Abstract
Disgust is an emotion that helps us deal with potential contamination (Rozin & Fallon, 1987). It produces a distinctive facial expression (e.g., wrinkled nose) and a physiological response that is accompanied by strong visceral sensations (e.g., nausea). Given the important role the anterior insula plays in processing and integrating visceral information (Craig, 2009), it is likely to be centrally involved in disgust. Despite this, few studies have examined the link between insular degeneration and the experience, physiology, and expression of disgust. We studied a group that was heterogeneous in terms of insular damage; 84 patients with neurodegenerative diseases (i.e., frontotemporal dementia, corticobasal syndrome, progressive supranuclear palsy, Alzheimer's disease) and 29 controls. Subjects viewed films that elicit high levels of disgust and sadness. Emotional reactivity was assessed using self-report, peripheral physiology, and facial behavior. Regional brain volumes (insula, putamen, pallidum, caudate, and amygdala) were determined from structural MRIs using the FreeSurfer method. Results indicated that smaller insular volumes were associated with reduced disgust responding in self-report and physiological reactivity, but not in facial behavior. In terms of the specificity of these findings, insular volume did not predict sadness reactivity, and disgust reactivity was not predicted by putamen, pallidum, and caudate volumes (lower self-reported disgust was associated with smaller amygdala volume). These findings underscore the central role of the insula in the experience and physiology of disgust.
Keywords: insula, disgust, neurodegenerative disease
Disgust is an emotion that functions to help the organism deal with potential contamination (Rozin & Fallon, 1987). This function is facilitated by actions mediated by the autonomic nervous system (e.g., gagging) that inhibit the ingestion of potentially harmful substances (Rozin, Haidt, & McCauley, 2008) and produce powerful visceral sensations (e.g., nausea). These visceral events and sensations are experienced as highly unpleasant and motivate additional avoidance behaviors. Disgust is thought to have a prototypic facial response, characterized by a nose wrinkle and upper lip raise – features that serve the function of closing off the nasal passages in order to protect against toxic substances and smells (Rozin & Fallon, 1987). Moreover, the resulting facial expression provides a useful signal to conspecifics that something harmful is nearby.
The Insula and Disgust
The insula is located deep in the Sylvian fissure. One role of the insula is gustatory, with important involvement in taste and smell (Rolls & Baylis, 1994). The insula has also been associated with a variety of complex higher order functions, some of the primary ones being emotional experience (e.g., induction, recall of emotions), empathy, attention, and language (Kurth, Zilles, Fox, Laird, & Eickhoff, 2010). The insula has been consistently associated with bodily awareness (e.g., Mutschler et al., 2009), helping translate bodily cues into subjective emotional feelings (Adolphs, 2002; Craig, 2009; Singer, Critchley, & Preuschoff, 2009). This latter function may be particularly important in emotions such as disgust in which visceral and bodily cues figure so prominently (Rozin et al., 2008).
Previous research has identified a number of associations between the insula and disgust. For example, in studies in which participants view images of mutilation and contamination, the insula appears to be one of the primary brain regions of activation (Wright, He, Shapira, Goodman, & Liu, 2004). Other research has found that the insula is activated when one person views another person experiencing disgust (Wicker et al., 2003). In a study of patients with bilateral insular lesions, loss of insular volume was associated with deficits in identifying disgust (Adolphs, Tranel, & Damasio, 2003).
Several recent meta-analytic studies have addressed the important question of whether particular emotions have different regional neural correlates, arriving at quite different conclusions. For example, Vytal and Hamann (2010) concluded that there was strong evidence for discrete neural correlates of “basic” emotions (e.g., amygdala, right cerebellum, and right insula for fear; bilateral insula for disgust; left inferior frontal gyrus and right parahippocampal gyrus for anger). In contrast, Lindquist et al. (2012) concluded that the bulk of the evidence supported common structures that are involved across the core emotions (e.g., amygdala, insula, orbitofrontal cortex, anterior cingulate cortex). We believe there is truth in both views. All basic emotions arise from the action of common generating structures (Rosen & Levenson, 2009); however, this does not preclude the possibility that particular emotions are associated with greater activation of particular regions by virtue of particular features (e.g., disgust may be more strongly linked with insular activation than other emotions because of greater visceral involvement in disgust). The different conclusions reached in these meta-analytic studies also make a strong case for the utility of research like the present study, which uses patient models and a multi-method (subjective, physiological, behavioral) assessment of emotion to evaluate the impact that atrophy of the insula (and other brain regions) has on disgust responding (and other emotions). Studies of this type have been rare despite their potential utility for illuminating fundamental issues concerning the specificity of brain-behavior relationships.
Insular Atrophy in Neurodegenerative Diseases
Behavioral variant frontotemporal dementia (bvFTD) may be particularly informative for studying the role of the insula in disgust responding. Among patients with bvFTD, the insula is frequently one of the first brain regions to show signs of neurodegeneration (Rosen et al., 2002). In terms of behavior, patients with bvFTD often show clear deficits in disgust responding. This is seen in everyday life, where bvFTD patients often engage in behaviors that others would find disgusting (e.g., eating food left out by others), and in laboratory studies using disgust-eliciting stimuli, where patients show low levels of reactivity to films depicting filth and contamination (Eckart, Sturm, Miller, & Levenson, 2012).
Insular atrophy is not unique to bvFTD; it is also found in several other neurodegenerative diseases including corticobasal syndrome (CBS), progressive supranuclear palsy (PSP), and late in the course of Alzheimer's disease (AD) (Belfor et al., 2006; Foundas, Leonard, Mahoney, Agee, & Heilman, 1997; Padovani et al., 2006). Despite this similarity in insular degeneration, there are important differences among these diseases. Whereas patients with bvFTD typically show atrophy in the frontal and anterior temporal lobes (McKhann et al., 2001), (a) CBS patients exhibit early atrophy primarily in the frontal and parietal lobes and in the striatum (Boxer et al., 2006); (b) PSP patients exhibit early atrophy in the midbrain, pons, thalamus, and striatum (Boxer et al., 2006); and (c) AD patients show atrophy in medial temporal regions and the hippocampus (H. Braak & Braak, 1995).
Present Study
The present study examined the relationship between total insular volume (to capture all insular subregions), and disgust reactivity (self-report, physiology, facial behavior) in a group of patients with neurodegenerative diseases (bvFTD, CBD, PSP, AD) that are characterized by varying degrees of insular atrophy, and a group of healthy controls. Including patients with different neurodegenerative diseases provides a high level of neuroanatomical heterogeneity, which is useful for mapping relationships between neural loss and deficits in emotional responding. Our primary hypothesis was that lower insular gray matter volume would be associated with less reactivity to disgust stimuli, as measured by self-reported emotional experience, physiological reactivity, and facial behavior.
Although examining additional brain regions and additional emotions would allow us to evaluate the extent of specificity more fully, the experimental design used in this study provides some support for specificity. First, to evaluate emotional specificity, we included a second emotion, sadness. Sadness was chosen because it shares several features with disgust: (a) both are considered to be “negative” emotions; (b) both involve activation of the parasympathetic branch of the autonomic nervous system (e.g., gastrointestinal responding in disgust, crying in sadness), and (c) neither is associated with the classic “fight or flight” behavioral response that is associated with other negative emotions such as anger or fear (Levenson, 2014). Second, to evaluate anatomical specificity, we measured gray matter integrity in several areas in addition to the insula, assessing total volumes of pallidum, putamen, caudate, and amygdala. These areas were chosen because they have been linked with emotional responding in general (e.g., the amygdala; LeDoux, 1992; Murphy, Nimmo-Smith, & Lawrence, 2003) and/or to disgust responding specifically (e.g., basal ganglia structures; Gray, Young, Barker, Curtis, & Gibson, 1997; Sprengelmeyer, Rausch, Eysel, & Przuntek, 1998).
Methods
Participants
Patients were recruited through the Memory and Aging Center at the University of California, San Francisco (UCSF), where they underwent extensive cognitive, neurological, and neuroimaging testing and received a diagnosis using consensus criteria: (a) bvFTD (Neary et al., 1998); (b) AD (McKhann et al., 1984); (c) CBD (Armstrong et al., 2013); and (d) PSP (Litvan et al., 1996). Control participants were recruited from the community and reported no history of neurological, psychiatric, or cognitive disturbances. The final participant sample consisted of 24 bvFTD patients, 20 AD patients, 9 CBD patients, 2 PSP patients, and 29 neurologically healthy controls (see Tables 1 and 2 for sociodemographic characteristics and brain volumes). All participants consented to participate in a daylong assessment of emotional functioning in our laboratory at Berkeley.
Table 1. Sociodemographic Characteristics, Disgust Reactivity, and Sadness Reactivity.
| bvFTD | AD | PSP | CBD | Controls | |
|---|---|---|---|---|---|
| N | 24 | 20 | 2 | 9 | 29 |
| Sex (male) | 22 | 12 | 2 | 2 | 14 |
| M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | |
| Age | 60.56 (5.55) | 61.50 (8.28) | 68.86 (3.88) | 63.41 (4.23) | 64.72 (12.02) |
| Disgust Facial Behavior | 11.52 (14.74) | 12.4 (13.79) | 17.3 (24.40) | 24.8 (30.56) | 20.7 (16.67) |
| Disgust Physiological Reactivity | -.14 (0.39) | -.09 (0.40) | -.17 (0.22) | -.08 (0.34) | 0.21 (0.58) |
| Disgust Self-report | 1.38 (0.71) | 1.45 (0.69) | 1.5 (0.71) | 1.33 (0.71) | 1.79 (0.49) |
| Sadness Facial Behavior | 6.02 (11.47) | 4.50 (9.85) | 0.00 (0.00) | 15.83 (14.25) | 8.46 (13.72) |
| Sadness Physiological Reactivity | 0.01 (0.39) | 0.13 (0.44) | -0.18 (0.21) | -0.03 (0.41) | -0.07 (0.45) |
| Sadness Self-Report | 1.17 (0.78) | 1.17 (0.78) | 1.50 (0.71) | 1.67 (0.71) | 1.31 (0.76) |
Notes. All calculated from raw data, except for physiological reactivity, which was standardized.
Table 2. Regions of Interest Brain Volumes.
| bvFTD | AD | PSP | CBD | Controls | |
|---|---|---|---|---|---|
| M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | |
| Insula | |||||
| Raw | 13711.63 (3492.76) | 13206.40 (2601.94) | 11596.00 (548.72) | 11166.00 (3799.36) | 15254.41 (2946.36) |
| Corrected | -0.07 (1.02) | -0.28 (0.77) | -0.84 (0.25) | -0.86 (1.24) | 0.57 (0.68) |
| Pallidum | |||||
| Raw | 2768.58 (534.91) | 2976.75 (668.14) | 2273.00 (101.82) | 2890.00 (754.56) | 3107.83 (540.0) |
| Corrected | -0.37 (0.85) | 0.03 (1.06) | -1.29 (.00) | 0.06 (1.26) | 0.36 (0.82) |
| Putamen | |||||
| Raw | 8072.63 (1392.62) | 9228.00 (1298) | 9351.50 (70.00) | 8086.89 (971.72) | 9888.38 (1333.74) |
| Corrected | -0.80 (0.91) | 0.17 (0.72) | 0.24 (0.17) | -0.33 (0.72) | 0.63 (0.81) |
| Caudate | |||||
| Raw | 6130.21 (1112) | 6984.05 (862.21) | 8385.00 (3350.27) | 5881.56 (756.43) | 6988.03 (836.79) |
| Corrected | -0.66 (0.94) | 0.30 (0.75) | 1.67 (3.04) | -0.43 (0.53) | 0.36 (0.71) |
| Amygdala | |||||
| Raw | 2594.50 (731.0) | 2793.60 (606.48) | 2793.50 (101.12) | 2844.00 (305.17) | 3336.66 (433.17) |
| Corrected | -0.55 (1.19) | -0.19 (0.87) | -0.31 (0.06) | -0.07 (0.55) | 0.63 (0.62) |
Notes. Raw volumes were corrected for scanner type and intracranial volume, presented as “corrected.”
Procedure
Laboratory assessment
Upon arrival at the Berkeley Psychophysiology Laboratory, participants completed additional consent forms and were seated in a 3 × 6 meter room. Stimuli were presented on a 21-inch monitor at a distance of 1.75 m from the participant. Participants completed a 6-hour laboratory session designed to provide a comprehensive assessment of emotional functioning that focuses on emotional reactivity (generating emotion), emotion regulation (down-regulating and up-regulating emotion) and recognizing emotion in others (Levenson et al., 2008). The present study focused on emotional reactivity, using data from two trials in which participants viewed films that are known to elicit disgust and sadness. The sample of participants used for this study was accumulated over a 10-year period. Most experimental procedures were unchanged across the 10 years except that the film used to elicit disgust and the length of the film used to elicit sadness differed (see below) for participants studied between 2003-2006 (N=38) and those studied between 2007-2012 (N=46).
After the consent forms were completed, non-invasive physiological sensors were attached to measure a broad range of autonomic nervous system functions as well as general somatic activity (see below for detailed description). The experimental protocol for film viewing consisted of a 60-second pre-film baseline period during which participants were instructed to “watch the X” that appeared on the screen and then watch the film. For the disgust trial, participants studied between 2003-2006 (wave 1) saw a 69-second excerpt from the film “Trainspotting” that depicts a man looking for drugs in a filthy toilet filled with excrement, and those studied between 2007-2012 (wave 2) saw a 105-second excerpt from the television show “Fear Factor” that depicts a man sucking fluids out of cow intestines and drinking a cup of the fluid. For the sadness trial, participants studied between 2003-2006 saw a 222-second excerpt from the film “The Champ” that depicts a young boy whose father dies after a boxing match. Participants studied between 2007-2012 saw a 92 second excerpt from the same film (both versions featured the same key sadness-inducing section). Physiological measures were monitored continuously during film viewing and facial behavior was recorded using partially hidden cameras. After the end of each film, participants were asked a number of questions about their subjective emotional experience during the film (see below for details). All participants were paid $30 at the end of the study for their participation and consent was obtained for the subsequent use of the video recordings.
Apparatus and Measures
Neuroimaging
Brain imaging was completed at UCSF prior to the testing at the Berkeley Psychophysiology Laboratory. Structural MRIs were obtained from each patient using either a 1.5 T, 3 T, or 4 T scanner (Boxer et al., 2011; Wilson et al., 2009). To ensure that results were not affected by scanner differences, scanner type was controlled for in all analyses (e.g., Wilson et al., 2009). For 69 participants, T1 images were acquired on a 1.5 T Siemens Magnetom VISION system (Siemens, Iselin, NJ) equipped with a standard quadrature head coil, using a magnetization prepared rapid gradient echo (MPRAGE) sequence (164 coronal slices; slice thickness = 1.5 mm; field of view (FOV) = 256 mm; matrix 256 × 256; voxel size 1.0 × 1.5 × 1.0 mm; repetition time (TR) = 10 ms; echo time (TE) = 4 ms; flip angle = 15°). For 9 participants, scans were obtained on a 3 T Trio Tim system (Siemens, Iselin, NJ) using high-resolution T1-weighted 3D MPRAGE sequences. The scan parameters were: TR/TE/T1, 2300/3/900 ms, flip angle 9°, 26 cm FOV, 256 × 256 in plane matrix, with a phase FOV of 0.94 and slice thickness of 1.0 mm. For 6 participants, images were acquired on a 4 T Bruker MedSpec system with an 8 channel head coil controlled by a Siemens Trio console, using an MPRAGE sequence (176 sagittal slices; slice thickness = 1 mm; FOV = 256 × 256 mm; matrix = 256 × 256; voxel size = 1.0 × 1.0 × 1.0 mm; TR = 2300 ms; TE = 3 ms; flip angle = 7°).
Brain volumes
Regional brain volumes were calculated using the FreeSurfer method. FreeSurfer is a semi-automated program that generates volumes for cortical and subcortical regions of interest (Desikan et al., 2006). This procedure has been shown to produce statistically indistinguishable results from those yielded by manual tracing (Fischl et al., 2002). For the present study, volumes were computed for bilateral insula, caudate, putamen, pallidum, and amygdala regions. A measure of total intracranial volume was also obtained and used as a covariate in analyses to control for head size. The FreeSurfer software authors request that the following explanatory paragraph be included in any study using this procedure:
Cortical reconstruction and volumetric segmentation was performed with the FreeSurfer image analysis suite, which is documented and freely available for download online (http://surfer.nmr.mgh.harvard.edu/). The technical details of these procedures are described in prior publications (Dale & Sereno, 1993; Desikan et al., 2006; Fischl et al., 2002; Fischl, Liu, & Dale, 2001; Fischl et al., 2004; Fischl, Sereno, & Dale, 1999a; Fischl, Sereno, Tootell, & Dale, 1999b; Han et al., 2006; Jovicich et al., 2006; Segonne et al., 2004). Briefly, this processing includes motion correction and averaging of multiple volumetric T1 weighted images (when more than one is available), removal of non-brain tissue using a hybrid watershed/surface deformation procedure (Segonne et al., 2004), automated Talairach transformation, segmentation of the subcortical white matter and deep gray matter volumetric structures (including hippocampus, amygdala, caudate, putamen, ventricles) (Fischl et al., 2002; 2004), intensity normalization (Sled, Zijdenbos, & Evans, 1998), tessellation of the gray matter white matter boundary, automated topology correction (Fischl et al., 2001; Segonne, Pacheco, & Fischl, 2007), and surface deformation following intensity gradients to optimally place the gray/white and gray/cerebrospinal fluid borders at the location where the greatest shift in intensity defines the transition to the other tissue class (Dale & Sereno, 1993; Dale, Fischl, & Sereno, 1999; Han et al., 2006). Once the cortical models are complete, a number of deformable procedures can be performed for in further data processing and analysis including surface inflation (Fischl, Sereno, & Dale, 1999a), registration to a spherical atlas which utilized individual cortical folding patterns to match cortical geometry across subjects (Fischl, Sereno, Tootell, & Dale, 1999b), parcellation of the cerebral cortex into units based on gyral and sulcal structure (Desikan et al., 2006; Fischl et al., 2004), and creation of a variety of surface based data including maps of curvature and sulcal depth. This method uses both intensity and continuity information from the entire three dimensional MR volume in segmentation and deformation procedures to produce representations of cortical thickness, calculated as the closest distance from the gray/white boundary to the gray/CSF boundary at each vertex on the tessellated surface (Han et al., 2006). The maps are created using spatial intensity gradients across tissue classes and are therefore not simply reliant on absolute signal intensity. The maps produced are not restricted to the voxel resolution of the original data thus are capable of detecting submillimeter differences between groups. Procedures for the measurement of cortical thickness have been validated against histological analysis (Rosas et al., 2002) and manual measurements (Kuperberg et al., 2003; Salat et al., 2004). FreeSurfer morphometric procedures have been demonstrated to show good test-retest reliability across scanner manufacturers and across field strengths (Han et al., 2006).
Physiological reactivity
Physiological signals were monitored using a Grass Model 7 polygraph (participants from 2003-2006) or a BIOPAC polygraph (participants from 2007-2012), a computer with analog-to-digital capability and an online data acquisition and analysis computer program written by Robert W. Levenson. The program calculated second-by-second averages for the following measures: (a) heart rate: the inter-beat interval was calculated as the interval, in milliseconds, between successive R waves, using Beckman miniature electrodes with Redux paste that were placed on opposite sides of the participant's chest; (b) finger pulse amplitude: a UFI photoplethysmograph recorded the amplitude of blood volume in the finger using a photocell taped to the distal phalanx of the index finger of the non-dominant hand; (c) ear pulse transmission time: a UFI photoplethysmograph recorded the volume of blood in the ear to measure the transmission time between the R wave of the electrocardiogram signal and the upstroke of pulse at the ear; (d) skin conductance level: the electrical conductance of the skin was calculated using a constant-voltage device to pass voltage between Beckman regular electrodes on the ring and index fingers of the non-dominant hand to calculate the sweat response; (e) finger temperature: records body temperature using a thermistor (Fahrenheit) attached to the tip of the little finger on the non-dominant hand; (f) respiration period: the inter-cycle interval in milliseconds between breaths was calculated using a pneumatic bellows stretched around the thoracic region; (g) general somatic activity: the amount of overall movement was determined using an electromechanical transducer attached to the platform under the participant's chair; (h) systolic blood pressure and (i) diastolic blood pressure: a cuff placed on the ring finger of the participants' non-dominant hand computed blood pressure on every heart beat using an Ohmeda Finapress 2300.
For each physiological measure, the average for the 60-second baseline was subtracted from the average obtained during a 30-second “hot spot” within each film (the most emotionally intense 30 seconds of the film as identified by a panel of raters) to create a difference score for physiological reactivity. Each of these scores was then normalized to obtain z-scores for comparability across the physiological channels. Z-scores for inter-beat interval, finger pulse amplitude, ear pulse transmission time, and respiration period were multiplied by -1 so that higher z-scores for all measures represented greater activation. Z-scores for all nine physiological measures were then averaged to create a single composite score to represent overall physiological reactivity during each film clip. We have used these composite scores in previous research to provide a comprehensive index of physiological responding while controlling for Type I error by reducing the number of dependent measures (see Gross & Levenson, 1997)1.
Facial behavior
Participants' facial behavior during both the disgust and sadness inducing film clips was coded using the Emotional Expressive Behavior Coding System (Gross & Levenson, 1993). Ten emotions (i.e., happiness/amusement, interest, embarrassment, surprise, disgust, anger, fear, contempt, confusion, sadness) were coded on a 0-3 intensity scale during the 30-second hot spot. Emotion intensity ratings were averaged across the 30 seconds as well as between coders. Inter-rater reliability between two to four coders was high (Cronbach's alpha = .91). In the present study, we focused solely on the targeted emotion for each film (i.e., disgust facial behavior for the disgust film, sadness facial behavior for the sadness film).
Self-reported emotion
Following each film, patients rated their subjective experience of a number of different emotions during the films (2003-2006: afraid, angry, happy, sad, disgusted, surprised, embarrassed, sexually aroused; 2007-2012: affectionate, afraid, amused, angry, ashamed, calm, disgusted, embarrassed, enthusiastic, proud, sad) using a 0-2 scale (0 = not at all; 1 = a little; 2 = a lot). As with the emotional behavior (see above), for the present study we focused on ratings of the target emotion for each film (i.e., self-reported disgust for the disgust film, self-reported sadness for the sadness film).
Results
Statistical Analyses
To examine relationships between regional brain volumes and emotional responding, we conducted multiple regressions with each of the three aspects of emotional responding (self-reported emotion, physiological reactivity, or facial behavior) for either the sadness film or the disgust film as the dependent measure. In these regressions total intracranial volume, participant age, participant sex, wave of the study, and scanner type (i.e., 1.5T, 3T, 4T) were entered together in the first step as covariates. Then in the second step, one of six regional gray matter volumes of interest was entered (insula, pallidum, putamen, caudate, or amygdala for disgust. Figure 1). For the sadness analyses, we only examined insular volume with the three aspects of emotional responding. Because the aim of these analyses was to examine neural loss both within and between diagnostic groups, we did not control for diagnosis in our analyses.
Figure 1.
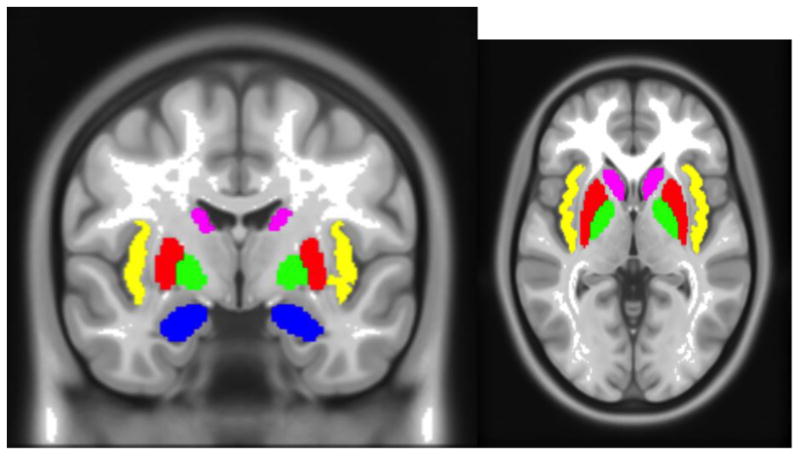
Regions of interest as delineated by Freesurfer. Insula (yellow), putamen (red), pallidum (green), caudate (violet), and amygdala (blue). ROIs shown in coronal and axial slices.
Effects of Film
Emotional reactivity (i.e., self-report, physiological reactivity, and facial behavior) for each film was compared using paired sample t-tests, in order to examine whether the two films were equivalent in their capacity to produce the target emotions (Figure 2). Films did not differ in their elicitation of self-report (t(83)=1.739, p=.086) or physiological reactivity (t(83)=-.017, p=.986), however the disgust film elicited significantly more facial behavior than the sadness film (t(83)=4.532, p<.001).
Figure 2.
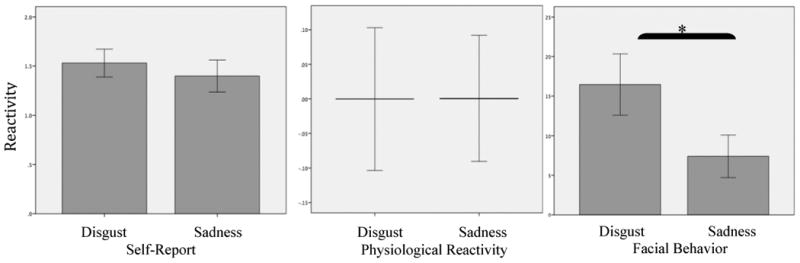
Main effects of physiological responding by film type, with confidence intervals (95%). *p<.05
Group Effects
We conducted univariate ANOVAS to determine whether there were group differences in the amount of self-reported disgust, physiological reactivity, and facial behavior between patient groups, controlling for age, gender, and wave of the study. Analyses revealed a main effect of diagnostic group for physiological reactivity during the disgust film, F(3,69)=4.123, p=.009) with the AD group showing less physiological reactivity than controls (means shown in Table 1). There was no main effect for diagnostic group in self-report and facial behavior for either the disgust or sad films, nor was there a main effect for diagnostic group in physiological reactivity for the sad film.
Hypothesis: Smaller Insular Volume Will Predict Decreased Disgust Responding
We predicted that smaller insular gray matter volume would predict less disgust responding in self-report, physiology, and facial behavior. Results supported this hypothesis for self-report and physiology (Table 3). Smaller insular volume significantly predicted less self-reported disgust (β = .383, t = 2.358, R2 change = .064, p = .021) and less overall physiological activation in response to the disgust film (β = .446, t = 2.890, R2 change = .087, p = .005)2. No relationship was found between insular volume and facial behavior in response to the disgust film (β = .197, t = 1.264, R2 change = .017, p = .210). In Table 4, mean insular volumes are presented for high and low responders (defined on the basis of those who were above the mean and those below the mean) in each of the three emotional domains during the disgust film.
Table 3. Relationships Between Disgust Reactivity and Brain Region Volume.
| β | t | ΔR2 | p | |
|---|---|---|---|---|
| Insula | ||||
| Self-report | .383 | 2.358 | .064 | .021 |
| Physiology | .446 | 2.890 | .087 | .005 |
| Facial Behavior | .197 | 1.264 | .017 | .210 |
| Pallidum | ||||
| Self-report | .026 | .207 | .001 | .837 |
| Physiology | .073 | .609 | .004 | .545 |
| Facial Behavior | -.100 | -.858 | .008 | .394 |
| Putamen | ||||
| Self-report | .108 | .863 | .009 | .391 |
| Physiology | .214 | 1.797 | .036 | .076 |
| Facial Behavior | .076 | .644 | .004 | .522 |
| Caudate | ||||
| Self-report | .124 | .135 | .014 | .282 |
| Physiology | .165 | 1.378 | .021 | .172 |
| Facial Behavior | -.017 | -.142 | .000 | .887 |
| Amygdala | ||||
| Self-report | .250 | 2.133 | .053 | .036 |
| Physiology | .181 | 1.572 | .028 | .120 |
| Facial Behavior | .139 | 1.240 | .016 | .219 |
Notes. Results from regression analyses with brain region volumes predicting disgust reactivity (i.e., self-report, physiology facial behavior) controlling for total intracranial volume, age, sex, wave of study, and scanner type.
Table 4. Insular Brain Volumes for High versus Low Disgust Responders.
| High Responders | Low Responders | |
|---|---|---|
| M (SD) | M (SD) | |
| Self-report | ||
| Raw | 14384.19 (3380.29) | 12852.91 (3003.28) |
| Corrected | 0.21 (0.97) | -0.34 (0.91) |
| Physiological Reactivity | ||
| Raw | 13993.03 (3175.88) | 13642.09 (3441.75) |
| Corrected | 0.12 (0.90) | -0.10 (1.05) |
| Facial Behavior | ||
| Raw | 13592.89 (3292.75) | 13949.39 (2246.56) |
| Corrected | 0.001 (0.96) | -0.001 (1.01) |
Notes. Raw insular volumes were corrected for scanner type and intracranial volume, presented as “corrected.” High vs. low responders were defined on the basis of those who were above the mean and those below the mean, for each of the three emotional reactivity measures.
Specificity of Findings
To evaluate the specificity of the relationship between insular volume and disgust responding, we examined the relationship between insular volume and sadness responding (emotional specificity), as well as the relationship between other relevant brain regions and disgust responding (anatomical specificity). Both analyses revealed evidence of specificity. In terms of emotional specificity, insular volume did not significantly predict self-reported sadness (β = -.033, t = -.197, R2 change = .000, p = .845), physiological activation (β = -.027, t = -.169, R2 change = .0010, p = .866), or sadness facial behavior (β = .168, t = 1.008, R2 change = .012, p = .316) in response to the sadness film (Table 5). In terms of anatomical specificity, pallidum, putamen, and caudate volumes did not significantly predict self-reported disgust, physiological activation, or disgust facial behavior in response to the disgust film (Table 3). The only evidence against anatomical specificity was found for the relationship between amygdala volume and disgust responding, with smaller amygdala volume predicting decreased self-reported disgust in response to the disgust film (β = .250, t = 2.133, R2 change = .053, p = .036).
Table 5. Relationship Between Sadness Reactivity and the Insula.
| β | t | ΔR2 | p | |
|---|---|---|---|---|
| Insula | ||||
| Self-report | -.033 | -.197 | .000 | .845 |
| Physiology | -.027 | -.169 | .000 | .866 |
| Facial Behavior | .168 | 1.008 | .012 | .316 |
Notes. Results from regression analyses with insula volume predicting sadness reactivity (i.e., self-report, physiology, facial behavior) controlling for total intracranial volume, age, sex, wave of study, and scanner type.
Summary
Our primary hypothesis that smaller insular volumes would be associated with diminished disgust responding received support for self-reported disgust and overall physiological reactivity in response to the disgust film. In terms of emotional specificity, insular volume was not related to the magnitude of self-reported sadness, physiological activation, or sadness facial behavior in response to the sad film. Importantly this specificity was not due to differences in the amount of emotion elicited, as there were no differences in self-reported emotion and physiological reactivity between films. In terms of anatomical specificity, there was no relationship between other key neural regions (putamen, pallidum, and caudate volumes) and self-reported disgust, physiological reactivity, or disgust facial behavior in response to the disgust film, or between amygdala volume and physiological reactivity or disgust facial behavior in response to the disgust film. The one exception to this pattern of anatomical specificity was the finding that smaller amygdala volume predicted less self-reported disgust in response to the disgust film. Thus, within the limits of the present design (i.e., small sample of emotions and brain regions), these findings provide some support for emotional and anatomical specificity.
Discussion
This is the first study to our knowledge that used a heterogeneous sample of patients with neurodegenerative disorders and normal controls and employed a multi-method assessment of emotional responding (self-report, physiology, facial behavior) to study the impact of neural loss in the insula on disgust reactivity. The findings that lower insular volume was associated with lower self-reported disgust and decreased physiological reactivity and the findings that these associations showed some emotional and anatomical specificity support the importance of the insula in disgust responding.
Our findings that insular volume is associated with self-report and physiological reactivity, but not with facial behavior, in response to a disgusting film underscores the strong links among autonomic responding, visceral sensations, and subjective experience in emotion. This is consistent with theories that posit that subjective emotional experience is constructed in large part from physiological sensations (e.g., James, 1884; Levenson, 2003); with empirical studies delineating the close relationship between physiological reactivity and self-reported experience (e.g., Sze, Gyurak, Yuan, & Levenson, 2010); and with neuroanatomical research indicating that one of the primary functions of the insula is to incorporate visceral sensations into subjective experience and emotional awareness (Craig, 2003). Although movement of the facial muscles clearly generates proprioceptive feedback, this is likely of much smaller magnitude than the information generated by the viscera. Moreover, there is some indication that visceral and somatic activity are processed by different parts of the insula (Craig, 2003; Farrer & Frith, 2002). Thus, it is possible that with a more detailed parcellation of insula regions (and larger sample size) relationships between insular volume loss and facial expressive behaviors may have emerged.
These findings may be particularly informative in helping understand the anatomical substrates of one of the prominent behavioral problems often seen in bvFTD patients. Anatomical studies indicate that the insula is often an early nidus of neurodegeneration in this disease (Rosen et al., 2002). Behavioral research has shown that these patients show diminished disgust reactivity (Eckart et al., 2012). The present study links these two literatures by establishing that insular atrophy is associated with less responding to disgusting stimuli in self-report and physiology.
Decrements in disgust responding associated with loss of insular volume may help explain some of the inappropriate social and emotional behaviors often reported in behavioral variant patients. For example, caregivers in our studies have reported anecdotally that some bvFTD patients rummage through trashcans and eat discarded food. Normally these situations would produce powerful disgust responding in subjective experience (e.g., nausea) and physiology (e.g., gagging), which would motivate avoidance and withdrawal behaviors. The known role of the insula in processing visceral information (Craig, 2009), combined with the present findings, suggests that diminished integration of visceral information and the concomitant blunting of disgust responding stemming from insular atrophy may underlie the reduction in avoidance and withdrawal responses in these patients.
An important remaining question is whether the deficits in disgust responding associated with insular atrophy result from diminished bottom-up signaling (diminished physiological responding) and/or diminished higher-level integration of this visceral information (e.g., diminished subjective experience). We expect that both processes are involved. Our findings that link smaller insular volume to both diminished physiological reactivity and diminished subjective experience are consistent with this view. Further research is clearly needed, however, to provide a more definitive answer to this question.
Finally, although our findings indicate some degree of emotional and anatomical specificity in the relationship between the insula and disgust responding, this should not be interpreted as indicating either that: (a) the insula is involved in processing disgust but no other emotions; or (b) disgust is processed in the insula but not in other brain regions. Rather, the insula is best viewed as being one of a number of brain regions that are part of a broader salience network (Seeley, Crawford, Zhou, Miller, & Greicius, 2009) that is involved in processing of all basic emotions. When that emotion processing is highly visceral (e.g., as in the kind of disgust processing assessed in the present study), then insular involvement will be relatively heightened. Although our findings suggest some specificity in the association between insular volume and disgust, the present study was limited in terms of the number of emotions and brain regions examined. Moreover, we also found evidence in support of the role of the amygdala in the subjective experience of disgust, a relationship that has been noted by others (Lindquist, Wager, Kober, Bliss-Moreau, & Barrett, 2012). Finally, the present study considered volume loss in the entire insular region. In future work it will be important to consider right and left insular regions, as well as anterior and posterior regions to obtain a more detailed picture of how the insula is associated with disgust reactivity.
Strengths and limitations
This study had a number of strengths, including the assessment of multiple aspects of emotional responding (self-report, physiology, facial behavior), inclusion of a heterogeneous group of neurological patients, quantitative analysis of brain volumes from structural brain images, and examination of multiple emotions. Limitations included our focus on a group of brain regions that were chosen a priori because of their previous association with disgust and/or emotional reactivity in the literature. In doing so, we may have omitted other important regions that are also involved in emotional responding in general and disgust responding in particular. In addition, because it took a decade to accumulate a sufficient number of patients to conduct these kinds of analyses, some changes in procedures (e.g., magnet strength, film excerpts) occurred over this period. We controlled for these changes statistically in our analyses, but it would have been preferable to have studied every patient under identical conditions. Including two negative emotions (disgust and sadness) was a strength of the study, enabling us to determine which findings were specific to disgust. In future work, it will be important to extend this work to other negative emotions (e.g., fear, which has been linked with the insula; Vytal & Hamann, 2010), as well as to positive (e.g., amusement) and self-conscious emotions (e.g., embarrassment). Finally, although the size of our sample was relatively large for a study of neurological patients, a larger sample with greater statistical power may have found less evidence of both emotional and anatomical specificity.
Conclusion
We found evidence supporting a strong relationship between neural loss in the insula and diminished disgust reactivity. This research contributes to growing body of literature on the importance of the insula for emotion in general and disgust in particular. Our findings also provide insights into how losses in the insula and in disgust responding may contribute to real-world deficits in emotional and social functioning in FTD and in other neurodegenerative diseases.
Footnotes
In response to a reviewer's request, we conducted exploratory analyses using scores derived from a factor analysis instead of the composite score. The findings using these factor scores were highly similar to those found for the composite score as reported in the Results section.
In an exploratory analysis, we examined the relationship between insula volume and self-reported disgust and physiological activation in response to the disgust film separately for four diagnostic groups (bvFTD, AD, PSP and CBD, controls). Analyses revealed that the direction of relationships was the same as in the overall analysis (i.e., smaller insula volumes associated with less reactivity) except for physiological reactivity in the bvFTD group. However, reflecting the reduced statistical power, the β values were non-significant (Self-report: bvFTD: β = .332, t = -.878, R2 change = .030, p = .393; AD: β = .220, t = .567, R2 change = .020, p = .580; PSP/CBD: β = .079, t = .073, R2 change = .001, p = .945; controls: β = .304, t = .717, R2 change = .022, p = .481. Physiological reactivity: bvFTD: β = -.226, t = -.579, R2 change = .014, p = .571; AD: β = .739, t = 2.036, R2 change = .223, p = .063; PSP/CBD: β = 1.289, t = 1.062, R2 change = .170, p = .337; controls: β = .377, t = .988, R2 change = .034, p = .335).
Author Note: Alice Verstaen, Janet A. Eckart, Luma Muhtadie, Marcela C. Otero, and Robert W Levenson, Department of Psychology and Institute of Personality and Social Research, Universty of California, Berkeley. Virginia E Sturm and Bruce L. Miller, Department of Neurology, University of California, San Francisco. Claudia M. Haase, School of Education and Social Policy and (by courtesy) Department of Psychology, Northwestern University.
References
- Adolphs R. Neural systems for recognizing emotion. Cognitive Neuroscience. 2002;12(2):169–177. doi: 10.1016/S0959-4388(02)00301-X. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio AR. Dissociable neural systems for recognizing emotions. Brain and Cognition. 2003;52(1):61–69. doi: 10.1016/S0278-2626(03)00009-5. [DOI] [PubMed] [Google Scholar]
- Armstrong MJ, Litvan I, Lang AE, Bak TH, Bhatia KP, Borroni B, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology. 2013;80(5):496–503. doi: 10.1212/WNL.0b013e31827f0fd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfor N, Amici S, Boxer AL, Kramier JH, Gorno-Tempini ML, Rosen HJ, et al. Clinical and neuropsychological features of corticobasal degeneration. Mechanism of Ageing and Development. 2006;127(2):203–207. doi: 10.1016/j.mad.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Boxer AL, Geschwind MD, Belfor N, Gorno-Tempini ML, Schauer GF, Miller BL, et al. Patterns of Brain Atrophy That Differentiate Corticobasal Degeneration Syndrome From Progressive Supranuclear Palsy. Archives of Neurology. 2006;63(1):81–86. doi: 10.1001/archneur.63.1.81. [DOI] [PubMed] [Google Scholar]
- Boxer AL, Mackensie IR, Boeve BF, Baker M, Seeley WW, Crook R, et al. Clinical, neuroimaging and neuropathological features of a new chromosome 9p-linked FTD-ALS family. Journal of Neurology, Neurosurgery, and Psychiatry. 2011;82(2):196–203. doi: 10.1136/jnnp.2009.204081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Staging of alzheimer's disease-related neurofibrillary changes. Neurobiology of Aging. 1995;16(3):271–278. doi: 10.1016/0197-4580(95)00021-6. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Current Opinion in Neurobiology. 2003;13(4):500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel—now? The anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Dale AM, Sereno MI. Improved Localizadon of Cortical Activity by Combining EEG and MEG with MRI Cortical Surface Reconstruction: A Linear Approach. Journal of Cognitive Neuroscience. 1993;5(2):162–176. doi: 10.1162/jocn.1993.5.2.162. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Eckart JA, Sturm VE, Miller BL, Levenson RW. Diminished disgust reactivity in behavioral variant frontotemporal dementia. Neuropsychologia. 2012;50(5):786–790. doi: 10.1016/j.neuropsychologia.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrer C, Frith CD. Experiencing oneself vs. another person as being the cause of an action: the neural correlates of the experience of agency. NeuroImage. 2002;15(3):596–603. doi: 10.1006/nimg.2001.1009. [DOI] [PubMed] [Google Scholar]
- Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Transactions of Medical Imaging. 2001;20(1):70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert MS, Dieterich M, Haselgrove C, et al. Whole Brain Segmentation. Neuron. 2002;33(3):341–355. doi: 10.1016/S0896-6273(02)00569-X. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe A, Makris N, Segonne F, Quinn BT, Dale AM. Sequence-independent segmentation of magnetic resonance images. NeuroImage. 2004;23:S69–S84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999a;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Human Brain Mapping. 1999b;8(4):272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foundas AL, Leonard CM, Mahoney SM, Agee OF, Heilman KM. Atrophy of the hippocampus, parietal cortex, and insula in Alzheimer's disease: a volumetric magnetic resonance imaging study. Neuropsychiatry, Neuropsychological, and Behavioral Neurology. 1997;10(2):81–89. [PubMed] [Google Scholar]
- Gray JM, Young AW, Barker WA, Curtis A, Gibson D. Impaired recognition of disgust in Huntington's disease gene carriers. Brain. 1997;120(Pt 11):2029–2038. doi: 10.1093/brain/120.11.2029. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Levenson RW. Emotional suppression: physiology, self-report, and expressive behavior. Journal of Personality and Social Psychology. 1993;64(6):970–986. doi: 10.1037/0022-3514.64.6.970. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Levenson RW. Hiding feelings: the acute effects of inhibiting negative and positive emotion. Journal of Abnormal Psychology. 1997;106(1):95–103. doi: 10.1037//0021-843x.106.1.95. [DOI] [PubMed] [Google Scholar]
- Han X, Jovicich J, Salat D, van der Kouwe A, Quinn BT, Czanner S, et al. Reliability of MRI-derived measurements of human cerebral cortical thickness: The effects of field strength, scanner upgrade and manufacturer. NeuroImage. 2006;32(1):180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- James W. What is an Emotion? Mind. 1884;9(34):188–205. doi: 10.2307/2246769?ref=no-x-route:4523db4974f8c0edb332b1ac5f3d432b. [DOI] [Google Scholar]
- Jovicich J, Czanner S, Greve D, Haley E, van der Kouwe A, Gollub R, et al. Reliability in multi-site structural MRI studies: Effects of gradient non-linearity correction on phantom and human data. NeuroImage. 2006;30(2):436–443. doi: 10.1016/j.neuroimage.2005.09.046. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa J, et al. Regionally Localized Thinning of the Cerebral Cortex in Schizophrenia. Archives of General Psychiatry. 2003;60(9):878–888. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Structure and Function. 2010;214(5-6):519–534. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. The amygdala: Neurobiological aspects of emotion, memory, and mental dysfunction. New York, NY: Wiley-Liss; 1992. Emotion and the amygdala. In J.P. Aggleton; pp. 339–351. [Google Scholar]
- Levenson RW. Blood, Sweat, and Fears. Annals of the New York Academy of Sciences. 2003;1000(1):348–366. doi: 10.1196/annals.1280.016. [DOI] [PubMed] [Google Scholar]
- Levenson RW. The Autonomic Nervous System and Emotion. Emotion Review. 2014;6(2):100–112. doi: 10.1177/1754073913512003. [DOI] [Google Scholar]
- Lindquist KA, Wager TD, Kober H, Bliss-Moreau E, Barrett LF. The brain basis of emotion: a meta-analytic review. The Behavioral and Brain Sciences. 2012;35(3):121–143. doi: 10.1017/S0140525X11000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996;47(1):1–9. doi: 10.1212/WNL.47.1.1. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Albert MS, Grossman M, Miller BL, Dickson D, Trojanowski JQ. Clinical and Pathological Diagnosis of Frontotemporal Dementia: Report of the Work Group on Frontotemporal Dementia and Pick's Disease. Archives of Neurology. 2001;58(11):1803–1809. doi: 10.1001/archneur.58.11.1803. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Drachman D, Folstein M, Katzman R, Price D, Stadlan E. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984 Jul; doi: 10.1212/WNL.34.7.939. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Nimmo-Smith I, Lawrence AD. Functional neuroanatomy of emotions: A meta-analysis. Cognitive, Affective, and Behavioral Neuroscience. 2003;3(3):207–233. doi: 10.3758/CABN.3.3.207. [DOI] [PubMed] [Google Scholar]
- Mutschler I, Wieckhorst B, Kowalevski S, Derix J, Wentlandt J, Schulze-Bonhage A, Ball T. Functional organization of the human anterior insular cortex. Neuroscience Letters. 2009;457(2):66–70. doi: 10.1016/j.neulet.2009.03.101. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology. 1998;51(6):1546–1554. doi: 10.1212/WNL.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Padovani A, Borroni B, Brambati SM, Agosti C, Broli C, Alonso R, et al. Diffusion tensor imaging and voxel based morphometry study in early progressive supranuclear palsy. Journal of Neurology, Neurosurgery, and Psychiatry. 2006;77(4):457–463. doi: 10.1136/jnnp.2005.075713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Baylis LL. Gustatory, olfactory, and visual convergence within the primate orbitofrontal cortex. The Journal of Neuroscience : the Official Journal of the Society for Neuroscience. 1994;14(9):5437–5452. doi: 10.1523/JNEUROSCI.14-09-05437.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, et al. Regional and progressive thinning of the cortical ribbon in Huntington's disease. Neurology. 2002;58(5):695–701. doi: 10.1212/WNL.58.5.695. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Levenson RW. The emotional brain: combining insights from patients and basic science. Neurocase. 2009;15(3):173–181. doi: 10.1080/13554790902796787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen HJ, Gorno-Tempini ML, Goldman WP, Perry RJ, Schuff N, Weiner M, et al. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology. 2002;58(2):198–208. doi: 10.1212/WNL.58.2.198. [DOI] [PubMed] [Google Scholar]
- Rozin P, Fallon AE. A perspective on disgust. Psychological Review. 1987;94(1):23–41. doi: 10.1037/0033-295X.94.1.23. [DOI] [PubMed] [Google Scholar]
- Rozin P, Haidt J, McCauley CR. Disgust. In: Lewis MD, Haviland-Jones JM, Barrett LF, editors. Handbook of Emotions. 3. New York: Guilford Press; 2008. pp. 757–776. [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve D, Desikan RS, Busa E, et al. Thinning of the Cerebral Cortex in Aging. Cerebral Cortex. 2004;14(7):721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62(1):42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B. A hybrid approach to the skull stripping problem in MRI. NeuroImage. 2004;22(3):1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Segonne F, Pacheco J, Fischl B. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Transactions of Medical Imaging. 2007;26(4):518–529. doi: 10.1109/TMI.2006.887364. [DOI] [PubMed] [Google Scholar]
- Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends in Cognitive Science. 2009;13(8):334–340. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A Nonparametric Method for Automatic Correctionof Intensity Nonuniformity in MRI Data. IEEE Transactions of Medical Imaging. 1998;17(1):87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Sprengelmeyer R, Rausch M, Eysel UT, Przuntek H. Neural structures associated with recognition of facial expressions of basic emotions. Proceedings of the Royal Society of London Series B Biological Sciences. 1998;265(1409):1927–1931. doi: 10.1098/rspb.1998.0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze JA, Gyurak A, Yuan JW, Levenson RW. Coherence between emotional experience and physiology: does body awareness training have an impact? Emotion. 2010;10(6):803–814. doi: 10.1037/a0020146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vytal K, Hamann S. Neuroimaging support for discrete neural correlates of basic emotions: a voxel-based meta-analysis. Journal of Cognitive Neuroscience. 2010;22(12):2864–2885. doi: 10.1162/jocn.2009.21366. [DOI] [PubMed] [Google Scholar]
- Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G. Both of us disgusted in My insula: the common neural basis of seeing and feeling disgust. Neuron. 2003;40(3):655–664. doi: 10.1016/S0896-6273(03)00679-2. [DOI] [PubMed] [Google Scholar]
- Wilson SM, Ogar JM, Laluz V, Growdon M, Jang J, Glenn S, et al. Automated MRI-based classification of primary progressive aphasia variants. NeuroImage. 2009;47(4):1558–1567. doi: 10.1016/j.neuroimage.2009.05.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright P, He G, Shapira NA, Goodman WK, Liu Y. Disgust and the insula: fMRI responses to pictures of mutilation and contamination. NeuroReport. 2004;15(15):2347–2351. doi: 10.1097/00001756-200410250-00009. [DOI] [PubMed] [Google Scholar]


