Abstract
Background
The correlation of changes (delta: Δ) of high-resolution computed tomography (HRCT) score with the Δ of other clinical variables has not been well studied. The purpose of this study was to determine the correlation of Δ HRCT score with Δ percent predicted forced vital capacity (%pFVC), Δ modified Rodnan Skin Score (mRSS), Δ erythrocyte sedimentation rate (ESR), and Δ percent of oxygen saturation at room air (%SpO2) in patients with early systemic sclerosis (SSc).
Methods
We used an inception cohort of early-SSc patients seen at the Rheumatology Clinic, Chiang Mai University, Thailand, between January 2010 and June 2014. All patients underwent HRCT at study entry and every 12 months thereafter. Thirty-one SSc patients who underwent pulmonary function test (PFT) within 12 weeks of their corresponding HRCT at baseline and last visit were identified. The extent of ground glass (GG), lung fibrosis (Fib), bronchiectasis (B), and honeycombing (HC) was scored, and then aggregated to produce a total (t) HRCT score.
Results
Mean ± SD age and disease duration from non-Raynaud’s phenomenon (NRP) to undergo HRCT at baseline were 52.2±8.8 years and 11.7±7.1 months, respectively. Seventeen (54.8%) patients were female and 20 (64.5%) were classified as dcSSc. The mean ± SD interval between the two HRCT tests was 16.0±7.2 months. The Δ HRCT scores [total fibrosis scores (t-Fib), total bronchiectasis scores (t-B), and total HRCT score (t-HRCT) scores] and Δ mRSS, but not Δ %pFVC, showed significant change over the observation period. We found significant correlation of Δ total honeycombing scores (t-HC) with Δ ESR (r=−0.44, P<0.05), and Δ t-Fib with Δ %SpO2 (r=−0.38, P<0.05). However, no significant correlation of any Δ HRCT scores with Δ %pFVC and Δ mRSS were observed.
Conclusions
In this study, the changes in the HRCT scores were greater than %pFVC; this, along with their correlations with the changes in ESR and %SpO2, suggest that HRCT scores are a useful and sensitive method for monitoring disease progression in early SSc-related ILD (SSc-ILD).
Keywords: Correlation, high-resolution computed tomography (HRCT), systemic sclerosis (SSc), interstitial lung disease (ILD)
Introduction
Interstitial lung disease (ILD) is a serious pulmonary complication of patients with systemic sclerosis (SSc). High-resolution computed tomography (HRCT) is a high-sensitivity diagnostic method useful for routine detection and evaluation of ILD complications of SSc patients (1-10). Recently, Suliman et al. (11) has proposed using HRCT as an additional imaging investigation for screening and early diagnosis of SSc-related ILD (SSc-ILD) in anti-centromere, antibody-negative patients with normal FVC values. Annual HRCT screening for early detection of ILD has also been suggested for early-SSc patients with high risk factors, including male gender, presence of anti-Scl-70, or absence of anti-centromere antibodies (4). Currently, HRCT, in conjunction with pulmonary function test (PFT), serves an important role in measuring SSc-ILD, making treatment decisions (10), and predicting treatment outcomes (5,12-14) and mortality (8).
While several HRCT scoring systems have been used to determine the extent of lung involvement in ILD, they basically divide into two quantification types: visual reader-based (4,10,15-17) and computer-based (18-21) scoring; although these have not yet been fully validated. Several researchers have reported a close correlation between visual reader-based and computer-based scores (2,18-20,22). Furthermore, Khanna et al. (5) suggested that the choice of staging system in a clinical trial should depend on the study feasibility and available expertise, rather than the quantification method used.
Cross-sectional studies have demonstrated significant correlations of different HRCT scores with other variables, including percent predicted forced vital capacity (%pFVC) (2-4,9,23), percent predicted diffusing capacity for carbon monoxide (%pDLco) (2,3,9), Health Assessment Questionnaire-Disability Index (HAQ-DI) (2), modified Rodnan Skin Score (mRSS) (3), percent of oxygen saturation at room air (%SpO2) (4,23), and erythrocyte sedimentation rate (ESR) (4).
However, to our knowledge, only one study has reported significant correlation between 12-month changes of computer-aided HRCT scores with changes of %pFVC, %pDLco, and mRSS (3), using data obtained from clinical trials (12). Therefore, this study aimed to determine the correlation of changes (delta: Δ) of our visual reader-based HRCT score (4) with the Δ of other clinical variables—Δ %pFVC, Δ mRSS, Δ ESR, and Δ %SpO2, from our previous inception cohort study.
Methods
Patients
This study was recruited from our previous inception cohort study (4) at Maharaj Nakorn Chiang Mai Hospital, Chiang Mai University, Thailand. This study included all consenting, consecutive, adult (≥18 years), early-diagnosed SSc patients [disease duration ≤3 years from the onset of the first non-Raynaud’s symptom attributable to SSc including swollen skin, skin thickening, digital pitting scar, digital ulcer or arthritis (non-Raynaud’s phenomenon: NRP)], from January 2010 to June 2014. All patients fulfilled the 1980 classification criteria of SSc (24); they were classified as dcSSc or lcSSc according to LeRoy and Medsger’s classification criteria (25). Patients were excluded if they had an overlap syndrome [SSc with rheumatoid arthritis or SSc with systemic lupus erythematosus (SLE)].
The onset of SSc was defined as the time of the first NRP, as reported by the patient. Disease duration was calculated as the interval between disease onset and the time at study entry. At cohort entry, we recorded demographic data, clinical manifestations, routine laboratory tests, tests for antinuclear and anti-centromere antibodies (by immunofluorescence on Hep2 cells) and anti-Scl-70 antibodies (enzyme linked immunosorbent assay; ELISA), and current medications. Self-reported functional status was determined by the Thai-version of a health assessment questionnaire (HAQ) (26).
All participants underwent HRCT, echocardiograph, and PFT at study entry, and annually thereafter. Patients were seen at regular intervals of one to three months; complete data, including clinical manifestations and routine blood tests, were recorded every 6 months. Patients received all medical treatments as recommended by the attending rheumatologist, following standards of care. ILD was determined by HRCT. Estimated systolic pulmonary artery pressure (SPAP) was determined by echocardiography.
Data were obtained from the subgroup of our previous inception cohort of SSc patients who underwent PFT within 12 weeks of their corresponding HRCT at baseline and last visit. Patients were excluded if they had ILD due to a condition other than SSc. The Research Ethics Committee, Faculty of Medicine, Chiang Mai University, approved this study. All participants gave informed consent at the time of cohort entry according to the Declaration of Helsinki.
Chest HRCT scoring system
Our previous visual reader-based HRCT scoring system (4) was used to determine the extent and severity of ILD using one of two MDCT platforms—Somatom Definition, Siemens, Forchheim, Germany or Aquilion 16, Toshiba, Tochigi-Ken, Japan. Volumetric scans were performed with a high spatial resolution (1-mm thick) and interval image reconstruction in the supine position with deep inspiration. Sampling the HRCT with 1-mm thick slices during expiration was constructed with at least six levels in order to cover the whole thorax. To exclude ground glass (GG) opacity from dependent atelectasis, prone inspiratory HRCT was performed to cover the suspected area. All images were reviewed by an experienced thoracic radiologist of 20 years (Juntima Euathrongchit), blinded to clinical and laboratory data. Soft-copy DICOM images were retrieved and reviewed with a picture archiving and communication system-PACS (Synapse FUJI-PACS; software version 4.2.200, Stamford, USA).
We used HRCT to categorize the pattern of the lung parenchyma findings representing ILD that were unexplainable by other causes. Parenchymal abnormalities were classified into four categories: ground-glass opacity (GG), lung fibrosis (Fib), bronchiectasis (B), and honeycombing (HC). The corresponding radiographic definitions were: ground-glass opacity (GG)—faint parenchymal opacity with preserved underlying bronchovascular structure without architectural distortion; Fib—thickening of interlobular septae or intralobular septae and traction B; B—dilatation of bronchial tree with peribronchial wall thickening; and HC—clustered air-filled cyst.
The radiographic definition of nonspecific interstitial pneumonitis (NSIP) and usual interstitial pneumonitis-usual nonspecific interstitial pneumonitis (UIP) on HRCT were modified from previous reports (27,28). The corresponding radiographic definitions were: NSIP—GG and reticular opacities (inter- or intralobular septal thickening) with or without micronodules, microcystic HC, and traction B (Figures 1,2,3); and UIP—reticular opacities and volume loss with macrocystic HC, traction B, and focal GG. NSIP distribution showed relatively symmetrical involvement of peripheral, subpleural, and basal lungs with subpleural sparing (Figure 3). UIP distribution was similar to NSIP, with its extent increasing from the apex to basal lungs (Figure 4).
Figure 1.
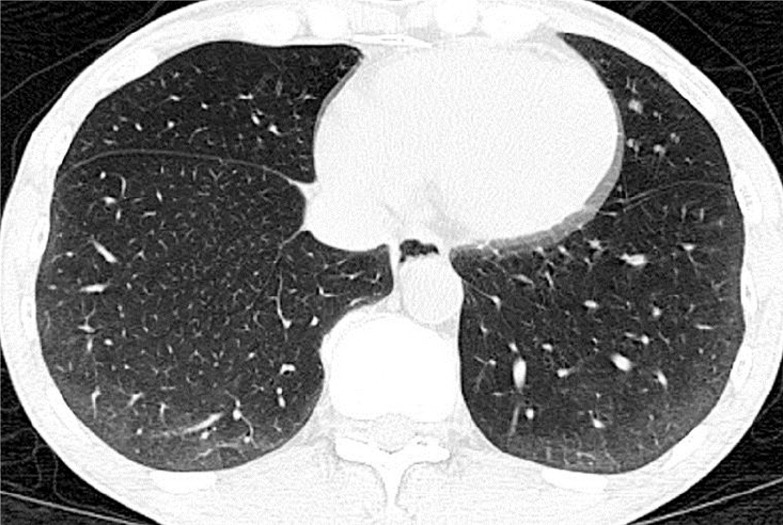
Axial high-resolution computed tomography (HRCT) image in a 56-year-old man with dcSSc reveals bilateral subpleural ground glass (GG) opacities in basal lungs, compatible with early nonspecific interstitial pneumonitis (NSIP) pattern.
Figure 2.
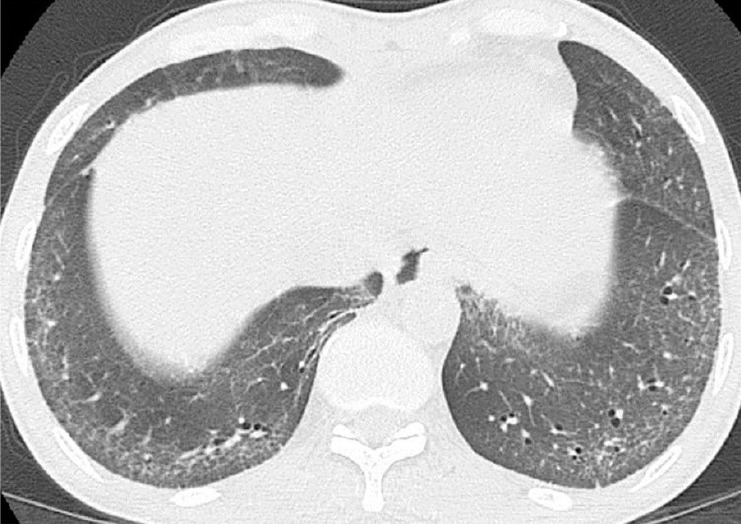
Axial high-resolution computed tomography (HRCT) image in a 53-year-old man with dcSSc reveals ground glass (GG) opacity, fine reticulation (Fib) and mild traction bronchiectasis (B) involving bilateral peripheral and posterior compartment of basal lungs and sparing of bilateral posterior subpleural regions, compatible with early fibrotic nonspecific interstitial pneumonitis (NSIP) pattern.
Figure 3.
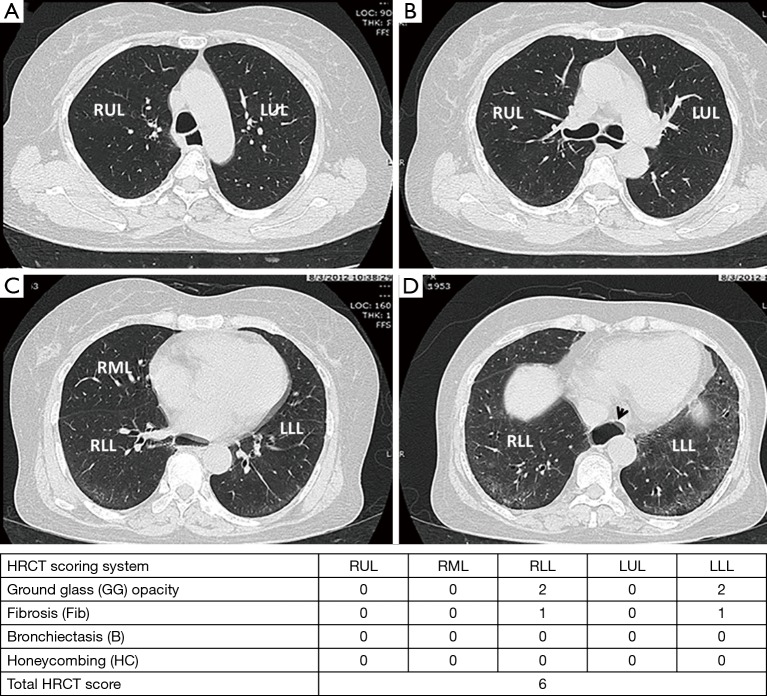
Four high-resolution computed tomography (HRCT) images in a 58-year-old man with dcSSc at level of aortic arch (A); carina (B); inferior pulmonary vein (C) and lung base (D) reveals bilateral peripheral ground glass (GG) opacities with fine reticulation (Fib) and relative subpleural sparing, which compatible with early fibrotic nonspecific interstitial pneumonitis (NSIP) pattern. There was esophageal dilatation (arrow head).
Figure 4.
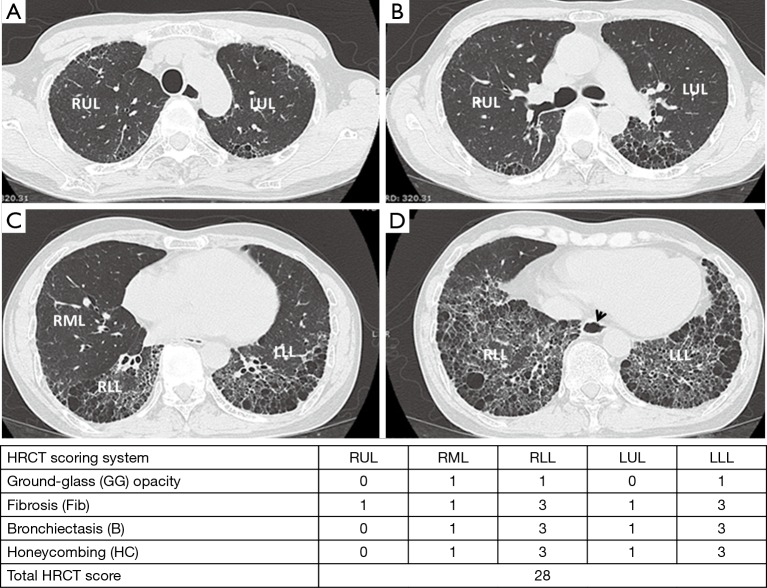
Four high-resolution computed tomography (HRCT) images in a 48-year-old man with dcSSc at level of aortic arch (A); carina (B); inferior pulmonary vein (C) and lung base (D) reveals peripheral diffuse coarse reticulation (Fib), honeycombing (HC) and traction bronchiectasis (B) in both lower lobes, and minimal ground glass (GG) opacity at RML, which compatible with usual nonspecific interstitial pneumonitis (UIP) pattern. There was esophageal dilatation (arrow head).
The extent of pulmonary parenchymal abnormality was scored from each lobe [right upper lobe (RUL), right middle lobe (RML), right lower lobe (RLL), left upper lobe (LUL), and left lower lobe (LLL)] using a Likert scale (0=absent; 1=1–25%; 2=26–50%; 3=51–75%; 4=76–100%) modified from Kazerooni et al. (15). The total (t)-GG, total fibrosis scores (t-Fib), total bronchiectasis scores (t-B), and total honeycombing scores (t-HC) were calculated by summing all of the scores from all five lung lobes, ranging from 0–20. All scores were aggregated to produce a total HRCT score, ranging from 0–80 (Figures 3,4).
Statistical analysis
The data were presented as percentage or mean ± SD. Comparison of continuous variables within the same population between baseline and last HRCT were analyzed using a repeated-measure mixed model that controlled for duration. The delta variables (Δ) were calculated as T2 values (last visit) minus T1 values (baseline); T2−T1. The correlation of Δ HRCT scores with Δ PFT results and Δ of the clinical variables—mRSS, ESR, and %SpO2—were performed using Spearman’s rank correlation coefficients. P values <0.05 were considered statistically significant. Statistical analyses were performed using Stata for Windows version 13.0 (Texas, USA).
Results
Demographic and clinical characteristics
Of the 117 early-SSc patients initially enrolled, 86 were excluded (3 did not undergo HRCT at study entry, 1 later developed an overlapping syndrome with SLE, and 82 did not have available PFT results within 12 weeks of their corresponding HRCT at either baseline or last visit), leaving a cohort of 31 patients for final analysis. The characteristics of the study population are summarized in Table 1. The mean interval between the two HRCT tests was 16.0±7.2 months.
Table 1. Baseline clinical characteristics of the study population.
| Variables | Total (n=31, %) |
|---|---|
| Demographics | |
| Age (years) | 52.3±8.8 |
| Female gender | 14 (45.2) |
| dcSSc | 20 (64.5) |
| Disease duration (months) | 11.7±7.1 |
| Clinical characteristics | |
| ILD | 22 (70.9) |
| NSIP | 17 (77.3) |
| UIP | 5 (22.7) |
| SPAP (mmHg) (n=23) | 30.5±6.9 |
| Dyspnea | |
| NYHA class I/II/III (n) | 4/20/7 |
| Arthritis | 6 (19.4) |
| Large joint contracture | 4 (12.9) |
| Small joint contracture | 7 (22.6) |
| Tendon friction rub | 4 (12.9) |
| Myositis | 5 (16.1) |
| GERD | 6 (19.4) |
| Dysphagia | 10 (32.3) |
| HAQ | 0.7±0.6 |
| Laboratory investigation | |
| Hemoglobin (g/dL) | 12.9±1.5 |
| Creatinine (mg/dL) | 0.8±0.2 |
| Creatine kinase (IU/L) | 233.6±192.4 |
| Anti-Scl-70 antibody | 25 (80.6) |
| Anti-centromere antibody | 2 (6.5) |
| Medication used | |
| Baseline | |
| Prednisolone | 7 (22.6) |
| Dose ≤7.5 mg/day | 4 (57.1) |
| Other immunosuppressants* | 9 (29.0) |
| Last visit | |
| Prednisolone | 23 (74.2) |
| Dose ≤7.5 mg/day | 22 (95.6) |
| Other immunosuppressants# | 22 (70.9) |
Values are presented in mean ± SD or n (%). *, cyclophosphamide (n=4), azathioprine (n=2), mycophenolate mofetil (n=2), methotrexate (n=1); #, cyclophosphamide (n=8), azathioprine (n=6), mycophenolate mofetil (n=5), methotrexate (n=3); ILD, interstitial lung disease; NSIP, nonspecific interstitial pneumonitis; UIP, usual nonspecific interstitial pneumonitis; SPAP, estimated systolic pulmonary artery pressure; NYHA, New York Heart Association functional class; GERD, gastroesophageal reflux disease; HAQ, Thai-version of a health assessment questionnaire.
Comparison of HRCT scores, PFT, and clinical variables between initial and last visit
After controlling for the different intervals between initial and last HRCT, we found that Δ t-Fib, Δ t-B, Δ total HRCT score (t-HRCT), and Δ mRSS showed significant differences over a mean ± SD interval of 16.0±7.2 months; in contrast, Δ %pFVC, Δ ESR, and Δ %SpO2 showed no significant differences (Table 2).
Table 2. Comparison of calculated HRCT scores and other variables between the initial (T1) and last (T2) HRCT (n=31).
| Variables | Initial visit (T1) | Last visit (T2) | Absolute Ä (T2−T1) | P value |
|---|---|---|---|---|
| HRCT Scores | ||||
| Total ground glass | 3.2±3.3 | 2.7±2.4 | 0.5±2.1 | 0.203 |
| Total fibrosis | 4.0±3.5 | 4.3±3.3 | 0.3±1.9 | 0.041* |
| Total bronchiectasis | 2.4±3.3 | 2.9±3.6 | 0.5±1.2 | 0.031* |
| Total honeycombing | 0.7±2.2 | 1.0±2.3 | 0.3±0.8 | 0.053 |
| Total HRCT score | 10.3±10.2 | 11.0±9.9 | 0.7±3.8 | 0.002* |
| Pulmonary function test | ||||
| FVC | 2.2±0.7 | 2.3±0.7 | 0.1±0.4 | 0.181 |
| %pFVC | 69.0±15.9 | 73.8±15.4 | 4.8±12.8 | 0.081 |
| FEV1/FVC | 87.0±7.4 | 86.4±8.2 | −0.7±6.3 | 0.713 |
| Clinical variables | ||||
| mRSS | 15.4±9.9 | 10.5±6.9 | −4.9±6.5 | 0.029* |
| ESR | 34.0±25.3 | 28.2±21.7 | −5.8±22.3 | 0.360 |
| %SpO2 | 96.7±2.6 | 97.2±1.6 | 0.6±2.6 | 0.081 |
Values are presented in mean ± SD; delta (Ä=T2−T1). P values are from a repeated-measures mixed model that controlled for duration; *, duration showed significant effect with Ä (T2−T1) values; HRCT, high-resolution computed tomography; FVC, forced vital capacity; %pFVC, percent predicted forced vital capacity; FEV1, forced expiratory volume in 1 s; mRSS, modified Rodnan Skin score; ESR, erythrocyte sedimentation rate; %SpO2, percent oxygen saturation at room air determined by digital probe pulse oximetry.
The correlation of Δ HRCT scores with Δ of other clinical variables
At baseline visit (n=31), we found significant negative correlation of %pFVC with total ground glass scores (t-GG) (r=−0.43, P<0.05), t-Fib (r=−0.56, P<0.01), t-B (r=−0.43, P<0.05), t-HRCT scores (r=−0.52, P<0.01), HAQ (r=−0.43, P<0.05), and ESR (r=−0.41, P<0.05). Furthermore, we observed significant correlation of t-HRCT scores with HAQ (r=0.37, P<0.05), ESR (r=0.38, P<0.05), and %SpO2 (r=−0.47, P<0.01). However, we found no significant correlation of mRSS with %pFVC, HRCT scores, HAQ, and ESR.
At last visit, the correlation of Δ HRCT scores with Δ FVC, Δ %pFVC, Δ mRSS, Δ ESR, and Δ %SpO2 are shown in Table 3. We found significant negative correlations of Δ t-HC with Δ ESR (r=−0.44, P<0.05) and Δ t-Fib with Δ %SpO2 (r=−0.38, P<0.05). However, we observed no significant correlation of any Δ HRCT scores with Δ %pFVC or Δ mRSS.
Table 3. Spearman’s rank correlation coefficients of Δ HRCT scores with Δ other variables.
| Variables (n=31) | Δ t-GG | Δ t-Fib | Δ t-B | Δ t-HC | Δ t-HRCT |
|---|---|---|---|---|---|
| Δ FVC | −0.07 | −0.09 | 0.03 | −0.16 | −0.03 |
| Δ %pFVC | −0.09 | −0.16 | 0.05 | −0.15 | −0.05 |
| Δ mRSS | 0.05 | −0.08 | 0.01 | −0.02 | −0.01 |
| Δ ESR | −0.02 | −0.09 | −0.29 | −0.44* | −0.33 |
| Δ %SpO2 | 0.14 | −0.38* | −0.19 | −0.26 | −0.15 |
*, P<0.05; t-GG, total ground glass scores; t-Fib, total fibrosis scores; t-B, total bronchiectasis scores; t-HC, total honeycombing scores; t-HRCT, total HRCT score.
Discussion
To date, HRCT is an important measure of the extent of SSc-ILD that can be used to screen for early ILD complications (4,11), initiate treatment decisions (10), and predict treatment outcomes (5,12-14) and mortality (8) in patients with SSc-ILD. Both visual reader-based (4,10,15-17) and computer-based (18-21) HRCT scores have been developed to determine the extent of ILD. Visual reader-based HRCT scores correlated well with computer-based HRCT scoring (2,18-20,22), PFT results (2,9,19,20,23), and other clinical variables (20,23) in cross-sectional studies. Furthermore, changes in computer-based HRCT scores measuring SSc-ILD provide a sensitive outcome measure of disease progression and response to treatment (3). However, visual reader-based HRCT scoring remains an essential diagnostic tool in treatment centers with HRCT scoring expertise, but where computer-aided scoring software is not a feasible option, as suggested by Khanna et al. (5).
Previously, we showed that visual reader-based HRCT scoring correlated well with %pFVC, ESR, and %SpO2 in an inception cohort study of 113 early-SSc patients with mean disease duration from NRP to first HRCT of 1 year (4). Our findings showed, in agreement with others (2,9,19,22), although using different scoring systems, that the extent of ILD measured by visual reader-based HRCT significantly correlated with %pFVC (2,9,19,23). However, we did not investigate the long-term changes in our visual reader-based HRCT scoring with changes in other clinical variables.
Therefore, in this study, we analyzed a subgroup of our previous inception cohort population; those with paired results of HRCT and PFT at study entry and last visit, with a mean observation period of 16 months. Twenty-two of 31 (70.9%) patients had ILD at study entry and last visit. No new ILD complications were detected during the observation period. At study entry, our results showed, in agreement with Salaffi et al. (2), although using different scoring systems, that visual reader-based HRCT scores showed significant correlation with %pFVC, but not mRSS. In contrast, Kim et al. (3) found significant correlation of HRCT score with mRSS using computer-based HRCT scoring. Different study populations and HRCT scoring methods may explain these discrepancies.
In this study, over the 2-year observation periods of early-SSc patients, the change in HRCT scores—consisting of t-Fib, t-B, and t-HRCT scores—increased significantly, while Δ mRSS decreased significantly; in contrast, Δ %pFVC, Δ ESR, and Δ %SpO2 were fairly stable. Kim et al. (3) demonstrated greater changes in computer-based HRCT scores—from fibrotic to either a GG or normal pattern—in the cyclophosphamide group compared to the placebo group of SSc-ILD patients (mean disease duration of 3 years) from a Scleroderma Lung Study-I (SLS-I) during a follow-up period of 12 months. Furthermore, in SLS-I, the changes in 12-month %pFVC and mRSS were higher in the cyclophosphamide group compared with the placebo group (12). Our findings, as supported by others (3,12), showed that changes in HRCT scores (3) and mRSS (12) provided a sensitive indication of disease progression and response to treatment in early-SSc patients. However, our observation of no significant change in %pFVC differed from a previous study (12), which may be due to different study populations and study design. Another possible explanation may be the low sensitivity to change of PFT results compared with HRCT scores for early detection of lung disease progression, similar with the low sensitivity of PFT to detect early ILD compared with HRCT, as reported by Suliman et al. (11).
We found significantly negative correlations of Δ t-HC with Δ ESR, reflecting that greater changes in HC scores were associated with less remaining inflammation. Furthermore, Δ t-Fib showed negative correlations with Δ %SpO2, reflecting that greater changes in fibrotic scores were associated with poorer oxygen saturation. To our knowledge, limited data exists on the correlation of change in HRCT score and change in ESR or %SpO2. However, our study observed no significant correlations of Δ HRCT scores with Δ %pFVC and mRSS, in contrast to Kim et al. (3); again, different study populations and HRCT scoring methods might be a possible explanation.
A major limitation of this study was the small sample size of early-SSc patients with sufficient PFT data at either cohort entry or last visit to correspond with the available HRCT data, given the limited availability of PFT at our institution. Another important limitation was that only a single experienced chest radiologist (blinded to clinical and laboratory data) read all of the HRCT results, although this is similar to some previous studies (4,17,23,29). Kazerooni et al. (15) has shown good inter-observer agreement in measuring the extent of pulmonary fibrosis; therefore, we do not think that the readings from a single radiologist bias the findings of this study. Our findings should also be interpreted with caution, given the limited follow-up period of 2 years; it is unknown whether the observed correlation persisted over longer periods. Furthermore, the use of immunosuppressive drugs after detection of ILD in our study cohort might have affected the change of HRCT score and PFT results compared to those not given immunosuppressive drugs. However, our study is the first to investigate the long-term relationship between the changes of simple, visual reader-based HRCT scores with the changes in other clinical variables commonly used in daily practice to monitor disease progression of early-SSc patients.
Conclusions
We found that greater changes of our visual reader-based HRCT scores, rather than %pFVC, along with their correlations with changes in ESR and %SpO2, suggested that visual reader-based HRCT scoring was a useful and sensitive method for monitoring disease progression in early SSc-ILD. A larger study population with longer follow-up is needed to confirm our findings.
Acknowledgements
The authors thank Mrs. Antika Wongthanee, medical statistician, for her statistical analysis and Mrs. Saowanee Puntana for her secretarial assistance.
Funding: This work was supported by the Chiang Mai University Endowment Fund (No. 27/2553), Faculty of Medicine, Chiang Mai University.
Ethical Statement: The study was approved by Research Ethics Committee, Faculty of Medicine, Chiang Mai University of No. 09NOV271015 and written informed consent was obtained from all patients.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Tashkin DP, Volkmann ER, Tseng CH, Kim HJ, Goldin J, Clements P, Furst D, Khanna D, Kleerup E, Roth MD, Elashoff R. Relationship between quantitative radiographic assessments of interstitial lung disease and physiological and clinical features of systemic sclerosis. Ann Rheum Dis 2016;75:374-81. 10.1136/annrheumdis-2014-206076 [DOI] [PubMed] [Google Scholar]
- 2.Salaffi F, Carotti M, Di Donato E, Di Carlo M, Ceccarelli L, Giuseppetti G. Computer-Aided Tomographic Analysis of Interstitial Lung Disease (ILD) in Patients with Systemic Sclerosis (SSc). Correlation with Pulmonary Physiologic Tests and Patient-Centred Measures of Perceived Dyspnea and Functional Disability. PLoS One 2016;11:e0149240. 10.1371/journal.pone.0149240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim HJ, Tashkin DP, Gjertson DW, Brown MS, Kleerup E, Chong S, Belperio JA, Roth MD, Abtin F, Elashoff R, Tseng CH, Khanna D, Goldin JG. Transitions to different patterns of interstitial lung disease in scleroderma with and without treatment. Ann Rheum Dis 2016;75:1367-71. 10.1136/annrheumdis-2015-208929 [DOI] [PubMed] [Google Scholar]
- 4.Wangkaew S, Euathrongchit J, Wattanawittawas P, Kasitanon N, Louthrenoo W. Incidence and predictors of interstitial lung disease (ILD) in Thai patients with early systemic sclerosis: Inception cohort study. Mod Rheumatol 2016;26:588-93. 10.3109/14397595.2015.1115455 [DOI] [PubMed] [Google Scholar]
- 5.Khanna D, Nagaraja V, Tseng CH, Abtin F, Suh R, Kim G, Wells A, Furst DE, Clements PJ, Roth MD, Tashkin DP, Goldin J. Predictors of lung function decline in scleroderma-related interstitial lung disease based on high-resolution computed tomography: implications for cohort enrichment in systemic sclerosis-associated interstitial lung disease trials. Arthritis Res Ther 2015;17:372. 10.1186/s13075-015-0872-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffmann-Vold AM, Aaløkken TM, Lund MB, Garen T, Midtvedt Ø, Brunborg C, Gran JT, Molberg Ø. Predictive value of serial high-resolution computed tomography analyses and concurrent lung function tests in systemic sclerosis. Arthritis Rheumatol 2015;67:2205-12. 10.1002/art.39166 [DOI] [PubMed] [Google Scholar]
- 7.Frauenfelder T, Winklehner A, Nguyen TD, Dobrota R, Baumueller S, Maurer B, Distler O. Screening for interstitial lung disease in systemic sclerosis: performance of high-resolution CT with limited number of slices: a prospective study. Ann Rheum Dis 2014;73:2069-73. 10.1136/annrheumdis-2014-205637 [DOI] [PubMed] [Google Scholar]
- 8.Moore OA, Goh N, Corte T, Rouse H, Hennessy O, Thakkar V, Byron J, Sahhar J, Roddy J, Gabbay E, Youssef P, Nash P, Zochling J, Proudman SM, Stevens W, Nikpour M. Extent of disease on high-resolution computed tomography lung is a predictor of decline and mortality in systemic sclerosis-related interstitial lung disease. Rheumatology (Oxford) 2013;52:155-60. 10.1093/rheumatology/kes289 [DOI] [PubMed] [Google Scholar]
- 9.Goldin JG, Lynch DA, Strollo DC, Suh RD, Schraufnagel DE, Clements PJ, Elashoff RM, Furst DE, Vasunilashorn S, McNitt-Gray MF, Brown MS, Roth MD, Tashkin DP, Scleroderma Lung Study Research Group . High-resolution CT scan findings in patients with symptomatic scleroderma-related interstitial lung disease. Chest 2008;134:358-67. 10.1378/chest.07-2444 [DOI] [PubMed] [Google Scholar]
- 10.Goh NS, Desai SR, Veeraraghavan S, Hansell DM, Copley SJ, Maher TM, Corte TJ, Sander CR, Ratoff J, Devaraj A, Bozovic G, Denton CP, Black CM, du Bois RM, Wells AU. Interstitial lung disease in systemic sclerosis: a simple staging system. Am J Respir Crit Care Med 2008;177:1248-54. 10.1164/rccm.200706-877OC [DOI] [PubMed] [Google Scholar]
- 11.Suliman YA, Dobrota R, Huscher D, Nguyen-Kim TD, Maurer B, Jordan S, Speich R, Frauenfelder T, Distler O. Brief Report: Pulmonary Function Tests: High Rate of False-Negative Results in the Early Detection and Screening of Scleroderma-Related Interstitial Lung Disease. Arthritis Rheumatol 2015;67:3256-61. 10.1002/art.39405 [DOI] [PubMed] [Google Scholar]
- 12.Tashkin DP, Elashoff R, Clements PJ, Goldin J, Roth MD, Furst DE, Arriola E, Silver R, Strange C, Bolster M, Seibold JR, Riley DJ, Hsu VM, Varga J, Schraufnagel DE, Theodore A, Simms R, Wise R, Wigley F, White B, Steen V, Read C, Mayes M, Parsley E, Mubarak K, Connolly MK, Golden J, Olman M, Fessler B, Rothfield N, Metersky M, Scleroderma Lung Study Research Group . Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med 2006;354:2655-66. 10.1056/NEJMoa055120 [DOI] [PubMed] [Google Scholar]
- 13.Khanna D, Brown KK, Clements PJ, Elashoff R, Furst DE, Goldin J, Seibold JR, Silver RM, Tashkin DP, Wells AU. Systemic sclerosis-associated interstitial lung disease-proposed recommendations for future randomized clinical trials. Clin Exp Rheumatol 2010;28:S55-62. [PubMed] [Google Scholar]
- 14.Roth MD, Tseng CH, Clements PJ, Furst DE, Tashkin DP, Goldin JG, Khanna D, Kleerup EC, Li N, Elashoff D, Elashoff RM, Scleroderma Lung Study Research Group . Predicting treatment outcomes and responder subsets in scleroderma-related interstitial lung disease. Arthritis Rheum 2011;63:2797-808. 10.1002/art.30438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kazerooni EA, Martinez FJ, Flint A, Jamadar DA, Gross BH, Spizarny DL, Cascade PN, Whyte RI, Lynch JP, 3rd, Toews G. Thin-section CT obtained at 10-mm increments versus limited three-level thin-section CT for idiopathic pulmonary fibrosis: correlation with pathologic scoring. AJR Am J Roentgenol 1997;169:977-83. 10.2214/ajr.169.4.9308447 [DOI] [PubMed] [Google Scholar]
- 16.Warrick JH, Bhalla M, Schabel SI, Silver RM. High resolution computed tomography in early scleroderma lung disease. J Rheumatol 1991;18:1520-8. [PubMed] [Google Scholar]
- 17.Zisman DA, Karlamangla AS, Ross DJ, Keane MP, Belperio JA, Saggar R, Lynch JP, 3rd, Ardehali A, Goldin J. High-resolution chest CT findings do not predict the presence of pulmonary hypertension in advanced idiopathic pulmonary fibrosis. Chest 2007;132:773-9. 10.1378/chest.07-0116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim HG, Tashkin DP, Clements PJ, Li G, Brown MS, Elashoff R, Gjertson DW, Abtin F, Lynch DA, Strollo DC, Goldin JG. A computer-aided diagnosis system for quantitative scoring of extent of lung fibrosis in scleroderma patients. Clin Exp Rheumatol 2010;28:S26-35. [PMC free article] [PubMed] [Google Scholar]
- 19.Shin KE, Chung MJ, Jung MP, Choe BK, Lee KS. Quantitative computed tomographic indexes in diffuse interstitial lung disease: correlation with physiologic tests and computed tomography visual scores. J Comput Assist Tomogr 2011;35:266-71. 10.1097/RCT.0b013e31820ccf18 [DOI] [PubMed] [Google Scholar]
- 20.Salaffi F, Carotti M, Bosello S, Ciapetti A, Gutierrez M, Bichisecchi E, Giuseppetti G, Ferraccioli G. Computer-aided quantification of interstitial lung disease from high resolution computed tomography images in systemic sclerosis: correlation with visual reader-based score and physiologic tests. Biomed Res Int 2015;2015:834262. [DOI] [PMC free article] [PubMed]
- 21.Kim HJ, Li G, Gjertson D, Elashoff R, Shah SK, Ochs R, Vasunilashorn F, Abtin F, Brown MS, Goldin JG. Classification of parenchymal abnormality in scleroderma lung using a novel approach to denoise images collected via a multicenter study. Acad Radiol 2008;15:1004-16. 10.1016/j.acra.2008.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ariani A, Carotti M, Gutierrez M, Bichisecchi E, Grassi W, Giuseppetti GM, Salaffi F. Utility of an open-source DICOM viewer software (OsiriX) to assess pulmonary fibrosis in systemic sclerosis: preliminary results. Rheumatol Int 2014;34:511-6. 10.1007/s00296-013-2845-6 [DOI] [PubMed] [Google Scholar]
- 23.Patiwetwitoon S, Wangkaew S, Euathrongchit J, Kasitanon N, Louthrenoo W. High-resolution computed tomographic findings in systemic sclerosis-associated interstitial lung disease: comparison between diffuse and limited systemic sclerosis. J Clin Rheumatol 2012;18:229-33. 10.1097/RHU.0b013e318261176f [DOI] [PubMed] [Google Scholar]
- 24.Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum 1980;23:581-90. 10.1002/art.1780230510 [DOI] [PubMed] [Google Scholar]
- 25.LeRoy EC, Medsger TA, Jr. Criteria for the classification of early systemic sclerosis. J Rheumatol 2001;28:1573-6. [PubMed] [Google Scholar]
- 26.Osiri M, Deesomchok U, Tugwell P. Evaluation of functional ability of Thai patients with rheumatoid arthritis by the use of a Thai version of the Health Assessment Questionnaire. Rheumatology (Oxford) 2001;40:555-8. 10.1093/rheumatology/40.5.555 [DOI] [PubMed] [Google Scholar]
- 27.Kim EA, Lee KS, Johkoh T, Kim TS, Suh GY, Kwon OJ, Han J. Interstitial lung diseases associated with collagen vascular diseases: radiologic and histopathologic findings. Radiographics 2002;22 Spec No:S151-65. 10.1148/radiographics.22.suppl_1.g02oc04s151 [DOI] [PubMed] [Google Scholar]
- 28.Webb WR, Muller NL, Naidich DP. High-resolution CT of the lung. 4th ed. Philadelphia: Lippincott Williams and Wilkins, 2009. [Google Scholar]
- 29.Pandey AK, Wilcox P, Mayo JR, Sin D, Moss R, Ellis J, Brown J, Leipsic J. Predictors of pulmonary hypertension on high-resolution computed tomography of the chest in systemic sclerosis: a retrospective analysis. Can Assoc Radiol J 2010;61:291-6. 10.1016/j.carj.2010.02.006 [DOI] [PubMed] [Google Scholar]