Abstract
Objective
The goal of this study was to retrospectively evaluate the clinical impact of an accurate autocorrecting blood glucose monitoring system (BGMS) in children with severe burns. BGMS accuracy is essential for providing appropriate intensive insulin therapy (IIT) and achieving tight glycemic control (TGC) in critically ill patients. Unfortunately, few comparison studies have been performed to evaluate the clinical impact of accurate BGMS monitoring in the high-risk pediatric burn population.
Design
Retrospective analysis of an electronic health record system.
Setting
Pediatric burn intensive care unit at an academic medical center.
Patients
Children (age<18 years) with severe burns (≥20% total body surface area [TBSA]) receiving IIT guided by either a non-correcting (BGMS-1) or an autocorrecting BGMS (BGMS-2).
Measurements and Main Results
Patient demographics, insulin rates, and BGMS measurements were collected. Frequency of hypoglycemia and glycemic variability was compared between the two BGMS groups. A total of 122 patient charts from 2001–14 were reviewed. Sixty-three patients received IIT using BGMS-1 and 59 via BGMS-2. Patient demographics were similar between the two groups. Mean insulin infusion rates (5.1±3.8 U/hour, n = 535 paired measurements vs. 2.4±1.3 U/hour, n = 511 paired measurements, P<0.001), glycemic variability, and frequency of hypoglycemic events (90 vs. 12, P<0.001) were significantly higher in BGMS-1 treated patients. Compared to laboratory measurements, BGMS-2 yielded the most accurate results (mean±SD bias: −1.7±6.9 mg/dL [−0.09±0.4 mmol/L] vs. 7.4±13.5 mg/dL [0.4±0.7 mmol/L]). BGMS-2 patients achieve glycemic control more quickly (5.7±4.3 hours vs. 13.1±6.9 hours, P<0.001) and stayed within the target glycemic control range longer compared to BGMS-1 patients (85.2±13.9% vs. 57.9±29.1%, P<0.001).
Conclusions
Accurate autocorrecting BGMS optimizes IIT, improves TGC, and reduces risk for hypoglycemia and glycemic variability. The use of an autocorrecting BGMS for IIT may improve glycemic control in severely burned children.
Keywords: Centers for Medicare and Medicaid Services, Food and Drug Administration, hypermetabolism, intensive insulin therapy, pediatrics, point-of-care testing
INTRODUCTION
Intensive insulin therapy (IIT) for tight glycemic control (TGC) in severely burned patients decreases infection rates and mortality and remains the standard of care in this high-risk population. (1) Normoglycemia accelerates donor site healing time and attenuates the acute phase response in burn patients. (1–4) Tight glycemic control aims to counteract the hyperglycemia and glycemic variability associated with the significant hypermetabolism and inflammation that occurs following burn injury. Increased glycemic variability has been shown to be predictive of mortality in critically ill burn patients—highlighting the importance of TGC in this population. (5) Unfortunately, IIT remains controversial due to reports of increased risk for hypoglycemia and mortality in non-burned intensive care unit (ICU) patients. (6,7) Follow-up studies suggest point-of-care (POC) blood glucose monitoring system (BGMS) inaccuracy contributes to hypoglycemia and glycemic variability during IIT. Klonoff et al. (8) revealed many BGMS’s involved with the seminal NICE-SUGAR (9) study were not approved for the critically ill population. This notion has been further codified by the United States Food and Drug Administration (FDA) draft guidelines released on January 2014 requiring BGMS manufacturers to prove acceptable performance in critically ill populations in order to be used in the ICU. (9) These FDA guidelines were later reinforced on November 21, 2014 by the Centers for Medicare and Medicaid Services (CMS) memorandum on BGMS’s not cleared for use in critically ill patients in the United States – placing health care facilities under threat of citation for using “off label devices”. (10)
Blood glucose monitoring system inaccuracy is attributed to endogenous and exogenous confounding factors summarized in Table 1. (11–15) Numerous studies have quantified the impact of these confounding factors on BGMS performance (11–15) and reporting poor outcomes such as severe hypoglycemia and significant glycemic variability (5). Advances in biosensor technology have enabled the development of BGMS’s that autocorrect for these confounding factors. (5, 16–18) The unique autocorrecting features of recent BGMS’s are based on the simultaneous measurement of hematocrit and oxidizing/reducing substances during the testing process—allowing these biosensors to adjust for interferences. Additionally, these autocorrecting BGMS’s incorporate modified glucose oxidase enzymes that are not susceptible to the same oxygen tension affects found in older generation devices. Despite numerous investigations supporting the use of autocorrecting BGMS’s critically ill patients, few have evaluated these devices in the high-risk pediatric burn population. To this end, the goal of our study is to evaluate the clinical impact of an autocorrecting BGMS compared to a traditional non-correcting BGMS in children with severe burn injury.
TABLE 1.
CONFOUNDING FACTORS OF GLUCOSE MONITORING SYSTEMS
| Factor | Mechanism of Action |
|---|---|
| Hematocrit | Red blood cell concentration alters the apparent glucose measured by biosensors. Specifically, anemic specimens yield falsely elevated results due to increased diffusion of glucose into the biosensor. Alternately, polycythemic specimens yield falsely low results due to mechanical impedance of glucose diffusion. |
| Non-glucose sugars | Sugars such as galactose, lactose, and maltose are Indistinguishable to certain glucose biosensors (e.g., glucose dehydrogenase) and generating falsely elevated results. Galactose may be encountered in neonates with hypergalactosemia. Maltose can be found in patients receiving peritoneal dialysis and icodextrin. |
| Oxidizing and reducing | Electrochemical glucose biosensors rely on oxidation and reduction reactions. The presence of oxidizing and reducing substances (e.g., ascorbic acid) affect these electrochemical reactions and produces erroneous results. |
| Oxygen tension (pO2) | Sample partial pressure of oxygen may alter electrochemical reaction kinetics in glucose biosensors that rely on an oxygen intermediate. |
| Sample pH | Glucose biosensors rely on enzymatic reactions to convert glucose into a readable signal. Abnormal pH may impair enzyme function and therefore impact glucose results. |
| Temperature | Both abnormal sample and environmental temperatures may affect glucose biosensors. Temperature may alter the enzymatic reaction required to produce glucose results. |
| User error | Inadequate blood volume, incorrect sample type, or collection of capillary blood samples in patients with systolic blood pressure of less than 90 mmHg yield inaccurate glucose results. |
MATERIALS AND METHODS
Patient Population
We conducted a retrospective study evaluating the clinical impact of accurate glucose monitoring in severely burned children admitted to Shriners Hospital for Children of Northern California (Sacramento, CA) between 2001 and 2014. The local Institutional Review Board committee approved the study. Patient medical chart inclusion criteria included: (a) age < 18 years and (b) burn size ≥ 20% TBSA. Chart exclusion criteria included: (a) incomplete medical record (i.e., missing laboratory data), (b) patients not requiring IIT, and (c) patients without BGMS testing.
Glucose Testing and Intensive Insulin Therapy Protocol
Point-of-care glucose measurements were collected using a non-correcting BGMS (BGMS-1, AccuChek Inform I, Roche Diagnostics, Indianapolis, IN) from 2001 to 2008 and an autocorrecting BGMS (BGMS-2, StatStrip Glucose Connectivity Meter, Nova Biomedical, Waltham, MA) from 2008 to 2014. Intensive insulin therapy targeted a TGC range of 80 to 130 mg/dL for both BGMS groups. Paired plasma glucose measurements (clinical laboratory) were made using a hexokinase-based Xpand Chemistry Analyzer (Siemens Medical Solutions, Malvern, PA).
Data Collection
Hourly BGMS results were collected for each patient over the course of their ICU stay. Patient demographics (age, gender), TBSA burned, presence of inhalation injury, diabetes status, hourly insulin rates, mechanical ventilator days, ICU length-of-stay (LOS), procedures (e.g., dialysis), nutritional support (i.e., parenteral vs. enteral feedings), medications (e.g., steroids, epinephrine infusions), and paired (± 5 minutes) laboratory plasma glucose results, and percent hematocrit were collected. Hypoglycemic events were also recorded and classified as moderate (40 to 69 mg/dL [2.2 to 3.8 mmol/L] or severe (< 40 mg/dL [2.2 mmol/L]). The mean multiple organ dysfunction score (MODS) was calculated for each patient group.
Data Analysis
Paired BGMS and laboratory measurements were compared using Bland-Altman plots. Bias was calculated for each paired measurement and is defined as the BGMS result minus the laboratory method. Glycemic variability between the BGMS-1 and -2 populations was determined by measuring the coefficient of variation (CV), continuous overall net glycemic action (CONGA), interquartile range (IQR), “M-value”, mean amplitude of glycemic excursions (MAGE), mean of daily differences (MODD), and standard deviation (SD) methods as reported previously using a MATLAB (Mathworks, Natick, MA) program (Table 2). (5) Data distribution was evaluated using the Ryan-Joiner test for normality. The 2-sample t-test compared independent variables (e.g., demographics, measures of glycemic variability). For non-parametric analysis, the Mann-Whitney U test was performed comparing medians between each group. Repeated measures analysis of variance (ANOVA) compared time-series data (hourly glucose measurements). Frequency of hypoglycemic events between groups was analyzed using the Fishers Exact Test. Multivariate logistic regression (MLR) determined mortality predictors controlling for age, TBSA, and presence of inhalation injury. Predictors for the logistic regression model included CV, CONGA, IQR, M-value, MAGE, MODD, SD, LOS, and hypoglycemia which were evaluated based on P < 0.10. Akaike and Schwarz information criteria were used to identify optimal MLR models.
Table 2.
Measures of Glycemic Variability
| Method | Calculation | Description | |
|---|---|---|---|
| CV |
|
Standard deviation of glucose values divided by the mean of glucose values. Advantages: Common measure of consistency (precision), and easy to calculate. Disadvantages: Does not account for glucose peaks and troughs over time and may be skewed by non-parametric distributions. | |
| CONGAn | , dt = xt − xt−n hours | Standard deviation of differences between adjacent glucose values for n hour intervals apart. Advantages: Conceptually easier to understand, relatively simple to calculate. Disadvantages: Does not account for glucose peaks and troughs over time, may be skewed by non-parametric distributions, and actually utility may depend on the “n” hour intervals. | |
| IQR | x75th−x25th | Difference between the 75th and 25th percentiles from a distribution of glucose values. Advantages: Common non-parametric measure of dispersion and easy to calculate. Disadvantages: Does not account for glucose peaks and troughs over time. | |
| M-value |
|
Complex log transformation of deviations from an assigned “ideal glucose value” where Mx, if T ≥ 24, Mx − MW, if T < 24. Advantages: Accounts for maximum and minimum glucose Values. Disadvantages: Difficult to calculate, hard to interpret, and dependent on defining an “ideal” glucose value. | |
| MAGE |
|
Average of absolute values of differences between adjacent glucose peaks and nadirs for all differences that are greater than one standard deviation. Advantages: Treats glucose peaks and nadirs equally, relatively easy to calculate and interpret. Disadvantage: Does not differentiate between peaks and nadirs. | |
| MODD |
|
Average of glucose differences across adjacent days. Advantages: Easy to calculate and interpret. Disadvantages: May be affected by non-parametric distributions, and does not account for peaks and nadirs. | |
| SD |
|
Average of the sum of squared deviations around mean glucose values. Advantages: Easy to calculate, well recognized, established in critical care for measuring glycemic variability, and easy to interpret. Disadvantages: May be affected by non-parametric distributions, and does not account for peaks and nadirs. |
Abbreviations: Ad, amplitude greater than 1 SD between the dth peak and dth nadir; CV, coefficient of variation; CONGAn, continuous overall net glycemic action for n hour intervals; D, total number of amplitudes greater than 1 SD; IQR, interquartile range; MAGE, mean amplitude of glycemic excursions; MODD, mean of daily differences; SD, standard deviation; T, number of time points; x̄, mean blood glucose; x25th, 25th percentile of blood glucose values; x75th, 75th percentile of blood glucose values; x1, ideal blood glucose value (set at 131 mg/dL for the study); xt, blood glucose at time point t; xt−n hours, blood glucose n hours before time t; xmax, maximum blood glucose value; xmin, minimum blood glucose value.
RESULTS
Patient Demographics and Outcomes
A total of 122 patient charts meeting the study inclusion criteria were reviewed. Sixty-three patients received IIT using BGMS-1 and 59 patients using BGMS-2. Mean (SD) age, TBSA, ICU LOS, mechanical ventilator days, MODS, inhalation injury status, nutritional support, relevant medications affecting glycemic control (e.g., steroids, vasopressors, immunosuppressants), and gender were similar between the two groups (Table 3). Mortality was similar (p = 0.764) for BGMS-1 (11.0%, 7/63) and BGMS-2 (8.4%, 5/59) groups. Mean hematocrit was also similar (25.7 ± 5.2% versus 23.2 ± 4.9%, p = 0.910).
TABLE 3.
STUDY RESULTS BETWEEN BGMS-1 AND BGMS-2 GROUPS
| Variable | BGMS-1 Group (n = 63) | BGMS-2 Group (n = 59) | P-Value |
|---|---|---|---|
| Demographics | |||
| Mean (SD) age (years) | 7.8 (5.6) | 7.1 (4.9) | 0.823 |
| Gender (M, F) | 35, 28 | 38, 21 | 0.318 |
| Mean (SD) TBSA (%) | 45.5 (15.2) | 42.1 (19.1) | 0.421 |
| Mean (SD) Weight (kg) | 30.0 (13.5) | 29.7 (11.4) | 0.794 |
| Intubated (%) | 79.3 | 76.3 | 0.897 |
| Inhalation injury (%) | 17.5 | 15.2 | <0.001 |
| Dialysis (%) | 1.6 | 0.0 | 0.915 |
| Diabetes (%) | 3.2 | 3.4 | 0.477 |
| Parenteral Nutrition (%) | 7.9 | 6.8 | 0.756 |
| Enteral Nutrition (%) | 66.7 | 64.4 | 0.813 |
| Mean hematocrit (%) | 25.7 (5.2) | 23.2 (4.9) | 0.329 |
| Mean (SD) MODS | 5.8 (6.4) | 5.4 (6.1) | 0.317 |
| Mean (SD) Glycemic Variability | |||
| CONGA (mg/dL) | 41.6 (16.6) | 17.3 (12.5) | <0.001 |
| CV (%) | 26.0 (9.0) | 23.1 (15.1) | 0.236 |
| IQR (mg/dL) | 33.0 (17.7) | 26.2 (20.9) | 0.101 |
| M-value | 0.83 (2.5) | 0.65 (3.3) | 0.125 |
| MAGE (mg/dL) | 64.0 (9.8) | 37.7 (28.2) | <0.001 |
| MODD (mg/dL) | 25.3 (9.8) | 10.2 (9.7) | <0.001 |
| SD (mg/dL) | 34.3 (15.0) | 28.9 (22.3) | 0.376 |
| Frequency of Hypoglycemic Events | |||
| Moderate Events (# of patients) | 64 (20) | 12 (4) | <0.001 |
| Severe Events (# of patients) | 26 (6) | 0 (0) | <0.001 |
| Patient Outcomes | |||
| Mortality (%) | 11.1 | 8.5 | 0.666 |
| Mean (SD) ICU LOS | 46.5 (46.7) | 32.9 (29.3) | 0.413 |
| Mean (SD) Ventilator days | 28.2 (35.6) | 23.5 (20.8) | 0.328 |
| Relevant Medications | |||
| Mean (SD) Insulin Rate (U/hr) | 5.1 (3.8) | 2.4 (1.3) | <0.001 |
| Vasopressors (%) | 34.9 | 28.7 | 0.221 |
Note: Glucose in mmol/L = 0.552 × glucose in mg/dL; moderate hypoglycemia: 40–69 mg/dL; severe hypoglycemia: < 40 mg/dL; vasopressors include epinephrine and norepinephrine; patients did not receive immunosuppressive agents or steroid therapy.
Abbreviations: CONGA, continuous overall net glycemic action; CV, coefficient of variation; F, female; ICU, intensive care unit; IQR, interquartile range; LOS, length of stay; M, male; MAGE, mean amplitude of glycemic excursions; MODD, mean of daily differences; MODS, multiple organ dysfunction score; NS; not significant; SD, standard deviation; and TBSA, total body surface area burned.
BGMS Performance and Mean Insulin Rates
Median (range) frequency of glucose measurements per day was similar between BGMS-1 versus BGMS-2 groups (2.0 [0 to 4] vs. 2.5 [0 to 3], P = 0.381). BGMS-1 performance significantly differed from the paired plasma glucose reference method (mean bias of 7.4 ± 13.5 mg/dL [0.41 ± 0.75 mmol/L], n = 535, P<0.001). In contrast, BGMS-2 results were similar to paired plasma glucose measurements (−1.7 ± 6.9 mg/dL [0.09 ± 0.38 mmol/L], n = 511, P = 0.349) (Figure 1). The average delay between paired BGMS measurements and plasma glucose testing by the laboratory was 2.5 ± 1.2 minutes. Mean insulin rates significantly differed between BGMS-1 and 2 groups (5.1 ± 3.8 U/hour, n = 535 vs. 2.4 ± 1.3 U/hour, n = 511, P<0.001). A significantly higher frequency of hypoglycemic events based on laboratory plasma glucose results was observed in the BGMS-1 group compared to the BGMS-2 group (90 vs. 12, P<0.001). Specifically, of the 90 hypoglycemic events recorded, 28 were less than 40 mg/dL (2.2 mmol/L). In contrast, the BGMS-2 group, 12 moderate hypoglycemic events were recorded. No severe hypoglycemic events were observed in the BGMS-2 treated group.
Figure 1. Bland-Altman Analysis of Paired Glucose Measurements.

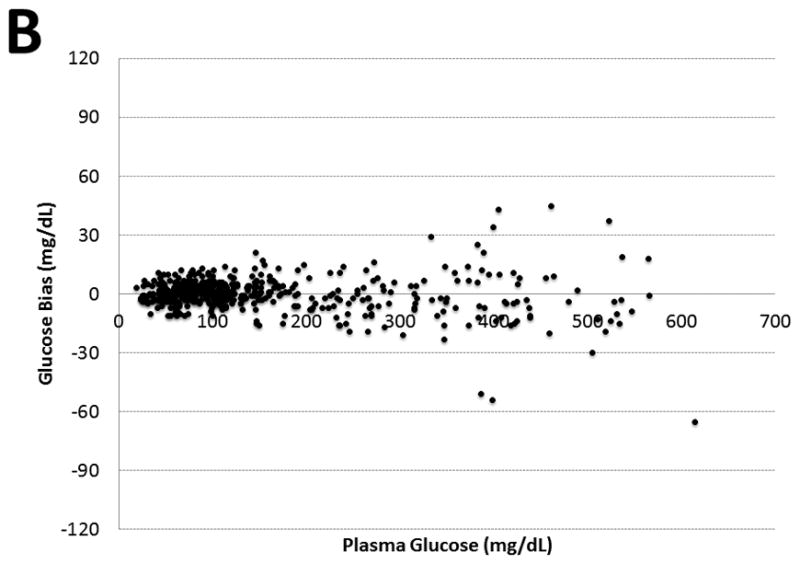
Figure 1 illustrates two Bland-Altman plots for BGMS-1 (Panel A, n = 535) versus BGMS-2 (Panel B, n = 511) respectively when compared to paired laboratory plasma glucose measurements through. Plasma glucose measurements are shown on the x-axis, and bias (BGMS – plasma glucose) are identified on the y-axis. The glucose in mmol/L = 0.552 x glucose in mg/dL.
Glycemic Variability
Compared to the BGMS-2 group, BGMS-1 patients exhibited significantly higher glycemic variability based on CONGA, MAGE, and MODD alone. In non-surviving BGMS-1 patients, glycemic variability was significantly higher as determined by CONGA (49.4 ± 29.6 mg/dL [2.7 ± 1.64 mmol/L] vs. 35.3 ± 14.5 mg/dL [2.0 ± 0.80 mmol/L], P = 0.011), CV (32.6 ± 16.0 % vs. 23.2 ± 15.1%, P < 0.001), MAGE (87.2 ± 56.1 mg/dL [4.8 ± 3.1 mmol/L] vs. 59.2 ± 21.4 mg/dL [3.3 ± 1.2 mmol/L], P < 0.001), and SD (42.9 ± 23.1 mg/dL [2.4 ± 1.3 mmol/L] vs. 30.3 ± 15.2 mg/dL [1.7 ± 0.83 mmol/L], P = 0.029). High glycemic variability was also seen in the non-survivor BGMS-2 subgroup based on CONGA (25.3 ± 3.1 mg/dL [1.4 ± 0.17 mmol/L] vs. 19.3 ± 13.1 mg/dL [1.07 ± 0.72 mmol/L], P<0.001), MAGE (82.8 ± 22.6 mg/dL [4.6 ± 1.25 mmol/L] vs. 35.7 ± 29.9 mg/dL [1.97 ± 1.65 mmol/L], P<0.001), and MODD (33.2 ± 4.3 mg/dL [1.83 ± 0.24 mmol/L] vs. 10.7 ± 8.1 mg/dL [0.59 ± 0.45 mmol/L], P<0.001). Multivariate logistic regression revealed MAGE was predictive of mortality when controlled for age, TBSA, and inhalation injury presence for both BGMS-1 (OR 1.10, 95% CI 1.0 – 1.09, P = 0.030) and BGMS-2 patient groups (OR 1.09, 95% CI 1.0 – 1.12, P = 0.022).
Glycemic Control Success
On average, BGMS-2 patients achieved glycemic control significantly faster than BGMS-1 patients (5.7 ± 4.3 hours vs. 13.1 ± 6.9 hours, P < 0.001). Additionally, a larger proportion of glucose measurements in the BGMS-2 group stayed within the targeted TGC range of 80 to 130 mg/dL (4.4 to 7.2 mmol/L) compared to patients in the BGMS-1 group (85.2 ± 13.9% vs. 57.9 ± 29.1%, P<0.001).
DISCUSSION
The goal of this study was to retrospectively determine the clinical impact of an autocorrecting BGMS in children with severe burns. Analytical performance of the autocorrecting BGMS was superior to its non-correcting counterpart. On average, BGMS-1 exhibited a significant positive bias likely due to known hematocrit and drug interference for this device (4, 11) when compared to the central laboratory. Consequently, patients receiving IIT based on BGMS-1 results, experienced significantly higher mean insulin rates, and a greater frequency of moderate and severe hypoglycemic events—potentially due to the device’s inherent falsely elevated glucose measurements. Conversely, we observed significantly lower mean insulin infusion rates and far fewer hypoglycemic events in BGMS-2 patients, and did not identify any values falling below 40 mg/dL (2.21 mmol/L). Glycemic variability was also significantly higher in the BGMS-1 group as determined by CONGA, MAGE, and MODD methods. MAGE was predictive of mortality as shown by our previous adult burn study—suggesting this measure of variability, which treats peaks and nadirs equally compared to other methods, better represents glycemic excursions encountered in severely burned children. (5) Interestingly, BGMS-2 patients achieved TGC more quickly and maintained patients within the targeted 80 to 130 mg/dL (4.4 to 7.2 mmol/L) range longer compared to individuals in the BGMS-1 group.
BGMS accuracy for IIT and TGC remains controversial due to recent FDA guidelines reinforcing existing laws as defined by the Clinical Laboratory Improvement Amendment of 1988 (CLIA). The CMS memorandum regarding “off-label” device use further exacerbates this controversy. Although the CMS memorandum has since been temporarily retracted and offered as a draft for public feedback, CLIA’88 requirements remains unchanged and are still enforced. Many facilities have changed their IIT protocols to target higher TGC intervals in an effort to minimize hypoglycemic risk. Unfortunately, these IIT protocols potentially increase rates of hyperglycemia and glycemic variability. (17) In the face of strict FDA and CMS BGMS guidelines and the concern for inaccurate bedside measurements, several institutions have removed these devices from patient care areas (19). Upon review of the FDA MAUDE database, we have found regulatory concerns to be well justified and highly relevant to critical care medicine. (20) The MAUDE database entries for the top five commercially available POC BGMS from 1997 to 2014 showed over 1,094 entries with 557 reports of erroneous measurements compared to central laboratory methods, and 28 device-associated adverse events including at least 13 deaths. Confounding interfering substances may have contributed to these events including those related to maltose interference and improper capillary fingerstick testing in patients with severe shock. Recent studies evaluating the effects of interfering substances on contemporary POC BGMS devices unfortunately continue to demonstrate inadequate performance by non-correcting devices. (21,22)
The implementation of an autocorrecting POC BGMS by our pediatric burn center has significantly improved glycemic control in this high-risk patient population. Automatic correction of interfering substances and abnormal hematocrit in critically ill patients enables BGMS’s to be comparable to central laboratory plasma glucose measurements. Additional studies have reported similar findings in adult patient populations. (5, 16, 17) As such, the MAUDE database entry for BGMS-2 yielded only 28 total entries, with four erroneous measurements and no adverse events or deaths since the device’s release in 2006. One of these four erroneous BGMS-2 measurements was due user error where capillary fingerstick specimens were improperly obtained in a patient with severe hypotension. In September 2015, BGMS-2 became the first and only device to receive FDA clearance for use in all hospital populations including critically ill patients. (9) It must be noted that the MAUDE database is a good post-market surveillance tool, however, there may be inherent biases due to the self-reporting nature of the database. For example, newly released devices (e.g., BGMS-3) or instruments that are not widely used (e.g., BGMS-4) may be underrepresented.
Point-of-care testing is not an excuse for inaccuracy. Blood glucose monitoring systems, although convenient, must meet performance requirements to facilitate safe dosing of intravenous insulin in the critically ill. Use of inaccurate BGMS’s and/or frame shifting TGC ranges to avoid hypoglycemia does not provide optimum care. Specifically, hospitals should validate inaccurate devices in a clearly defined critically ill populations, remove off-label devices from the bedside, or adopt an FDA cleared autocorrecting BGMS. The future of IIT and TGC may involve the use of emerging continuous glucose monitoring (CGM) devices, however, there are substantial analytical limitations that remain unaddressed including accuracy, precision, drift, and need to calibrate after a certain time. Firstly, subcutaneous systems measure glucose from interstitial fluid, which are not adequate for TGC. Conversely, CGM from indwelling catheters provides higher quality results. Unfortunately, the overall analytical performance characteristics of CGM devices are not comparable to existing laboratory methods or even BGMS’ studied in this paper due to biosensor degradation. Biosensor degradation is the result of prolonged interaction in the complex milieu of the human body—resulting in analytical drift and potential erratic performance (23,24). Our study provides the first, to our knowledge, data evaluating the clinical impact of accurate glucose measurements using an autocorrecting biosensor in the severely burned pediatric population. Study limitations include the retrospective nature of our analysis the time span of the data collection required to have sufficient number of severely burned children who received IIT, and small sample size associated with a single-site investigation. Although BGMS-2 exhibited more accurate performance, the clinical ramifications of accurate versus inaccurate BGMS measurements remain highly controversial.
CONCLUSIONS
Glucose remains one of the most important and frequently measured analytes in the clinical setting. In critically ill patients, the use of IIT and TGC has potential benefits; however, this ideally requires accurate POC devices. Our study reports the clinical impact of accurate glucose monitoring in a high-risk pediatric burn population and provides healthcare providers with alternative evidence-based solutions for the recent FDA and CMS requirements. Improved glucose monitoring optimizes insulin therapy and reduces risk for hypoglycemia and glycemic variability as determined by the MAGE method. We recommend pediatric burn centers to work closely with their clinical laboratory to identify appropriate BGMS devices that meet the FDA guidelines and improve the quality of patient care, while being aware of potential confounding factors that may compromise glucose monitoring performance.
Acknowledgments
Financial Support: National Institutes of Health and Department of Defense
This investigator-initiated project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health (Award Number: UL1TR000002), a National Heart Lung and Blood Institute Emergency Medicine K12 Career Award (Dr. Tran, Award Number: 5K12HL108964), a Department of Defense sub-award (#126333 125762), and intramural funds from the UCD Biomedical Engineering Department. We thank the UCD Data Coordination Center, and Dr. Charlie Wade for their support.
Footnotes
Copyright form disclosures:
Dr. Tran received support for article research from the National Institutes of Health (NIH) and Department of Defense. His institution received funding from the NIH and Department of Defense. Dr. Wolf is the Principal Investigator of the overall grant TATRC, W81XWH-09-2-0194), and this work is from one of the projects. He does not receive salary support for the grant, but has received travel reimbursement for grant management activities. Dr. Wolf disclosed other support: Potential Conflicts Elsevier Editor – Burns (salary $40,000/year) Nov 2004 – present Merck Manual Contributor ($1050) Oct 2012–Sept 2015 Grants Steven E Wolf (PI) TATRC, W81XWH-12-2-0074-02 ($500,000) 2012–2016 Steven E Wolf (PI) CDMRP 11231488 ($415,972) 2013–2015 Steven E Wolf (PI) TATRC, W81XWH-09-2-0194 ($7,866,937) 2013–2015 Reviewer and Executive Boards Shriners’ Hospitals for Children Research Advisory Board ($3000/year) 2006 – present American Burn Association Verification Committee ($0) 2010 – present American Burn Association Program Committee ($0) 2012–2016 American Burn Association Board of Trustees 2013 – present Shock Society Executive Council ($0) 2010 – present Sons of the Flag Board of Directors ($0) 2013 – present DHHS – FDA Special Employee ($0) 2014 – present Patents 2011 Decision-Assist Method of Resuscitation of Patients (US 7,879,020 B1) Jose Salinas, George C Kramer, Leopoldo C Cancio, Kevin Chung, Elizabeth Mann, Steven E Wolf, Guy A Drew 2013 Decision-Assist Method of Resuscitation of Patients (US 8,585,675 B2) Jose Salinas, George C Kramer, Leopoldo C Cancio, Kevin Chung, Elizabeth Mann, Steven E Wolf, Guy A Drew. The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Pham TN, Warren AJ, Phan HH, et al. Impact of tight glycemic control in severely burned children. J Trauma. 2005;59:1148–1154. doi: 10.1097/01.ta.0000188933.16637.68. [DOI] [PubMed] [Google Scholar]
- 2.Pierre EJ, Barrow RE, Hawkins HK, et al. Effects of insulin on wound healing. J Trauma. 1998;44:342–345. doi: 10.1097/00005373-199802000-00019. [DOI] [PubMed] [Google Scholar]
- 3.Cree MG, Aarsland A, Herndon DN, et al. Role of fat metabolism in burn trauma-induced skeletal muscle insulin resistance. Crit Care Med. 2007;35:S476–483. doi: 10.1097/01.CCM.0000278066.05354.53. [DOI] [PubMed] [Google Scholar]
- 4.Jeschke MG, Kulp GA, Kraft R, et al. Intensive insulin therapy in severely burned pediatric patients: a prospective randomized trial. Am J Respir Crit Care Med. 2010;182:351–359. doi: 10.1164/rccm.201002-0190OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tran NK, Godwin ZR, Bockhold JC, et al. Clinical impact of sample interference on intensive insulin therapy in severely burned patients: a pilot study. J Burn Care Res. 2014;35:72–79. doi: 10.1097/BCR.0b013e31829b3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finfer S, Chittock DR, Su SY, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 7.Finfer S, Liu B, Chittock DR, et al. Hypoglycemia and risk of death in critically ill patients. N Engl J Med. 2012;367:1108–1118. doi: 10.1056/NEJMoa1204942. [DOI] [PubMed] [Google Scholar]
- 8.Klonoff DC. Regulatory controversies surround blood glucose monitoring devices. J Diabetes Sci Technol. 2010;4:231–235. doi: 10.1177/193229681000400201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.FDA. [Accessed on December 3, 2014];Draft Guidance Document for Blood Glucose Monitoring Test Systems for Prescription Point-of-Care Use. http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM380325.pdf.
- 10.Centers for Medicare and Medicaid Services (CMS) Memorandum. Directions on the Off-Label/Modified Use of Waived Blood Glucose Monitoring Systems (BGMS) Nov 21, 2014. [Google Scholar]
- 11.Dungan K, Chapman J, Braithwaite SS, et al. Glucose measurement: confounding issues in seting targets for inpatient management. Diabetes Care. 2007;30:403–409. doi: 10.2337/dc06-1679. [DOI] [PubMed] [Google Scholar]
- 12.Tang Z, Louie RF, Payes M, et al. Oxygen effects on glucose measurements with a reference analyzer and three handheld meters. Diabetes Technol Ther. 2000;2:349–362. doi: 10.1089/15209150050194215. [DOI] [PubMed] [Google Scholar]
- 13.Tang Z, Lee JH, Louie RF, et al. Effects of different hematocrit levels on glucose measurements with handheld meters for point-of-care testing. Arch Pathol Lab Med. 2000;124:577–582. doi: 10.5858/2000-124-1135-EODHLO. [DOI] [PubMed] [Google Scholar]
- 14.Tang Z, Du X, Louie RF, et al. Effects of pH on glucose measurements with handheld glucose meters and a portable glucose analyzer for point-of-care testing. Arch Pathol Lab Med. 2000;124:577–582. doi: 10.5858/2000-124-0577-EOPOGM. [DOI] [PubMed] [Google Scholar]
- 15.Tang Z, Louie RF, Lee JH, et al. Oxygen effects on glucose meter measurements with glucose dehydrogenase- and oxidase-based test strips for point-of-care testing. Crit Care Med. 2001;29:1062–1070. doi: 10.1097/00003246-200105000-00038. [DOI] [PubMed] [Google Scholar]
- 16.Karon BS, Blashan CT, Deobald GR, et al. Retrospective evaluation of the accuracy of Roche AccuChek Inform and Nova StatStrip glucose meters in critically ill patients. Diabetes Technol Ther. 2014;16:828–832. doi: 10.1089/dia.2014.0074. [DOI] [PubMed] [Google Scholar]
- 17.Karon BS, Bryant SK. Impact of glucose meter accuracy on the efficacy of glycemic control in critically ill patients after cardiovascular surgery. Critical Care Point-of-Care Testing Meeting; San Diego, CA. September 17–19, 2014. [Google Scholar]
- 18.Mann EA, Mora AG, Podcoke HF, et al. Glycemic control in the burn intensive care unit: focus on the role of anemia in glucose measurement. J Diabet Sci Technol. 2009;3:1319–1328. doi: 10.1177/193229680900300612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.New York State Department of Health Letter. [Accessed on December 7, 2014]; http://www.wadsworth.org/labcert/clep/files/Glucose_meters_off_label_use_1_13_14.pdf.
- 20.FDA MAUDE Database. [Accessed on August 20, 2014]; http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/search.cfm.
- 21.Steele AN, Godwin Z, Howes M, et al. Extensive evaluation of sample interferences on point-of-care glucose meters. American Association for Clinical Chemistry Annual Meeting; July 30, 2014; Chicago, IL. [Google Scholar]
- 22.Steele AN, Godwin Z, Howes M, et al. Extensive evaluation of hematocrit interferences on point-of-care glucose meters. American Association for Clinical Chemistry Annual Meeting; July 30, 2014; Chicago, IL. [Google Scholar]
- 23.Signal M, Pretty CG, Chase JG, et al. Continuous glucose monitors and the burden of tight glycemic control in critical care: can they cure the time cost? J Diabetes Sci Technol. 2010;4:625–635. doi: 10.1177/193229681000400317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schierenbeck F, Franco-Cereceda A, Liska J. Evaluation of a continuous blood glucose monitoring system using central venous microdialysis. J Diabetes Sci Technol. 2012;6:1365–1371. doi: 10.1177/193229681200600615. [DOI] [PMC free article] [PubMed] [Google Scholar]


