.1 | 181±28 |
| F (%) | - | 72±11 |
NADPH, reduced nicotinamide adenine dinucleotide phosphate; PBS, phosphate-buffered saline; t1/2, terminal half life; UDPG, uridine diphosphate glucose; AUC, area under the curve; Cmax, maximal plasma concentration; F%, oral bioavailability; Tmax, time at which Cmax is reached.
83± 4% remaining at 120 min;
70± 7% remaining at 60 min;
96% remaining at 60 min.
Scheme 1.

a) (2S)-2-methylpiperazine, NaHCO3, EtOH/ H2O, reflux, 15 h, quant.; b) EtI, 2M NaOH, EtOH, rt, 15 h, quant.; c) Oxone, H2O, 40°C, 15 h, 96%; d) HATU, Et3N, CH3CN, rt, 15 h, 43%.
Current NAAA inhibitors react covalently with the enzyme's catalytic cysteine.[5] To probe the interaction of 8 with NAAA, we used four approaches. First, we incubated purified human (h) NAAA (2 μM) with 8 (50 μM), digested it with trypsin and searched for covalent adducts using liquid chromatography mass spectrometry (LC-MS). As positive control, we included the β-lactam 4 (20 μM), whose covalent interaction with NAAA is documented.[5b] Incubating hNAAA with 4 yielded the expected acylated peptide, whereas no such adduct was found when hNAAA was exposed to either 8 or vehicle (Figure 3A). Similarly, no covalent adducts were retrieved by a search through the entire peptide map of hNAAA (Figure S1, Supporting Info). Second, we incubated hNAAA (4.0 μM) with 4 or 8 (1.0 μM), precipitated the protein, and measured compounds in the supernatant by LC-MS. Whereas 4 was precipitated with NAAA, as expected from its covalent binding to the enzyme, 8 was entirely recovered in the supernatant (Figure 3B). Third, we asked whether 8 prevents the binding of the covalent activity-based probe 5 to NAAA.[9] As previously shown,[9] 5 labeled hNAAA in cell extracts, and this effect was blocked by incubation with 4 (Figure 3C). By contrast, incubation with 8 antagonized hNAAA labeling by 5 only partially and at short incubation times (Figure 3C). Lastly, kinetic analyses revealed that 8 inhibits NAAA via an uncompetitive mechanism (Figure 4A, B). The results identify 8 as the first non-covalent NAAA inhibitor disclosed to date.
Figure 3.

Compound 8 inhibits NAAA via a non-covalent mechanism. (A) LC-MS tracings showing (top) that the covalent inhibitor 4 forms an adduct with a peptide containing NAAA's catalytic C126 (C126TSIVAQDSR), as illustrated in the inset, whereas (bottom) 8 or its vehicle (DMSO) have no such effect. (B) Covalent inhibitor 4 (top) or 8 (bottom) was incubated with NAAA (filled bars) or buffer alone (open bars) and quantified in supernatant after protein precipitation. Bars: mean ± SEM, n =3. (C) Lysosomal extracts of hNAAA-overexpressing HEK293 cells were incubated with vehicle (2% DMSO), 4 or 8 for 2 h before addition of probe 5. A rhodamine fluorophore was inserted by click chemistry. The arrowhead indicates NAAA. Top, fluorescence; bottom, Coomassie blue staining (loading control).
Figure 4.

(A) Michaelis–Menten analysis of the reaction of hNAAA in the presence of vehicle (DMSO, black circle) or 8 (50 nM, filled triangle; 200 nM, filled square). (B) Lineweaver–Burk plot indicates uncompetitive inhibition (inset: magnification of the plot close to its origin).
The unprecedented mechanistic profile of 8 prompted us to test the compound's usefulness as an oral agent. In vitro studies demonstrated that 8 is soluble in aqueous buffer (pH 7.4) and stable in mouse plasma (Table 5). Similarly, 8 is stable in mouse liver microsomes (MLM).
Experiments in mice showed that 8 is extensively absorbed after oral administration (Figure 5A). The pharmacokinetic (PK) parameters listed in Table 5 indicate that 8 had excellent oral bioavailability. Importantly, 8 crossed the blood-brain barrier, reaching a brain-to-plasma ratio of 0.2 and causing a substantial accumulation of PEA and OEA in brain tissue (Figure 5A, B). No changes were seen in the levels of the anandamide, an endocannabinoid lipid amide that is degraded by fatty acid amide hydrolase (FAAH) rather than by NAAA.[3]
Figure 5.

PK and pharmacodynamic profiles of 8 in mice. (A) Levels of 8 in plasma (black circles) or brain (gray triangles) after oral administration (3 mg/kg). (B) Time course of the effects of 8 (30 mg/kg) on PEA, OEA and anandamide (AEA) levels in brain. Results are expressed as mean ± SEM, n = 3. * P< 0.05; **, P < 0.01; ***, P < 0.001, one-way ANOVA.
A selectivity screen showed that 8 (10 μM) had little or no effect on a panel of >50 receptors, ion channels and transporters (Table S2, Supporting Info). Moreover, as expected from our target engagement studies (Figure 5B), 8 had only a weak inhibitory effect on FAAH (IC50 ≈10 μM),[10] and no effect on either acid ceramidase, a cysteine amidase that has 33–34% identity with NAAA,[1] or monoacylglycerol lipase, a serine hydrolase that degrades the endocannabinoid 2-arachidonoyl-sn-glycerol.[11]
MS is a neuroinflammatory disorder accompanied by alterations in cerebrospinal and plasma levels of PEA and OEA.[12] Because PEA administration attenuates spasticity in the experimental allergic encephalomyelitis (EAE) model of MS,[13] we tested whether accrual of PEA/OEA by treatment with 8 might be beneficial in this model. EAE mice and sham-immunized controls were treated with 8 (30 mg/kg, twice daily) or vehicle for 28 days while recording clinical scores and body weight gain. Clinical scores were generated using a symptom scale that ranged from 0 (no clinical signs) to 5 (moribund).[14]
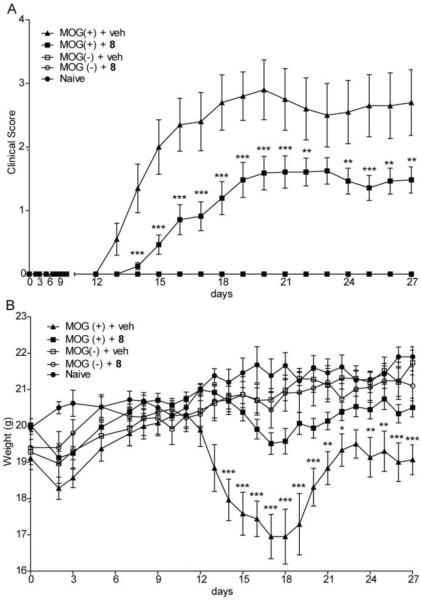
Figure 6 shows that treatment with 8 did not affect sham-immunized mice, whereas it delayed disease onset, attenuated symptom intensity, and normalized body weight in EAE animals. Moreover, 8 reduced mononuclear cell infiltration and microglia activation - two anatomical correlates of disease - in spinal cord of EAE mice (Figure S2, Supporting Info). Underscoring the superiority of 8 over existing NAAA inhibitors, the highly potent irreversible β-lactam derivative 4 had no effect in the EAE model (Figure S3, Supporting Info).
Figure 6.
Time-course of the effects of 8 or vehicle on (A) clinical score and (B) body weight in EAE mice and sham-immunized controls. 8 was administered at 30 mg/kg twice daily. MOG: myelin oligodendrocyte glycoprotein 35–55, antigen used to induce EAE. Results are expressed as mean ± SEM, n = 30. * P< 0.05; **, P < 0.01; ***, P < 0.001, two-way ANOVA followed by Bonferroni post hoc test.
In sum, the present study describes a novel class of benzothiazole-piperazine derivatives that inhibit NAAA through a non-covalent, uncompetitive mechanism. A representative member of this class, 8, shows excellent oral PK properties and good brain penetration. Its strong protective activity in a mouse model of MS stands comparison with other agents used to target MS.[15] Compound 8 exemplifies a new generation of non-covalent NAAA inhibitors that may find therapeutic applications in the treatment of neuroinflammation.
Supplementary Material
Acknowledgements
The work was partially supported by a grant from the National Institute on Drug Abuse (to DP). The authors thank Dr Angelo Reggiani for discussion; Dr Annalisa Fiasella, Dr Andrea Nuzzi and Dr Stefano Ponzano for the synthesis of 4 and 5; Natasha Margaroli and Ilaria Penna for assay support and Silvia Venzano for compound handling.
References
- [1].a) Tsuboi K, Sun YX, Okamoto Y, Araki N, Tonai T, Ueda N. J Biol Chem. 2005;280:11082–11092. doi: 10.1074/jbc.M413473200. [DOI] [PubMed] [Google Scholar]; b) Tsuboi K, Takezaki N, Ueda N. Chem Biodivers. 2007;4:1914–1925. doi: 10.1002/cbdv.200790159. [DOI] [PubMed] [Google Scholar]; c) Ueda N, Tsuboi K, Uyama T. Prog Lipid Res. 2010;49:299–315. doi: 10.1016/j.plipres.2010.02.003. [DOI] [PubMed] [Google Scholar]
- [2].Pontis S, Ribeiro A, Sasso O, Piomelli D. Crit Rev Biochem Mol Biol. 2016;51:7–14. doi: 10.3109/10409238.2015.1092944. [DOI] [PubMed] [Google Scholar]
- [3].Piomelli D, Sasso O. Nat Neurosci. 2014;17:164–174. doi: 10.1038/nn.3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bandiera T, Ponzano S, Piomelli D. Pharmacol Res. 2014;86:11–17. doi: 10.1016/j.phrs.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].a) Armirotti A, Romeo E, Ponzano S, Mengatto L, Dionisi M, Karacsonyi C, Bertozzi F, Garau G, Tarozzo G, Reggiani A, Bandiera T, Tarzia G, Mor M, Piomelli D. ACS Med Chem Lett. 2012;3:422–426. doi: 10.1021/ml300056y. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Ribeiro A, Pontis S, Mengatto L, Armirotti A, Chiurchiu V, Capurro V, Fiasella A, Nuzzi A, Romeo E, Moreno-Sanz G, Maccarrone M, Reggiani A, Tarzia G, Mor M, Bertozzi F, Bandiera T, Piomelli D. ACS Chem Biol. 2015;10:1838–1846. doi: 10.1021/acschembio.5b00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Duranti A, Tontini A, Antonietti F, Vacondio F, Fioni A, Silva C, Lodola A, Rivara S, Solorzano C, Piomelli D, Tarzia G, Mor M. J Med Chem. 2012;55:4824–4836. doi: 10.1021/jm300349j. [DOI] [PubMed] [Google Scholar]
- [7].a) Ariza A, Mayorga C, Fernandez TD, Barbero N, Martin-Serrano A, Perez-Sala D, Sanchez-Gomez FJ, Blanca M, Torres MJ, Montanez MI. J Investig Allergol Clin Immunol. 2015;25:12–25. [PubMed] [Google Scholar]; b) Karlsson I, Samuelsson K, Ponting DJ, Tornqvist M, Ilag LL, Nilsson U. Sci Rep. 2016;6:21203. doi: 10.1038/srep21203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Alhouayek M, Bottemanne P, Subramanian KV, Lambert DM, Makriyannis A, Cani PD, Muccioli GG. FASEB J. 2015;29:650–661. doi: 10.1096/fj.14-255208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Romeo E, Ponzano S, Armirotti A, Summa M, Bertozzi F, Garau G, Bandiera T, Piomelli D. ACS Chem Biol. 2015;10:2057–2064. doi: 10.1021/acschembio.5b00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ahn K, McKinney MK, Cravatt BF. Chem Rev. 2008;108:1687–1707. doi: 10.1021/cr0782067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Blankman JL, Simon GM, Cravatt BF. Chem Biol. 2007;14:1347–1356. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].a) Di Filippo M, Pini LA, Pelliccioli GP, Calabresi P, Sarchielli P. J Neurol Neurosurg Psychiatry. 2008;79:1224–1229. doi: 10.1136/jnnp.2007.139071. [DOI] [PubMed] [Google Scholar]; b) Jean-Gilles L, Feng S, Tench CR, Chapman V, Kendall DA, Barrett DA, Constantinescu CS. J Neurol Sci. 2009;287:212–215. doi: 10.1016/j.jns.2009.07.021. [DOI] [PubMed] [Google Scholar]
- [13].Baker D, Pryce G, Croxford JL, Brown P, Pertwee RG, Makriyannis A, Khanolkar A, Layward L, Fezza F, Bisogno T, Di Marzo V. FASEB J. 2001;15:300–302. doi: 10.1096/fj.00-0399fje. [DOI] [PubMed] [Google Scholar]
- [14].Stromnes IM, Goverman JM. Nat Protoc. 2006;1:1810–1819. doi: 10.1038/nprot.2006.285. [DOI] [PubMed] [Google Scholar]
- [15].a) Choi JW, Gardell SE, Herr DR, Rivera R, Lee CW, Noguchi K, Teo ST, Yung YC, Lu M, Kennedy G, Chun J. Proc Natl Acad Sci U S A. 2011;108:751–756. doi: 10.1073/pnas.1014154108. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Deng H, Bernier SG, Doyle E, Lorusso J, Morgan BA, Westlin WF, Evindar G. ACS Med Chem Lett. 2013;4:942–947. doi: 10.1021/ml400194r. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Chen H, Assmann JC, Krenz A, Rahman M, Grimm M, Karsten CM, Kohl J, Offermanns S, Wettschureck N, Schwaninger M. J Clin Invest. 2014;124:2188–2192. doi: 10.1172/JCI72151. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.