Abstract
Aims/Introduction
Dipeptidyl peptidase‐4 inhibitors (DPP‐4i) are a common first‐line treatment for type 2 diabetes in Japan. However, little is known about patients’ medication adherence, persistence and discontinuation in this setting.
Materials and Methods
This was a retrospective cohort study of new DPP‐4i users in a Japanese claims database. Adult patients (age 18–65 years) with type 2 diabetes diagnosis and no diagnosis of other diabetes or pregnancy during the study period were included if they were prescribed a DPP‐4i as monotherapy or combination oral therapy. Adherence to therapy was measured using the proportion of days covered method over a fixed period of 1 year. The proportion of days covered of ≥80% was considered adherent. Persistence was defined as continuing index DPP‐4i treatment with <90‐day gap between refills. Patient baseline characteristics were explored as potential predictors of DPP‐4i discontinuation and adherence in multivariable models.
Results
The final sample contained 2,874 monotherapy and 3,016 dual therapy patients. The mean age was approximately 51 years, and 75% were men. The mean proportion of days covered was 76.6% among monotherapy patients and 82.5% among dual therapy patients, with 67.2% of monotherapy and 74.4% of dual therapy patients classified as adherent. At 12 months, 72.2% of monotherapy and 79.2% of dual therapy patients were persistent. In adjusted models, younger age and having fewer concomitant medications were significantly associated with lower adherence and higher discontinuation, in both treatment groups.
Conclusions
Those under the age of 45 years, and those with fewer concomitant medications were less likely to be adherent and persistent, and more likely to discontinue DPP‐4i therapy.
Keywords: Dipeptidyl peptidase‐4 inhibitors, Medication adherence, Type 2 diabetes
Introduction
Diabetes prevalence is on the rise worldwide, and Japan has the 10th highest number of individuals who have the disease among adults aged 20–79 years1. Over the period from 1997 to 2007, diabetes incidence increased from 8.0% to 14.0% in men, and from 7.9% to 15.9% in women2. The Japan Ministry of Health, Labor and Welfare now estimates that 9.5 million Japanese have diabetes, and 11.0 million meet the criteria for prediabetes3. Type 1 diabetes is very rare in Japan; therefore, the vast majority of these cases are understood to be type 2 diabetes.
Treating diabetes is costly, with annual expenditures for type 2 diabetes in Japan estimated to be $4,054 per person in 20131. Diabetes spending is driven largely by diabetes‐related complications and comorbid conditions, such as nephropathy, neuropathy, retinopathy and cardiovascular disease4, 5. Specifically, diabetes‐related complications, and peripheral vascular disease in particular, increase hospital length of stay and total hospital charges for patients with diabetes in Japan4.
Such complications arise from inadequate glucose control over the long term, which might be exacerbated by poor medication adherence6. Patients in the real world are often not adherent to or persistent with part, or all, of their therapy regimen. Adherence rates to oral type 2 diabetes therapy range across the North American and European literature from 52% to 88%7. A meta‐analysis of diabetes medication adherence found a mean adherence rate of 75%, with almost one‐third (32%) of patients considered to be non‐adherent8. Likewise, persistence to oral diabetes therapy has been reported to range from 16% to 80% over a 6–24‐month period.6 The impact of improved adherence to type 2 diabetes treatment could be substantial, potentially saving $5 billion per year in the USA by way of reducing hospitalizations and emergency room visits9.
The Japan Diabetes Society guidelines recommend a target glycated hemoglobin (HbA1c) value of <7% to prevent complications of diabetes; however, physicians can individualize treatment goals for patients within a range from <6.0% to <8%10. The Japan Diabetes Society recommends initiating low‐dose oral therapy when a trial of diet and exercise fails to reduce HbA1c within 2–3 months10. A variety of oral therapies are approved for use in Japan including biguanides, sulfonylureas, thiazolidinediones, glinides, dipeptidyl peptidase‐4 inhibitors (DPP‐4i) and alpha‐glucosidase inhibitors. Of these, the DPP‐4i class, introduced in Japan in 2009, is the most commonly prescribed oral treatment in the first month of therapy11. There are no data on adherence and persistence to these widely‐used medications in Japan. The objectives of the present study were to estimate medication adherence and persistence to, and discontinuation of, DPP‐4is in Japan, and examine factors predicting adherence to and discontinuation of DPP‐4is.
Methods
We carried out a retrospective cohort study of Japanese type 2 diabetes patients who initiated a DPP‐4i as monotherapy or dual therapy between 1 January 2010 and 31 July 2013 (index period). Data were obtained from the Japan Medical Data Center, which houses a database of inpatient, outpatient and pharmacy claims from enrollees of employer‐sponsored health plans throughout all prefectures of Japan12. Several studies have been carried out and published in peer‐reviewed journals using the Japan Medical Data Center database to estimate disease incidence and prevalence, describe patient populations, model health care costs, and evaluate the quality of healthcare services13, 14, 15, 16, 17, 18, 19.
The index date was defined as the first prescription for DPP‐4i during the index period. The baseline period was defined as 12 months before the index date. Patients were included in the analysis if they were between the ages of 18 and 64 years at index, had a diagnosis of type 2 diabetes based on International Classification of Diseases, 10th Revision codes and were initiating DPP‐4i treatment as monotherapy or dual therapy. Dual therapy was defined as a DPP‐4i prescribed with another oral antihyperglycemic medication, whether coadministered or as a fixed‐dose combination. We required at least 12 months of continuous enrolment before the index date to assess baseline characteristics. Patients were excluded if they had type 1 diabetes, ketoacidosis, malnutrition‐associated diabetes, drug‐induced diabetes, gestational diabetes or were pregnant at any time during the study period. Patients who used insulin or DPP‐4i during the baseline period were also excluded.
New prescription medications in Japan are restricted to a 2‐week refill period during their first year on the market, which might increase the likelihood of missing a refill. Patients initiating a DPP‐4i in its first year on the market (113 monotherapy patients and 139 dual therapy patients) were excluded to avoid the impact of the 2‐week refill requirement on measures of adherence.
Participants were followed up for a minimum of 3 months and a maximum of 24 months to track their prescription‐filling behavior. We defined discontinuation as an observed gap of ≥90 days between prescription fills for the index DPP‐4i to remain consistent with previous studies of discontinuation of oral type 2 diabetes therapies, which used the same threshold20, 21. Those who switched to another DPP‐4i during follow up were considered to be continuing on DPP‐4i therapy, but switching to another class of medication resulted in censoring. Time to discontinuation was censored for patients who reached the end of the follow‐up time, whether due to end of continuous enrolment or end of study period, before discontinuation was observed.
We measured adherence over a fixed time interval by the proportion of days covered (PDC). PDC was calculated based on the total number of days during follow up with medication on hand within the first 12 months of follow up divided by the follow‐up time (365 days), for those who had at least 1 year of follow up. A PDC value of ≥80% was considered ‘adherent.’ We defined persistence as the proportion of patients remaining on the index medication at the end of 12 and 24 months, among those with 12 and 24 months of follow‐up time, respectively, with no gap in therapy ≥90 days.
Descriptive statistics for baseline characteristics were calculated for overall population and by subgroups (age category, sex and number of concomitant medications). Factors associated with discontinuation were identified with a Cox proportional hazards model. Mean and median PDC, and percent adherent were calculated for all participants who had at least 1 year of follow‐up time. Logistic regression models were used to explore baseline patient characteristics that were associated with adherence and persistence. Age (categorized as <45 years and ≥45 to <65 years), sex, number of concomitant medications (0, 1, 2, ≥3) and individual comorbid conditions at baseline were added in the models in a stepwise manner, with the threshold for inclusion set at P < 0.05, and at P < 0.05 to remain in the model. Concomitant medications included those that were likely to be prescribed for chronic conditions that are frequently comorbid with type 2 diabetes (e.g., hypertension): diuretics, antiplatelet agents, cardiac medications, dyslipidemic drugs and antihypertensive medications. All analyses were carried out separately by treatment complexity (monotherapy or dual therapy).
Results
We observed 14,449 enrollees who initiated a DPP‐4i (i.e., new users) during the index period. After applying all inclusion and exclusion criteria, there were 2,874 monotherapy and 3,016 dual therapy patients included in the final sample, with 441 and 480 mean days of follow‐up time, respectively. The sample population was approximately 75% men, and the mean age at index was approximately 51 years. Over half of the study population had hypertension, and approximately 30% had cardiovascular disease. Dyslipidemia medications, antihypertensive medications and prescription non‐steroidal anti‐inflammatory drugs (NSAIDs) were common concomitant medications, used by >40% of participants. Patient characteristics are fully described in Table 1.
Table 1.
Patient characteristics at baseline
| Overall (n = 5,890) | ||
|---|---|---|
| Mean (SD) or n (%) | ||
| Monotherapy (n = 2,874) | Dual therapy (n = 3,016) | |
| Mean age (years) | 51.3 (8.2) | 50.8 (8.5) |
| Male | 2,153 (74.9) | 2,244 (74.4) |
| Comorbidities | ||
| Cancer† | 129 (4.5) | 132 (4.4) |
| Cardiovascular disease‡ | 866 (30.1) | 940 (31.2) |
| Hypertension | 1,479 (51.5) | 1,561 (51.8) |
| Cerebrovascular conditions§ | 357 (12.4) | 321 (10.6) |
| Neuropathy | 214 (7.4) | 264 (8.8) |
| Diabetic retinopathy | 237 (8.2) | 462 (15.3) |
| Depression | 165 (5.7) | 151 (5.0) |
| Chronic kidney disease¶ | 235 (8.2) | 425 (14.1) |
| DPP‐4i use | ||
| Standard price for DPP‐4i (Japanese yen) | 171.36 (40.03) | 173.01 (39.34) |
| Twice daily dosing frequency | 249 (8.7) | 218 (7.2) |
| Concomitant medications | ||
| NSAID | 1,408 (49.0) | 1,473 (48.8) |
| Disopyramide | 1 (0.0) | 4 (0.1) |
| Coumadin | 36 (1.3) | 34 (1.1) |
| MAOI | 0 (0.0) | 0 (0.0) |
| Beta‐blockers | 242 (8.4) | 267 (8.9) |
| Loop diuretics | 47 (1.6) | 50 (1.7) |
| Dyslipidemia medications | 1,183 (41.2) | 1,373 (45.5) |
| Diuretics | 236 (8.2) | 255 (8.5) |
| Antiplatelet agents | 245 (8.5) | 225 (7.5) |
| Cardiac medications | 691 (24.0) | 614 (20.4) |
| Antihypertensive medications | 1,315 (45.8) | 1,413 (46.9) |
| No. concomitant medications†† | ||
| None | 845 (29.4) | 812 (26.9) |
| One | 954 (33.2) | 1,051 (34.8) |
| Two | 653 (22.7) | 744 (24.7) |
| Three or more | 422 (14.7) | 409 (13.6) |
†Includes bladder, breast, non‐skin, metastatic, pancreatic and thyroid cancer. ‡Includes acute coronary syndrome, acute myocardial infarction, angina, heart failure and peripheral arterial disease. §Includes cerebrovascular disease, hemiplegia and stroke/transient ischemic attack. ¶Includes chronic kidney disease, end‐stage renal disease and nephropathy. ††Concomitant medications include diuretics, antiplatelet agents, cardiac medications, dyslipidemic drugs and antihypertensive medications. DPP‐4i, dipeptidyl peptidase‐4 inhibitor; drug; MAOI, monoamine oxidase inhibitor; NSAID, non‐steroidal anti‐inflammatory; SD, standard deviation.
Table 2 shows adherence and percent adherent by treatment complexity and subgroups (age, sex and concomitant medications). Mean PDC was 76.6% for all monotherapy patients, and 82.5% for all dual therapy patients. Among monotherapy patients, 67.2% were considered adherent, and 74.6% of dual therapy patients were adherent. Overall, mean adherence rates and the proportion adherent were lower for patients in younger age groups or taking fewer concomitant medications among both monotherapy and dual therapy patients. There was no significant difference in adherence by sex.
Table 2.
Adherence and percent adherent among patients with at least 1 year of follow up by age group, sex and number of concomitant medications
| Monotherapy | Dual therapy | |||
|---|---|---|---|---|
| Mean PDC (95% CI) | Percent adherent (95% CI) | Mean PDC (95% CI) | Percent adherent (95% CI) | |
| Overall | 76.6 (75.1–78.2) | 67.2 (64.9–69.5) | 82.5 (81.2–83.7) | 74.6 (72.6–76.5) |
| Age | ||||
| <45 years | 67.6 (64.0–71.3) | 53.4 (48.0–58.7) | 74.8 (71.9–77.7) | 63.1 (58.5–67.4) |
| ≥45 and <65 years | 79.1 (77.4–80.8) | 71.0 (68.4–73.4) | 84.9 (83.6–86.3) | 78.3 (76.1–80.4) |
| Sex | ||||
| Male | 76.2 (74.4–78.0) | 66.8 (64.1–69.4) | 82.7 (81.3–84.1) | 74.7 (72.4–76.9) |
| Female | 78.0 (74.8–81.1) | 68.5 (63.7–73.1) | 81.9 (79.3–84.6) | 74.3 (70.1–78.1) |
| Concomitant medications | ||||
| None | 69.2 (66.2–72.3) | 56.7 (52.3–61.1) | 76.9 (74.2–79.5) | 66.3 (62.0–70.4) |
| One | 75.7 (72.9–78.5) | 66.9 (62.6–70.9) | 81.6 (79.5–83.8) | 73.6 (70.2–76.9) |
| Two | 81.7 (78.8–84.6) | 73.6 (68.9–78.0) | 87.1 (84.9–89.3) | 80.6 (76.8–84.1) |
| Three or more | 86.4 (83.0–89.7) | 80.3 (74.7–85.2) | 87.2 (84.3–90.2) | 82.2 (77.2–86.5) |
The proportion of days covered (PDC) of ≥80% was considered adherent. CI, confidence interval.
At 12 months, 72.2% of monotherapy patients and 79.2% of dual therapy patients were persistent with their DPP‐4i medication (Table 3). Among those with follow up to 24 months, 63.3% of monotherapy patients and 74.2% of dual therapy patients remained on their DPP‐4i medication. Persistence at 12 and 24 months was lower for younger patients and those with fewer concomitant medications.
Table 3.
Dipeptidyl peptidase‐4 inhibitor persistence at 12 and 24 months among those with continuous enrolment for 12 and 24 months, respectively, by age group, sex and number of concomitant medications
| Monotherapy | Dual therapy | |
|---|---|---|
| No. patients at 12 months | 1,647 | 1,950 |
| Overall persistence (%) | 72.2 | 79.2 |
| Age | ||
| <45 years | 59.7 | 69.6 |
| ≥45 and <65 years | 75.6 | 82.2 |
| Sex | ||
| Male | 72.1 | 79.3 |
| Female | 72.3 | 78.8 |
| Concomitant medications | ||
| None | 61.4 | 71.1 |
| One | 72.3 | 79.2 |
| Two | 78.1 | 84.3 |
| Three or more | 85.8 | 85.4 |
| No. patients at 24 months | 594 | 818 |
| Overall persistence | 63.3 | 74.2 |
| Age | ||
| <45 years | 50.4 | 63.6 |
| ≥45 and <65 years | 67.0 | 78.0 |
| Sex | ||
| Male | 61.5 | 74.4 |
| Female | 69.3 | 73.7 |
| Concomitant medications | ||
| None | 53.6 | 66.5 |
| One | 61.5 | 73.5 |
| Two | 69.5 | 78.9 |
| Three or more | 81.6 | 84.6 |
DPP‐4i, dipeptidyl peptidase‐4 inhibitor.
Results of multivariable Cox regression models examining baseline factors associated with discontinuation of DPP‐4i medication are shown in Table 4. Among monotherapy patients, older age, greater number of concomitant medications, acute myocardial infarction (MI), and diabetic retinopathy were independently associated with a lower rate of discontinuation of DPP‐4i medication. For dual therapy patients, older age, greater number of concomitant medications and chronic kidney disease were independently associated with a lower rate of discontinuation of DPP‐4i medication, whereas urinary tract infection was associated with a higher rate of discontinuation.
Table 4.
Cox regression models for baseline characteristics associated with discontinuation of dipeptidyl peptidase‐4 inhibitor therapy by treatment complexity
| Monotherapy | Dual therapy | |
|---|---|---|
| Hazard ratio (95% CI) | Hazard ratio (95% CI) | |
| Age (reference: ≤45 years) | 0.68 (0.57–0.80) | 0.62 (0.52–0.74) |
| Concomitant medications (reference: 0) | ||
| One concomitant medication | 0.67 (0.56–0.80) | 0.73 (0.60–0.80) |
| Two concomitant medications | 0.51 (0.41–0.63) | 0.61 (0.49–0.77) |
| Three or more concomitant medications | 0.47 (0.36–0.63) | 0.53 (0.39–0.71) |
| Acute myocardial infarction | 0.26 (0.08–0.84) | – |
| Diabetic retinopathy | 0.70 (0.52–0.94) | – |
| Urinary tract infection | – | 1.55 (1.09–2.21) |
| Chronic kidney disease | – | 0.72 (0.55–0.95) |
Only variables that were found to be statistically significant by stepwise selection (level of significance for entry into the model = 0.05; to stay in the model = 0.05) are shown. The full model tested age, sex, individual comorbidities, number of concomitant medications, dipeptidyl peptidase‐4 inhibitor (DPP‐4i) dosing frequency and DPP‐4i standard price. CI, confidence interval.
The logistic regression models for baseline factors associated with being adherent at follow‐up are shown in Figure 1. Among monotherapy patients, older age, greater number of concomitant medications, acute MI and diabetic retinopathy were independently associated with being adherent. Among dual therapy patients, older age, greater number of concomitant medications and chronic kidney disease were associated with being adherent, whereas urinary tract infection was negatively associated with being adherent.
Figure 1.
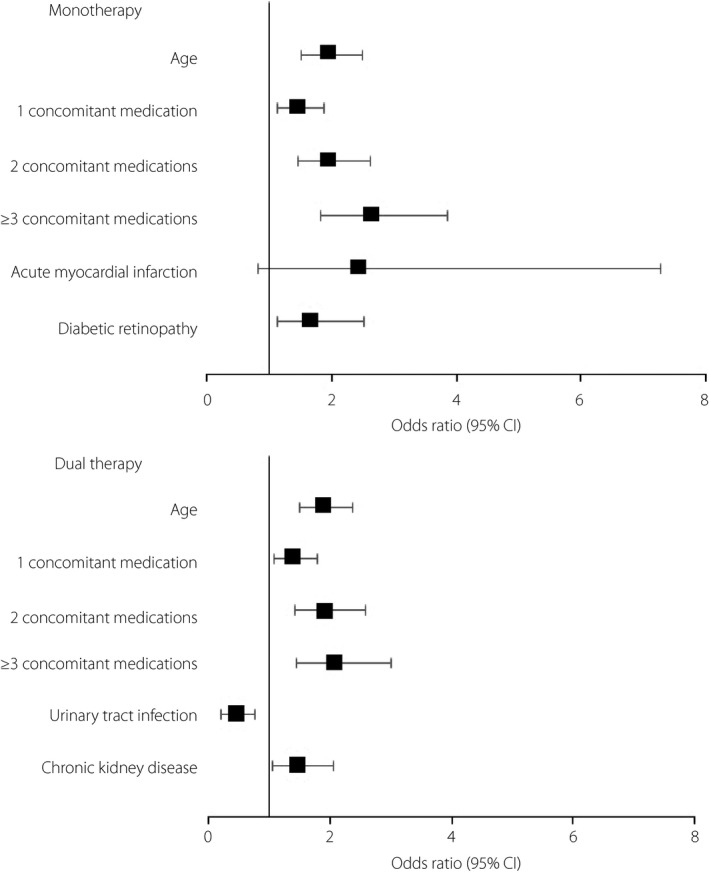
Logistic regression model for baseline characteristics associated with being adherent to dipeptidyl peptidase‐4 inhibitor therapy. Only variables that were found to be statistically significant by stepwise selection (level of significance for entry into the model = 0.05; to stay in the model = 0.05) are shown. The full model tested age, sex, individual comorbidities, number of concomitant medications, dipeptidyl peptidase‐4 inhibitor dosing frequency and dipeptidyl peptidase‐4 inhibitor standard price. The reference category for age is ≤45 years and for concomitant medications is none. CI, confidence interval.
Discussion
In the current study, approximately one‐quarter to one‐third of type 2 diabetes patients were non‐adherent to their DPP‐4i monotherapy and dual therapy, respectively. In the global context, these patients appeared to be more adherent than those in the USA, where just 45.1% of monotherapy and dual therapy patients22 to 59% of monotherapy patients23 are estimated to be adherent to DPP‐4i therapy. Persistence at 24 months (63.3% in monotherapy and 74.2% in dual therapy patients) was also somewhat higher than a German study that found 61% of type 2 diabetes patients taking DPP‐4is to be persistent at 24 months20.
In the present analysis, younger patients or those with fewer concomitant medications were more likely to discontinue, and less likely to be adherent to and persistent with DPP‐4is. This is consistent with a previous adherence study of claims data in the USA, which found that just 37% of patients aged <45 years were adherent to oral antihyperglycemic therapy, compared with 55% for those aged 45–64 years23. The same study found that mean PDC and percent adherent increased with categories of increasing number of concomitant medications, with the highest PDC of 80% observed among those with three or more concomitant medications, and the lowest PDC of 60% observed among those with no concomitant medications23. Other studies have not identified age as an independent risk factor for poor adherence24, yet our finding that younger age is associated with medication non‐adherence might point to another factor that cannot be captured in claims data that is driving the association between age and adherence.
Numerous factors have been proposed to influence patients’ medication‐taking behaviors. The features of a particular diabetes therapy itself might impact how well patients adhere to it. For instance, more frequent dosing and polytherapy have both been shown to contribute to poorer adherence6. However, in the present study, polytherapy was associated with better adherence. Psychosocial challenges, such as lack of social support and socioeconomic barriers like low income, make adherence more difficult6. Ultimately, whatever the attributes of the disease or the medication at hand, adherence is understood to be a dynamic decision‐making process in which patients rationally weigh their experiences and beliefs about their disease and their medication24.
Type 2 diabetes might be perceived as a disease that primarily affects aging people. Nevertheless, diabetes is not an insignificant problem among working Japanese. A study of several large Japanese corporations in various industries found the prevalence of diabetes to be 8.0% in men and 3.3% in women in the workforce, with 14.1% of men and 9.2% of women meeting the criteria for prediabetes25. Compared with those who are diagnosed with diabetes later in life, these patients will require many years or decades of glucose control, and diabetes complications might impact them sooner, during their productive years. Improving diabetes medication adherence in working adults might be a worthwhile strategy to prevent lost productivity in the near‐term and costly long‐term complications.
All patients in the present study were new initiators of DPP‐4i therapy, and therefore adherence might be lower than what would be expected among established patients. Those who are new to a therapy might never fill their prescription (primary non‐adherence), or might discontinue shortly after the first prescription (‘early non‐persistence’). These patterns of non‐adherence are fundamentally different from persistent patients who comply poorly with regular dosing26. Much of the non‐adherence in this analysis can be attributed to discontinuation during the first year after the initial prescription. Understanding adherence patterns to DPP‐4i therapy in Japan is important, because it is a commonly prescribed class of first‐line therapy, and represents an opportunity for developing good health behaviors early in disease progression.
We identified several individual comorbid conditions at baseline that were associated with medication adherence during follow up, including MI, retinopathy, urinary tract infection, and chronic kidney disease. In line with our findings, Curkendall et al.22 also found renal disease to be an independent predictor of oral type 2 diabetes medication adherence in a population from the USA (odds ratio 1.14, 95% confidence interval 1.10–1.20). Beliefs about disease severity and the importance of medication could account for this difference in adherence. Alternatively, these patients might be followed more closely by their physicians, which could help reinforce positive health behaviors.
The present study had several limitations to note. First, because of the nature of employer‐sponsored healthcare plans, too few enrollees in the Japan Medical Data Center database were aged 65 years and older. We excluded those aged older than 65 years from the analysis, because they were not likely to be generalizable to the Japanese population in this age range. In previous studies carried out in the USA, older patients tended to have better adherence to their medications than younger patients22, 23. Therefore, we might expect that medication adherence and persistence would be higher in older Japanese patients, but further research is required to confirm this hypothesis. Furthermore, the present study population was largely male, which might limit our ability to generalize these results to female type 2 diabetes patients.
Pharmacy claims‐based measures of adherence offer limitations and advantages. They are a small window into medication‐taking behavior – one cannot observe whether those who faithfully refill their prescriptions on time take them exactly as prescribed, nor can one determine which patients have primary non‐adherence. Yet, refill data produce adherence estimates that are comparable with electronic monitoring27. Furthermore, there is no consensus on the level of adherence required for optimal, or even minimal, glycemic control, but nevertheless PDC ≥80% is a commonly used threshold in the adherence literature for chronic diseases28. Further investigation is required to fully understand the impact of discrete levels of adherence on glycemic control.
Finally, the comorbid conditions and concomitant medications found to be associated with adherence, persistence or discontinuation only represent the patient profile before initiating a DPP‐4i. Data on comorbid conditions originated from claims, and were not confirmed with medical record data. The present study was not designed to observe changes in the participants’ health states that might have affected medication‐taking behaviors over the course of follow up. Therefore, one must be cautious in attributing causation to any comorbidities diagnosed or medications prescribed during the baseline period, as they relate to DPP‐4i adherence or persistence.
In conclusion, although adherence and persistence to DPP‐4i therapy in Japan appears to be somewhat higher than those reported for the USA and other developed countries, there is still room for improvement. Subgroups with the greatest likelihood of discontinuing DPP‐4i were those aged <45 years or those with fewer concomitant medications. Likewise, adherence was lowest in these two subgroups. Given that younger patients in particular will have many years of blood glucose maintenance ahead of them, it is important to find solutions to help patients maximize their medication adherence for the best chance of avoiding or minimizing complications as their diabetes progresses.
Disclosure
The present study was funded by Merck & Co., Inc., Kenilworth, New Jersey, USA. KB, KT and YC have disclosed that they are/were full‐time employees of Merck & Co., Inc. at the time of the analysis, and could potentially own stock and/or hold stock options in the company. CI and RN have disclosed that they received research support from Merck & Co., Inc. for carrying out this study. KK has disclosed that her work was carried out under a fellowship sponsored by Merck & Co., Inc., in partnership with Rutgers University School of Public Health, Piscataway, New Jersey, USA.
Acknowledgment
The present study was previously presented at the 2014 International Diabetes Federation – Western Pacific Region Congress.
J Diabetes Investig 2016; 7: 737–743
References
- 1. International Diabetes Federation . IDF Diabetes Atlas, 6th edn Brussels, Belgium: International Diabetes Federation, 2013. Available from: http://www.idf.org/diabetesatlas Accessed January 15, 2014. [Google Scholar]
- 2. Morimoto A, Nishimura R, Tajima N. Trends in the Epidemiology of Patients with Diabetes in Japan. Japan Med Assoc J 2010; 53: 36–40. [Google Scholar]
- 3. Japan Ministry of Health Labor and Welfare . National report on Japanese people health and nutrition, 2012. Available from: http://www.mhlw.go.jp/stf/houdou/0000032074.html Accessed January 15, 2014.
- 4. Lkhagva D, Kuwabara K, Matsuda S, et al Assessing the impact of diabetes‐related comorbidities and care on the hospitalization costs for patients with diabetes mellitus in Japan. J Diabetes Complications 2012; 26: 129–136. [DOI] [PubMed] [Google Scholar]
- 5. Neville SE, Boye KS, Montgomery WS, et al Diabetes in Japan: a review of disease burden and approaches to treatment. Diabetes Metab Res Rev 2009; 25: 705–716. [DOI] [PubMed] [Google Scholar]
- 6. Lerman I. Adherence to treatment: the key for avoiding long‐term complications of diabetes. Arch Med Res 2005; 36: 300–306. [DOI] [PubMed] [Google Scholar]
- 7. Cramer JA, Benedict A, Muszbek N, et al The significance of compliance and persistence in the treatment of diabetes, hypertension and dyslipidaemia: a review. Int J Clin Pract 2004; 62: 76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Iglay K, Cartier SE, Rosen VM, et al Meta‐analysis of studies examining medication adherence, persistence, and discontinuation of oral antihyperglycemic agents in type 2 diabetes. Curr Med Res Opin 2015; 31: 1283–1296. doi:10.1185/03007995.2015.1053048. [DOI] [PubMed] [Google Scholar]
- 9. Jha AK, Aubert RE, Yao J, et al Greater adherence to diabetes drugs is linked to less hospital use and could save nearly $5 billion annually. Health Aff (Millwood) 2012; 31: 1836–1846. [DOI] [PubMed] [Google Scholar]
- 10. Japan Diabetes Society . Evidence‐based Practice Guideline for the Treatment for Diabetes in Japan, 2013. Available from: http://www.jds.or.jp/modules/en/index.php?content_id=44 Accessed January 15, 2014.
- 11. Kohro T, Yamazaki T, Sato H, et al Trends in antidiabetic prescription patterns in Japan from 2005 to 2011: impact of the introduction of Dipeptidyl Peptidase‐4 inhibitors. Int Heart J 2013; 54: 93–97. [DOI] [PubMed] [Google Scholar]
- 12. Japan Medical Data Center Co., Ltd . JMDC Claims Database. n.p. n.d. Web. 12 Dec 2015. Available from: www.jmdc.co.jp/en/srv_pharma/jdm.html Accessed December 12, 2015.
- 13. Takeuchi M, Tomomasa T, Yasunaga H, et al Descriptive epidemiology of children hospitalized for inflammatory bowel disease in Japan: inpatient database analysis. Pediatr Int 2015; 57: 443–448. [DOI] [PubMed] [Google Scholar]
- 14. Fujimoto M, Higuchi T, Hosomi K, et al Association between statin use and cancer: data mining of a spontaneous reporting database and a claims database. Int J Med Sci 2015; 12: 223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Otsubo T, Goto E, Morishima T, et al Regional variations in in‐hospital mortality, care processes, and spending in acute ischemic stroke patients in Japan. J Stroke Cerebrovasc Dis 2015; 24: 239–251. [DOI] [PubMed] [Google Scholar]
- 16. Nakamura Y, Sugawara T, Kawanohara H, et al Evaluation of estimated number of influenza patients from national sentinel surveillance using the national database of electronic medical claims. Jpn J Infect Dis 2015; 68: 27–29. [DOI] [PubMed] [Google Scholar]
- 17. Kunisawa S, Tange C, Shimozuma K. Realities in cost‐effectiveness analyses: a study of castration‐resistant prostate cancer patients using a medical claims database. Springerplus 2015; 4: 624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sako A, Yasunaga H, Horiguchi H, et al Prevalence and in‐hospital mortality of gastrostomy and jejunostomy in Japan: a retrospective study with a national administrative database. Gastrointest Endosc 2014; 80: 88–96. [DOI] [PubMed] [Google Scholar]
- 19. Saito AM, Landrum MB, Neville BA, et al Hospice care and survival among elderly patients with lung cancer. J Palliat Med 2011; 14: 929–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rathmann W, Kostev K, Gruenberger JB, et al Treatment persistence, hypoglycaemia and clinical outcomes in type 2 diabetes patients with dipeptidyl peptidase‐4 inhibitors and sulphonylureas: a primary care database analysis. Diabetes Obes Metab 2012; 15: 55–61. [DOI] [PubMed] [Google Scholar]
- 21. Karter AJ, Parker MM, Moffet HH, et al New prescription medication gaps: a comprehensive measure of adherence to new prescriptions. Health Serv Res 2009; 44: 1640–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Curkendall SM, Thomas N, Bell KF, et al Predictors of medication adherence in patients with type 2 diabetes mellitus. Curr Med Res Opin 2013; 29: 1275–1286. [DOI] [PubMed] [Google Scholar]
- 23. Tunceli K, Zhao C, Davies MJ, et al Factors associated with adherence to oral antihyperglycemic monotherapy in patients with type 2 diabetes. Patient Prefer Adherence. 2015; 9: 191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McHorney CA. The Adherence Estimator: a brief, proximal screener for patient propensity to adhere to prescription medications for chronic disease. Curr Med Res Opin 2009; 25: 215–238. [DOI] [PubMed] [Google Scholar]
- 25. Uehara A, Kurotani K, Kochi T, et al Prevalence of diabetes and pre‐diabetes among workers: Japan Epidemiology Collaboration on Occupational Health Study. Diabetes Res Clin Pract 2014; 106: 118–127. [DOI] [PubMed] [Google Scholar]
- 26. Blackburn DF, Swidrovich J, Lemstra M. Non‐adherence in type 2 diabetes: practical considerations for interpreting the literature. Patient Prefer Adherence 2013; 7: 183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lehmann A, Aslani P, Ahmed R, et al Assessing medication adherence: options to consider. Int J Clin Pharm 2014; 36: 55–69. [DOI] [PubMed] [Google Scholar]
- 28. Karve S, Cleves MA, Helm M, et al Good and poor adherence: optimal cut‐point for adherence measures using administrative claims data. Curr Med Res Opin 2009; 25: 2303–2310. [DOI] [PubMed] [Google Scholar]


