Abstract
Background
Helicobacter pylori (H. pylori) prevalence in Western countries has been declining simultaneously with increases in childhood asthma and allergic diseases; prior studies have linked these phenomena.
Aims
We aimed to examine the association between H. pylori colonization in children and risk of asthma and related conditions at school age. We secondly examined additional effects of maternal H. pylori status by pairing with children's status.
Methods
This study was embedded in a multi-ethnic population-based cohort in Rotterdam, The Netherlands. We measured anti-H. pylori and anti-CagA antibodies in serum of children obtained at age 6 years, and of their mothers obtained during mid-pregnancy. Asthma or related conditions were reported for children at age 6 years. We used multivariate logistic regression analyses among 3,797 subjects.
Results
In children, the H. pylori positivity rate was 8.7%, and 29.2% of these were CagA-positive. A child's colonization with a CagA-negative-H. pylori strain was associated with an increased risk of asthma (Odds ratio 2.11; 95% CI 1.23-3.60, but this differed for European (3.64; 1.97-6.73) and non-European (0.52; 0.14-1.89) children. When taking into account maternal H. pylori status, only H. pylori positive children with an H. pylori negative mother had increased risk of asthma (2.42; 1.11-5.27), accounting for 3.4% of the asthma risk.
Conclusions
Colonization of a European child with a CagA-negative-H. pylori strain at age 6 was associated with an increased prevalence of asthma, but there was no association for non-European children. The underlying mechanisms for the observed risk differences require further research.
Keywords: disappearing microbiota, asthma, atopy, allergy, birth-cohort effects
Introduction
Reduced exposure to exogenous microbes and their products have been suggested to be involved in the pathogenesis of asthma and related conditions, such as eczema and allergies (1). An alternate hypothesis is that the epidemic rise in asthma and related conditions may be partially explained by altered composition of our indigenous microbiota due to changes in human ecology (2). The gastric bacterium Helicobacter pylori (H. pylori) has been used as a proxy for this modern phenomenon (2). Studies in mice have demonstrated that experimental infection with H. pylori prevents allergic asthma through the induction of regulatory T cells (Tregs) (3). Direct contact between H. pylori and dendritic cells (DCs) was found to be essential for the induction of tolerogenic DCs. These DCs produce IL-18, important for the conversion of naïve T-cells into Tregs (3). Eventually, these Tregs may suppress asthmatic immune responses in the airways (4). This interaction between H. pylori and the immune system may be affected by its genotype: expression of the cagA gene (cytotoxin-associated gene A) has been associated with a more marked cellular and humoral immune response, with persists throughout human life (5).
During recent decades the prevalence of H. pylori colonization has dropped dramatically in Western countries (6). Currently, fewer than 10% of children born in Western countries are H. pylori positive (7) (8). Several epidemiologic studies examined the relation between H. pylori colonization and asthma and asthma-related conditions in childhood (9-14). However, results from these studies appear contradictory. This may have been due to differences in study design (9, 10, 14), low number of participants (9, 10, 13), differing methods and timing of H. pylori status identification (11, 14), and insufficient accounting for potential confounders (12, 14). It also is unclear whether maternal H. pylori colonization during pregnancy affects the child's risk for asthma and related conditions. Maternal H. pylori colonization during pregnancy may be a risk factor for foetal growth retardation (15, 16), which subsequently might lead to increased risk for asthma (17). Children of an H. pylori-positive mother may be more likely to acquire colonization at younger age, but it is not known whether this also may affect risk of asthma and related conditions.
Therefore, we first examined the associations of asthma and related conditions with H. pylori colonization in children, and secondly we focused on the effect of paired maternal and child's H. pylori status on these outcomes. These analyses were facilitated by a large multi-ethnic population-based prospective cohort study in Rotterdam, The Netherlands.
Methods
Design and setting
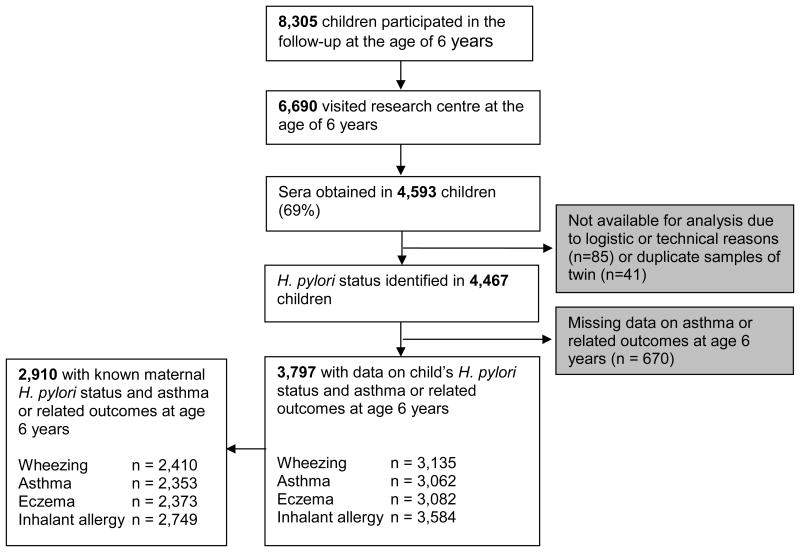
This study was embedded in the Generation R Study, a population-based prospective cohort study of pregnant women and their children in Rotterdam, The Netherlands (18). All children were born between April 2002 and January 2006. The Medical Ethics Committee of the Erasmus University Medical Centre approved the study protocol and parents gave written informed consent for themselves and their children. For the current study, data from 3,797 children with information on H. pylori colonization and any asthma or related conditions were available (Figure 1).
Figure 1. Study design and number of participants.
H. pylori colonization in children and their mothers
H. pylori colonization of children was defined by measuring IgG antibody levels in serum using an enzyme-linked immunosorbent assay (ELISA) (19). Sera samples were obtained at age 6 years. A separate ELISA was performed to determine serum IgG antibodies against a specific recombinant truncated cytotoxin-associated gene A (CagA) protein (20). Both ELISAs were validated locally, by adapting the ELISA properties based on positive and negative controls. H. pylori and CagA colonization in mothers was measured from serum samples obtained during mid-pregnancy (gestational age 18-25 weeks) (21). Similar ELISAs were used for mothers and children, with specific properties known from use in previous birth cohorts (19, 22).
Asthma and related conditions at school age
Wheezing in the prior 12 months (no, yes), physician-diagnosed asthma ever (no, yes) and physician-diagnosed eczema in the last 12 months (no, yes) were assessed using questions adapted from the International Study on Asthma and Allergy in Childhood (ISAAC) core questionnaires at age 6 years (23). Inhalant allergy was assessed by questionnaire at age 6 years; a positive history was defined as inhalant allergy to pollens, mites, or pets in the prior 12 months (no, yes), diagnosed by a physician.
Covariates
Information on birth weight, gestational age, and sex of the children were obtained from midwife and hospital registries at birth. Postnatal questionnaires at ages of 6 and 12 months supplied information on breastfeeding, and at age of 6 years about lower respiratory tract infections during the prior 12 months. Use of antibiotics in the prior 12 months was assessed by questionnaires yearly at the ages of 1 to 6 years. Information on maternal age, anthropometrics, ethnicity, socio-economic status, history of asthma or atopy, parity, and pet keeping were obtained by questionnaire, completed by the mother at enrolment. Socio-economic status was assessed using the educational level of mother on the basis of her highest level of completed education. Smoking during pregnancy was reported at enrolment. Maternal psychological distress in the second trimester of pregnancy was defined using a global severity index (GSI) (24).
Statistical analyses
The prevalence of asthma and related conditions in relation to child's H. pylori and CagA status were examined using Chi-square tests. We used multivariate logistic regression analysis to examine the association of child's H. pylori and CagA status with asthma and related conditions, taking potential confounders into account. Missing data in the covariates were imputed with multiple imputations using chained equations (25).
If available, a child's H. pylori status was paired with maternal H. pylori status, resulting in four different groups: mother and child both H. pylori negative, mother H. pylori negative and child positive, mother H. pylori positive and child negative, both mother and child H. pylori positive. We used multivariate logistic regression analysis to examine the association of combined H, pylori status of mother and child, with asthma and related conditions. Potential confounders were taken into account. Due to the small numbers of cases we were not able to differentiate with respect to CagA-status. We calculated the population attributable fraction of H. pylori colonization for asthma, using adjusted ORs estimated from logistic regression models (26).
All measures of associations are presented as Odds Ratios (OR) with their 95% Confidence Intervals (CI). Statistical analyses were performed using SPSS version 23.0 for Windows (SPSS Inc., Chicago, IL, USA). An extensive description of the methods is provided in the Supplementary material.
Results
Population characteristics
Characteristics of included children and their mothers are provided in Table 1 and Table S1 shows the comparison of the included (n = 3,797) with the excluded (n = 4,508) subjects. At a mean age of 6.1 years (SD 0.5), 8.7% of the children were H. pylori-positive. H. pylori status was determined in their mothers at a mean age of 31.1 years (SD 4.9), and 38.0% tested positive. The proportion of CagA-positive strains among H. pylori-positive mothers and children was 33.0% and 29.2%, respectively. As expected, the colonization rate of children with an H. pylori-positive mother was higher than in children with an H. pylori-negative mother (14.3% vs. 5.4%, p<0.05).
Table 1. Characteristics of the study population.
| Population for analysis (n = 3,797) | ||
|---|---|---|
|
|
||
| Observed | Imputed | |
| Sex (%) | ||
| Female | 48.1 (1,828) | 48.1 (1,828) |
| Male | 51.9 (1,969) | 51.9 (1,969) |
| Gestational age at birth (weeks) | 40.1 (35.7-42.3) | 40.1 (35.7-42.3) |
| Missing | 0.8 (29) | - |
| Birth weight (grams) | 3,446 (550) | 3,445 (551) |
| Missing | 0.1 (4) | - |
| Ethnicity1 (%) | ||
| European | 65.8 (2,492) | 65.6 (2,492) |
| Non-European | 34.2 (1,294) | 34.4 (1,305) |
| Missing | 0.3 (11) | - |
| Breastfeeding (%) | ||
| Never | 9.0 (230) | 12.9 (491) |
| Ever | 91.0 (2,331) | 87.1 (3,306) |
| Missing | 32.6 (1,236) | - |
| Pet keeping (%) | ||
| No | 66.3 (1,977) | 64.8 (2,460) |
| Yes | 33.7 (1,007) | 35.2 (1,337) |
| Missing | 21.4 (813) | - |
| Lower respiratory tract infections at 6 years (%) | ||
| No | 95.5 (3,458) | 95.2 (3,616) |
| Yes | 4.5 (162) | 4.8 (181) |
| Missing | 4.7 (177) | - |
| Antibiotic use (%) | ||
| Never | 17.6 (508) | 17.3 (657) |
| For 1-2 time periods | 55.5 (1,599) | 36.3 (1,378) |
| For 3 or more time periods | 26.8 (772) | 46.4 (1,762) |
| Missing | 24.2 (918) | |
| Maternal education level (%) | ||
| Primary, or secondary | 48.8 (1,723) | 50.6 (1,922) |
| Higher | 51.2 (1,811) | 49.4 (1,875) |
| Missing | 6.9 (263) | - |
| Maternal age2 (years) | 31.1 (4.9) | 31.1 (4.9) |
| Maternal body mass index2 (kg/m2) | 23.7 (18.9-35.5) | 23.8 (18.7-35.2) |
| Missing | 10.5 (398) | - |
| Maternal history of asthma or atopy (%) | ||
| No | 61.7 (1,904) | 61.8 (2,346) |
| Yes | 38.3 (1,183) | 38.2 (1,451) |
| Missing | 18.7 (710) | - |
| Parity2 (%) | ||
| 0 | 56.2 (2,057) | 55.3 (2,100) |
| ≥1 | 43.8 (1,601) | 44.7 (1,697) |
| Missing | 3.7 (139) | - |
| Smoking during pregnancy (%) | ||
| No | 85.4 (2,859) | 85.1 (3,235) |
| Yes | 14.6 (490) | 14.9 (562) |
| Missing | 11.8 (448) | - |
| Psychological distress during pregnancy (%) | ||
| No | 92.4 (2,646) | 91.0 (3,455) |
| Yes | 7.6 (218) | 9.0 (342) |
| Missing | 24.6 (933) | - |
|
| ||
| Children's H. pylori colonization rate (%) | ||
| Hp- | 91.3 (3,465) | - |
| Hp+CagA- | 6.2 (235) | |
| Hp+CagA+ | 2.6 (97) | |
| Maternal H. pylori colonization rate3 (%) | ||
| Hp- | 62.0 (1,804) | - |
| Hp+CagA- | 25.5 (741) | |
| Hp+CagA+ | 12.5 (365) | |
| Missing | 23.4 (887) | |
| Paired maternal and child's H. pylori status (%) | ||
| mHp-cHp- | 58.6 (1,706) | - |
| mHp+cHp- | 32.6 (948) | |
| mHp-cHp+ | 3.4 (98) | |
| mHp+cHp+ | 5.4 (158) | |
| Missing | 23.4 (887) | |
Values are means (SD), medians (2.5-97.5th percentile) or percentages (absolute numbers).
Ethnic background is based on data of mother
Data measured at moment of enrolment of pregnant mother
Data on H. pylori colonization is not imputed
Child's H. pylori colonization and asthma and related conditions
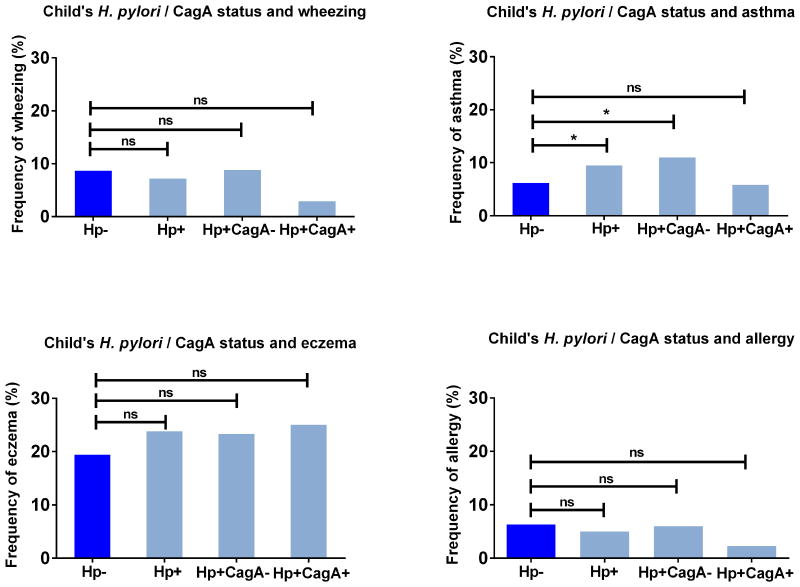
We observed a higher prevalence of recent asthma, but not of wheezing, eczema, or inhalant allergy, in H. pylori-positive compared with H. pylori-negative children (asthma prevalence 9.5% vs. 5.2% respectively, p=0.045) (Figure 2, Supplementary Table 2). Multivariate analyses showed an association between H. pylori and increased risk of asthma (OR 1.75; 95% CI 1.07-2.87) (Table 2). Compared with H. pylori-negative children, those colonized with a CagA-negative strain had an increased risk of asthma (OR 2.11; 95% CI 1.23-3.60), but those colonized with a CagA-positive strains were not (OR 0.94; 95% CI 0.32-2.79). The size of the effect estimates did not change after additional adjustment for maternal H. pylori status. Carriage of an Hp+CagA+ strain tended to be inversely related to wheezing and inhalant allergy, but effects were not significant (OR 0.24; 95% CI 0.05-1.05 for wheezing, OR 0.26; 95%CI 0.06-1.07 for inhalant allergy). Stratification for ethnicity showed that the association of H. pylori colonization with asthma was only present in children of European background (OR 3.11; 95% CI 1.70-5.72), and not in those of non-European background (OR 0.72; 95% CI 0.29-1.74), with a p-value for interaction of 0.009 (Table 3, Supplementary Table 4). Consistent trends were observed for wheezing, eczema, and inhalant allergy, however, the interaction terms were non-significant (Supplementary Table 4). Other tests for interactions were non-significant (p-values >0.05).
Figure 2. Prevalence of wheezing, asthma, eczema, and inhalant allergy in 6-year old children, according to H. pylori and corresponding CagA status of children.
The y-axis reflects the proportion of children with asthma or related outcome at age 6 years. H. pylori status is shown on the x-axis, divided by CagA- and CagA+ strains. The reference group consists of H. pylori-negative children.
Values are percentages. Mean proportions are compared using Chi-square tests (ns = non-significant).
* p<0.05
Table 2. Associations of children's H. pylori and corresponding CagA status with asthma and asthma-related outcomes at age 6 years.
| Wheezing | Asthma | Eczema | Inhalant allergy | |
|---|---|---|---|---|
| Odds Ratio | Odds Ratio | Odds Ratio | Odds Ratio | |
| (95% CI) | (95% CI) | (95% CI) | (95% CI) | |
|
|
||||
| n = 3,135 | n = 3,062 | n = 3,082 | n = 3,584 | |
| Hp- | Reference | Reference | Reference | Reference |
| n = 251 / 2,886 | n = 175 / 2,820 | n = 551 / 2,834 | n = 206 / 3,282 | |
| Hp+ | 0.73 | 1.75 | 1.04 | 0.69 |
| (0.43, 1.27) | (1.07, 2.87)* | (0.75, 1.43) | (0.39, 1.20) | |
| n = 18 / 249 | n = 23 / 242 | n = 59 / 248 | n = 15 / 302 | |
| Hp+CagA- | 0.98 | 2.11 | 1.12 | 0.91 |
| (0.55, 1.75) | (1.23, 3.60)** | (0.77, 1.61) | (0.50, 1.66) | |
| n = 16 / 181 | n = 19 / 173 | n = 42 / 180 | n = 13 / 216 | |
| Hp+CagA+ | 0.24 | 0.94 | 0.87 | 0.26 |
| (0.05, 1.05) | (0.32, 2.79) | (0.48, 1.55) | (0.06, 1.07) | |
| n = 2 / 68 | n = 4 / 69 | n = 17 / 68 | n = 2 / 86 | |
Values are odds ratios for wheezing, asthma, eczema, and inhalant allergy (95% confidence interval) from logistic regression models.
Models were adjusted for maternal age, ethnicity, pre-pregnancy body mass index, educational level, history of asthma or atopy, parity, pet keeping, smoking during pregnancy, psychological stress during pregnancy, and child's birth weight, gestational age at birth, sex, breastfeeding, lower respiratory tract infections, and antibiotic use.
n = number of cases (i.e. children with asthma or related conditions) per total group (i.e. according to H. pylori/CagA status)
p < 0.05,
p<0.01.
Table 3. Associations of child's H. pylori status with asthma stratified by ethnicity.
| Asthma | Asthma | ||
|---|---|---|---|
| Odds Ratio (95% CI) | Odds Ratio (95% CI) | ||
|
| |||
| European | n = 2,140 | Non-European | n = 922 |
| Hp- | Reference | Hp- | Reference |
| n = 111 / 2,0261 | n = 65 / 794 | ||
| Hp+ | 3.11 | Hp+ | 0.72 |
| (1.70, 5.72)* | (0.29, 1.74) | ||
| n = 16 / 114 | n = 7 / 128 | ||
| Hp+CagA- | 3.64 | Hp+CagA- | 0.52 |
| (1.97, 6.73)* | (0.14, 1.89) | ||
| n = 16 / 100 | n = 3 / 73 | ||
| Hp+CagA+ | NA | Hp+CagA+ | 0.98 |
| (0.31, 3.11) | |||
| n = 0 / 14 | n = 4 / 55 | ||
Values are odds ratios for asthma (95% confidence interval) from logistic regression models.
Models were adjusted for maternal age, pre-pregnancy body mass index, educational level, history of asthma or atopy, parity, psychological stress during pregnancy, and child's sex, gestational age at birth, birth weight, breastfeeding, pet keeping, lower respiratory tract infections, and antibiotic use.
Overall PInteraction ethnicity * H. pylori: <0.05.
n = number of cases (i.e. children with asthma) per total group (i.e. according to H. pylori/CagA status
p < 0.01, NA = not applicable.
Paired mother-child H. pylori status and asthma and related conditions in children
In 2,910 (77%) of the children, the H. pylori status of their mother was known. We observed no differences in prevalence of wheezing, asthma, eczema and inhalant allergy of the children according to the combined H. pylori status of mother and child Supplemental table S5) (p-values>0.05). Multivariate analysis revealed a positive association with asthma in children who were H. pylori positive, but with a negative mother (OR 2.42; 95% CI 1.11- 5.27) (Table 4). The proportion of asthma attributable to H. pylori-positivity in children with an H. pylori-negative mother was 3.4% (95% CI 0.6-4.7) Stratification for ethnicity showed that the association with asthma was only present in children of European background, both for H. pylori positive children with a H. pylori negative or positive mother (OR 3.25; 95% CI 1.41-7.54 for mHp-cHp+, and OR 4.07; 95% CI 1.47-11.31 for mHp+cHp+) (Supplementary Table 6). Interactions of paired H. pylori status with maternal asthma or atopy, and child's sex, gestational age or weight at birth, or antibiotic use were not associated with asthma or related conditions (p-values for interaction >0.05).
Table 4. Associations of combined maternal and children's H. pylori status with asthma and related outcomes at the child's age of 6 years.
| Wheezing | Asthma | Eczema | Inhalant allergy | |
|---|---|---|---|---|
| Odds Ratio | Odds Ratio | Odds Ratio | Odds Ratio | |
| (95% CI) | (95% CI) | (95% CI) | (95% CI) | |
|
|
||||
| n = 2,410 | n = 2,353 | n = 2,373 | n = 2,749 | |
|
|
||||
| mHp-cHp- | Reference | Reference | Reference | Reference |
| n = 122 / 1,4761 | n = 85 / 1,441 | n = 268 / 1,451 | n = 97 / 1,626 | |
| mHp+cHp- | 0.99 | 1.11 | 0.85 | 0.86 |
| (0.68, 1.43) | (0.73, 1.68) | (0.66, 1.09) | (0.59, 1.24) | |
| n = 68 / 740 | n = 50 / 726 | n = 163 / 731 | n = 65 / 888 | |
| mHp-cHp+ | 0.55 | 2.42 | 1.04 | 0.55 |
| (0.18, 1.63) | (1.11, 5.27) | (0.59, 1.84) | (0.17, 1.79) | |
| n = 4 / 82 | n = 9 / 79 | n = 17 / 82 | n = 3 / 94 | |
| mHp+cHp+ | 0.91 | 1.76 | 0.89 | 0.62 |
| (0.43, 1.93) | (0.81, 3.79) | (0.55, 1.45) | (0.28, 1.38) | |
| n = 11 / 112 | n = 10 / 107 | n = 28 / 109 | n = 8 / 141 | |
Values are odds ratios for wheezing, asthma, eczema, and inhalant allergy (95% confidence interval) from logistic regression models. Models were adjusted for maternal age, ethnicity, pre-pregnancy body mass index, educational level, history of asthma or atopy, parity, pet keeping, smoking during pregnancy, psychological stress during pregnancy, and child's birth weight, gestational age at birth, sex, breastfeeding, lower respiratory tract infections, and antibiotic use.
n = number of cases (i.e. children with asthma or related conditions) per total group (i.e. according to H. pylori status)f
Discussion
In this large population-based prospective cohort, we observed that H. pylori colonization in children was associated with an increased risk of asthma at the age of 6 years. Differentiation of H. pylori-positivity into CagA-negative or CagA-positive strains showed that the effects were explained by CagA-negative-H. pylori strains only. This association of CagA-negative H. pylori colonization with asthma was present in children of European ethnic background, and not in those of non-European ethnic background. In non-stratified analysis, maternal H. pylori colonization seems to have a protective effect on asthma in their children, as an increased risk of asthma was only found in H. pylori-positive children with an H. pylori-negative mother. Although the relative risk was higher, the attributable risk for this association only explained 3.4% of the asthma in this population.
Comparison with previous studies
The positive association between H. pylori and physician-diagnosed asthma is in contrast with most prior studies in adults and children (9-14, 27, 28). A meta-analysis of pooled data (n= 34,018 subjects) from these studies showed an inverse association between H. pylori and asthma in children (OR 0.81; 95% CI 0.72-0.91) (29). However, the pooled OR of included (birth) cohort studies was non-significant (10, 11, 13) (OR 0.82; 95% CI 0.53-1.27) (29). Two of these studies had comparable study designs, but smaller sample size; the Dutch study included 575 children between 7 years and 9 years old, and showed an OR of 0.87 (95% CI 0.37-2.08) for the relation between H. pylori and physician-diagnosed asthma (13). The second study, from Ethiopia, assessed the association with current H. pylori colonization at age 3 years and allergic disease in a birth cohort of 878 children (11). Due to low asthma prevalence, calculations could not be performed associating H. pylori and asthma; however, for H. pylori and wheezing, the outcome was a trend towards an inverse association, which is consistent with our study. A second evaluation within the cohort from Ethiopia, revealed an inverse association between H. pylori and eczema in children at the age of 5 years, but not for wheezing and rhinitis (30). The contrast with other studies implies that so far no universal conclusion can be made on the relation between H. pylori and asthma.
In contrast to asthma outcome, the observed tendency of inverse associations of H. pylori status with wheezing and inhalant allergy were consistent with prior studies, and also consistent with the hypothesis that the endemic rise in asthma and allergy may be causally related to compositional changes in our indigenous microbiota (31), probably as a result of a modern lifestyle (2). Since H. pylori colonization persists for life, it may be considered as a model to examine the effects of once-common microorganisms on the development of asthma and related disease.
Interpretation of results
Considering this paradigm and the data from prior studies, our finding of CagA-negative H. pylori colonization in 6-year old children of European origin as a risk factor for asthma is notable, and requires further explanation. First, the positive association between H. pylori and asthma was explained by CagA-negative-strains in children with a European ethnic background only. In this group, none of the asthmatic children were colonized with a CagA-positive strain. This suggests that the asthma risk may be lower in CagA-positive children compared with H. pylori-negatives. The low number of cases in this group reflects the overall low H. pylori-prevalence in children of European ethnic background, and therefore limits our conclusions based on these findings. Due to lack of CagA-positives in asthmatic children in this subgroup, the overall detected effect of H. pylori-positivity might be skewed to an increased risk for asthma. It has been shown that CagA-positive strains have a stronger interaction with their hosts, leading to pronounced immune responses (5). Prior studies have observed more pronounced effects of CagA-positivity in lowering the risk on asthma and wheezing (32). Accordingly, we found larger effects towards an inverse trend for CagA-positive strains than for CagA-negative strains in relation to all outcomes examined. Second, although an association between H. pylori and asthma was only present in children of European ethnic background, consistent trends were observed for wheezing, eczema, and inhalant allergy, either for CagA-negative or CagA-positive strains. Such differences may reflect variation of the gut microbiome by ethnicity, in both richness and composition. Children (33) and adults living in developing countries have significantly greater faecal diversity than those in developed countries (34). Among subjects differing in ethnic background, but migrating to the same country, gut microbiome composition also varies (35). In animal models, there is growing evidence that H. pylori colonization not only affects the gastric microbiome (36), but also the composition of the lower gastro-intestinal tract (37). In addition, the composition of the gut microbiome itself also may modulate the development of H. pylori-associated disease (38). Within this context, we speculate that the effects of H. pylori on asthma or related disease may depend on both the richness and composition of the gut microbiome. Depending on its composition, it might sometimes promote and other times mitigate H. pylori disease (39). Taken together, these observations from the literature might explain the differences between ethnic groups in the present study. Third, any potential protective effect of H. pylori on asthma may differ for allergic asthma and non-allergic asthma. However, our questionnaires did not distinguish between allergic and non-allergic asthma, and hence we cannot explore this issue further. Fourth, the effect estimates for asthma were in the opposite direction compared with wheezing and inhalant allergy, which are usually closely related with allergic asthma. This can be explained by the difference in definition between asthma, and wheezing and inhalant allergy used in this study. Asthma was diagnosed as having ever occurred during the age period of 1 to 6 years, while data on wheezing and inhalant allergy referred to complaints and diagnosis during the last 12 months at the age of 6 years. Ever asthma could reflect various phenotypes including early wheezing, mostly induced by respiratory tract infections and transient, and multitrigger wheezing. Current wheezing at age 6 years most likely reflects multitrigger wheezing only. The difference in definition means that H. pylori acquisition could have occurred before or after the first asthma period, while for wheezing and inhalant allergy it is more likely that H. pylori acquisition may have preceded these outcomes.
In the unstratified paired group, we observed no increased risk of asthma in H. pylori-positive children with an H. pylori-positive mother (Table 4). This finding suggests that maternal H. pylori carriage affects the risk of asthma, possibly by facilitating early H. pylori colonization in their children. Maternal IgG antibodies against H. pylori can be transferred to the child via the placenta, but seem not to protect the child against colonization (40). Direct contact between mother and child is most intense during the first years of life, which may result in early acquisition of H. pylori by a child. Children with an H. pylori-positive mother were more likely to become colonized with H. pylori themselves, confirming a recent analysis of an overlapping subset of mothers and children (41). Whether H. pylori-positive children with an H. pylori-negative mother received the infection from other family members or children at day care is not known. Moreover, in stratified analysis according to ethnicity, the increased risk of asthma was the same both for European children with an H. pylori-negative and positive mother.
Strengths and limitations
The strengths of this study in comparison to prior studies include the large number of subjects within a birth cohort of children within the same age period, all living in one urban region with detailed prospectively collected information on socioeconomic characteristics, and numerous potential confounding factors. Limitations of the study include that missing data on outcomes, determinants, and several covariates might have resulted in biased effect estimates. The population of analysis might reflect a selection towards a more healthy and affluent population (18). Included subjects more often were of higher socio-economic status, from European ethnic background, and less often exposed to adverse lifestyle factors. Of all children participating in the follow-up at age 6 years, 54.4% did not have data on both H. pylori colonization and asthma or related conditions. Based on the differences in characteristics between those included and not included in the study and previous literature (41), we speculate that the prevalence of H. pylori and asthma could have been higher in the excluded subjects, compared to the population of analysis. We have aimed to reduce the potential selection bias by the use of multiple imputation of covariates. Second, although both ELISAs have been validated in adults and children, and have been previously used in Dutch children (22), they have not been separately validated in Dutch children of different origins. Third, all outcomes largely relied on data obtained from questionnaires completed by the parents. Although the questions were previously validated and commonly used for epidemiological studies (42), we cannot rule out the possibility of misclassification or misinterpretation. Forth, data on antibiotic use were not available about specific types, nor validated by pharmacy records. Fifth, lacking of detailed data of allergic features in mothers made direct comparison with their children in relation to H. pylori status impossible. Finally, despite the large sample size of this study, the number of children with H. pylori colonization, especially CagA-positive strains, and asthma or related conditions was limited. In total, only a small proportion of the asthma risk could be attributed to H. pylori. Therefore, conclusions based on these numbers should be interpreted with caution.
Conclusions
In conclusion, we observed no significant protective association of H. pylori status at age 6 with asthma and related conditions. Instead, colonization with a CagA-negative H. pylori strain at age 6 was a risk factor for asthma in children, but only in those of European ethnic background. Explanations of underlying mechanisms for the differences between ethnic groups are still speculative, and therefore need further research. Such studies also should include the role of the gut microbiome in relation to H. pylori colonization and ethnic background, which may indicate new directions for asthma prevention.
Supplementary Material
Acknowledgments
The Generation R Study is conducted by the Erasmus Medical Centre in close collaboration with the School of Law and Faculty of Social Sciences of the Erasmus University Rotterdam; the Municipal Health Service Rotterdam area, Rotterdam; the Rotterdam Homecare Foundation, Rotterdam; and the Stichting Trombosedienst and Artsenlaboratorium Rijnmond (STAR), Rotterdam. The authors gratefully acknowledge the contribution of participating parents, children, general practitioners, hospitals, midwives, and pharmacies in Rotterdam.
WH is acting as the submission's guarantor. WH, AS, MB, LD, EK contributed to the conception and design, acquisition of data, analyses and interpretation of the data, drafted the article, revised it critically for important intellectual content and gave final approval of the version to be published. LH, JJ, VJ, AH, GP, HM contributed to the conception and design and acquisition of data, revised the article critically for important intellectual content and gave final approval of the version to be published.
Financial Support: The Generation R Study is made possible by financial support from the Erasmus Medical Centre, Rotterdam, the Erasmus University Rotterdam and the Netherlands Organization for Health Research and Development. The researchers are independent from the funders. The study sponsors had no role in the study design, data analysis, interpretation of data, or writing of this report. Dr. Liesbeth Duijts received funding from the Lung Foundation Netherlands (no 3.2.12.089; 2012). Supported in part by R01DK090989 from the National Institutes of Health, the Diane Belfer Program for Human Microbial Ecology, and by the Knapp Family Foundation.
LD reports grants from Lung Foundation Netherlands, during the conduct of the study; personal fees from Nestlé, outside the submitted work. All other authors declare that they have no competing interests.
References
- 1.Hansel TT, Johnston SL, Openshaw PJ. Microbes and mucosal immune responses in asthma. Lancet. 2013;381:861–73. doi: 10.1016/S0140-6736(12)62202-8. [DOI] [PubMed] [Google Scholar]
- 2.Blaser MJ, Falkow S. What are the consequences of the disappearing human microbiota? Nat Rev Microbiol. 2009;7:887–94. doi: 10.1038/nrmicro2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oertli M, Sundquist M, Hitzler I, et al. DC-derived IL-18 drives Treg differentiation, murine Helicobacter pylori-specific immune tolerance, and asthma protection. J Clin Invest. 2012;122:1082–96. doi: 10.1172/JCI61029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnold IC, Hitzler I, Muller A. The immunomodulatory properties of Helicobacter pylori confer protection against allergic and chronic inflammatory disorders. Front Cell Infect Microbiol. 2012;2:10. doi: 10.3389/fcimb.2012.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atherton JC, Blaser MJ. Coadaptation of Helicobacter pylori and humans: ancient history, modern implications. J Clin Invest. 2009;119:2475–87. doi: 10.1172/JCI38605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kusters JG, van Vliet AH, Kuipers EJ. Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev. 2006;19:449–90. doi: 10.1128/CMR.00054-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weyermann M, Rothenbacher D, Brenner H. Acquisition of Helicobacter pylori infection in early childhood: independent contributions of infected mothers, fathers, and siblings. Am J Gastroenterol. 2009;104:182–9. doi: 10.1038/ajg.2008.61. [DOI] [PubMed] [Google Scholar]
- 8.Sykora J, Siala K, Varvarovska J, et al. Epidemiology of Helicobacter pylori infection in asymptomatic children: a prospective population-based study from the Czech Republic. Application of a monoclonal-based antigen-in-stool enzyme immunoassay. Helicobacter. 2009;14:286–97. doi: 10.1111/j.1523-5378.2009.00689.x. [DOI] [PubMed] [Google Scholar]
- 9.Imamura S, Sugimoto M, Kanemasa K, et al. Inverse association between Helicobacter pylori infection and allergic rhinitis in young Japanese. J Gastroenterol Hepatol. 2010;25:1244–9. doi: 10.1111/j.1440-1746.2010.06307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cam S, Ertem D, Bahceciler N, et al. The interaction between Helicobacter pylori and atopy: does inverse association really exist? Helicobacter. 2009;14:1–8. doi: 10.1111/j.1523-5378.2009.00660.x. [DOI] [PubMed] [Google Scholar]
- 11.Amberbir A, Medhin G, Erku W, et al. Effects of Helicobacter pylori, geohelminth infection and selected commensal bacteria on the risk of allergic disease and sensitization in 3-year-old Ethiopian children. Clin Exp Allergy. 2011;41:1422–30. doi: 10.1111/j.1365-2222.2011.03831.x. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Blaser MJ. Helicobacter pylori colonization is inversely associated with childhood asthma. J Infect Dis. 2008;198:553–60. doi: 10.1086/590158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holster IL, Vila AM, Caudri D, et al. The impact of Helicobacter pylori on atopic disorders in childhood. Helicobacter. 2012;17:232–7. doi: 10.1111/j.1523-5378.2012.00934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zevit N, Balicer RD, Cohen HA, et al. Inverse association between Helicobacter pylori and pediatric asthma in a high-prevalence population. Helicobacter. 2012;17:30–5. doi: 10.1111/j.1523-5378.2011.00895.x. [DOI] [PubMed] [Google Scholar]
- 15.Eslick GD, Yan P, Xia HH, et al. Foetal intrauterine growth restrictions with Helicobacter pylori infection. Aliment Pharmacol Ther. 2002;16:1677–82. doi: 10.1046/j.1365-2036.2002.01333.x. [DOI] [PubMed] [Google Scholar]
- 16.Cardaropoli S, Rolfo A, Piazzese A, et al. Helicobacter pylori's virulence and infection persistence define pre-eclampsia complicated by fetal growth retardation. World J Gastroenterol. 2011;17:5156–65. doi: 10.3748/wjg.v17.i47.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mu M, Ye S, Bai MJ, et al. Birth Weight and Subsequent Risk of Asthma: A Systematic Review and Meta-Analysis. Heart Lung Circ. 2014 doi: 10.1016/j.hlc.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 18.Jaddoe VW, van Duijn CM, Franco OH, et al. The Generation R Study: design and cohort update 2012. Eur J Epidemiol. 2012;27:739–56. doi: 10.1007/s10654-012-9735-1. [DOI] [PubMed] [Google Scholar]
- 19.Everhart JE, Kruszon-Moran D, Perez-Perez GI, et al. Seroprevalence and ethnic differences in Helicobacter pylori infection among adults in the United States. J Infect Dis. 2000;181:1359–63. doi: 10.1086/315384. [DOI] [PubMed] [Google Scholar]
- 20.Blaser MJ, Perez-Perez GI, Kleanthous H, et al. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55:2111–5. [PubMed] [Google Scholar]
- 21.den Hollander WJ, Holster IL, den Hoed CM, et al. Ethnicity is a strong predictor for Helicobacter pylori infection in young women in a multi-ethnic European city. J Gastroenterol Hepatol. 2013;28:1705–11. doi: 10.1111/jgh.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.den Hoed CM, Vila AJ, Holster IL, et al. Helicobacter pylori and the birth cohort effect: evidence for stabilized colonization rates in childhood. Helicobacter. 2011;16:405–9. doi: 10.1111/j.1523-5378.2011.00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pearce N, Weiland S, Keil U, et al. Self-reported prevalence of asthma symptoms in children in Australia, England, Germany and New Zealand: an international comparison using the ISAAC protocol. Eur Respir J. 1993;6:1455–61. [PubMed] [Google Scholar]
- 24.de Beurs E. Brief Symptom Inventory, handleiding addendum. Leiden, The Netherlands: PITS BV; 2009. [Google Scholar]
- 25.Sterne JA, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. American journal of public health. 1998;88:15–9. doi: 10.2105/ajph.88.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corrado G, Luzzi I, Pacchiarotti C, et al. Helicobacter pylori seropositivity in children with atopic dermatitis as sole manifestation of food allergy. Pediatr Allergy Immunol. 2000;11:101–5. doi: 10.1034/j.1399-3038.2000.00028.x. [DOI] [PubMed] [Google Scholar]
- 28.Jaber SM. Helicobacter pylori seropositivity in children with chronic disease in Jeddah, Saudi Arabia. Saudi J Gastroenterol. 2006;12:21–6. doi: 10.4103/1319-3767.27740. [DOI] [PubMed] [Google Scholar]
- 29.Wang Q, Yu C, Sun Y. The association between asthma and Helicobacter pylori: a meta-analysis. Helicobacter. 2013;18:41–53. doi: 10.1111/hel.12012. [DOI] [PubMed] [Google Scholar]
- 30.Amberbir A, Medhin G, Abegaz WE, et al. Exposure to Helicobacter pylori infection in early childhood and the risk of allergic disease and atopic sensitization: a longitudinal birth cohort study. Clin Exp Allergy. 2014;44:563–71. doi: 10.1111/cea.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shanahan F. The gut microbiota-a clinical perspective on lessons learned. Nat Rev Gastroenterol Hepatol. 2012;9:609–14. doi: 10.1038/nrgastro.2012.145. [DOI] [PubMed] [Google Scholar]
- 32.Chen Y, Blaser MJ. Inverse associations of Helicobacter pylori with asthma and allergy. Arch Intern Med. 2007;167:821–7. doi: 10.1001/archinte.167.8.821. [DOI] [PubMed] [Google Scholar]
- 33.De Filippo C, Cavalieri D, Di Paola M, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010;107:14691–6. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–7. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prideaux L, Kang S, Wagner J, et al. Impact of ethnicity, geography, and disease on the microbiota in health and inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:2906–18. doi: 10.1097/01.MIB.0000435759.05577.12. [DOI] [PubMed] [Google Scholar]
- 36.Maldonado-Contreras A, Goldfarb KC, Godoy-Vitorino F, et al. Structure of the human gastric bacterial community in relation to Helicobacter pylori status. ISME J. 2011;5:574–9. doi: 10.1038/ismej.2010.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heimesaat MM, Fischer A, Plickert R, et al. Helicobacter pylori induced gastric immunopathology is associated with distinct microbiota changes in the large intestines of long-term infected Mongolian gerbils. PLoS One. 2014;9:e100362. doi: 10.1371/journal.pone.0100362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lofgren JL, Whary MT, Ge Z, et al. Lack of commensal flora in Helicobacter pylori-infected INS-GAS mice reduces gastritis and delays intraepithelial neoplasia. Gastroenterology. 2011;140:210–20. doi: 10.1053/j.gastro.2010.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martin ME, Solnick JV. The gastric microbial community, Helicobacter pylori colonization, and disease. Gut Microbes. 2014;5:345–50. doi: 10.4161/gmic.28573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bunn JE, Thomas JE, Harding M, et al. Placental acquisition of maternal specific IgG and Helicobacter pylori colonization in infancy. Helicobacter. 2003;8:568–72. doi: 10.1046/j.1523-5378.2003.00178.x. [DOI] [PubMed] [Google Scholar]
- 41.den Hollander WJ, Holster IL, van Gilst B, et al. Intergenerational reduction in Helicobacter pylori prevalence is similar between different ethnic groups living in a Western city. Gut. 2014 doi: 10.1136/gutjnl-2014-307689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jenkins MA, Clarke JR, Carlin JB, et al. Validation of questionnaire and bronchial hyperresponsiveness against respiratory physician assessment in the diagnosis of asthma. Int J Epidemiol. 1996;25:609–16. doi: 10.1093/ije/25.3.609. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.