Abstract
Background
Pepsin plays a role in gastroesophageal reflux (GER). Aims of this study were to verify if pepsin could be the cause of frequent bronchial exacerbations and to check if the persistence of chronic respiratory symptoms were correlated with pre-existing respiratory diseases.
Methods
From January to May 2016, 42 patients underwent a diagnostic bronchoscopy. All patients had a history of at least one bronchial exacerbation during the previous year. Bronchial lavage fluid specimens were obtained. A semiquantitative assessment of pepsin in the samples was carried out based on the intensity of the test sample.
Results
Pepsin was present in 37 patients (88%), but in patients with bronchial asthma and chronic obstructive pulmonary disease (COPD), the finding of pepsin in the bronchoalveolar fluid was 100%. There was a strong positive statistical correlation between pepsin detection and radiological signs of GER (ρ=0.662), and between pepsin detection and diagnosis (ρ=0.682). No correlation was found between the bacteriology and the presence of pepsin in the airways (ρ=0.006).
Conclusions
The presence of pepsin in the airways shows the occurrence of reflux. The persistence of respiratory symptoms by at least 2 months suggest an endoscopic bronchial examination. This straightforward test confirms the cause possible irritation of the airways and may prevent further diagnostic tests, such as an EGD or pH monitoring esophageal.
Keywords: Bronchial reacutization, gastroesophageal reflux (GER), pepsin, bronchoscopy
Introduction
In the Montreal Classification, gastroesophageal reflux disease (GERD) is a condition developing when the reflux of stomach contents causes troublesome symptoms and complications (1). GERD is a term referring to the clinical symptoms, which is caused by advanced gastroesophageal reflux (GER). According to the position statement of the Committee on Speech, Voice, and Swallowing Disorders of the American Academy of Otolaryngology-Head and Neck Surgery, the laryngopharyngeal reflux (LPR) is the backflow of stomach contents into the laryngopharynx (2). Even if heartburn and regurgitation represent the most common clinical manifestation, sometimes GERD is asymptomatic and causes extraesophageal symptoms such as asthma, dysphonia, sore or burning throat, excessive throat clearing, chronic cough, globes pharyngeus, apnea, laryngospasm, laryngitis, dysphagia, and postnasal drip (3). Nevertheless, GERD is a risk factor for bronchial exacerbations in patients with chronic obstructive pulmonary disease (COPD) (4). LPR differs from GERD in many aspects, such as symptoms, treatment modalities, and pathophysiology. Patients with chronic pulmonary diseases could have a high prevalence of aspiration-associated extraesophageal reflux disease (AERD) (5), but, regrettably, the diagnosis and therapy have focused, as first monitoring parameter and treatment targets, on gastric acid (5). Recent studies have shown that the damage to the respiratory system can be due to acidity, but can be attributed to the bile and pepsin (6). These findings could explain the non-optimal nature of diagnostic tests in GERD, especially in the extraesophageal reflux (EER), thereby, causing a delay in diagnosis and treatments (7,8). Furthermore, current techniques for assessing the reflux, such as oesophagal pH monitoring and esophagoscopy with oesophagal biopsy, identify only an increased risk of pulmonary aspiration patients, but obtaining proof of aspiration remains elusive (9). Pepsin plays the leading role in the formation of GER and related diseases since studies claim the key role of pepsin in mucosal lesions. Therefore, pepsin can be a good alternative diagnostic method, particularly in patients with non-acid reflux (2). The gastric enzyme pepsin is a member of the family of aspartic proteins, synthesized by the chief cells of the gastric fundus epithelium. Pepsin exhibits maximal activity at pH 2.0 and is inactive at pH >6.5. However, pepsin is not fully denatured or irreversibly inactivated until pH 8.0 (2,9). The presence of pepsin is then a better predictor of AERD in patients with respiratory symptoms. The gastric pepsin is obviously absent in respiratory secretions, and the detection of pepsin in bronchoalveolar lavage fluid specimens could be a biomarker for AERD (5,9).
The aims of this pilot study were to verify if pepsin could be the cause of frequent bronchial exacerbations (>1 year) and to check if the persistence (>2 months) of chronic respiratory symptoms were correlated with pre-existing respiratory diseases, like asthma or COPD. Therefore, the intensification of these symptoms could be the expression of an EER.
Methods
Study design and study population
From January to May 2016, 42 patients with a history of a chronic cough, plug or dyspnea and abnormal lung examination underwent a diagnostic bronchoscopy were recruited. Patients were enrolled for the worsening of respiratory symptoms of the pre-existing pulmonary disease in spite of regular treatment (Table 1). All enrolled patients had a history of at least one bronchial exacerbation during the previous year. This cohort had no history of GERD or any prior diagnosis or therapy for acid reflux; all patients had clinically suspected atypical GERD (Table 2). After the collections of written informed consents, patients underwent fiberoptic bronchoscopy for the detection of the gastric pepsin (Pep-test) and the bacteriological examination of the bronchial secretions (Figure 1). Bronchoscopy was performed under conscious sedation with Midazolam IV <5 mL. The early digestive tract of patients was also studied through a roentgenogram for the GER. The radiological study routinely included: an upright left posterior oblique double-contrast views of the oesophagus and a prone position right anterior oblique single-contrast views. The left posterior oblique double-contrast views were obtained with a 250% weight/volume high-density barium suspension and an effervescent agent (Baros, Lafayette Pharmaceuticals). The prone position right anterior oblique single-contrast views were obtained with a 50% weight/volume low-density barium suspension (Entrobar, Lafayette Pharmaceuticals). Oesophageal motility was evaluated with multiple separated swallows of low-density barium while in the prone right anterior oblique position. At the end of the study, the patient was routinely rotated to the supine position and then to the right lateral position for assessment of spontaneous GER (11). All radiological studies were performed with digital fluoroscopic equipment (Sirescop SX 40, Fluorospot top; Siemens AG).
Table 1. Respiratory diseases diagnosis at the enrollment.
| Diagnosis | No. (%) |
|---|---|
| Bronchial asthma mild/moderate | 10 (23.8) |
| Severe asthma | 2 (4.7) |
| COPD (total number) | 12 (28.5) |
| COPD degree A | 2 (4.7) |
| COPD degree B | 7 (16.7) |
| COPD degree C | 2 (4.7) |
| Bronchial exacerbation | 10 (23.8) |
| Acute pneumonia | 4 (9.5) |
| No resolving pneumonia | 4 (9.5) |
| Lung cancer | 1 (2.4) |
| Basal bronchiectasis | 1 (2.4) |
| UIP | 1 (2.4) |
COPD, chronic obstructive pulmonary disease; UIP, usual interstitial pneumonia.
Table 2. Symptoms associated with LPR.
| Hoarseness |
| Dysphonia |
| Sore or burning throat |
| Excessive throat clearing |
| Chronic cough |
| Globus pharyngeus |
| Apnea |
| Laryngospasm |
| Dysphagia |
| Postnasal drip |
| Neoplasm |
LPR, aryngopharyngeal reflux.
Figure 1.

Fiberoptic bronchoscopy for the detection of the gastric pepsin (Pep-test) (10). The main risk of pepsin detection in bronchial secretions is the sucking of the salivary secretions. Secretions enter in the bronchial tree during the anaesthesia of the vocal cords and the tracheal introduction of the bronchoscope. It is necessary to standardise the procedure to reduce this risk: (I) perform an abundant nasal local anaesthetic (Lidocaine spray 10% in aqueous solution), not in the larynx; (II) insert the bronchoscope into the trachea slowly and, if possible, not introduce anaesthetic into the trachea; (III) proceed aspiration supporting the tip of the instrument on the front wall of trachea; (IV) if the specimen is not significant, wash the saline into the trachea and aspire in the middle and lower lobe and then repeat the manoeuvre on the left side; (V) after the Pep-test, complete the exam with Bronchoalveolar lavage for bacteriological examination. Available online: http://www.asvide.com/articles/1119
Sample collection
Bronchial lavage fluid specimens were obtained from the anterior wall of the trachea and main bronchi, avoiding the aspiration of secretions on the pars membranacea, in which could be present the saliva penetrates during the tracheal introduction of the bronchoscope. Bronchial secretions were placed in a 15 mL sterile plastic test tube, stored on ice, and promptly transferred to the refrigerator at 4 °C. In the second half of the bronchoscopy, bronchial washing and bronchial aspirate for bacteriological examination was performed in all patients (Figure 1).
Pepsin analysis
The Pep-test (Peptest™, RD Biomed Ltd., USA) is an immunological test that consists of a Lateral Flow Device (LFD) containing two human monoclonal antibodies: the first to detect and the second to capture pepsin. The Pep-test identifies sub-units of pepsin A, exclusively of gastric origin, in particular in its isoforms 3 (3a, 3b and 3c) (12). The test can detect the presence of pepsin, not its activity. Test specificity is close to 100%, and the sensitivity is 81%. Positive predictive value is 100%, and negative predictive value is 60%. The Bronchial Lavage fluid specimen (added with citric acid 0.1 M 0.5 mL) was vortexed and centrifuged for 5 minutes at 4,000 rotations per minute. Then the supernatant was collected, transferred to 1.5 mL microcentrifuge tube and again centrifuged to obtain clear material. At this point, 80 µL of sample was transferred into microtubes containing 240 µL of migration buffer and mixed by vortex. 80 µL was then deposited on the device, waiting about 15 minutes for the complete migration. In this study, a semiquantitative assessment of pepsin in the samples was carried out based on the intensity of the test sample (line T compared to the control line C) (Figure 2).
Figure 2.
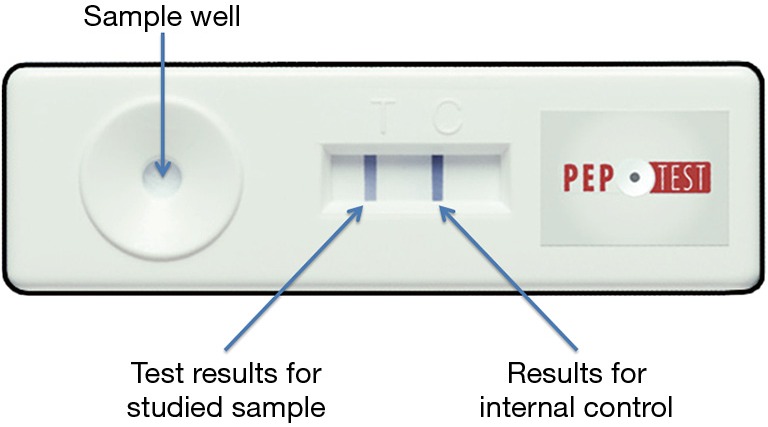
Pep-test. A semiquantitative assessment of pepsin was carried out based on the intensity of the test sample (line T compared to the control line C).
Statistical analysis
Statistical analysis was realized with the bootstrap method using 1,000 simple bootstrap samples with 95% confidence interval. Bootstrap analysis was proposed as a breakthrough method for internal validation of surgical regression models (13). Two-tailed t-tests (normally distributed data) or Wilcoxon-Mann-Whitney U-test (not normally distributed data) were used to test for significance of differences in the mean values for the groups. Correlation between variables was analyzed using the Spearman’s Rank Order non-parametric correlation coefficient (ρ). The bootstrap can be used to construct confidence intervals for Spearman’s Rank Order correlation coefficients. A 95% confidence interval for P can be defined as the interval spanning from the 2.5th to the 97.5th percentile of the resampled values. The following points are the accepted guidelines (14) for interpreting the correlation coefficient: values between 0 and 0.3 (0 and −0.3) indicate a weak positive (negative) linear relationship via a shaky linear rule; values between 0.3 and 0.7 (0.3 and −0.7) indicate a moderate positive (negative) linear relationship via a fuzzy-firm linear rule; values between 0.7 and 1.0 (−0.7 and −1.0) indicate a strong positive (negative) linear relationship via a firm, linear rule. The main advantage of this technique is that the entire dataset can be used for model building, which would yield more robust models, especially in moderate-size databases and for rare outcomes. We employed R-software for statistical analysis (15).
Results
A total of 42 patients were enrolled in this pilot study during the 5 months’ period. Demographic characteristics were described in Table 3. Fifty percent were men. The mean age was 56 years (range, 20–84 years). Thirty (71%) reported exacerbations annually with an average of at least three episodes by year. All patients have at least one symptom for atypical GERD. Half of the subjects reported an allergy to common allergens. Four patients were former smokers, only one patient was an active smoker. No patient was involved in respiratory risk jobs. Patients enrolled underwent bronchoscopy for the persistence of the respiratory symptoms: 16 (38%) patients reported coughs, 16 (38%) coughs and phlegm for at least 2 months, and 3 (7%) patients exertional dyspnea (Table 3). Bronchial asthma was mild in 10 patients, moderate in 23.8% and severe in 2 (4.7%). Twelve patients had COPD (28.5%): degree A 2 (4.7%), degree B 7 (16.7%), degree C 2 (4.7%), and degree D 1 (2.4%) (Table 4). Ten patients (23.8%) with persistent respiratory symptoms after a diagnosis of bronchial exacerbation did not have a previous diagnosis of chronic respiratory disease. Four patients (9.5%) had a radiological diagnosis of non-resolving pneumonia, 1 (2.4%) lung cancer, 1 (2.4%) basal bronchiectasis, 1 (2.4%) a usual interstitial pneumonia (UIP) treated with pirfenidone, and 1 (2.4%) a suspected pulmonary tuberculosis infection. All patients had one or more symptoms correlated to an EER, while only eight patients (19%) had symptoms of regurgitation/heartburn. The reflux was radiologically detected in 25 patients (59.5%) and in 21 (56.8%) a hiatal hernia was also demonstrated. In 83% patients with asthma, the simultaneous presence of spontaneous GER and hiatal hernia was observed. In 12 patients with COPD, 75% had a spontaneous reflux with a hiatal hernia associated (66.7%). In more than half of subjects with persistent respiratory symptoms due to bronchial exacerbation, the first digestive tract X-ray has encountered spontaneous reflux and a hiatal hernia in 20%. Reflux was also found in patients with bronchiectasis and with UIP. There were a radiologic reflux and herniation in a patient with non-resolving pneumonia and a patient with lung cancer. In patients with pulmonary TB, the radiological examination was not performed. Pep-test analysis was performed on a bronchoalveolar fluid sample from each patient: pepsin was present in 37 patients (88%), but in patients with bronchial asthma and COPD the finding of pepsin in the bronchoalveolar fluid was 100%. The positive feedback of pepsin was also found in eight patients with bronchial exacerbation (80%), in two patients with non-resolving pneumonia (50%), but also in a patient with lung cancer, in those with bronchiectasis and finally in what has performed the bronchoscopy investigation for suspected pulmonary. After bronchial fluid intake for Pep-test, a bronchoalveolar lavage for bacteriological examination was performed. Bacteriological exams showed the growth of Haemophilus influenzae and Streptococcus pneumoniae in 5 patients (0.9%), while the direct and culture for BK was negative. There was a strong positive statistical correlation between pepsin detection and radiological signs of GER (ρ=0.662), and between pepsin detection and diagnosis (ρ=0.682). There was a moderate positive statistical correlation in reactivations by years and pepsin detection (ρ=0.570). There was only a mild correlation between pepsin detection and radiological signs of a hiatal hernia (ρ=0.513). No correlation was found between the bacteriology and the presence of pepsin in the airways (ρ=0.006).
Table 3. Demographic data of the study population.
| Characteristics | No. |
|---|---|
| Gender (% male) | 50 |
| Median age (range, years) | 56 [20–84] |
| Current smokers (%) | 1.05 |
| Ex-smokers (%) | 10.5 |
| Atopic (%) | 50 |
| Cough (%) | 38 |
| Cough, Plug (%) | 38 |
| Dyspnea (%) | 7 |
| Median number of re-exacerbations/yr | 3 |
| Numbers of re-exacerbations (%) | 72 |
Table 4. Pre-existing diagnosis at enrollment, radiological findings of GERD, hiatal hernia and positivity to the Pep-test.
| Respiratory diagnosis | No. (%) | GER (Rx) (%) | Hiatal hernia (Rx) (%) | GER typical symptoms (%) | Pep-test positive (%) |
|---|---|---|---|---|---|
| Bronchial asthma | |||||
| Mild/moderate | 10 (23.8) | 8 (19.4) | 8 (19.4) | 4 (9.5) | 10 (23.8) |
| Severe | 2 (4.7) | 2 (4.7) | 2 (4.7) | 0 | 2 (4.7) |
| COPD | |||||
| Degree A | 2 (4.7) | 2 (4.7) | 2 (4.7) | 0 | 2 (4.7) |
| Degree B | 7 (16.7) | 5 (11.9) | 4 (9.5) | 0 | 7 (16.7) |
| Degree C | 2 (4.7) | 1 (2.3) | 1 (2.3) | 0 | 1 (2.3) |
| Degree D | 1 (2.3) | 1 (2.3) | 1 (2.3) | 0 | 1 (2.3) |
| Bronchial exacerbation | 10 (23.8) | 4 (9.5) | 2 (4.7) | 0 | 8 (19.4) |
| Non-resolving pneumonia | 4 (9.5) | 0 | 0 | 2 (4.7) | 2 (4.7) |
| Lung cancer | 1 (2.4) | 0 | 0 | 0 | 1 (2.4) |
| Bronchiectasis | 1 (2.4) | 1 (2.4) | – | 1 (2.4) | 1 (2.4) |
| Tubercolosis | 1 (2.4) | – | – | 1 (2.4) | 1 (2.4) |
| UIP | 1 (2.4) | 1 (2.4) | 1 (2.4) | 0 | 1 (2.4) |
GERD, gastroesophageal reflux disease; GER, gastroesophageal reflux; COPD, chronic obstructive pulmonary disease; UIP, usual interstitial pneumonia.
Discussion
In bronchial secretions, pepsin was positive in 88%. In 0.9%, there was a bacterial infection of the airways. Eight patients (19%) have typical reflux symptoms (heartburn and acid regurgitation). All had at least one atypical symptom of GER. This finding could explain the persistence of respiratory symptoms as an extraesophageal manifestation of asymptomatic GER (EEA). These results are in disagreement when evaluating the upper digestive radiological studies since more than half of the participants had spontaneous GER (59.4%) and hiatal hernia (56.8%) in the absence of typical GER symptoms. Unfortunately, our method does not allow establishing a causal link between reflux and symptoms of the patients, because only direct measurement of the acid reflux can be achieved with prolonged pH monitoring, which is the only method able to provide guidance on causation (16). Pepsin assay alone does not indicate a causal relationship to airway damage, but the presence of pepsin in the airway, per se, indicating reflux because it cannot be present in the airways, if not through a microaspiration of gastric contents. The hypothesis that, in the examined population, the GER could be the cause of the persistence of respiratory symptoms is mainly supported by the finding of a strong positive correlation between the detection of pepsin and the suspected diagnosis and the lack of correlation between the bacteriological test and the presence of pepsin in the airways. Nevertheless, the radiological diagnosis of GER through the study of the upper digestive double contrast showed a high correlation with the presence of pepsin in bronchial secretions. The mild relationship between the number of bronchial exacerbations and the positive pepsin in the airways could support the hypothesis that a cough, in well-selected cases, may be the expression of an EER (EAR). These findings are interesting because the correlation between EER and airway disease is gradually gaining recognition increasing the need for new standards and diagnostic standards for EER related to GER (2,17). In fact, as with other diseases affecting the respiratory system, this study does strongly suggesting a relationship of occult aspiration bronchial exacerbations (6).
Conclusions
Although pepsin alone does not indicate a causal relationship to the airway damage, the reflux is showed by the presence of pepsin in the airways. While the correlation between EER and airway disease is gradually gaining recognition, it has also increased the need for new regulatory standards and diagnostic EER related to GER. For this reason, the gastric enzyme pepsin could be a reliable marker for the diagnosis of EER, especially in patients with persistent respiratory symptoms not otherwise justifiable. The persistence of respiratory symptoms by at least 2 months suggests an endoscopic bronchial examination. This straightforward and reliable test could confirm the cause possible irritation of the airways and may prevent further diagnostic tests, such as an EGD or pH monitoring esophageal.
Acknowledgements
None.
Ethical Statement: The study was approved by the institutional ethics board.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Vakil N, van Zanten SV, Kahrilas P, et al. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 2006;101:1900-20; quiz 1943. [DOI] [PubMed]
- 2.Ocak E, Kubat G, Yorulmaz İ. Immunoserologic pepsin detection in the saliva as a non-invasive rapid diagnostic test for laryngopharyngeal reflux. Balkan Med J 2015;32:46-50. 10.5152/balkanmedj.2015.15824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore JM, Vaezi MF. Extraesophageal manifestations of gastroesophageal reflux disease: real or imagined? Curr Opin Gastroenterol 2010;26:389-94. 10.1097/MOG.0b013e32833adc8d [DOI] [PubMed] [Google Scholar]
- 4.Kim J, Lee JH, Kim Y, et al. Association between chronic obstructive pulmonary disease and gastroesophageal reflux disease: a national cross-sectional cohort study. BMC Pulm Med 2013;13:51. 10.1186/1471-2466-13-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelly EA, Parakininkas DE, Werlin SL, et al. Prevalence of pediatric aspiration-associated extraesophageal reflux disease. JAMA Otolaryngol Head Neck Surg 2013;139:996-1001. 10.1001/jamaoto.2013.4448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Savarino E, Carbone R, Marabotto E, et al. Gastro-oesophageal reflux and gastric aspiration in idiopathic pulmonary fibrosis patients. Eur Respir J 2013;42:1322-31. 10.1183/09031936.00101212 [DOI] [PubMed] [Google Scholar]
- 7.Ahmed T, Vaezi MF. The role of pH monitoring in extraesophageal gastroesophageal reflux disease. Gastrointest Endosc Clin N Am 2005;15:319-31. 10.1016/j.giec.2004.10.006 [DOI] [PubMed] [Google Scholar]
- 8.Saritas Yuksel E, Hong SK, Strugala V, et al. Rapid salivary pepsin test: blinded assessment of test performance in gastroesophageal reflux disease. Laryngoscope 2012;122:1312-6. 10.1002/lary.23252 [DOI] [PubMed] [Google Scholar]
- 9.Krishnan U, Mitchell JD, Messina I, et al. Assay of tracheal pepsin as a marker of reflux aspiration. J Pediatr Gastroenterol Nutr 2002;35:303-8. 10.1097/00005176-200209000-00012 [DOI] [PubMed] [Google Scholar]
- 10.Pomari C, Mauroner L, Paiano S, et al. Fiberoptic bronchoscopy for the detection of the gastric pepsin (Pep-test). Asvide 2016;3:350. Available online: http://www.asvide.com/articles/1119
- 11.Samadi F, Levine MS, Rubesin SE, et al. Feline esophagus and gastroesophageal reflux. AJR Am J Roentgenol 2010;194:972-6. 10.2214/AJR.09.3352 [DOI] [PubMed] [Google Scholar]
- 12.Bardhan KD, Strugala V, Dettmar PW. Reflux revisited: advancing the role of pepsin. Int J Otolaryngol 2012;2012:646901. [DOI] [PMC free article] [PubMed]
- 13.Blackstone EH. Breaking down barriers: helpful breakthrough statistical methods you need to understand better. J Thorac Cardiovasc Surg 2001;122:430-9. 10.1067/mtc.2001.117536 [DOI] [PubMed] [Google Scholar]
- 14.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307-10. 10.1016/S0140-6736(86)90837-8 [DOI] [PubMed] [Google Scholar]
- 15.R Core Team. R: The R Project for Statistical Computing Vienna, Austria. Available online: https://www.r-project.org/
- 16.Kahrilas PJ, Quigley EM. Clinical esophageal pH recording: a technical review for practice guideline development. Gastroenterology 1996;110:1982-96. 10.1053/gast.1996.1101982 [DOI] [PubMed] [Google Scholar]
- 17.Samuels TL, Johnston N. Pepsin as a marker of extraesophageal reflux. Ann Otol Rhinol Laryngol 2010;119:203-8. [DOI] [PubMed] [Google Scholar]


