Abstract
Introduction
The results of a clinical trial to evaluate the efficacy and safety of initial combination therapy with sitagliptin and metformin in Chinese patients with type 2 diabetes and inadequate glycemic control are reported here.
Materials and Methods
This was a multicenter, randomized, double‐blind, placebo‐controlled, parallel group, 24‐week clinical trial carried out in China. Patients (n = 744) with type 2 diabetes and inadequate glycemic control (glycated hemoglobin ≥7.5 and ≤11.0%) who were either drug‐naïve or washed out of previous therapy were randomized in equal ratios to sitagliptin 100 mg once daily (q.d.; S100), metformin 500 mg twice daily (b.i.d.; M1000), metformin 850 mg b.i.d. (M1700), sitagliptin 50 mg b.i.d. plus metformin 500 mg b.i.d. (S100/M1000), sitagliptin 50 mg b.i.d. plus metformin 850 mg b.i.d. (S100/M1700), or placebo.
Results
The mean baseline glycated hemoglobin in randomized patients was 8.7%. Least squares mean changes from baseline in glycated hemoglobin were −0.59% (placebo), −0.99% (S100), −1.29% (M1000), −1.56% (M1700), −1.67% (S100/M1000) and −1.83% (S100/M1700) (P < 0.05 for each active group vs placebo, for S100/M1700 and S100/M1000 vs S100, and for S100/M1000 vs M1000). All treatments were generally well‐tolerated. The overall incidence of hypoglycemia (symptomatic or asymptomatic) was higher in the two co‐administration groups (S100/M1700 and S100/M1000) compared with the placebo. The incidence of symptomatic hypoglycemia was low, and similar, across all treatment groups. The incidences of gastrointestinal adverse events were generally higher in high‐dose metformin groups than in the placebo group.
Conclusions
In Chinese patients with type 2 diabetes, initial combination therapy with sitagliptin and metformin was generally well‐tolerated, and provided improvement in glycemic control.
Keywords: Dipeptidyl peptidase‐4 inhibitor, Glycemic control, Incretin therapy
Introduction
It is estimated that by 2030, approximately 130 million people in China will have type 2 diabetes1; consequently, the number of individuals with diabetes‐related complications will create an increasing economic burden2. Rigorous glycemic control can help minimize the risk of complications, but initial treatment with a single antihyperglycemic agent (AHA) frequently does not achieve treatment goals3, necessitating consideration of initial combination therapy.
In patients for whom combination therapy is appropriate, use of AHAs with complementary mechanisms of action should result in augmented improvement in glycemic control. Two such agents are sitagliptin and metformin. Sitagliptin, an orally active, highly selective dipeptidyl peptidase‐4 (DPP‐4) inhibitor, increases the plasma concentrations of active forms of the incretins, glucagon‐like peptide‐1 (GLP‐1) and glucose‐dependent insulinotropic peptide, which increase insulin levels in a glucose‐dependent manner4; active GLP‐1 also reduces hepatic glucose output by lowering glucagon levels4, 5. Sitagliptin has been shown to be generally well‐tolerated and efficacious in a large number of clinical trials6. Metformin, a generally well‐tolerated, orally administered biguanide, reduces blood glucose by decreasing hepatic glucose output, insulin resistance and gastrointestinal absorption of glucose7. In a previous global clinical trial, 24 weeks of treatment with the initial combination of sitagliptin and metformin was found to be generally well‐tolerated and efficacious8.
The pathophysiological mechanisms that lead to type 2 diabetes in Asians (e.g., insulin resistance, β‐cell failure and overproduction of hepatic glucose) are similar to those observed in other racial and ethnic groups; however, although Asian patients have higher amounts of total and visceral fat for any given body mass index compared with Caucasians9, 10, there is conflicting information on the relative degree of insulin resistance in Asians. Chiu et al.11 reported that Asian Americans show more insulin resistance even at lower body mass index levels compared with other ethnicities, whereas Jensen et al.12 reported that high‐risk Asian Americans might be less insulin resistant than other racial and ethnic groups. Some studies suggest an important role for β‐cell dysfunction in the pathogenesis of type 2 diabetes in Asian patients13, 14, whereas at least one study suggests heterogeneity in both insulin sensitivity and β‐cell function among Asian populations15. With regard to treatment of type 2 diabetes in Asian patients, a meta‐analysis of studies predominantly carried out in Japan suggests that Asians might be more sensitive than non‐Asians to the glucose‐lowering effects of DPP‐4 inhibitors16, suggesting that Chinese patients might show greater improvement in glycemic control when treated with these agents compared with non‐Asian populations.
Sitagliptin and metformin are approved in China for the treatment of type 2 diabetes. The present study tested the hypothesis that initial combination therapy with sitagliptin and metformin will result in better glycemic control than component monotherapy in Chinese patients with type 2 diabetes being treated in China.
Methods
Study design
This was a multicenter, randomized, double‐blind, factorial, parallel‐group study carried out in China in patients with type 2 diabetes who had inadequate glycemic control with diet and exercise alone (glycated hemoglobin [HbA1c] ≥7.5 and ≤11.0%), or while on a single oral AHA other than a thiazolidinedione (HbA1c ≥7.0 and ≤10.5%) or on low dose combination AHA (i.e., ≤50% maximum labeled dose of each agent) with HbA1c ≥7.0 and ≤10.0% (Figure 1). Study duration was up to 35 weeks, including an 8‐week washout period for patients on an AHA at screening. Patients were male or female, and aged >18 and ≤78 years. Patients were excluded if they had type 1 diabetes, a history of ketoacidosis, active liver disease, significant and active cardiovascular disease or hematological disorders, had been treated with a DPP‐4 inhibitor or a GLP‐1 receptor agonist, or had been treated with a thiazolidinedione or insulin within 12 weeks before screening. Patients with serum creatinine ≥1.4 mg/dL (men) or ≥1.3 mg/dL (women), estimated glomerular filtration rate <60 mL/min/1.73 m2 (calculated using the Modification of Diet in Renal Disease equation17), alanine aminotransferase or aspartate aminotransferase more than twofold the upper limit of normal, hemoglobin <11 g/dL (men) or <10 g/dL (women), triglycerides >600 mg/dL or thyroid‐stimulating hormone outside the normal range were also excluded from the study.
Figure 1.
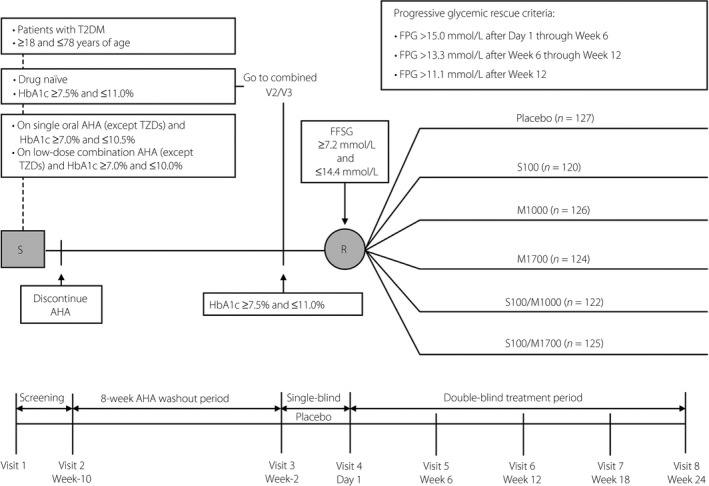
Study design. AHA, antihyperglycemic agent; FFSG, fasting finger‐stick glucose; FPG, fasting plasma glucose; M1000, metformin 500 mg b.i.d.; M1700, metformin 850 mg b.i.d.; R, randomization; S, screening; S100, sitagliptin 100 mg q.d.; S100/M1000, sitagliptin/metformin 50/500 mg b.i.d.; S100/M1700, sitagliptin/metformin 50/850 mg b.i.d.; T2DM, type 2 diabetes mellitus; TZDs, thiazolidinediones.
Patients who were not taking an AHA for ≥8 weeks, with HbA1c ≥7.5 and ≤11.0%, and who met all other enrolment criteria could enter a 2‐week single‐blind placebo run‐in. Patients receiving single or low‐dose combination AHA therapy discontinued their AHA(s) and entered an 8‐week washout period before beginning the placebo run‐in.
At visit 2/week 10, patients were counseled to adhere to a diet consistent with the American Diabetes Association and other standard diabetes guidelines, containing approximately 45–65% carbohydrate, 10–35% protein and 20–35% fat, for the duration of the study. At each subsequent visit, patients' diets were monitored and reinforced by counseling to enhance adherence to the recommended diet. Patients were counseled to limit alcohol use to moderate amounts and to maintain a medically appropriate, routine exercise program; consistency in physical activity levels was encouraged throughout the study.
Patients meeting all study enrolment criteria, including a fasting finger‐stick glucose ≥7.2 and ≤14.4 mmol/L at the time of randomization, could enter the 24‐week double‐blind treatment period. Patient randomization was stratified based on AHA status at the screening visit (on AHA vs not on AHA). Patients were randomly allocated, according to a computer‐generated schedule created by the sponsor, in a 1:1:1:1:1:1 ratio to sitagliptin 100 mg once daily (q.d.; S100), metformin 500 mg twice daily (b.i.d.; M1000), metformin 850 mg b.i.d. (M1700), sitagliptin 50 mg b.i.d. plus metformin 500 mg b.i.d. (S100/M1000), sitagliptin 50 mg b.i.d. plus metformin 850 mg b.i.d. (S100/M1700), or placebo. Sitagliptin, metformin and matching placebos were supplied to patients as oral tablets in a blinded manner under in‐house blinding procedures. To improve tolerability, doses of metformin were uptitrated over a period of up to 2 weeks from 500 and 850 mg q.d. to 500 and 850 mg b.i.d.
Patients not meeting specific glycemic thresholds (fasting plasma glucose [FPG] >15.0 mmol/L after day 1 through week 6; FPG >13.3 mmol/L after week 6 through week 12; FPG >11.1 mmol/L after week 12) initiated rescue therapy with open‐label glipizide 5 mg/day, which could be uptitrated to 20 mg/day at the discretion of the investigator.
The study (Merck & Co., Inc. Protocol 121; ClinicalTrials.gov: NCT01076088) was carried out in accordance with the principles of Good Clinical Practice, and was approved by the appropriate institutional review boards and regulatory agencies. Written informed consent was obtained from all patients before undergoing any study procedure.
Study objectives
The primary objectives of the present study were to assess the effects on HbA1c of initial co‐administration of sitagliptin and metformin compared with initial treatment with component monotherapy, and to assess the safety and tolerability of initial co‐administration of sitagliptin and metformin compared with placebo.
The primary hypothesis of the present study was that after 24 weeks, initial co‐administration with sitagliptin and metformin provides greater reduction in HbA1c compared with initial treatment with each component monotherapy.
Secondary objectives were assessment of the effects of initial co‐administration of sitagliptin and metformin compared with initial treatment with each component monotherapy on 2‐h post‐meal glucose after a meal tolerance test, and on FPG. Additional efficacy end‐points were assessment of the percentages of patients who meet HbA1c goals (<7.0 and <6.5%) after 24 weeks.
The meal tolerance test (consisting of approximately 460 kcal, with 75 g carbohydrate, 9 g fat and 18 g protein) was carried out at randomization (study day 1), before initiation of study treatment, and at week 24, 30 min after the morning dose of study treatment. Patients were on their usual diet for the 72 h preceding the test and reported to the clinic fasting (no food or drink except water for at least 12 h), without taking their morning double‐blind study medication. A blood sample was collected 120 min after the start of a meal. Efficacy end‐points were measured at the central laboratory facilities of Covance, Shanghai, China.
Safety and tolerability were assessed through physical examination, vital signs, standard laboratory evaluations (including blood chemistry, hematology and urinalysis), and the collection and analysis of adverse experiences (AEs). All AEs were rated by the study investigators for intensity and relationship to the study drug. Prespecified safety end‐points of special interest were AEs of hypoglycemia (asymptomatic and symptomatic), selected gastrointestinal (GI) AEs (nausea, vomiting, diarrhea and abdominal pain) and change from baseline in body weight at week 24.
Any episode with symptoms consistent with hypoglycemia (e.g., weakness, dizziness, shakiness, increased sweating, palpitations or confusion) was reported as an episode of symptomatic hypoglycemia without a requirement for confirmatory blood glucose values. Asymptomatic hypoglycemia was defined as an episode without symptoms of hypoglycemia, but with finger‐stick glucose level ≤3.9 mmol/L (≤70 mg/dL). Severe hypoglycemia was defined as any episode requiring assistance, either medical or non‐medical. Episodes with a markedly depressed level of consciousness, loss of consciousness or seizure were to be classified as having required medical assistance, whether or not medical assistance was obtained.
Statistical analysis
The primary population for efficacy analyses was the full analysis set (FAS), which included all randomized patients who received at least one dose of study treatment and had a measurement of the analysis end‐point at baseline, as well as at one or more post‐baseline time‐points. The prespecified methodology for addressing the primary hypothesis was analysis of covariance (ANCOVA), with the change from baseline in HbA1c at week 24 as the outcome variable, and terms for treatment, prior antihyperglycemic therapy status (yes/no) and baseline HbA1c (continuous). Superiority of co‐administration of sitagliptin and metformin compared with each component monotherapy in decreasing HbA1c at a given metformin dose level was assessed using the appropriate contrasts under the ANCOVA model. The following testing procedure was planned to control the type 1 error rate at 0.05 (two‐sided) for the end‐point of HbA1c. The primary hypothesis test for a given co‐administration group was to be declared successful only if superiority was shown (P < 0.05) vs each of its component monotherapies. The hypothesis test was to be carried out for the low‐dose co‐administration group only if success was first achieved at the high dose. P‐values were to be provided for efficacy end‐points that were not part of the type 1 error control strategy as a measure of strength of association rather than an indication of statistical significance.
Because of the presence of an extreme, biologically implausible HbA1c value for one patient that was discovered after the database was locked, and that resulted in a non‐normal distribution of the HbA1c data (described in the Results section), the validity of the prespecified ANCOVA methodology was questionable. Several post‐hoc analyses were carried out to supplement the prespecified analysis in addressing the primary hypothesis. The first post‐hoc analysis used the primary analysis methodology (ANCOVA) in the FAS excluding the patient with the biologically implausible HbA1c value. The second post‐hoc analysis was a robust regression approach using Huber's bounded influence function18. The third post‐hoc analysis was a non‐parametric analysis in which HbA1c change from baseline data were sorted and assigned their corresponding ranks. The primary ANCOVA model was then used, substituting the ranks for the changes from baseline as the outcome variable. The second and third post‐hoc approaches, which are valid for non‐normally distributed data, included the entire FAS.
Continuous secondary efficacy end‐points were analyzed using the prespecified ANCOVA method described for HbA1c, substituting the relevant baseline efficacy measurement for HbA1c.
Missing values at week 24 were imputed from the last observed post‐baseline measurement. To avoid the confounding influence of rescue therapy, efficacy analyses treated data obtained after the initiation of rescue therapy as missing.
Analyses of the proportion of patients at HbA1c goals of <7.0 and <6.5% at week 24 were carried out using a logistic regression model with the same terms as the ANCOVA model used for the analyses of continuous end‐points.
Analyses of AEs included all randomized patients who received at least one dose of study medication. Statistical testing of between‐group differences (active treatment groups vs placebo) was prespecified for AEs of special interest (all hypoglycemia, symptomatic hypoglycemia, severe hypoglycemia, and GI AEs of diarrhea, nausea, abdominal pain and vomiting). The Miettinen and Nurminen method19 was used for between‐group comparisons.
For body weight, change from baseline was analyzed using the ANCOVA method described above, substituting baseline body weight for HbA1c. Patients without a week 24 body weight measurement were excluded from this analysis.
The primary approach to analyzing safety data treated data obtained after the initiation of rescue therapy as missing; a secondary approach included all data, regardless of rescue therapy.
The study was powered assuming a total enrolment of 720 patients, yielding 120 patients randomized to each of the six treatment arms, and 103 patients per group available for the analysis for the primary hypothesis at week 24. Using a standard deviation of 1.1%, this sample size provided 90% power to detect a true difference of 0.5% in the mean change from baseline in HbA1c between any two individual treatment groups (α = 0.05, two‐sided).
Results
A total of 1,441 patients were screened, and 744 patients were randomized to double‐blind treatment. The most common reason screened patients were not randomized was screen failure (85.1%). The most common reason for screen failure was that the patient did not meet HbA1c entry criteria (77.7%). There were 120–127 patients in each of the six treatment arms of the study (Table S1), distributed across 23 treatment sites in China. A majority of patients were drug‐naïve in all treatment groups (between 87.9 and 91.3%; Table 1). The first dose of study medication was taken on 29 November 2010 and the last dose was taken on 24 December 2012. The study was completed by 86.6% of randomized patients (Table S1).
Table 1.
Baseline demographic and anthropometric characteristics
| Parameter | Placebo n = 127 | S100 n = 120 | M1000 n = 126 | M1700 n = 124 | S100/M1000 n = 122 | S100/M1700 n = 125 |
|---|---|---|---|---|---|---|
| Age (years) | 53.6 ± 9.7 | 51.7 ± 10.2 | 52.6 ± 9.5 | 53.0 ± 10.3 | 52.6 ± 11.3 | 52.4 ± 9.3 |
| Sex (male) | 87 (68.5) | 74 (61.7) | 69 (54.8) | 75 (60.5) | 85 (69.7) | 67 (53.6) |
| Race (Asian) | 127 (100) | 120 (100) | 126 (100) | 124 (100) | 122 (100) | 125 (100) |
| Body weight (kg) | 70.8 ± 12.5 | 71.8 ± 12.1 | 71.1 ± 13.7 | 71.1 ± 11.8 | 72.4 ± 12.1 | 69.4 ± 10.8 |
| Body mass index (kg/m2) | 25.4 ± 3.4 | 26.0 ± 3.5 | 26.0 ± 3.7 | 25.8 ± 3.5 | 26.1 ± 3.4 | 25.4 ± 3.1 |
| Duration of type 2 diabetes, years | 1.1 ± 0.2 | 1.1 ± 0.2 | 1.0 ± 0.2 | 1.1 ± 0.2 | 1.1 ± 0.3 | 1.1 ± 0.3 |
| Patients who were drug‐naïve† | 116 (91.3) | 108 (90.0) | 115 (91.3) | 109 (87.9) | 108 (88.5) | 111 (88.8) |
Data are expressed as mean or mean ± standard deviation or frequency, n (%). †The remaining patients were washed out of the antihyperglycemic medication they were on at screening.
Demographic and anthropometric traits and baseline disease characteristics in each treatment group were balanced among the treatment groups (Table 1).
Efficacy
HbA1c
At week 24, despite a substantial decrease in HbA1c observed in the placebo group (least squares (LS) mean change from baseline = −0.59%), all active treatments provided robust reductions from baseline in HbA1c that were clinically meaningful compared with the placebo (Table 2). The LS mean reduction from baseline in the S100/M1700 group was superior compared with the S100 group (Table 2; P < 0.001), but not compared with the M1700 group (Table 2; between‐group difference of −0.27, P = 0.087) using the prespecified primary analysis. Treatment‐associated changes in HbA1c were apparent by the first post‐dose measurement at week 6, and were near maximal by week 18 (Figure S1). These analyses included a patient in the S100/M1700 group whose final post‐baseline HbA1c value taken on study day 90, 13.2%, was 4.9% higher than baseline and 5.2% higher than the value obtained at a visit on study day 45. Because the patient's changes in FPG and absence of body weight loss were not consistent with a large increase in HbA1c, and no AE reports suggested symptoms of hyperglycemia, this value was considered biologically implausible. In an ANCOVA analysis excluding this patient from the FAS, the LS mean reduction from baseline in HbA1c at week 24 in the S100/M1700 group was superior to that of each of the S100 and the M1700 groups (P < 0.05 for both). In addition, in a robust regression analysis including the participant with the implausible HbA1c (Table 2), all active treatments provided clinically meaningful reductions from baseline in HbA1c compared with the placebo and compared with the component monotherapies (including a 0.36% reduction in HbA1c from baseline in the S100/M1700 group compared with the M1700 group [P = 0.008]). The sensitivity analysis using non‐parametric methods showed results similar to the other sensitivity analyses (P < 0.05 for all comparisons). At week 24, when analyzed by ANCOVA, robust regression or non‐parametric analysis, the S100/M1000 group showed a greater reduction from baseline in HbA1c compared with its component monotherapies (P < 0.05 in all cases).
Table 2.
Efficacy end‐points
| Parameter | Placebo | S100 | M1000 | M1700 | S100/M1000 | S100/M1700 |
|---|---|---|---|---|---|---|
| HbA1c, % (n) | 117 | 113 | 116 | 117 | 118 | 114 |
| Baseline | 9.0 ± 1.1 | 8.7 ± 1.1 | 8.7 ± 1.0 | 8.7 ± 1.1 | 8.5 ± 1.0 | 8.6 ± 0.9 |
| Week 24 | 8.1 ± 1.8 | 7.6 ± 1.5 | 7.3 ± 1.1 | 7.0 ± 1.2 | 6.8 ± 1.0 | 6.7 ± 1.3 |
| ANCOVA analysis change from baseline† | −0.59 (−0.84, −0.34) | −0.99 (−1.24, −0.75) | −1.29 (−1.54, −1.04) | −1.56 (−1.80, −1.32) | −1.67 (−1.92, −1.43) | −1.83 (−2.07, −1.58) |
| Difference from placebo† | – |
−0.40 (−0.71, −0.09) P = 0.011 |
−0.70 (−1.01, −0.39) P < 0.001 |
−0.97 (−1.28, −0.66) P < 0.001 |
−1.08 (−1.39, −0.78) P < 0.001 |
−1.24 (−1.55, −0.93) P < 0.001 |
| Difference from sitagliptin‡ | – | – | – | – |
−0.68 (−0.99, −0.37) P < 0.001 |
−0.84 (−1.15, −0.52) P < 0.001 |
| Difference from component metformin‡ | – | – | – | – |
−0.39 (−0.69, −0.08) P = 0.014 |
−0.27 (−0.58, 0.04) P = 0.087 |
| Robust analysis change from baseline† | −0.71 (−0.92, −0.50) | −1.10 (−1.31, −0.88) | −1.35 (−1.56, −1.14) | −1.61 (−1.81, −1.40) | −1.71 (−1.92, −1.50) | −1.96 (−2.17, −1.75) |
| Difference from placebo§ | – |
−0.39 (−0.65, −0.12) P = 0.004 |
−0.64 (−0.90, −0.37) P < 0.001 |
−0.90 (−1.16, −0.63) P < 0.001 |
−1.00 (−1.26, −0.74) P < 0.001 |
−1.25 (−1.52, −0.99) P < 0.001 |
| Difference from sitagliptin§ | – | – | – | – |
−0.61 (−0.88, 0.35) P < 0.001 |
−0.87 (−1.13, −0.60) P < 0.001 |
| Difference from component metformin§ | – | – | – | – |
−0.36 (−0.63, −0.10) P = 0.007 |
−0.36 (−0.62, −0.09) P = 0.008 |
| 2‐h post‐meal glucose, mmol/L (n) | 107 | 107 | 112 | 108 | 110 | 110 |
| Baseline | 17.0 (4.7) | 16.4 (3.9) | 16.6 (4.2) | 16.5 (4.0) | 16.3 (4.6) | 16.3 (3.8) |
| Week 24 | 15.3 (4.7) | 13.6 (4.0) | 12.7 (3.7) | 11.3 (3.2) | 10.8 (3.1) | 10.1 (3.5) |
| ANCOVA analysis change from baseline† | −1.21 (−1.91, −0.52) | −2.67 (−3.35, −1.99) | −3.64 (−4.32, −2.97) | −5.05 (−5.72, −4.37) | −5.39 (−6.05, −4.72) | −6.07 (−6.74, −5.41) |
| Difference from placebo‡ | – |
−1.46 (−2.31, −0.60) P < 0.001 |
−2.43 (−3.27, −1.59) P < 0.001 |
−3.83 (−4.68, −2.98) P < 0.001 |
−4.17 (−5.02, −3.32) P < 0.001 |
−4.86 (−5.71, −4.01) P < 0.001 |
| Difference from sitagliptin‡ | – | – | – | – |
−2.72 (−3.56, −1.87) P < 0.001 |
−3.40 (−4.25, −2.56) P < 0.001 |
| Difference from component metformin‡ | – | – | – | – |
−1.74 (−2.58, −0.90) P < 0.001 |
−1.03 (−1.87, −0.18) P = 0.017 |
| Fasting plasma glucose (mmol/L), n | 119 | 114 | 119 | 119 | 118 | 116 |
| Baseline | 10.3 (2.6) | 10.0 (2.3) | 10.2 (2.6) | 10.1 (2.6) | 9.8 (2.3) | 10.1 (2.4) |
| Week 24 | 9.5 (3.1) | 8.8 (2.7) | 8.3 (2.1) | 7.9 (2.0) | 7.8 (1.8) | 7.4 (2.1) |
| ANCOVA analysis change from baseline† | −0.66 (−1.05, −0.27) | −1.21 (−1.60, −0.82) | −1.87 (−2.26, −1.48) | −2.20 (−2.58, −1.82) | −2.19 (−2.57, −1.80) | −2.65 (−3.04, −2.26) |
| Difference from placebo‡ | – |
−0.55 (−1.04, −0.06) P = 0.027 |
−1.21 (−1.69, −0.72) P < 0.001 |
−1.54 (−2.02, −1.05) P < 0.001 |
−1.52 (−2.01, −1.04) P < 0.001 |
−1.99 (−2.47, −1.50) P < 0.001 |
| Difference from sitagliptin‡ | – | – | – | – |
−0.97 (−1.46, −0.48) P < 0.001 |
−1.44 (−1.93, −0.95) P < 0.001 |
| Difference from component metformin‡ | – | – | – | – |
−0.32 (−0.80, 0.17) P = 0.198 |
−0.45 (−0.94, 0.04) P = 0.069 |
Values are mean ± standard deviation unless noted. To convert mmol/L to mg/dL, multiply by 18. †Least squares mean (95% CI). ‡Difference in least squares mean changes from baseline. §Estimates of between‐treatment differences in change from baseline and P‐values are based on Huber's robust procedure. ANCOVA, analysis of covariance.
Participants meeting glycemic goals (HbA1c <7 and <6.5%)
At week 24, the percentages of patients with HbA1c <7.0% and with HbA1c <6.5% were highest in the S100/M1700 group, followed by the S100/M1000 group, and lowest in the placebo group (Figure 2). The percentages were higher in both co‐administration groups compared with the S100 group (P < 0.001), and compared with the corresponding metformin dose groups (<7%, P = 0.007 for both; <6.5%, P = 0.005 for M1000 and P = 0.002 for M1700).
Figure 2.
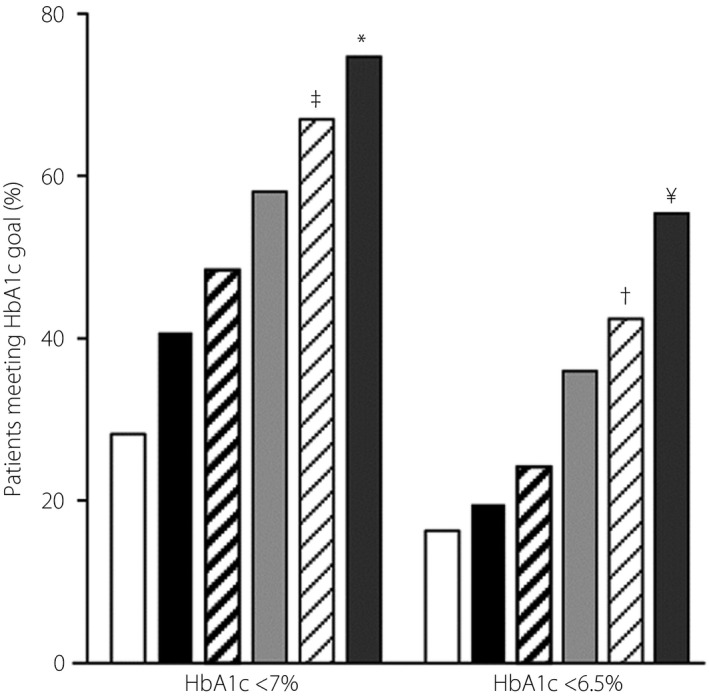
Percentage of patients with glycated hemoglobin (HbA1c) <7.0 and <6.5% at Week 24 or the time of discontinuation. ‡vs S100 P < 0.001; vs M1000 P = 0.007; *vs S100 P = 0.001; vs M1700 P = 0.007; †vs S100 P < 0.001; vs M1000 P = 0.005; ¥vs S100 P < 0.001; vs M1700 P = 0.002. Placebo ( ); S100 (
); S100 ( ); M1000 (
); M1000 ( ); M1700 (
); M1700 ( ); S100/M1000 (
); S100/M1000 ( ); S100/M1700 (
); S100/M1700 ( ).
).
Post‐meal glucose
At week 24, reductions from baseline in 2‐h post‐meal glucose were observed in all active treatment groups compared with the placebo (Table 2), with a greater reduction in both combination groups compared with the S100 group (P < 0.001) and compared with the corresponding metformin monotherapy groups (P = 0.017 and P < 0.001, respectively for the M1700 and M1000 groups).
Fasting plasma glucose
At week 24, reductions from baseline in FPG were noted in all active treatment groups compared with the placebo (Table 2), and there was a greater reduction from baseline in FPG after either combination treatment compared with S100 (P < 0.001). Neither combination treatment provided significantly better reduction from baseline in FPG compared with the corresponding metformin monotherapy group.
Initiation of glycemic rescue
Over the 24‐week course of the study, 12.6, 7.5, 3.2, 1.6, 1.6, and 0% of the patients initiated glycemic rescue in the placebo, S100, M1000, M1700, S100/M1000 and S100/M1700 groups, respectively.
Safety
Using the primary approach to analyzing safety data, in which data obtained after the initiation of rescue therapy were treated as missing, the percentage of participants reporting one or more AEs ranged from 28.6 in the M1000 group to 35.5 in the M1700 group (Table 3). Among summary measures of AEs, the 95% confidence intervals (CI) around the between‐treatment differences for each active treatment vs placebo included 0, with the exception of drug‐related AEs, for which the 95% CI for the M1700 group was (0.8, 17.5), largely because of differences in GI‐ and metabolism‐related drug‐related AEs (M1700 vs placebo: 8.9% vs 3.2% and 4.0% vs 0.8%, respectively). The percentages of participants reporting serious AEs were low, with no serious AEs reported for patients in the co‐administration groups (Table 3). No deaths were reported during the study.
Table 3.
Adverse events summary
| Participants, n (%) | Placebo n = 126 | S100 n = 120 | M1000 n = 126 | M1700 n = 124 | S100/M1000 n = 122 | S100/M1700 n = 125 |
|---|---|---|---|---|---|---|
| With one or more | ||||||
| AEs | 38 (30.2) | 39 (32.5) | 36 (28.6) | 44 (35.5) | 40 (32.8) | 40 (32.0) |
| Drug‐related† AEs | 10 (7.9) | 6 (5.0) | 10 (7.9) | 21 (16.9) | 13 (10.7) | 17 (13.6) |
| Serious AEs | 1 (0.8) | 3 (2.5) | 1 (0.8) | 1 (0.8) | 0 (0.0) | 0 (0.0) |
| Serious drug‐related† AEs | 0 (0.0) | 1 (0.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Who died | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Who discontinued due to | ||||||
| An AE | 3 (2.4) | 2 (1.7) | 3 (2.4) | 2 (1.6) | 1 (0.8) | 6 (4.8) |
| A drug‐related† AE | 2 (1.6) | 2 (1.7) | 2 (1.6) | 2 (1.6) | 1 (0.8) | 5 (4.0) |
| A serious AE | 1 (0.8) | 1 (0.8) | 1 (0.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| A serious drug‐related† AE | 0 (0.0) | 1 (0.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Prespecified AEs of interest | ||||||
| One or more | ||||||
| Events of hypoglycemia | 4 (3.2) | 5 (4.2) | 3 (2.4) | 9 (7.3) | 13 (10.7) | 16 (12.8) |
| Symptomatic‡ | 1 (0.8) | 2 (1.7) | 0 (0.0) | 3 (2.4) | 3 (2.5) | 3 (2.4) |
| Severe§ | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Selected gastrointestinal AEs | ||||||
| Diarrhea | 4 (3.2) | 2 (1.7) | 4 (3.2) | 9 (7.3) | 3 (2.5) | 4 (3.2) |
| Nausea | 0 (0.0) | 0 (0.0) | 1 (0.8) | 4 (3.2) | 2 (1.6) | 8 (6.4) |
| Abdominal pain¶ | 2 (1.6) | 0 (0.0) | 4 (3.2) | 5 (4.0) | 4 (3.3) | 4 (3.2) |
| Vomiting | 0 (0.0) | 0 (0.0) | 1 (0.8) | 1 (0.8) | 0 (0.0) | 3 (2.4) |
†Determined by the investigator to be related to study drug. ‡Clinical symptoms attributed to hypoglycemia, without regard to glucose level. §Episode that required assistance, either medical or non‐medical. ¶Abdominal pain adverse events (AEs) include the following terms: abdominal pain lower, abdominal pain upper, abdominal pain, abdominal discomfort and epigastric discomfort.
The percentages of patients with at least one event of hypoglycemia (symptomatic or asymptomatic) were significantly higher in the S100/M1000 and S100/M1700 groups compared with the placebo group (Table 3; P = 0.010 and P = 0.002, respectively). This between‐group difference was predominantly the result of differences in the proportions of patients with asymptomatic hypoglycemia; percentages of participants with AEs of symptomatic hypoglycemia were low and similar across the treatment groups, and no patients were reported to have had an AE of severe hypoglycemia (Table 3).
With the exception of the incidences of AEs of nausea, which were significantly higher in both high‐dose metformin groups (i.e., S100/M1700 and M1700) compared with the placebo (P = 0.002 and P = 0.021, respectively), incidences of prespecified GI AEs were similar among the active treatment and placebo groups (Table 3). All of the selected GI AEs were of mild or moderate intensity, and most were considered related to the study treatment.
The secondary method for analysis of safety end‐points including data after glycemic rescue (data not shown) yielded results similar to those reported above, from which data after glycemic rescue were excluded.
At week 24, modest reductions in body weight were observed in the placebo, M1000 and M1700 groups (change [95% CI] = −1.2 kg, 95% CI [−2.0, −0.4]; −0.9 kg, 95% CI [1.6, −0.2]; and −1.0 kg, 95% CI [−1.7, ‐0.3]; respectively), whereas no meaningful changes from baseline were observed in the S100, S100/M1000 and S100/M1700 groups (change [95% CI] = 0.6 kg, 95% CI [‐0.2, 1.3]; 0.6 kg, 95% CI [‐0.1, 1.3] and ‐0.2 kg, 95% CI [‐0.9, 0.5]; respectively).
Discussion
In this 24‐week placebo‐controlled study, initial combination treatment of sitagliptin and metformin provided clinically meaningful reductions in HbA1c compared with each monotherapy alone at corresponding dose strengths and compared with the placebo.
In the prespecified primary analysis, S100/M1700 was statistically superior to S100, but not M1700 in reducing HbA1c. A post‐hoc diagnostic analysis of the HbA1c data showed one patient in the S100/M1700 group with an extreme outlier value that was highly influential on the primary efficacy analysis. The patient's changes in FPG and weight were not consistent with a large increase in HbA1c, and the patient had no AE reports suggestive of hyperglycemia. For these clinical reasons, and because the outlier value resulted in a violation of the normality assumption required for valid ANCOVA, three post‐hoc analyses were carried out to assess its impact on the primary analysis, and to aid in the interpretation of the study results. These post‐hoc analyses either removed the biologically implausible value or used statistical methodology that was valid in the presence of non‐normal data. The results from all three sensitivity analyses were consistent with one another in providing evidence that co‐administration of sitagliptin with high‐dose metformin is superior to each component monotherapy. S100/M1000 was superior to each component monotherapy in reducing HbA1c.
Consistent with this conclusion, at week 24, a greater percentage of patients in the S100/M1700 group met HbA1c goals (<7 and <6.5%) compared with the M1700 group. Similar patterns were observed in the comparisons of the S100/M1000 group with the M1000 group. Additionally, the proportions of patients who initiated glycemic rescue across the treatment groups also supports this conclusion.
After a standard meal, greater reductions in 2‐h post‐meal glucose at week 24 compared with baseline were observed in both co‐administration groups, compared with each respective component monotherapy. The co‐administration groups provided greater reductions in FPG from baseline compared with the sitagliptin group, but not compared with the corresponding metformin groups, which is consistent with the complementary mechanisms of action of the component medications (i.e., metformin's predominant effect on fasting glucose and sitagliptin's predominant effect on post‐meal glucose).
Although the placebo‐adjusted changes in key glycemic end‐points in the current study were consistent with a previous global study comparing initial sitagliptin/metformin combination therapy with each component monotherapy8, the magnitude of the difference between the co‐administration groups and each of the placebo and monotherapy groups was smaller in the current study. One difference in the results of the current and previous studies of initial combination treatment with sitagliptin and metformin is the 0.59% decrease from baseline in LS mean HbA1c observed in the placebo group in the current study at week 24, compared with the 0.17% increase from baseline in the previous study8. This larger than expected response in the placebo group suggests a clinical trial effect in the present study that might be related to differences in the study population or study design, and that could have impacted the difference in observed treatment effects.
In the current study, the efficacy of S100/M1000 was similar to M1700. This is consistent with the finding from another study in Chinese participants with type 2 diabetes comparing the efficacy between M2000 and the combination of another DPP‐4 inhibitor and M100020.
Another important observation made in the present study relates to the efficacy of metformin in a Chinese population. Although metformin is recommended as a first‐line option for the treatment of type 2 diabetes in the Chinese diabetes guideline, a placebo‐controlled study to evaluate the efficacy of metformin in Chinese and other Asian populations has not been reported. In the present study, statistically significant, placebo‐adjusted reductions from baseline in HbA1c were observed in both the M1000 and M1700 groups. The efficacy of metformin observed in the current study is comparable with the study results observed in other populations21. At each dose of metformin tested, co‐administration of sitagliptin and metformin was generally well‐tolerated. The incidences of overall AEs, serious AEs and AEs leading to discontinuation in the active treatment groups were similar to the placebo. Consistent with previous reports of sitagliptin used as monotherapy and in combination with other oral antihyperglycemic agents22, 23, 24, the incidences of symptomatic AEs of hypoglycemia were low and similar across the treatment groups. As expected23, in general patients treated with high‐dose metformin had the greatest incidence of pre‐specified GI AEs, although with the exception of the AE of nausea, none of the between‐group differences in the incidences relative to the placebo were statistically significant or meaningful.
The glucose‐lowering efficacy of DPP‐4 inhibitors or GLP‐1 receptor agonists has been reported to be greater in Asians than in non‐Asians16. However, in the study reported in this manuscript, the placebo‐controlled reductions from baseline in HbA1c in all treatment groups were not notably different from those of similar treatments reported in the previous global study of initial combination therapy with sitagliptin and metformin8, despite the racial differences in the study populations. The majority of the studies analyzed by Kim et al.16 took place in Japan, not China, and other studies analyzed included patients from India and Korea; thus it might be that relatively few patients in the studies used in the meta‐analysis of Kim et al. are directly relevant to the effects of treatment on Chinese patients reported in the current study. Differences in the pathophysiology of type 2 diabetes (e.g., insulin production or insulin sensitivity), body composition, genetic makeup, and/or dietary factors might contribute to this apparent discrepancy25, 26.
The safety profile observed in the current study is generally comparable with the safety profile observed in the previous study carried out in a mostly non‐Asian population8. The overall incidence of hypoglycemia appears higher in the current study (2.4–12.8%) compared with the previous study (0.5–2.2%), because only events of symptomatic hypoglycemia were reported for the previous study. The incidence of AEs of symptomatic hypoglycemia in the current study was low (ranging from 0.0 to 2.5%), and similar to that seen previously (ranging from 0.5 to 2.2%8). As expected, because of the use of metformin, a modestly higher incidence of GI AEs relative to placebo was observed in both studies.
In the present study, carried out in Chinese patients with type 2 diabetes, initial co‐administration of sitagliptin and metformin was associated with better glycemic control compared with individual component monotherapies, and was generally well‐tolerated.
Disclosure
L Ji reports grants from MSD outside the submitted work. YM Jou, EA O'Neill, GT Golm, SS Engel, KD Kaufman and RR Shankar are current or former employees of Merck Sharp & Dohme, Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and might own stock/stock options in the company. YM Jou also discloses employment and stock at Bristol‐Myers Squibb, Hopewell, NJ, USA. P Han, J Liu, X Wang and S Zheng declare no conflict of interest.
Supporting information
Table S1 | Patient disposition
Figure S1 | Change from baseline in glycated hemoglobin (%) by week.
Acknowledgments
Funding for the present study was provided by Merck & Co., Inc., Kenilworth, NJ, USA. The authors acknowledge the assistance of Yong Wang and Yajie Li of MSD China in administration of the study, and Kristen Lewis of Merck & Co., Inc., Kenilworth, NJ, USA in the preparation and submission of this manuscript.
J Diabetes Investig 2016; 7: 727–736
References
- 1. Whiting DR, Guariguata L, Weil C, et al IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract 2011; 94: 311–321. [DOI] [PubMed] [Google Scholar]
- 2. Narayan KM, Gregg EW, Fagot‐Campagna A, et al Diabetes – a common, growing, serious, costly, and potentially preventable public health problem. Diabetes Res Clin Pract 2000; 50 (Suppl 2): S77–S84. [DOI] [PubMed] [Google Scholar]
- 3. Karter AJ, Moffet HH, Liu J, et al Achieving good glycemic control: initiation of new antihyperglycemic therapies in patients with type 2 diabetes from the Kaiser Permanente Northern California Diabetes Registry. Am J Manag Care 2005; 11: 262–270. [PMC free article] [PubMed] [Google Scholar]
- 4. Drucker DJ. The biology of incretin hormones. Cell Metab 2006; 3: 153–165. [DOI] [PubMed] [Google Scholar]
- 5. Plosker GL. Sitagliptin: a review of its use in patients with type 2 diabetes mellitus. Drugs 2014; 74: 223–242. [DOI] [PubMed] [Google Scholar]
- 6. Engel SS, Round E, Golm GT, et al Safety and tolerability of sitagliptin in type 2 diabetes: pooled analysis of 25 clinical studies. Diabetes Ther 2013; 4: 119–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hundal RS, Inzucchi SE. Metformin: new understandings, new uses. Drugs 2003; 63: 1879–1894. [DOI] [PubMed] [Google Scholar]
- 8. Goldstein BJ, Feinglos MN, Lunceford JK, et al Effect of initial combination therapy with sitagliptin, a dipeptidyl peptidase‐4 inhibitor, and metformin on glycemic control in patients with type 2 diabetes. Diabetes Care 2007; 30: 1979–1987. [DOI] [PubMed] [Google Scholar]
- 9. Deurenberg P, Deurenberg‐Yap M, Guricci S. Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obes Rev 2002; 3: 141–146. [DOI] [PubMed] [Google Scholar]
- 10. Deurenberg P, Yap M, van Staveren WA. Body mass index and percent body fat: a meta analysis among different ethnic groups. Int J Obes Relat Metab Disord 1998; 22: 1164–1171. [DOI] [PubMed] [Google Scholar]
- 11. Chiu KC, Cohan P, Lee NP, et al Insulin sensitivity differs among ethnic groups with a compensatory response in beta‐cell function. Diabetes Care 2000; 23: 1353–1358. [DOI] [PubMed] [Google Scholar]
- 12. Jensen CC, Cnop M, Hull RL, et al Beta‐cell function is a major contributor to oral glucose tolerance in high‐risk relatives of four ethnic groups in the U.S. Diabetes 2002; 51: 2170–2178. [DOI] [PubMed] [Google Scholar]
- 13. Ohn JH, Kwak SH, Cho YM, et al 10‐year trajectory of beta‐cell function and insulin sensitivity in the development of type 2 diabetes: a community‐based prospective cohort study. Lancet Diabetes Endocrinol 2016; 4: 27–34. [DOI] [PubMed] [Google Scholar]
- 14. Taniguchi A, Fukushima M, Sakai M, et al Insulin secretion, insulin sensitivity, and glucose effectiveness in nonobese individuals with varying degrees of glucose tolerance. Diabetes Care 2000; 23: 127–128. [DOI] [PubMed] [Google Scholar]
- 15. Tan VM, Lee YS, Venkataraman K, et al Ethnic differences in insulin sensitivity and beta‐cell function among Asian men. Nutr Diabetes 2015; 5: e173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim YG, Hahn S, Oh TJ, et al Differences in the glucose‐lowering efficacy of dipeptidyl peptidase‐4 inhibitors between Asians and non‐Asians: a systematic review and meta‐analysis. Diabetologia 2013; 56: 696–708. [DOI] [PubMed] [Google Scholar]
- 17. Levey AS, Bosch JP, Lewis JB, et al A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999; 130: 461–470. [DOI] [PubMed] [Google Scholar]
- 18. Huber PJ. Robust regression: asymptotics, conjectures and Monte Carlo. Ann Stat 1973; 1: 799–821. [Google Scholar]
- 19. Miettinen O, Nurminen M. Comparative analysis of two rates. Stat Med 1985; 4: 213–226. [DOI] [PubMed] [Google Scholar]
- 20. Ji L, Zinman B, Patel S, et al Efficacy and safety of linagliptin co‐administered with low‐dose metformin once daily versus high‐dose metformin twice daily in treatment‐naive patients with type 2 diabetes: a double‐blind randomized trial. Adv Ther 2015; 32: 201–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Saenz A, Fernandez‐Esteban I, Mataix A, et al Metformin monotherapy for type 2 diabetes mellitus. Cochrane Database Syst Rev 2005; CD002966. [DOI] [PubMed] [Google Scholar]
- 22. Charbonnel B, Karasik A, Liu J, et al Efficacy and safety of the dipeptidyl peptidase‐4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care 2006; 29: 2638–2643. [DOI] [PubMed] [Google Scholar]
- 23. Inzucchi SE. Oral antihyperglycemic therapy for type 2 diabetes: scientific review. JAMA 2002; 287: 360–372. [DOI] [PubMed] [Google Scholar]
- 24. Raz I, Hanefeld M, Xu L, et al Efficacy and safety of the dipeptidyl peptidase‐4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia 2006; 49: 2564–2571. [DOI] [PubMed] [Google Scholar]
- 25. Cho YM. Incretin physiology and pathophysiology from an Asian perspective. J Diabetes Investig 2015; 6: 495–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yabe D, Kuwata H, Iwasaki M, et al Why are incretin‐based therapies more efficient in East Asians? Perspectives from the pathophysiology of type 2 diabetes and East Asian dietary habits. Eur Med J Diabetes 2015; 3: 57–65. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 | Patient disposition
Figure S1 | Change from baseline in glycated hemoglobin (%) by week.


