Abstract
Aims/Introduction
To investigate the effects of vitamin D and its receptor on cytokines expression and podocytes apoptosis.
Materials and Methods
Cultured mouse podocytes were pre‐incubated with vitamin D or transiently transfected with small interfering ribonucleic acid (RNA) to knock down the vitamin D receptor. Lipopolysaccharide was used to mimic the inflammation status of diabetes.
Results
In a lipopolysaccharide‐induced state, expressions of transforming growth factor‐β, angiotensinogen and vascular endothelial growth factor were similarly increased. Transforming growth factor‐β and angiotensinogen levels originally elevated by lipopolysaccharide challenge were distinctly reduced after pre‐incubation with vitamin D. Whereas after vitamin D receptor small interfering (si)RNA transfection, the aforementioned cytokines had opposite changes as expected. However, neither vitamin D pretreatment nor vitamin D receptor siRNA transfection influenced the previously increased vascular endothelial growth factor expression at messenger RNA or protein levels. When pretreated with vitamin D, decreases were observed for phosphorylated inhibitor‐κB and the inhibitor kinase proteins. After siRNA transfection, those proteins levels were further elevated. The originally increased transforming growth factor‐β and angiotensinogen levels as a result of lipopolysaccharide stimulation were reduced at both the messenger RNA and protein levels after the specific inhibition of the nuclear factor‐κB pathway with pyrrolidine dithiocarbamate. The apoptosis rate of podocytes was decreased in a parallel manner after vitamin D pre‐incubation, and increased after siRNA transfection, which was also suppressed by pyrrolidine dithiocarbamate.
Conclusions
Vitamin D and its receptor might be involved in the progression of diabetic nephropathy by regulating transforming growth factor‐β, angiotensinogen expression and apoptosis of podocytes. The processes are mediated through the signaling of nuclear factor‐κB pathway.
Keywords: Nephropathy, Podocyte, Vitamin D
Introduction
Along with the prevalence of diabetes, the number of patients suffering diabetic nephropathy has been growing year by year. Diabetic nephropathy and other chronic kidney diseases are commonly characterized by proteinuria, which predicts cardiovascular events and all‐cause mortality1. Increasing evidence shows that podocytes act as the last barrier of glomeruli, whose functional change and damage are likely the key factor for urine protein filtration2, 3. It is presently thought that the activation of the renin–angiotensin–aldosterone system and various inflammatory cytokines (e.g. transforming growth factor‐β [TGF‐β], vascular endothelial growth factor [VEGF], platelet‐derived growth factor and tumor necrosis factor‐α) play important roles in proteinuria development and kidney disease progression4, 5, 6, 7, 8. Podocytes can synthesize and secrete the aforementioned cytokines, and thus might have a critical role in chronic kidney disease. In addition, podocyte numbers are also the key factor in preventing urinary protein excretion9. A decline of podocyte abundance as a result of apoptosis or necrosis will result in proteinuria.
Kidney disease is in close association with vitamin D (VD). On the one hand, 1α‐hydroxylase in kidney mitochondria is the rate‐limiting enzyme for the synthesis of active VD10; on the other hand, the VD receptor (VDR) exists in mesangial cells and podocytes of the kidney, which is among the major target organs of VD11. An increasing number of studies have shown that VD plays other roles in addition to regulating calcium, phosphorus and bone metabolism12. Low VD levels are associated with kidney disease progression and the risk of death, whereas the supplementation of VD can reduce the level of proteinuria in diabetic kidney disease13, 14. The biological effect of VD is mediated by specific intracellular or intranuclear VDR. VDR is widely present in various human tissues and cells, with high expression in podocytes as well.
Research has shown that VD inhibits angiotensinogen (AGT) expression through the nuclear factor‐κB (NF‐κB) pathway in podocytes cultured with high glucose15. However, that research has a certain limitation: podocytes were cultured in Roswell Park Memorial Institute‐1640 medium with a glucose content of 200 mg/dL (11.1 mmol/L); treatments with glucose contents of 450 mg/dL (25 mmol/L) and 110 mg/dL (6.1 mmol/L) as the respective high‐ and normal‐glucose levels might be inappropriate. Low‐dose lipopolysaccharide (LPS) induction can result in chronic kidney structural changes and proteinuria of typical diabetic nephropathy. Therefore in the present study, we used LPS to induce podocytes for simulating the inflammatory challenge state in diabetic nephropathy. Additionally, VD can regulate TGF‐β expression in animals with kidney disease. However, the effect of VD on TGF‐β expression in podocytes has rarely been reported. It remains inconclusive whether VD is also involved in regulating the expression of other important cytokines (e.g. VEGF) in podocytes. Furthermore, whether expression changes of VDR itself would influence the expression of various cytokines in podocytes is still unknown. Furthermore, few experiments focused on the effects of VD or VDR on the apoptosis of podocytes. To address the aforementioned issues, the present study investigated the regulatory roles of VD and VDR in podocytes.
NF‐κB is a nuclear transcription factor that extensively regulates cell growth, apoptosis and inflammatory cytokine expression16. There are a number of target genes for NF‐κB, and the promoter regions of TGF‐β, AGT and VEGF all contain NF‐κB binding sites. Therefore, the present study aimed to investigate whether VD and its receptor regulate TGF‐β, AGT, VEGF expression and podocytes apoptosis through the NF‐κB pathway.
Materials and Methods
Cell culture
Immortalized mouse podocytes (MPC‐5) were kindly provided by Professor Yu from the first affiliated Hospital of Sun Yat‐sen University, Guangzhou, China. Podocytes were cultured as previously described17, 18, 19. Podocytes in the proliferation state were small in cell body, with a round or oval nucleus, and were either mononuclear or binuclear. When growing to a certain density, cells presented a paving stone‐like pattern. Differentiated and matured podocytes apparently became larger in the cell body, with multiple protrusions projecting. Neighboring podocytes formed interconnections and contained relatively large nucleuses.
LPS and VD treatments of podocytes
Mouse podocytes were synchronized with Roswell Park Memorial Institute‐1640 medium containing 1% fetal bovine serum for 24 h. The podocytes were pre‐incubated in a culture medium containing 20 nmol/L 1, 25‐(OH)2VD3 for 24 h, and then challenged with 100 ng/mL LPS for 4 h15, 20, 21.
VDR small interfering ribonucleic acid transfection
The N‐TER peptide transfection agent was used for small interfering ribonucleic acid (siRNA) transfection according to the supplier's instructions. The VDR‐siRNA sequence of target 1 was 5′‐AAGCAGGACAAUCUGGUCA‐3′; target 2 was 5′‐UACGUCUGCACGAAUUGGAG‐3′. Negative control siRNA was designed so as not to target any known mouse genes. The final concentration of siRNA was 20 nmol/L. VDR and negative control siRNAs were designed and synthesized by Sigma‐Aldrich company (St. Louis, MO, USA).
NF‐κB inhibitor treatment of podocytes
The NF‐κB inhibitor, pyrrolidine dithiocarbamate (PDTC), was dissolved in dimethylsulfoxide and added to the culture medium at a concentration of 50 μmol/L. After mixing, podocytes were pre‐incubated for 1 h.
RNA extraction and reverse transcription polymerase chain reaction
Primer design
Primers were designed by following the general principles of real‐time polymerase chain reaction primer design. The complete sequences of relevant RNA genes were retrieved from the GenBank database. TGF‐β, VEGF, AGT and β‐actin primers were designed using PRIMER 5.0 (Biosoft International, Palo Alto, CA, USA). The primer sequences are as follows:
VDR (forward) AGGAGCAACAGCACATTATCG, VDR (reverse) ATCGGAGCCTTCTTCATTCAG, TGF‐β1 (forward) GCTGACCCCCACTGATAC, TGF‐β1 (reverse) GAAAGCCCTGTATTCCGTCTC, AGT (forward) CTTGCCACTGAGAAAATCAACA, AGT (reverse) TTGAAAAGTAGGGTGCTGTCTG, VEGF (forward) GTAACGATGAAGCCCTGGAGT, VEGF (reverse) CTCTCCTATGTGCTGGCTTTG, β‐actin (forward) GTCCCTCACCCTCCCAAAAG, β‐actin (reverse) GCTGCCTCAACACCTCAACCC.
RNA isolation and real‐time reverse transcription polymerase chain reaction
Total RNA from cultured podocytes was extracted by using the Trizol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) in accordance with the manufacturer's instructions. The extracted RNA was first pretreated with RNAase‐free deoxyribonuclease and then used for complementary deoxyribonucleic acid synthesis primed with random hexamers. Complementary deoxyribonucleic acids were subsequently amplified and quantified by using SYBR Premix Ex Taq (Takara Bio Inc., Kusatsu, Japan) in the ABI Prism 7500 Sequence Detection System (Applied Biosystems, Carlsbad, CA, USA).
Western blotting
Cells were washed with phosphate‐buffered saline, and then lysed in the protein extraction reagent radio immunoprecipitation assay (Beyotime, Jiangsu, China) with phenylmethanesulfonyl fluoride, and protease and phosphatase inhibitors (Roche Diagnostics). Proteins were separated in sodium dodecyl sulfate polyacrylamide gel electrophoresis, transferred to polyvinylidene fluoride membrane and immunoblotted with antibodies. The following primary antibodies were used: anti‐TGF‐β, anti‐β‐actin, anti‐IκB kinase phosphorylation (P‐IKK) and anti‐IκB phosphorylation (P‐IκB; Cell Signaling, Beverly, MA, USA), anti‐AGT (Epitomics, Burlingame, CA, USA), anti‐VEGF (Merck & Co. Inc., Whitehouse Station, NJ, USA). After being rinsed with buffer solution, the polyvinylidene fluoride membrane was incubated with a horseradish peroxidase‐linked secondary antibody (Cell Signaling).
Apoptosis assay
LPS challenge duration was extended to 24 h to evaluated podocyte apoptosis. The number of apoptotic cells was calculated by scoring cells showing a fragmented nucleus and/or pycnotic nucleus visualized with Hoechst 33 258 as previously described22.
Flow cytometry analysis
Flow cytometry analysis of apoptotic podocytes was carried out by using an Annexin V fluorescein isothiocyanate/propidium iodide (FITC/PI) staining kit (BD Biosciences, San Diego, CA USA). After washes, the cells were resuspended in the buffer followed by staining with Annexin V‐FITC/PI. Apoptotic cells were then evaluated by gating both PI and Annexin V‐positive cells on a flow cytometer (BD Biosciences).
Statistical analysis
All normally distributed data are expressed as means ± standard deviations. Statistical analysis was carried out using the SPSS 15.0 statistical software package (SPSS, Chicago, IL, USA). Normally distributed data were analyzed by using the t‐test (two groups) or single‐factor analysis of variance (multiple groups), and non‐normally distributed data were analyzed by using the rank–sum test to test the significance of differences between the groups. A P‐value of ≤0.05 was considered statistically significant.
Results
Effect of VD on LPS‐mediated expression of cytokines in podocytes
LPS challenge significantly elevated TGF‐β, AGT, and VEGF expression at both messenger RNA (mRNA) and protein levels in podocytes (Figure 1). After pre‐incubation with VD, TGF‐β and AGT mRNA expression levels originally elevated by LPS challenge were reduced by 50.61 and 48.14%, respectively (P < 0.05). After pre‐incubation with VD, the elevated TGF‐β and AGT expression levels were both reduced to levels similar to the negative controls. However, neither the VEGF mRNA nor protein expression level changed significantly (Figure 2).
Figure 1.
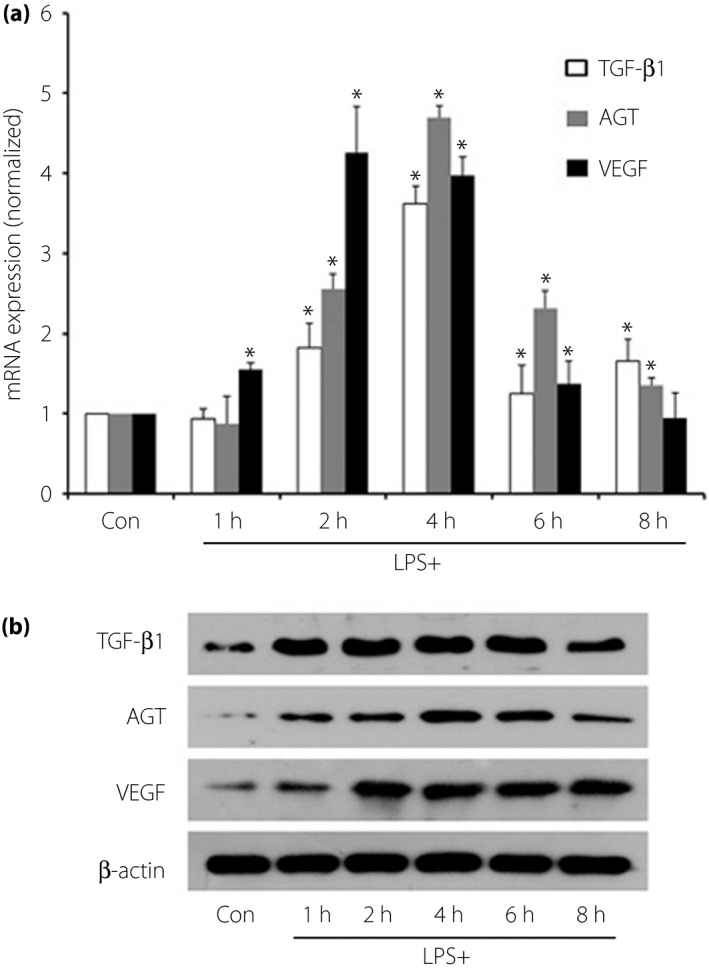
Lipopolysaccharide (LPS) challenge elevated transforming growth factor‐β (TGF‐β), angiotensinogen (AGT) and vascular endothelial growth factor (VEGF) expression in podocytes. (a) TGF‐β, AGT and VEGF expression was significantly elevated at messenger ribonucleic acid (mRNA) level after 2–4 h of LPS stimulation (P < 0.05). (b) TGF‐β and AGT protein expression was increased after 2–4 h of LPS challenge, simultaneously (P < 0.05). Therefore, we took the stimulation time of 4 h as the subsequent stimulus duration. Expression levels were normalized to β‐actin and the error bars represented standard deviations, which were calculated from three parallel experiments. *P < 0.05.
Figure 2.
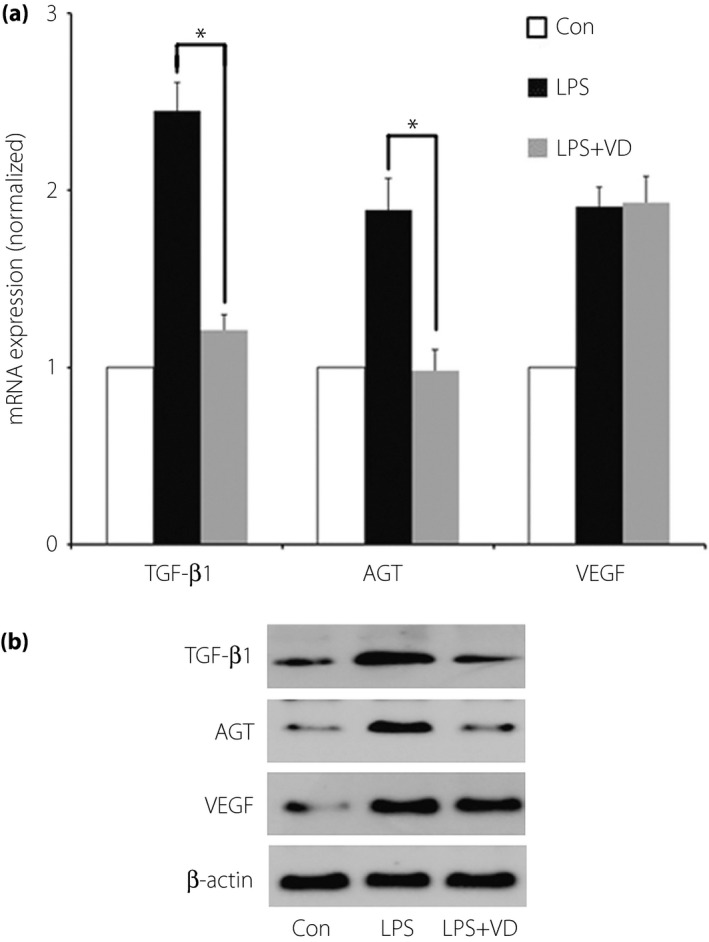
Effect of vitamin D (VD) on lipopolysaccharide (LPS)‐mediated expression of transforming growth factor‐β (TGF‐β), angiotensinogen (AGT) and vascular endothelial growth factor (VEGF) in podocytes. (a) After pre‐incubation with VD, TGF‐β and AGT mRNA expression levels originally elevated by LPS challenge were reduced by 50.61 and 48.14%, respectively (P < 0.05). However, the VEGF messenger ribonucleic acid (mRNA) expression level did not change as the other factors did. (b) After pre‐incubation with VD, the elevated protein expression levels of TGF‐β and AGT, but not VEGF, were both reduced to levels similar to the negative controls. *P < 0.05.
Effect of VDR‐siRNA transfection on LPS‐mediated cytokine expression in podocytes
Podocytes were transfected with transfection solution at the concentrations of 20 nmol/L based on the manufacturer's instructions and the gradient selection. After transfection with VDR‐siRNA of target 1 or target 2, decreases were observed both in VDR mRNA and protein expression compared with those of mock transfection, respectively (P < 0.05; Figure 3a,b). Thus, the siRNA of target 2 was chosen as the proper VDR‐siRNA for podocyte transfection.
Figure 3.
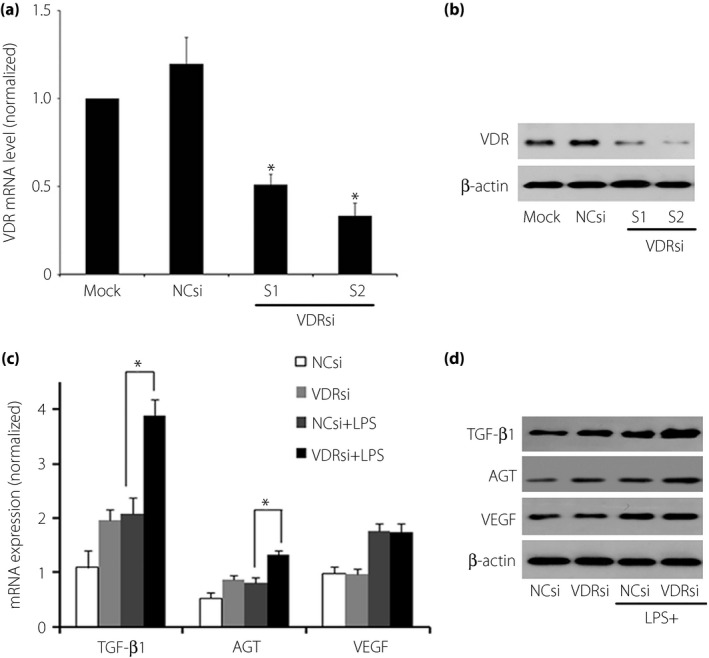
Effect of vitamin D receptor (VDR) small interfering ribonucleic acid (siRNA) transfection on podocytes. After transfecting with VDR‐siRNA of target 1 or target 2 at the concentrations of 20 nmol/L transfection solution, decreases were observed both in (a) VDR messenger RNA (mRNA) and (b) protein expression compared with those of mock or negative siRNA transfection, respectively (P < 0.05). The target 2 siRNA was chosen as the experimental VDR‐siRNA because of a more thorough inhibition of VDR. After VDR‐siRNA transfection, transforming growth factor‐β (TGF‐β) and angiotensinogen (AGT) expression were further elevated at both (c) mRNA and (d) protein levels (P < 0.05), whereas the vascular endothelial growth factor (VEGF) expression levels did not change significantly (P > 0.05). NCsi, negative control‐siRNA; VDRsi, VDR‐siRNA.
After VDR‐siRNA transfection, TGF‐β and AGT expression were further elevated at both mRNA (by 24.24 and 40.35% respectively, P < 0.05) and protein levels (by 43.09 and 88.29% respectively, P < 0.05), whereas the VEGF expression levels did not change significantly compared with the siRNA‐transfected negative control group (Figure 3c,d; P > 0.05).
Role of the NF‐κB pathway in VDR regulation of LPS‐mediated cytokine expression in podocytes
After LPS challenge, P‐IκB, P‐IKK and P‐P65 protein expression levels were significantly elevated in podocytes (Figure 4a). When pretreated with VD, decreases were observed in P‐IκB and P‐IKK protein levels (Figure 4b).
Figure 4.
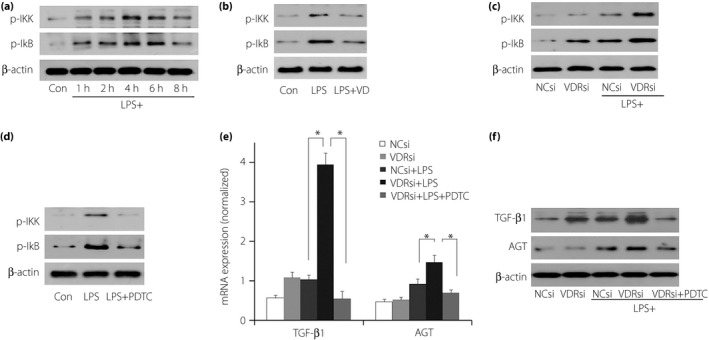
The role of the nuclear factor‐κB (NF‐κB) pathway in vitamin D (VD) and vitamin D receptor (VDR) regulation of lipopolysaccharide (LPS)‐mediated transforming growth factor‐β (TGF‐β), angiotensinogen (AGT) and vascular endothelial growth factor (VEGF) expression. (a) IκB phosphorylation (P‐IκB) and IκB kinase phosphorylation (P‐IKK) were significantly elevated after LPS challenge. (b) Under the existence of LPS, when pretreated with VD, decreases were observed in P‐IκB and P‐IKK protein levels. (c) Whereas after VDR small interfering ribonucleic acid (siRNA) transfection, the increased levels were further elevated. That meant that NF‐κB pathways were involved in the regulation of podocytes by VD and VDR. (d) Pyrrolidine dithiocarbamate (PDTC) suppressed the elevated P‐IKK and P‐IκB greatly. (e,f) When PDTC was added to VDR‐siRNA transfected podocytes, the originally increased TGF‐β and AGT levels due to LPS challenge were significantly reduced at both the (e) messenger RNA (mRNA) and (f) protein levels (P < 0.05). *P < 0.05. NCsi, negative control‐siRNA; VDRsi, VDR‐siRNA.
LPS challenge resulted in a significant elevation of P‐IκB, P‐IKK and P‐P65 protein expression levels in podocytes. After VDR‐siRNA transfection, P‐IκB and P‐IKK protein levels were further elevated by LPS challenge (Figure 4c).
PDTC, an inhibitor of the NF‐κB pathway, suppressed the elevated P‐IKK and P‐IκB greatly (Figure 4d). When it was added to podocytes transfected with VDR‐siRNA, the originally increased TGF‐β and AGT levels due to LPS challenge were significantly reduced at both the mRNA (Figure 4e) and protein levels (Figure 4f).
Effect of VD and VDR‐siRNA transfection on LPS‐mediated podocyte apoptosis
The podocyte apoptosis rate rose significantly after 24‐h stimulation of LPS. Pre‐incubation with VD presented to protect podocytes from excessive apoptosis (Figure 5a,c). Whereas VDR‐siRNA transfection resulted in a further strikingly elevated apoptosis rate after LPS challenge. When the NF‐κB pathway was blocked by PDTC, it showed an extreme reduction of the apoptosis rate detected through flow cytometry analysis of Annexin V‐FITC/PI staining cells, as well as microscopy of Hoechst 33 258‐staining podocytes (Figure 5b,d).
Figure 5.
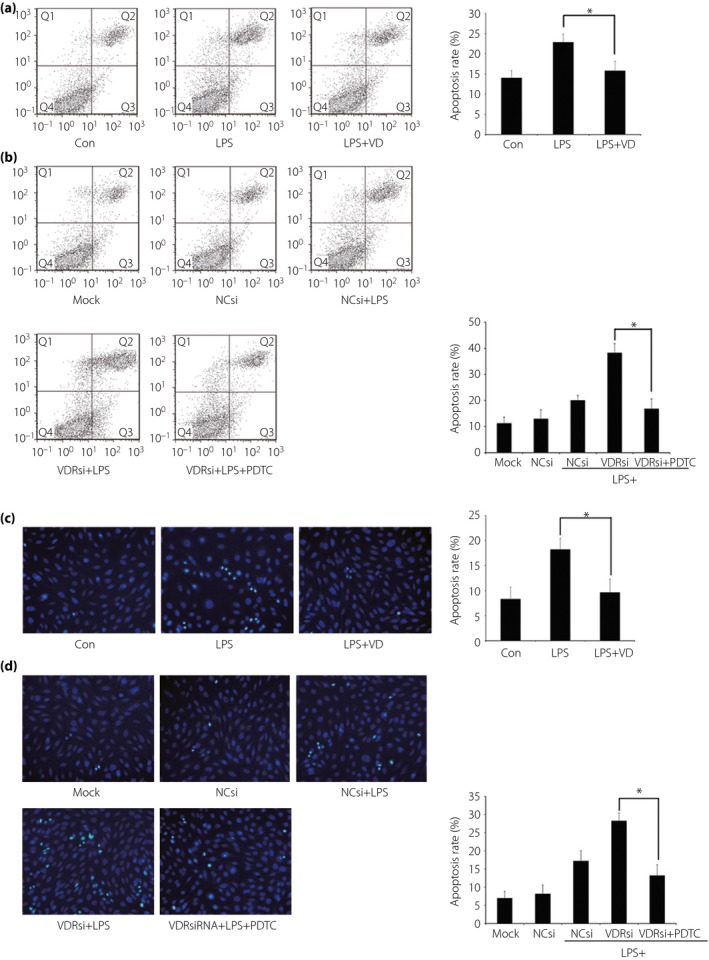
Effect of vitamin D (VD) and vitamin D receptor (VDR) small interfering ribonucleic acid (siRNA) transfection on lipopolysaccharide (LPS)‐mediated podocyte apoptosis. (a) Pre‐incubation with VD presented to protect podocytes from excessive apoptosis induced by LPS. (b) VDR‐siRNA transfection in podocytes resulted in further elevated apoptosis after LPS challenge. When the nuclear factor‐κB (NF‐κB) pathway was blocked by pyrrolidine dithiocarbamate (PDTC), the apoptosis rate showed an extreme reduction. (a,b) The results detected through flow cytometry by using an Annexin V‐FITC/PI staining kit. Q1, Q2, Q3 and Q4 in the figures represented dead cells, late apoptotic cells, early apoptotic cells and viable cells. Cell proportions of Q2 together with Q3 were calculated. (c,d) The results found through Hoechst 33 258 staining, which showed similar outcomes of (a) and (b), respectively. The apoptosis rate of cells was evaluated by scoring the cell numbers showing a pycnotic nucleus or fragmented nucleus. *P < 0.05. NCsi, negative control‐siRNA; VDRsi, VDR‐siRNA.
Discussion
In the present study, LPS‐challenged podocytes were tested. As can be observed, TGF‐β1, AGT, and VEGF mRNA and protein expression levels were significantly increased in podocytes after LPS stimulation. LPS, also called endotoxin, is the product of Gram‐negative bacteria lysis after death. Research has recently shown that LPS could play a critical role in diabetes progression23, 24, 25.
Clinical trials have found that a high‐fat diet can elevate and change LPS in the blood circulation26. Diabetic patients are associated with significantly increased intestinal LPS absorption. Greely et al.27 reported that compared with healthy subjects with matching sex, body mass index and age, type 2 diabetes patients had a 76% increase in blood LPS levels. Even for type 2 diabetes patients receiving hypoglycemic therapy, their blood LPS levels were still significantly higher than those in healthy subjects28. LPS induction of animal models has been used to simulate changes in diabetic nephropathy, resulting in more typical pathological and functional changes compared with traditional methods of induction of diabetic nephropathy29, 30. Additionally, MPC‐5 mouse podocytes were commonly cultured using Roswell Park Memorial Institute‐1640 medium containing glucose at a concentration of 200 mg/dL (11.1 mmol/L). The treatment with glucose at 110 mg/dL (6.1 mmol/L) as a normal control would be inappropriate. In the present study, LPS was used to challenge MPC‐5 mouse podocytes cultured in vitro to overcome the limitations of the previous studies.
Furthermore, the present study showed that VD inhibited TGF‐β and AGT expression induced by LPS challenge, whereas LPS challenge after VDR inhibition further enhanced TGF‐β and AGT expression. Neither activation nor inhibition of VDR strongly influenced VEGF levels. Because TGF‐β and AGT play important roles in the progression of proteinuria, renal fibrosis, and renal failure, VD and VDR might implement their roles in kidney protection by changing the levels of the aforementioned two cytokines.
TGF‐β is a key regulator of renal fibrosis; pathologically, its production can be increased by the elevation of blood glucose, the accumulation of glycation end‐products, and oxidative stress, along with inflammatory stimuli. TGF‐β1 is most important in the induced fibrosis process of diabetic nephropathy and other kidney diseases, such as obstructive nephropathy, as it mediates the changes in a number of biochemical factors and cytokines, leading to kidney damage6, 31. In the present study, the activation of VDR by VD inhibited TGF‐β1 expression in podocytes; on the contrary, VDR suppression promoted the increase of TGF‐β1 expression. These results suggest that VD and VDR are likely to play important roles in chronic kidney diseases by acting on TGF‐β1. AGT is a polypeptide consisting of 453 amino acids, and is the only known substrate of renin. Increased AGT expression directly reflects renin–angiotensin system (RAS) activation, which can induce changes in renal hemodynamics and other factors32. The results of the present study showed that VDR adjusted local RAS levels, consistent with other studies on the influence of VD on AGT levels in podocytes in a high‐glucose state. Furthermore, VEGF plays a critical role in kidney disease. Clinical studies have shown that urinary VEGF levels are significantly elevated in the early stage of kidney disease, and are closely associated with urinary protein excretion33. Animal experiments have also found that the expression of VEGF and its high‐affinity receptor are obviously upregulated in rats with diabetic nephropathy in either an early or an advanced stage34. Associated pathological changes in the kidneys can be alleviated by neutralizing the effect of VEGF through the application of anti‐VEGF antibodies. However, an animal experiment showed that despite the increased level of VEGF receptor‐2 in diabetic mice, blocking this receptor led to severe renal damage by diabetic nephropathy35. Thus, the exact role of VEGF in kidney disease needs to be further studied. In the present study, LPS challenge increased VEGF levels in podocytes, suggesting that VEGF is involved in the inflammation process. However, the application of VD had no obvious effect on VEGF expression, indicating that VD improves the function of podocytes through factors other than VEGF.
Podocyte damage and loss might be important factors in the development and progression of kidney diseases. It is presumed that podocytes are incapable of regeneration and have a quite limited potential for cytothesis, therefore once lost they cannot be repaired36. The podocyte is also an integral part of the glomerular filtration barrier, and higher a apoptosis rate of podocytes means increased leakiness of urine protein. In the present study, VD effectively inhibited cell apoptosis. In addition, suppression of VDR caused greater damage to podocytes. Therefore, VD and VDR were indispensible factors in protecting the integrated structure of the filtration barrier.
NF‐κB is a nucleoprotein factor that can specifically bind to the sequence of the light chain enhancer κB of the immunoglobulin κ. Widely present in various eukaryotic cells, NF‐κB regulates various cellular responses, such as cell growth, differentiation, apoptosis and inflammation. In unchallenged cells, NF‐κB interacts with IκB‐inhibitory protein and exists in the cytoplasm in the form of aninactive complex (p50‐p65‐IkB). This complex can prevent the transfer of NF‐κB into the nucleus. In the classical activation pathway, various stimuli cause P‐IKB by acting on the IKK (IκB kinase) complex, resulting in IκB degradation. The released NF‐κB is then translocated to the nucleus to activate the expression of a series of target genes. P‐IKK is a key step in the activation of NF‐κB37. Thus, our research focus was to clarify whether VDR regulates TGF‐β and AGT expression by influencing P‐IKK and P‐IκB, the two key enzymes in the NF‐κB pathway.
NF‐κB has a number of target genes, and there are binding sites for this transcription factor in the promoter regions of both TGF‐β and AGT. The existing research has shown that podocytes challenged by high glucose can activate RAS through the NF‐κB pathway, contributing to the progression of diabetic nephropathy. Other studies have indicated that after the activation of the TGF‐β pathway, podocytes can further activate the downstream NF‐κB pathway, promoting the progression of kidney disease. Because the promoter region of TGF‐β contains the NF‐κB‐binding site, whether the NF‐κB pathway in podocytes acts on TGF‐β to form a loop signaling pathway remains unknown; whether the NF‐κB pathway acts as a common pathway of TGF‐β and RAS to influence the role of VDR in podocytes is also unclear. In the present study, PDTC, a classical NF‐κB pathway inhibitor, was used to inhibit the activation of this pathway. Under the condition of LPS challenge, the original elevations of TGF‐β, AGT and apoptosis rate were well suppressed in podocytes after VDR‐siRNA transfection. These observations suggested that VDR regulates LPS‐induced changes in TGF‐β, and AGT expression and cell apoptosis in podocytes through the common NF‐κB pathway.
The present study used VDR‐siRNA to inhibit VDR expression in podocytes to observe its effect. We propose that in an inflammation state, both VD and VDR play their roles by acting on TGF‐β, AGT and cell apoptosis, and the process is regulated by the NF‐κB pathway. Additionally, the results show that the influence of VD and VDR on kidney podocytes is not achieved through VEGF. This finding suggests that VD and its receptor are both involved in the protection of kidney podocytes, providing reference information for the prevention and treatment of chronic kidney diseases. However, the present study had certain limitations, and its conclusions can be further verified if the role of VD and its receptor can be confirmed in other kidney cell lines.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
This work was supported by the Natural Science Foundation of China (no. 81070659); the Natural Science Foundation of Guangdong Province of China (no. 1251008901000030); the Science and Technique Research Project of Guangzhou Municipality, Guangdong Province, China (no. 2010J‐E521); and the Guangdong Provincial Key Laboratory of Medicine. LJX and PYZ designed and carried out the experiments and analyzed the data. The other authors participated in the coordination and review of the manuscript. We carried out the study under the guidance of Professor YBL who wrote the final manuscript.
J Diabetes Investig 2016; 7: 680–688
References
- 1. De Zeeuw D, Remuzzi G, Parving HH, et al Albuminuria, a therapeutic target for cardiovascular protection in type 2 diabetic patients with nephropathy. Circulation 2004; 110: 921–927. [DOI] [PubMed] [Google Scholar]
- 2. Shankland SJ. The podocyte's response to injury: role in proteinuria and glomerulosclerosis. Kidney Int 2006; 69: 2131–2147. [DOI] [PubMed] [Google Scholar]
- 3. Faul C, Donnelly M, Merscher‐Gomez S, et al The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med 2008; 14: 931–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen H‐b, Jun‐xi L, Li Q, et al The protective effect of the RAS inhibitor on diabetic patients with nephropathy in the context of VEGF suppression. Acta Pharmacol Sin 2009; 30: 242–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Veron D, Reidy KJ, Bertuccio C, et al Overexpression of VEGF‐A in podocytes of adult mice causes glomerular disease. Kidney Int 2010; 77: 989–999. [DOI] [PubMed] [Google Scholar]
- 6. Wang S, Wilkes MC, Leof EB, et al Noncanonical TGF‐β pathways, mTORC1 and Abl, in renal interstitial fibrogenesis. Am J Physiol Renal Physiol 2010; 298: F142–F149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chung ACK, Zhang H, Kong Y‐Z, et al Advanced Glycation End‐Products Induce Tubular CTGF via TGF‐β‐Independent Smad3 Signaling. J Am Soc Nephrol 2010; 21: 249–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schordan S, Schordan E, Endlich N, et al Alterations of the podocyte proteome in response to high glucose concentrations. Proteomics 2009; 9: 4519–4528. [DOI] [PubMed] [Google Scholar]
- 9. Dalla Vestra M, Masiero A, Roiter AM, et al Is podocyte injury relevant in diabetic nephropathy? Studies in patients with type 2 diabetes. Diabetes 2003; 52: 1031–1035. [DOI] [PubMed] [Google Scholar]
- 10. Peleg S, Posner GH. Vitamin D analogs as modulators of vitamin D receptor action. Curr Top Med Chem 2003; 3: 1555–1572. [DOI] [PubMed] [Google Scholar]
- 11. Yamauchi K, Takano Y, Kasai A, et al Screening and identification of substances that regulate nephrin gene expression using engineered reporter podocytes. Kidney Int 2006; 70: 892–900. [DOI] [PubMed] [Google Scholar]
- 12. Samuel S, Sitrin MD. Vitamin D's role in cell proliferation and differentiation. Nutr Rev 2008; 66: S116–S124. [DOI] [PubMed] [Google Scholar]
- 13. De Zeeuw D, Agarwal R, Amdahl M, et al Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet 2010; 376: 1543–1551. [DOI] [PubMed] [Google Scholar]
- 14. Liu LJ, Lv JC, Shi SF, et al Oral calcitriol for reduction of proteinuria in patients with IgA nephropathy: a randomized controlled trial. Am J Kidney Dis 2012; 59: 67–74. [DOI] [PubMed] [Google Scholar]
- 15. Deb DK, Chen Y, Zhang Z, et al 1,25‐ Dihydroxyvitamin D3 suppresses high glucose‐induced angiotensinogen expression in kidney cells by blocking the NF‐κB pathway. Am J Physiol Renal Physiol 2009; 296: F1212–F1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shen HM, Tergaonkar V. NF‐κB signaling in carcinogenesis and as a potential molecular target for cancer therapy. Apoptosis 2009; 14: 348–363. [DOI] [PubMed] [Google Scholar]
- 17. Reddy GR, Pushpanathan MJ, Ransom RF, et al Identification of the glomerular podocyte as a target for growth hormone action. Endocrinology 2007; 148: 2045–2055. [DOI] [PubMed] [Google Scholar]
- 18. Mundel P, Reiser J, Zuniga Mejia Borja A, et al Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res 1997; 236: 248–258. [DOI] [PubMed] [Google Scholar]
- 19. Saleem M, O'Hare MJ, Reiser J, et al A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol 2002; 13: 630–638. [DOI] [PubMed] [Google Scholar]
- 20. Yuan W, Pan W, Kong J, et al 1,25‐dihydroxyvitamin D3 suppresses renin gene transcription by blocking the activity of the cyclic AMP response element in the renin gene promoter. J Biol Chem 2007; 282: 29821–29830. [DOI] [PubMed] [Google Scholar]
- 21. Mashmoushi AK, Oates JC. Lipopolysaccharide induces inducible nitric oxidesynthase‐dependent podocyte dysfunction via a hypoxia‐inducible factor 1α and cell division control protein 42 and Ras‐related C3 botulinum toxin substrate 1 pathway. Free Radic Biol Med 2015; 84: 185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Abderrahmani A, Niederhauser G, Favre D, et al Human high‐density lipoprotein particles prevent activation of the JNK pathway induced by human oxidised low‐density lipoprotein particles in pancreatic beta cells. Diabetologia 2007; 50: 1304–1314. [DOI] [PubMed] [Google Scholar]
- 23. Min KB, Min JY. Household endotoxin exposure and increased risk of diabetes in older adults. Diabet Med 2015; 32: 1667–1669. [DOI] [PubMed] [Google Scholar]
- 24. Vagaja NN, Binz N, McLenachan S, et al Influence of endotoxin‐mediated retinal inflammation on phenotype of diabetic retinopathy in Ins2 Akita mice. Br J Ophthalmol 2013; 97: 1343–1350. [DOI] [PubMed] [Google Scholar]
- 25. Jayashree B, Bibin YS, Prabhu D, et al Increased circulatory levels of lipopolysaccharide (LPS) and zonulin signify novel biomarkers of proinflammation in patients with type 2 diabetes. Mol Cell Biochem 2014; 388: 203–210. [DOI] [PubMed] [Google Scholar]
- 26. Erridge C, Attina T, Spickett CM, et al A high‐fat meal induces low‐ grade endotoxemia: evidcnce of a novel mechanism of postprandial inflammation. Am J Clin Nutr 2007; 86: 1286–1292. [DOI] [PubMed] [Google Scholar]
- 27. Creely SJ, McTernan PG, Kusminski CM, et al Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am J Physiol Endocrinol Metab 2007; 92: E740–E747. [DOI] [PubMed] [Google Scholar]
- 28. Al‐Attas OS, Al‐Daghri NM, Al‐Rubeaan K, et al Changes in endotoxin levels in T2DM subjects on anti‐diabetic therapies. Cardiovasc Diabetol 2009; 8: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lorenzen J, Shah R, Biser A, et al The role of osteopontin in the development of albuminuria. J Am Soc Nephrol 2008; 19: 884–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sun Y, He L, Takemoto M, et al Glomerular transcriptome changes associated with lipopolysaccharide‐induced proteinuria. Am J Nephrol 2009; 29: 558–570. [DOI] [PubMed] [Google Scholar]
- 31. Basu Rajit K, Hubchak S, Hayashida T, et al Interdependence of HIF‐1αand TGF‐β/Smad3 signaling in normoxic and hypoxic renal epithelial cell collagen expression. Am J Physiol Renal Physiol 2011; 300: F898–F905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brosius FC, Khoury CC, Buller CL, et al Abnormalities in signaling pathways in diabetic nephropathy. Expert Rev Endocrinol Metab 2010; 5: 51–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Veron D, Bertuccio CA, Marlier A, et al Podocyte vascular endothelial growth factor (Vegf164) overexpression causes severe nodular glomerulosclerosis in a mouse model of type 1 diabetes. Diabetologia 2011; 54: 1227–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Naskret D, Zozulinska‐Ziolkiewicz DA, Dankowski R, et al Albuminuria and VEGF as early markers of cardiovascular disturbances in young type 1 diabetic patients. Microvasc Res 2010; 80: 440–444. [DOI] [PubMed] [Google Scholar]
- 35. Kim HW, Lim JH, Kim MY, et al Long‐term blockade of vascular endothelial growth factor receptor‐2 aggravates the diabetic renal dysfunction associated with inactivation of the Akt/eNOS‐NO axis. Nephrol Dial Transplant 2011; 26: 1173–1188. [DOI] [PubMed] [Google Scholar]
- 36. Kriz W, Gretz N, Lemley KV. Progression of glomerular diseases: is the podocyte the culprit? Kidney Int 1998; 54: 687–697. [DOI] [PubMed] [Google Scholar]
- 37. Cl'ement JF, Meloche S, Servant MJ. The IKK‐related kinases: from innate immunity to oncogenesis. Cell Res 2008; 18: 889–899. [DOI] [PubMed] [Google Scholar]


