Abstract
Aims/Introduction
The aim of the present prospective observational study was to assess long‐term efficacy and safety of insulin degludec as a part of a basal–bolus therapy for Japanese patients with type 1 or type 2 diabetes in routine clinical practice.
Materials and Methods
In the present study, 93 type 1 diabetes patients and 135 type 2 diabetes patients treated with insulin glargine or detemir were switched from their basal insulin to insulin degludec. The primary end‐points were the changes in glycated hemoglobin (HbA1c) from baseline at 3, 6 and 12 months. The secondary end‐points were changes in body mass index, insulin dose, frequency of hypoglycemia and adverse events.
Results
HbA1c levels from baseline were significantly reduced at 3, 6, and 12 months by 0.4, 0.4 and 0.3% in type 1 diabetes patients, respectively, and by 0.5, 0.5 and 0.3% in type 2 diabetes patients, respectively. Body mass index in type 1 diabetes patients increased significantly (P < 0.05), whereas that in type 2 diabetes patients did not change. Basal insulin dose decreased significantly at 3 months after switching (P < 0.05), and returned baseline dose at 12 months in type 1 diabetes and type 2 diabetes patients. The frequency of both total and nocturnal hypoglycemia decreased significantly in type 1 diabetes and type 2 diabetes patients (P < 0.05). The result of multiple regression analysis showed that baseline HbA1c was a significant independent variable of the percentage change in HbA1c with switching.
Conclusion
In both type 1 diabetes and type 2 diabetes patients, switching from insulin glargine or insulin detemir to insulin degludec led to improvement of glycemic control with a significant reduction of hypoglycemia.
Keywords: Insulin degludec, Insulin detemir, Insulin glargine
Introduction
Insulin degludec (IDeg), a new long‐acting insulin analog, has a relatively flat glucose‐lowering profile that was shown by using the euglycemic clamp technique1, 2. IDeg is a soluble dihexamer in preparation that forms stable soluble multihexamers after subcutaneous injection. The gradual separation of IDeg monomers from the multihexamers results in a slow and continuous delivery of IDeg from the subcutaneous injection site into the circulation2.
Phase III clinical studies showed that IDeg showed non‐inferiority to the control drug, insulin glargine (IGlar), in the magnitude of glycated hemoglobin (HbA1c) reduction of non‐Japanese subjects3, 4. However, these phase III studies tested a specific group of patients who were selected according to strict criteria regarding baseline HbA1c, body mass index (BMI) and prior medication period, based on the regulatory authority's guidelines. The insulin dose in the studies was adjusted with a target fasting blood glucose level of 70–90 mg/dL, and direct application of this dose adjustment method might be unsuitable for diabetic patients in routine clinical practice. Furthermore, in the previous reports5, 6, 7, 8, 9 under routine clinical conditions, few studies have reported on the long‐term efficacy and safety of IDeg as switching from basal insulin. Therefore, it is still unclear whether IDeg would be effective and safe for diabetic patients under the conditions of routine clinical practice.
The present study was carried out to evaluate glycemic control and the incidence of adverse reactions during a 1‐year period when well‐experienced diabetologists switched from IGlar or insulin detemir (IDet) to IDeg in both type 1 diabetes and type 2 diabetes outpatients.
Materials and Methods
Participants and study design
The Kumamoto Insulin DeglUdec observatioNAl study (KIDUNA study) was designed as a 1‐year, prospective, open‐label, multicenter, non‐randomized, observational study. In the present study, adult Japanese outpatients with type 1 diabetes or type 2 diabetes treated with the basal–bolus insulin therapy (BBT) using rapid‐acting insulin analog and long‐acting insulin analog at 26 medical institutions were enrolled, between April 2013 and June 2014. The diagnosis of type 1 diabetes and type 2 diabetes was on the basis of the criteria of the Japan Diabetes Society for the diagnosis of diabetes10, 11. The exclusion criteria were pregnant or nursing women and subjects in whom the physician deemed IDeg treatment inappropriate.
At the start of the study, the basal insulin was switched from IGlar or IDet to IDeg once a day. IDeg was injected at bedtime in all participants. The initial dose of IDeg was determined by the attending physician, and ranged from 80 to 100% of the dose of IGlar or IDet. The dose of bolus insulin was not changed at the start of the study. Target plasma glucose level was set between 80 and 129 mg/dL before breakfast, and between 80 and 179 mg/dL at 2 h after meals without causing hypoglycemia, which had been recommended by the Japan Diabetes Society12, 13, to achieve HbA1c <6.9%. Insulin titration was then carried out according to the attending physician's instruction to achieve the target plasma glucose level. Doses of concomitant antidiabetic agents were not changed until the end of the study.
HbA1c, BMI and insulin dose were recorded at the time of switching to IDeg, as well as at 1, 2, 3, 6, 9 and 12 months after switching.
Safety was assessed on the basis of hypoglycemic episodes and adverse events (AEs). The frequency of hypoglycemic episodes per month from 3 to 6 months, after stable glycemic control and stable insulin dose were achieved by active titration, after switching was compared with that for 1 month before switching. Hypoglycemia was defined as any of the following criteria: (i) the presence of symptoms that were alleviated by oral ingestion of carbohydrates, an intramuscular injection of glucagon or other resuscitative actions; and (ii) a blood glucose <70 mg/dL, regardless of the presence or absence of symptoms14. Nocturnal hypoglycemia was defined as hypoglycemia developing between 00.01 and 05.59 hours. Severe hypoglycemia was defined as hypoglycemia accompanied by severe central nervous system symptoms that could not be resolved by the patient and required assistance15. AEs included all events excluding hypoglycemia temporally associated with the use of IDeg, whether or not considered related to IDeg.
All participants provided written, informed consent to participate in this study, which was carried out in accordance with the principles stated in the Declaration of Helsinki (amended in 2008 at Seoul). The study protocol was approved by the ethics committee of Kumamoto University (approval number 1,580).
Study measurements
The primary end‐points were the changes in HbA1c from baseline at 3, 6 and 12 months after switching. The secondary end‐points were changes in BMI, insulin dose (bolus, basal and total), frequency of hypoglycemia after switching and AEs excluding hypoglycemia.
Statistical analysis
Data are expressed as mean ± standard derivation. Of the participants registered, only those who completed the study were included in the analysis. Changes in clinical parameters were evaluated by paired t‐tests or unpaired t‐tests. Stepwise multiple regression analysis was used to identify independent determinants of the percentage change in HbA1c with switching, calculated as (value at month 6 − value at baseline) ×100/baseline. All variables considered to be clinically meaningful were used as independent variables in the multivariate analysis; namely, sex, age, duration of diabetes, baseline HbA1c, treated basal insulin (IGlar or IDet), treated basal insulin dose, bolus insulin dose and total insulin dose before switching, injection times of basal insulin before switching, percentage change in basal insulin dose at the time of switching, and frequency of total and nocturnal hypoglycemia. The values of β and Stdβ in multiple regression analysis represent the partial regression coefficient and standard regression coefficient, respectively. P‐values of <0.05 were considered to be statistically significant. Data analysis was carried out using SPSS software version 11.5 for Windows (SPSS Inc., Chicago, IL, USA).
Results
Clinical characteristics
The baseline characteristics of the participants are shown in Table1. All participants were treated with BBT three times daily with bolus insulin and once or twice a day with basal insulin, once at bedtime or twice at pre‐breakfast and bedtime, with or without oral hypoglycemic agents. Among 243 participants, 96 participants had type 1 diabetes and 147 participants had type 2 diabetes. Of the 243 participants enrolled in the KIDUNA study, 228 participants (93.8%) completed the study. A total of 15 participants (3 type 1 diabetes patients and 12 type 2 diabetes patients) withdrew. The reasons for discontinuation were transfer to other hospital (3 type 1 diabetes patients and 5 type 2 diabetes patients), conversion to other regimens (6 type 2 diabetes patients) and lost to follow up (one type 2 diabetes patient; Figure 1).
Table 1.
Demographic and baseline characteristics of the study participants
| Characteristics | Type 1 (n = 93) | Type 2 (n = 135) |
|---|---|---|
| Men/women | 43/50 | 74/61 |
| Age (years) | 53.3 ± 13.6 | 63.3 ± 12.6 |
| BMI (kg/m2) | 22.2 ± 3.3 | 25.0 ± 4.0 |
| Creatinine (mg/dL) | 0.77 ± 0.30 | 0.88 ± 0.32 |
| eGFR (mL/min per 1.73 m2) | 83.6 ± 33.5 | 66.3 ± 22.1 |
| HbA1c (%) | 8.7 ± 1.4 | 8.1 ± 1.4 |
| Duration of diabetes (years) | 12.9 ± 9.8 | 15.0 ± 8.1 |
| Basal insulin, n | ||
| Glargine | 59 (63.4%) | 59 (43.7%) |
| Detemir | 34 (36.6%) | 76 (56.3%) |
| Injection twice a day | 32 (34.4%) | 7 (5.2%) |
| Glargine/detemir | 21/11 | 2/5 |
| Antidiabetic agents (n) | ||
| α‐Glucosidase inhibitor | 14 (15.1%) | 20 (14.8%) |
| Biguanide | – | 27 (20.0%) |
| Thiazolidinedione | – | 7 (5.2%) |
| DPP4 inhibitor | – | 47 (34.8%) |
Data are mean ± SD or n. BMI, body mass index; DPP4, dipeptidyl peptidase‐4; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin.
Figure 1.
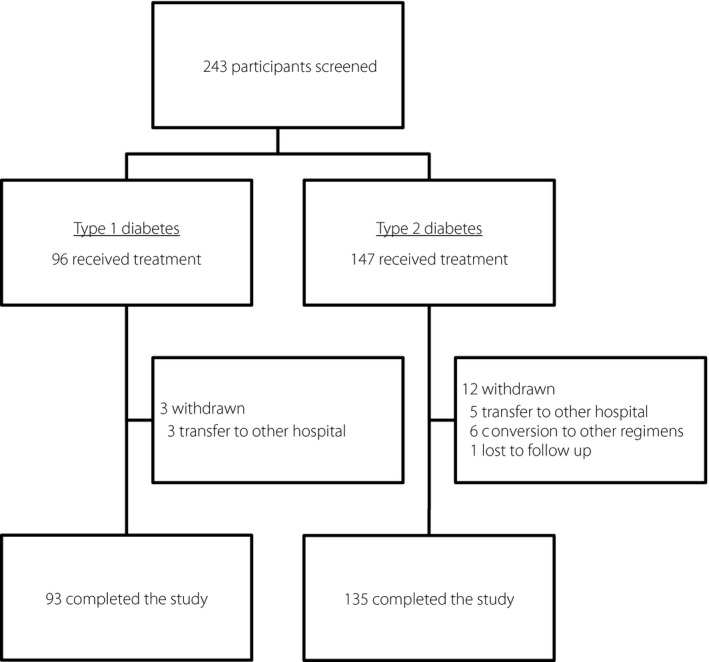
Flow chart of study participants throughout the trial. Data are the number of study participants.
Among 228 participants who completed the study, 59 type 1 diabetes patients (63.4%) and 59 type 2 diabetes patients (43.7%) were treated with IGlar. In both type 1 diabetes and type 2 diabetes patients, there was no statistically significant difference in baseline characteristics between the IGlar group and IDet group. Although we did not ask the all the participants to carry out self‐monitoring of blood glucose (SMBG), overall 85% of participants used a SMBG meter. However, participants were not obliged to record their glucose levels with SMBG in the present study.
Before switching from basal insulin to IDeg, 32 type 1 diabetes participants (34.4%) and seven type 2 diabetes participants (5.2%) injected basal insulin twice a day.
Glycemic control
During the 1‐year observation period, HbA1c levels improved significantly from 8.7 ± 1.4% at baseline to 8.4 ± 1.4% at the end of the study in type 1 diabetes participants (P < 0.01), and from 8.1 ± 1.4% at baseline to 7.8 ± 1.3% in type 2 diabetes participants (P < 0.001; Figure 2). The change in HbA1c from baseline at 3, 6, and 12 months was −0.4, −0.4 and −0.3% in type 1 diabetes patients, respectively, and −0.5, −0.5 and −0.3% in type 2 diabetes patients, respectively. The percentage changes in HbA1c of type 1 diabetes and type 2 diabetes patients were −3.6 ± 9.6% and −5.8 ± 10.6%, respectively.
Figure 2.
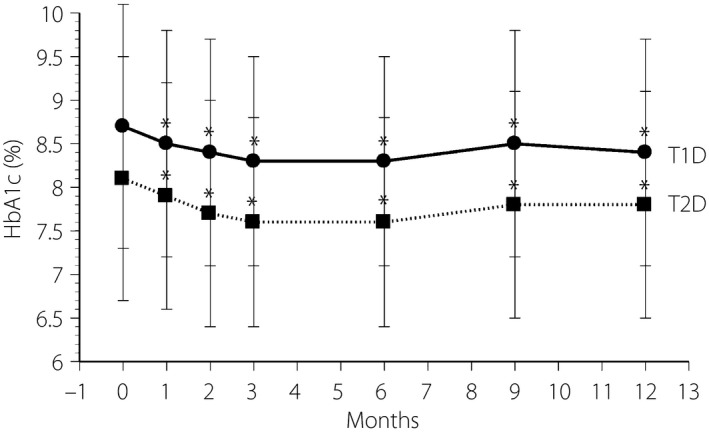
Time‐course of glycated hemoglobin (HbA1c) during the 1‐year study in type 1 diabetes (T1D) patients (circles, solid line) and type 2 diabetes (T2D) patients (squares, dotted line). *P < 0.01 vs baseline.
The percentage change in HbA1c was significantly larger in the IGlar group (−8.0 ± 12.5%) than in the IDet group (−4.7 ± 9.3%; P < 0.01) among type 2 diabetes patients, but not significantly among type 1 diabetes patients.
Regarding type 1 diabetes patients, the percentage change in HbA1c in the participants who had previously received twice‐daily basal insulin injections (T1D‐BID) and the participants who had previously received once‐daily basal insulin injection (T1D‐OD) were −4.3 ± 11.1% and −3.2 ± 8.7%, respectively. Statistical analysis in type 2 diabetes patients was not carried out because of the limited number of type 2 diabetes patients (n = 7) who had previously received twice‐daily basal insulin injections.
BMI change
The BMI level was significantly increased at the end of the study in type 1 diabetes patients (22.2 ± 3.3 kg/m2 to 22.6 ± 3.5 kg/m2, P < 0.01), but not in type 2 diabetes patients (25.0 ± 4.0 kg/m2 to 25.2 ± 4.5 kg/m2, P = 0.522).
There was no statistically significant difference in baseline BMI level between IGlar and IDet groups in both type 1 diabetes and type 2 diabetes patients. However, the BMI level at the end of the study was significantly larger in the IDet group (23.4 ± 3.1 kg/m2) than in the IGlar group (22.1 ± 3.7 kg/m2; P < 0.05) among type 1 diabetes patients, which was not observed in type 2 diabetes patients.
Insulin requirement profiles
The daily insulin requirement profiles are summarized in Table2. At the time of switching, the percentage changes in basal insulin doses were −6.9 ± 11.0% in type 1 diabetes and −2.7 ± 9.8% in type 2 diabetes. However, there was no significant difference between the basal insulin doses at baseline and that at the end of study in both groups. The bolus insulin dose decreased significantly in type 1 diabetes patients at the end of study (P < 0.05), but not significantly in type 2 diabetes patients.
Table 2.
Mean changes of the daily insulin requirement profiles (unit/kg/day) at baseline and during 1 year after switching from insulin glargine or insulin detemir to insulin degludec
| Baseline (before switching) | 0 month (starting dose) | 1 month | 2 months | 3 months | 6 months | 9 months | 12 months | |
|---|---|---|---|---|---|---|---|---|
| Type 1 (n = 93) | ||||||||
| Basal | 0.213 ± 0.103 | 0.195 ± 0.088* | 0.196 ± 0.087* | 0.199 ± 0.087* | 0.202 ± 0.088* | 0.200 ± 0.086* | 0.209 ± 0.093 | 0.211 ± 0.089 |
| Bolus | 0.374 ± 0.164 | 0.374 ± 0.164 | 0.372 ± 0.160 | 0.372 ± 0.156 | 0.367 ± 0.157 | 0.360 ± 0.157* | 0.362 ± 0.158 | 0.359 ± 0.156* |
| Breakfast | 0.126 ± 0.058 | 0.126 ± 0.058 | 0.126 ± 0.058 | 0.124 ± 0.057 | 0.122 ± 0.058 | 0.119 ± 0.059* | 0.123 ± 0.060 | 0.120 ± 0.060 |
| Lunch | 0.117 ± 0.054 | 0.117 ± 0.054 | 0.117 ± 0.053 | 0.117 ± 0.051 | 0.115 ± 0.051 | 0.114 ± 0.051 | 0.113 ± 0.051 | 0.114 ± 0.052 |
| Dinner | 0.131 ± 0.081 | 0.131 ± 0.081 | 0.130 ± 0.081 | 0.131 ± 0.079 | 0.130 ± 0.079 | 0.127 ± 0.076 | 0.127 ± 0.076 | 0.125 ± 0.076 |
| Total | 0.587 ± 0.218 | 0.568 ± 0.212* | 0.568 ± 0.207* | 0.571 ± 0.204* | 0.569 ± 0.202* | 0.560 ± 0.200* | 0.571 ± 0.204 | 0.570 ± 0.202 |
| Type 2 (n = 135) | ||||||||
| Basal | 0.203 ± 0.121 | 0.195 ± 0.112* | 0.193 ± 0.109* | 0.195 ± 0.106* | 0.194 ± 0.106* | 0.196 ± 0.107 | 0.201 ± 0.124 | 0.212 ± 0.159 |
| Bolus | 0.311 ± 0.157 | 0.311 ± 0.157 | 0.307 ± 0.155* | 0.302 ± 0.151* | 0.305 ± 0.153 | 0.303 ± 0.153* | 0.310 ± 0.173 | 0.316 ± 0.202 |
| Breakfast | 0.108 ± 0.067 | 0.108 ± 0.067 | 0.106 ± 0.066 | 0.102 ± 0.068* | 0.104 ± 0.067* | 0.103 ± 0.067* | 0.105 ± 0.072 | 0.108 ± 0.082 |
| Lunch | 0.096 ± 0.055 | 0.096 ± 0.055 | 0.095 ± 0.055 | 0.095 ± 0.055 | 0.095 ± 0.054 | 0.095 ± 0.054 | 0.097 ± 0.058 | 0.097 ± 0.066 |
| Dinner | 0.107 ± 0.060 | 0.107 ± 0.060 | 0.106 ± 0.059 | 0.104 ± 0.059 | 0.105 ± 0.059 | 0.105 ± 0.059 | 0.108 ± 0.067 | 0.111 ± 0.077 |
| Total | 0.514 ± 0.224 | 0.505 ± 0.217* | 0.500 ± 0.213* | 0.498 ± 0.207* | 0.499 ± 0.211* | 0.499 ± 0.212* | 0.511 ± 0.256 | 0.528 ± 0.326 |
Data are mean ± standard deviation. *P < 0.05 vs baseline (before replacement).
There was no statistically significant difference in basal, bolus and total insulin dose during the 1‐year period between the IGlar‐ and IDet‐treated groups among type 1 diabetes patients. The basal insulin dose in the IDet group was significantly higher than that in the IGlar group at month 9 and 12 among the type 2 diabetes patients (P < 0.05; Table S1).
Regarding basal insulin dose in type 1 diabetes, the percentage change in basal insulin doses at the time of switching was −13.1 ± 11.1% in the T1D‐BID group and −3.6 ± 9.5% in the T1D‐OD group. T1D‐BID group maintained a significantly smaller basal insulin dose than that before switching during the 1‐year period (P < 0.05). In contrast, in the T1D‐OD group, the basal insulin doses at month 9 and 12 were significantly higher than that before switching (P < 0.05; Table S2).
Multiple regression analysis
As shown in Table S3, various factors were adjusted by the stepwise procedure. Multiple regression analyses for percentage change in HbA1c identified baseline HbA1c in type 1 diabetes patients, and baseline HbA1c and treated basal insulin (IGlar or IDet) in type 2 diabetes patients as independent determinants of percentage change in HbA1c (R 2 = 0.066, P = 0.013 in type 1 diabetes patients, and R 2 = 0.222, P < 0.001 in type 2 diabetes patients).
Hypoglycemia
A reduction in the frequency of total hypoglycemia and nocturnal hypoglycemia was shown in both type 1 diabetes and type 2 diabetes patients (Table 3). Severe hypoglycemia was observed before and after switching in one participant with type 1 diabetes. There was no significant difference in the frequency of severe hypoglycemia between before and after switching.
Table 3.
Change of the frequency of hypoglycemic episodes
| Type 1 (n = 93) | Type 2 (n = 135) | |||
|---|---|---|---|---|
| Before (−1 month to 0 month) | After (3 months to 6 months) | Before (−1 month to 0 month) | After (3 months to 6 months) | |
| Overall | ||||
| Participants (n) | 63 (67.7%) | 61 (65.6%) | 51 (37.8%) | 52 (38.5%) |
| Episodes | 378 | 719 | 134 | 187 |
| Rate † | 4.06 ± 5.60 | 2.58 ± 4.49* | 0.99 ± 1.97 | 0.46 ± 1.42* |
| Nocturnal | ||||
| Participants (n) | 22 (23.7%) | 19 (20.4%) | 8 (5.9%) | 4 (3.0%) |
| Episodes | 53 | 38 | 17 | 6 |
| Rate † | 0.57 ± 1.36 | 0.14 ± 0.32* | 0.13 ± 0.58 | 0.02 ± 0.10* |
| Severe | ||||
| Participants (n) | 1 (1.1%) | 1 (1.1%) | 0 (0.0%) | 0 (0.0%) |
| Episodes | 1 | 2 | 0 | 0 |
| Rate † | 0.01 ± 0.10 | 0.01 ± 0.07 | 0.00 ± 0.00 | 0.00 ± 0.00 |
Data are mean ± standard deviation or n. *P < 0.05 vs baseline. †The rate of hypoglycemic episodes per patient‐month of exposure.
There was no statistically significant difference in the frequency of total hypoglycemia and nocturnal hypoglycemia between the IGlar and IDet groups in both type 1 diabetes and type 2 diabetes patients.
Adverse events
The percentage of participants reporting AEs excluding hypoglycemia was 4.0% (n = 9). AEs excluding hypoglycemia were generally mild to moderate, and there was no withdrawal because of AEs. Acute bronchitis (0.9%, n = 2), elevation of blood pressure (0.9%, n = 2), infectious enteritis (0.4%, n = 1), lumbago (0.4%, n = 1), hyperkalemia (0.4%, n = 1), reflux esophagitis (0.4%, n = 1), symptoms of dumping syndrome (0.4%, n = 1) and first metatarsal bone fracture (0.4%, n = 1) were reported. No injection‐site reactions were reported.
Discussion
In the present study, Japanese outpatients with type 1 diabetes or type 2 diabetes whose existing long‐acting basal insulin was switched to IDeg, a new long‐acting insulin analog, were observed for 1‐year under the routine clinical practice. During the observation period, both type 1 diabetes and type 2 diabetes patients showed a significant reduction in both HbA1c levels and the frequency of hypoglycemia.
In previous phase III clinical studies comparing IDeg with IGlar in BBT, the IDeg group showed a reduction of hypoglycemia, particularly at night, while showing non‐inferiority to IGlar in its blood glucose‐lowering effect3, 4, 16. In a 2‐year follow‐up study, the basal and total insulin doses were lower in the IDeg group compared with the IGlar group17. Recently, Kobuke et al.9 reported that the switching from conventional long‐acting basal insulin to IDeg in Japanese patients with type 2 diabetes was effective in lowering HbA1c at an equal insulin dose over 24 weeks, whereas improvement of HbA1c was not shown in patients with type 1 diabetes. There were significant reductions in basal, bolus and total insulin doses at 24 weeks after switching in the type 1 diabetes patients. The percentage changes in basal, bolus and total insulin doses after 24 weeks were approximately −20.0, −7.5 and −12.3%, respectively9. In contrast, in the present study, the percentage changes in basal, bolus, and total insulin doses after 6 months in type 1 diabetes patients were −6.0, −3.7 and −4.6%, respectively. Furthermore, baseline HbA1c level in type 1 diabetes patients in the present study (8.7%) differed from that in the previous study (7.8%). Because the amount of reduced insulin dose varies according to the difference of baseline characteristics, it is possible that the disagreement on the results was caused by the differences in the background of the participants.
In the present study, the basal insulin dose was decreased by 3.6% in T1D‐OD, by 13.1% in T1D‐BID and by 2.7% in type 2 diabetes patients, when switching from current basal insulin to IDeg. After switching, the glycemic control was improved in all types of patients. In previous studies7, 9, when participants were switched to IDeg, the basal insulin dose was adjusted, and dose reduction was greater in these reports than in the present study. Such greater dose reduction might be one of the reasons for unimproved glycemic control for participants that switched to IDeg in the previous studies7, 9. Therefore, our finding suggested that smaller basal insulin dose reduction might provide better glycemic control when patients with difficult glycemic control using IGlar or IDet were transferred to IDeg.
This is the first report showing that baseline HbA1c was a significant and an independent determinant of percentage change in HbA1c when switching from IGlar or IDet to IDeg in both type 1 diabetes and type 2 diabetes patients. In the present study, the multiple regression analysis showed greater decreases in HbA1c with higher baseline HbA1c. Although there was no statistically significant difference in baseline characteristics and insulin dose until 6 months after switching between the IGlar and IDet groups, it also showed greater decreases in percentage change in HbA1c with the IGlar group than that with the IDet group among type 2 diabetes patients. However, the precise reason remains unknown, and further study is required to evaluate this issue.
Phase III clinical studies showed that the frequency of nocturnal hypoglycemia was significantly lower in type 1 diabetes or type 2 diabetes patients treated with IDeg than those with IGlar3, 4, 16, 18. In the present study, we did observe a significant reduction in the rates of total and nocturnal hypoglycemia after switching in both type 1 diabetes and type 2 diabetes patients, despite decreasing HbA1c levels. Lower HbA1c levels of insulin treatment would normally be expected to be accompanied by a higher rate of hypoglycemia, but IDeg reduced the rate of hypoglycemia with decreasing HbA1c levels. This could most likely be attributed to the stable and consistent profile of IDeg, with its long duration of action and lower day‐to‐day pharmacodynamic variability compared with other conventional basal insulin2, 19, 20. The molecular design and mechanism of protracting the pharmacodynamics of IDeg differ from the existing basal insulin analogs. Therefore, it is critically important to understand how the properties of insulin degludec might influence its clinical use.
The present study had a few limitations. First, there was the small sample size, single‐arm and non‐controlled observational study design in the study. Second, we did not evaluate insulin secretion status and the daily glucose profile. Third, there was a low percentage of participants reporting AEs excluding hypoglycemia in the present study, that might mean the participants had a limited understanding of their AEs with their treatment. Furthermore, there is the possibility that the absence of SMBG in some participants affected the detection rate of hypoglycemia, because we did not ask all the participants to carry out SMBG in our study.
In conclusion, switching from IGlar or IDet to IDeg significantly improved glycemic control with fewer incidents of hypoglycemia in both type 1 diabetes and type 2 diabetes patients inadequately controlled with BBT. As a novel basal insulin analog, IDeg might provide a benefit for diabetic patients who require BBT in clinical practice.
Disclosure
No funding or sponsorship was received for this study or publication of this article. SS has received lecture fees from Daiichi Sankyo Inc and MSD K.K. EA has received lecture fees from Astellas Pharma Inc, Astra Zeneca PLC, MSD K.K., Ono Pharmaceutical Co. Ltd., Kowa, Kowa Pharmaceutical Co. Ltd., Sanofi‐Aventis, Takeda Pharmaceutical Company, Mitsubishi Tanabe Pharma, Eli Lilly Japan, Novo Nordisk Pharma and Taisho Toyama Pharmaceutical, and research funding from Astellas Pharma Inc, Astra Zeneca PLC, MSD K.K., Ono Pharmaceutical Co. Ltd., Kyowa Hakko Kirin Co. Ltd., Sanofi‐Aventis, Shionogi Inc, Takeda Pharmaceutical Company, Daiichi Sankyo Inc, Mitsubishi Tanabe Pharma, Novo Nordisk Pharma and Pfizer Inc. The other authors declare no conflict of interest.
Supporting information
Table S1 ¦ Mean changes of the daily insulin requirement profiles (unit/kg/day) in the insulin glargine and insulin detemir groups
Table S2 ¦ Mean changes of the daily insulin requirement profiles (unit/kg/day) in once‐daily and twice‐daily groups among type 1 diabetes patients
Table S3 ¦ Stepwise multiple regression analysis to identify factors associated with percentage change in glycated hemoglobin
Acknowledgments
In addition to the authors, The KIDUNA Study Group are: Takeshi Nishikawa MD PhD, Noboru Furukawa MD PhD, Takeshi Matsumura MD PhD, Tatsuya Kondo MD PhD, Junji Kawashima MD PhD, Takafumi Senokuchi MD PhD, Motoyuki Igata MD PhD (Kumamoto University Hospital), Kenro Nishida MD PhD (Minamata City Hospital and Medical Center), Tomohiko Yano MD PhD, Tetsuya Taguchi MD PhD (Kikuchi Medical Association Hospital), Hirofumi Matsuda MD PhD (Tamana Central Hospital), Kaku Tsuruzoe MD PhD (Amakusa City Sumoto Hospital), Yoshiaki Hirashima MD PhD (Amakusa Medical Center), Kengo Kaneko MD PhD (Kumamoto Rosai Hospital), Sayaka Kitanao MD (Arao Municipal Hospital), Iwaho Hazekawa MD PhD (Kumamoto City Hospital), Kenji Ebihara MD PhD (National Hospital Organization Saisyunso National Hospital), Yusuke Murata MD PhD (Yamaga Medical Center), Eiichiro Watanabe MD (Kumamoto Chuo Hospital), Norio Ishii MD PhD (Mimori Hospital), Hiroko Nishioka MD, Kaori Horio MD, Masatsugu Furusho MD, Michiko Ikema MD, Kae Otsu MD, Tomomi Yano MD, Tomohiro Shirao MD (Sugimura Hospital), Rina Matsuyama MD PhD (Konan Hosiptal), Toshihiko Nishiyama MD PhD (Sakuradori Clinic), Akiko Matsuyoshi MD (Matsubase Otolaryngology and Internal Medicine Clinic), Haruo Takeda MD PhD (Uki General Hospital), Masaya Kasho MD PhD (Asahino Hospital), Etsuro Tsutsumi MD PhD and Kaoru Ono MD (Tsutsumi Hospital).
J Diabetes Investig 2016; 7: 703–710
Clinical Trial Registry
UMIN‐CTR
UMIN000021569
References
- 1. Jonassen I, Havelund S, Hoeg‐Jensen T, et al Design of the novel protraction mechanism of insulin degludec, an ultra‐long‐acting basal insulin. Pharm Res 2012; 29: 2104–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Heise T, Nosek L, Bøttcher SG, et al Ultra‐long‐acting insulin degludec has a flat and stable glucose‐lowering effect in type 2 diabetes. Diabetes Obes Metab 2012; 14: 944–950. [DOI] [PubMed] [Google Scholar]
- 3. Heller S, Buse J, Fisher M, et al Insulin degludec, an ultralongacting basal insulin, versus insulin glargine in basal bolus treatment with mealtime insulin aspart in type 1 diabetes (BEGIN Basal‐Bolus Type 1): a phase 3, randomised, open‐label, treat‐to‐target non‐inferiority trial. Lancet 2012; 379: 1489–1497. [DOI] [PubMed] [Google Scholar]
- 4. Garber AJ, King AB, Del Prato S, et al Insulin degludec, an ultra‐long acting basal insulin, versus insulin glargine in basal‐bolus treatment with mealtime insulin aspart in type 2 diabetes (BEGIN Basal‐Bolus Type 2): a phase 3, randomised, open‐label, treat‐to‐target non‐inferiority trial. Lancet 2012; 379: 1498–1507. [DOI] [PubMed] [Google Scholar]
- 5. Yamada K, Nakayama H, Sato S, et al A randomized crossover study of the efficacy and safety of switching from insulin glargine to insulin degludec among patients with type 1 diabetes. Diabetol Int 2014; 5: 74–77. [Google Scholar]
- 6. Kusunoki Y, Katsuno T, Miyakoshi K, et al Effects of switching from insulin glargine or detemir to insulin degludec in patients with type 1 diabetes mellitus. Diabetes Ther 2013; 4: 461–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nakae R, Kusunoki Y, Katsuno T, et al Medium‐term effects of insulin degludec on patients with type 1 diabetes mellitus. Drugs R D 2014; 14: 133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Komuro M, Inoue G, Tabata M, et al Insulin degludec requires lower bolus insulin doses than does insulin glargine in Japanese diabetic patients with insulin‐dependent state. J Diabetes Sci Technol 2015; 9: 632–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kobuke K, Yoneda M, Nakanishi S, et al Efficacy and safety of insulin degludec in Japanese patients with type 1 and type 2 diabetes: 24‐week results from the observational study in routine clinical practice. J Diabetes Investig 2016; 7: 94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kuzuya T, Nakagawa S, Satoh J, et al Report of the Committee on the classification and diagnostic criteria of diabetes mellitus. Diabetes Res Clin Pract 2002; 55: 65–85. [DOI] [PubMed] [Google Scholar]
- 11. Seino Y, Nanjo K, Tajima N, et al Report of the Committee on the Classification and Diagnostic Criteria of Diabetes Mellitus. J Diabetes Investig 2010; 1: 212–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Editorial committee for the treatment guide for diabetes . Treatment and diet therapy In: Japan Diabetes Society (ed.). Treatment Guide for Diabetes. Tokyo: Bunkodo, 2007; 21–37. [Google Scholar]
- 13. Kashiwagi A, Kasuga M, Araki E, et al Committee on the Standardization of Diabetes Mellitus‐Related Laboratory Testing of the Japan Diabetes Society International clinical harmonization of glycated hemoglobin in Japan: From Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Investig 2012; 3: 39–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Workgroup on Hypoglycemia, American Diabetes Association . Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care 2005; 28: 1245–1249. [DOI] [PubMed] [Google Scholar]
- 15. Ratner RE, Gough SC, Mathieu C, et al Hypoglycaemia risk with insulin degludec compared with insulin glargine in type 2 and type 1 diabetes: a pre‐planned meta‐analysis of phase 3 trials. Diabetes Obes Metab 2013; 15: 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mathieu C, Hollander P, Miranda‐Palma B, et al Efficacy and safety of insulin degludec in a flexible dosing regimen vs insulin glargine in patients with type 1 diabetes (BEGIN: Flex T1): a 26‐week randomized, treat‐to‐target trial with a 26‐week extension. J Clin Endocrinol Metab 2013; 98: 1154–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bode BW, Buse JB, Fisher M, et al Insulin degludec improves glycaemic control with lower nocturnal hypoglycaemia risk than insulin glargine in basal‐bolus treatment with mealtime insulin aspart in Type 1 diabetes (BEGIN Basal‐Bolus Type 1): 2‐year results of a randomized clinical trial. Diabet Med 2013; 30: 1293–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zinman B, Philis‐Tsimikas A, Cariou B, et al Insulin degludec versus insulin glargine in insulin‐naive patients with type 2 diabetes: a 1‐year, randomized, treat‐to‐target trial (BEGIN Once Long). Diabetes Care 2012; 35: 2464–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heise T, Hermanski L, Nosek L, et al Insulin degludec: four times lower pharmacodynamic variability than insulin glargine under steady‐state conditions in type 1 diabetes. Diabetes Obes Metab 2012; 14: 859–864. [DOI] [PubMed] [Google Scholar]
- 20. Nakamura T, Sakaguchi K, So A, et al Effects of insulin degludec and insulin glargine on day‐to‐day fasting plasma glucose variability in individuals with type 1 diabetes: a multicentre, randomised, crossover study. Diabetologia 2015; 58: 2013–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 ¦ Mean changes of the daily insulin requirement profiles (unit/kg/day) in the insulin glargine and insulin detemir groups
Table S2 ¦ Mean changes of the daily insulin requirement profiles (unit/kg/day) in once‐daily and twice‐daily groups among type 1 diabetes patients
Table S3 ¦ Stepwise multiple regression analysis to identify factors associated with percentage change in glycated hemoglobin


