Abstract
Aims/Introduction
To investigate the effect of pitavastatin on glucose control in patients with type 2 diabetes.
Materials and Methods
Medical records of 340 patients with type 2 diabetes treated with pitavastatin or atorvastatin between 1 August 2013 and 31 May 2014 were reviewed. A total of 96 patients who had not received statins were treated with pitavastatin (N to P group). A total of 100 patients who had previously used atorvastatin were switched to pitavastatin (A to P group). A total of 144 patients continued with atorvastatin treatment. Data were collected at baseline, 3 and 6 months of treatment. Changes in glycated hemoglobin (HbA1c) level were analyzed in 222 patients who did not change their antidiabetic agent during 6 months of treatment.
Results
A negative correlation between baseline HbA1c and delta HbA1c at 6 months was found in the pitavastatin‐treated patients (N to P group: ρ = −0.329, P = 0.006; A to P group: ρ = −0.480, P < 0.001). The correlation remained similar after adjusting for age, body mass index, dose of pitavastatin, estimated glomerular filtration rate and high‐density lipoprotein cholesterol. After 6 months of treatment, the benefit of pitavastatin on HbA1c in the patients with poorly controlled diabetes was significant in both the N to P (8.1 vs 7.4%, P = 0.018) and A to P (9.7 vs 9.0%, P = 0.015) groups.
Conclusions
Pitavastatin decreases HbA1c in patients with type 2 diabetes with a higher baseline HbA1c level. The benefit on HbA1c was also observed in patients with previous use of atorvastatin.
Keywords: Glycemic control, Statin, Type 2 diabetes mellitus
Introduction
Hydroxymethylglutaryl coenzyme A (HMG‐CoA) reductase inhibitor (statin) treatment for patients with dyslipidemia has been proven to decrease low‐density lipoprotein cholesterol (LDL‐C) and the risk of cardiovascular diseases, regardless of being the primary or secondary prevention1, 2. Recently, the risk of incident diabetes among patients treated with statins has gained attention1, 2, 3, 4, 5, 6, 7. In the Stroke Prevention by Aggressive Reduction in Cholesterol Levels trial, a higher frequency of new‐onset type 2 diabetes was found in patients randomized to receive atorvastatin 80 mg/day (hazard ratio [HR] 1.37, 95% confidence interval [CI] 1.08–1.75) compared with those who received a placebo3. In one population‐based cohort study, atorvastatin (adjusted HR 1.22, 95% CI 1.15–1.29), rosuvastatin (adjusted HR 1.18, 95% CI 1.10–1.26) and simvastatin (adjusted HR 1.10, 95% CI 1.04–1.17) were found to have an overall 10–22% increased risk of new‐onset diabetes compared with pravastatin4, which is consistent with the findings from previous meta‐analyses5, 6. However, the effect of pitavastatin treatment on the risk of new‐onset diabetes mellitus is controversial. Pitavastatin has been reported to reduce the risk of new‐onset diabetes in patients with impaired glucose tolerance by 18%, although research is still ongoing8, 9. Two prospective studies reported no deterioration in glucose metabolism in patients with metabolic syndrome and type 2 diabetes after treatment with pitavastatin for 1 year10, 11. Another recent study reported a higher risk of new‐onset diabetes in patients receiving pitavastatin compared with simvastatin (HR 2.68, P = 0.011)12.
As statins are frequently prescribed for patients with diabetes and dyslipidemia, the effect of statins on glycemic control in patients with type 2 diabetes is a concern. Although a few studies have reported no measurable effect of atorvastatin on the clinical course of diabetes mellitus13, 14, potential adverse effects with atorvastatin on glycemic control in patients with type 2 diabetes have been reported15, 16, 17, 18, 19, 20. Yamakawa et al.15 reported a significant increase in glycated hemoglobin (HbA1c) in patients with diabetes receiving atorvastatin treatment for 3 months (7.0 ± 1.1% to 7.4 ± 1.2%, P < 0.001), whereas only a minimal change in HbA1c in patients receiving pravastatin or pitavastatin. Takano et al.16 reported a similar adverse influence on HbA1c with atorvastatin treatment for 3 months (6.8 ± 0.9% to 7.2 ± 1.1%, P < 0.001). A substudy of the Pravastatin or Atorvastatin Evaluation in Myocardial Infarction study reported that atorvastatin 80 mg daily was associated with a significant risk of developing worse glycemic control in patients with diabetes (adjusted HR 2.36, 95% CI 1.45–3.86, P = 0.0006)16. In a subanalysis of the collaborative study on hypercholesterolemia drug intervention and their benefits for atherosclerosis prevention study, glycoalbumin was found to be significantly increased after 3 months of atorvastatin treatment (19.0 ± 3.4% to 19.7 ± 3.8%, P = 0.026)18. Another study showed that atorvastatin 20–40 mg daily for 12 weeks increased fasting blood glucose from baseline (7.2% increase, P < 0.05) in patients with diabetes, whereas pitavastatin 4 mg had no significant effect18. Mita et al.20 reported a favorable outcome of pitavastatin treatment on glycemic control in patients with type 2 diabetes compared with atorvastatin (the difference in HbA1c level was −0.18, 95% CI −0.34 to −0.02, P = 0.03).
The aim of the present study was to investigate the effect of pitavastatin, a moderately potent HMG‐CoA reductase inhibitor, on glycemic control in patients with type 2 diabetes who were either statin‐naïve or had previously been treated with atorvastatin.
Materials and Methods
Study participants
The present study was carried out at the diabetes outpatient clinics of Chang Gung Memorial Hospital at Linkou, a tertiary care center in Taiwan. Ethical approval was given by the Chang Gung Medical Foundation institutional review board (103‐4345). The medical records of 340 patients with type 2 diabetes treated with pitavastatin or atorvastatin for dyslipidemia were reviewed. From 1 August 2013 to 31 May 2014, 196 patients with type 2 diabetes who received their first treatment with pitavastatin for dyslipidemia were enrolled. A total of 96 patients who were not taking medication for dyslipidemia and who began pitavastatin treatment were defined as the N to P group. A total of 100 patients who had previously used atorvastatin for at least half a year and then switched treatment from atorvastatin to pitavastatin were defined as the A to P group. In the same period from 1 August 2013 to 31 May 2014, 144 patients who had been treated with atorvastatin for at least half a year and who continued the atorvastatin treatment were enrolled and defined as the A to A group. We excluded patients aged younger than 20 years, those with type 1 diabetes mellitus, those with major organ failure and those who had undergone an organ transplant. All patients received counseling with regard to lifestyle modifications including exercise and diet during the study period.
Study methods
The demographics and baseline laboratory data of all patients were collected from medical records. Serum lipid profiles and HbA1c levels were recorded at enrolment (baseline), and after 3 and 6 months of pitavastatin or atorvastatin treatment.
In the A to P group, the dose of treatment was converted either from atorvastatin 5 mg daily to pitavastatin 1 mg daily, or from atorvastatin 10 mg daily to pitavastatin 2 mg daily. For patients in the N to P group, the dose of pitavastatin (1 or 2 mg daily) was given according to clinical judgment to achieve a treatment goal of LDL‐C <100 mg/dL. For patients in the A to A group, the dose of atorvastatin was maintained at the same level.
Among the 340 patients, 222 (73 in the N to P group, 65 in the A to P group and 84 in the A to A group) did not change or adjust the dosage of their antidiabetic agents during the 6 months of the study period. Changes in glucose control after statin treatment were studied in these patients. Correlations between baseline HbA1c and delta HbA1c at 6 months (i.e., the HbA1c level at 6 months minus the HbA1c level at baseline) in the three study groups were analyzed. To further analyze changes in HbA1c between the patients with different baseline levels of HbA1c, we subgrouped the baseline HbA1c level into tertiles in each group.
Statistical analysis
The demographic and baseline laboratory data in the three groups were compared using the Kruskal–Wallis test and χ2‐test where appropriate. Changes in lipid and HbA1c levels between baseline and 3 months, and baseline and 6 months were assessed using the Wilcoxon signed‐rank test. Differences in lipid and HbA1c levels between the patients treated with pitavastatin 1 mg or 2 mg daily at baseline, 3, and 6 months were examined using the Mann–Whitney U‐test. Correlations between baseline HbA1c and delta HbA1c at 6 months were evaluated using Spearman's rank correlation. We adjusted for age, body mass index (BMI), estimated glomerular filtration rate, and high‐density lipoprotein cholesterol (HDL‐C) using partial correlation. The baseline HbA1c values were equally subgrouped into three tertiles (i.e., according to the 33rd and 66th percentiles) in each study group. In each tertile subgroup, differences in the level of HbA1c between baseline and 3 months, and between baseline and 6 months were examined using the Wilcoxon signed‐rank test. All statistical tests were carried out at a two‐tailed significance level of 0.05 using spss software version 22 (IBM SPSS Inc., Chicago, IL, USA).
Results
Demographic and baseline laboratory data
There were no significant differences in the baseline BMI, HDL‐C, triglycerides or HbA1c among the three groups (Table 1). Age, estimated glomerular filtration rate and duration of diabetes were significantly different among the three groups. Higher baseline levels of total cholesterol and LDL‐C were observed in the patients in the A to P and N to P groups compared with the patients in the A to A group. Metformin was the most commonly used antidiabetic agent in all three groups. The patients in the A to A group were treated more commonly with sulfonylurea, dipeptidyl peptidase‐4 inhibitors and insulin compared with the other two groups. There were no significant differences among the three groups in the percentages of using renin–angiotensin system (RAS) inhibitors (P = 0.315). In the 222 patients who did not change their antidiabetic agents during the study period, differences in the use of telmisartan or RAS inhibitors remained insignificant among the three studied groups (P = 0.457 and P = 0.753, respectively). The BMI after 6 months was also insignificantly different compared with baseline BMI in the three studied groups of the 222 patients (in the N to P group: P = 0.561; in the A to P group: P = 0.363; in the A to A group: P = 0.067).
Table 1.
Demographics of 340 patients with type 2 diabetes in the three groups divided by statin treatment
| Group | A to A | A to P | N to P | P‐value |
|---|---|---|---|---|
| n | 144 | 100 | 96 | |
| Age (years) | 65.0 (57.3–74.0) | 64.0 (58.0–70.8) | 61.0 (53.8–69.0) | 0.004 |
| Duration of diabetes (years) | 10.5 (7.3–15.0) | 10.1 (6.1–13.9) | 5.1 (2.0–8.0) | <0.001 |
| Sex (male) | 53.5% | 49.0% | 45.8% | 0.496 |
| Baseline bodyweight (kg) | 67.0 (61.1–75.0) | 67.0 (59.0–74.8) | 67.5 (59.6–78.0) | 0.716 |
| BMI, baseline (kg/m2) | 26.3 (25.0–28.6) | 25.6 (23.7–28.9) | 26.5 (23.8–28.9) | 0.638 |
| BMI, at 6 months (kg/m2) | 26.8 (25.2–28.6) | 25.7 (23.7–29.0) | 25.8 (23.2–28.9) | 0.453 |
| Creatinine (mg/dL) | 0.74 (0.57–0.92) | 0.79 (0.62–0.99) | 0.68 (0.53–0.84) | 0.010 |
| eGFR (mL/min/1.73 m2) | 104.5 (88.3–127.8) | 87.3 (69.3–106.5) | 103.3 (82.3–123.8) | <0.001 |
| ALT (U/L) | 21.0 (15.5–31.5) | 18.0 (15.0–24.0) | 19.0 (14.0–31.0) | 0.066 |
| Baseline TC (mg/dL) | 156.0 (141.0–174.0) | 171.0 (150.0–194.0) | 195.5 (182.8–221.0) | <0.001 |
| Baseline LDL‐C (mg/dL) | 88.0 (78.0–02.0) | 101.0 (88.3–115.5) | 137.5 (125.8–158.8) | <0.001 |
| Baseline TG (mg/dL) | 115.0 (79.3–158.8) | 111.0 (74.3–148.3) | 116.0 (90.0–152.0) | 0.642 |
| Baseline HDL‐C (mg/dL) | 43.0 (36.0–51.8) | 47.5 (39.3–56.0) | 46.0 (39.0–54.0) | 0.090 |
| Male | 39.0 (34.0–45.0) | 39.0 (33.0–46.0) | 39.0 (33.0–46.0) | 0.016 |
| Female | 47.0 (41.0–60.0) | 51.5 (44.8–56.3) | 51.5 (44.8–56.3) | 0.297 |
| TG/HDL‐C | 2.7 (1.7–4.1) | 2.4 (1.4–3.6) | 2.7 (1.7–3.6) | 0.395 |
| Baseline HbA1c (%) | 7.4 (6.8–8.3) | 7.4 (6.9–8.3) | 7.2 (6.7–7.9) | 0.112 |
| Atorvastatin dose | ||||
| 5 mg/day | 62.5% | 39.4% | – | – |
| 10 mg/day | 37.5% | 60.6% | – | – |
| Antidiabetic agents | ||||
| Metformin | 86.7% | 72.0% | 79.2% | 0.017 |
| Sulfonylurea | 76.2% | 39.0% | 33.3% | <0.001 |
| DPP4 inhibitors | 51.0% | 9.0% | 9.4% | <0.001 |
| Acarbose | 4.2% | 6.0% | 12.5% | 0.043 |
| Pioglitazone | 4.9% | 6.0% | 1.0% | 0.182 |
| Insulin | 26.6% | 5.0% | 4.2% | <0.001 |
| RAS inhibitors | 56.3% | 48.5% | 47.4% | 0.315 |
| Telmisartan | 3.7% | 2.1% | 1.5% | 0.671 |
Values not specified are median and interquartiles. The P‐values were calculated by the Kruskal–Wallis test except for sex, antidiabetic agents and renin–angiotensin system (RAS) inhibitors. RAS inhibitors included valsartan, irbesartan, losartan, olmesartan, telmisartan, candisartan, fosinopril, enalapril, captopril and aliskiren. A to A group, patients who continued atorvastatin treatment; A to P group, patients who were switched from atorvastatin to pitavastatin treatment; N to P group, patients who had no medication for dyslipidemia and began pitavastatin treatment. ALT, alanine aminotransferase; BMI, body mass index; DPP4, dipeptidyl peptidase‐4; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; TC, total cholesterol; TG, triglyceride.
Changes in lipid profile
In the N to P group, cholesterol‐lowering effects were found at 3 months (total cholesterol: 195.5 vs 161.5 mg/dL, P < 0.001; LDL‐C: 137.5 vs 97.0 mg/dL, P < 0.001) extending to 6 months of pitavastatin treatment (total cholesterol: 162.5 mg/dL, P < 0.001; LDL‐C: 97.0 mg/dL, P < 0.001; Table 2). A decrease in triglyceride level was also observed at 3 months (116.0 vs 108.0 mg/dL, P = 0.033) and 6 months (103.0 mg/dL, P = 0.008). An increase in HDL‐C was shown in patients with HDL‐C <40 mg/dL in the N to P group after both 3 and 6 months of treatment (Table 2). Differences in the increase in HDL‐C between male and female patients were further analyzed, and the results showed that the increase in HDL‐C occurred in male, but not female patients, with HDL‐C <40 mg/dL in the N to P group after both 3 months (33.5 vs 38.0 mg/dL, P = 0.016) and 6 months of treatment (36.0 mg/dL, P = 0.030). A significant decrease in triglyceride/HDL‐C level was noted at 3 months (2.7 vs 2.2, P = 0.024) extending to 6 months (2.1, P = 0.034).
Table 2.
Comparison of data between baseline and values at 3 or 6 months among the three statin groups
| Group | A to A (n = 144) | A to P (n = 100) | N to P (n = 96) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 3 months | 6 months | Baseline | 3 months | 6 months | Baseline | 3 months | 6 months | |
| n | 144 | 134 | 144 | 100 | 98 | 94 | 96 | 94 | 87 |
| HbA1c (%) | 7.4 (6.8–8.3) | 7.6 (6.9–8.4) | 7.5 (6.8–8.6) | 7.4 (6.9–8.3) | 7.4 (6.9–8.4) | 7.4 (6.8–8.6) | 7.2 (6.7–7.9) | 7.1 (6.5–8.0) | 7.1 (6.6–7.8) |
| TC (mg/dL) | 156.0 (141.0–174.0) | 152.5 (141.3–170.0) | 154.0 (141.0–172.0) | 171.0 (150.0–194.0) | 167.0 (148.5–186.5) | 163.0 (146.3–187.5) | 195.5 (182.8–221.0) | 161.5 (147.8–183.3)** | 162.5 (150.0–185.3)** |
| LDL‐C (mg/dL) | 88.0 (78.0–102.0) | 88.0 (79.0–103.0) | 91.0 (78.3–98.0) | 101.0 (88.3–115.5) | 100.5 (83.0–109.0) | 97.5 (83.0–109.0) | 137.5 (125.8, 158.8) | 97.0 (82.8–110.0)** | 97.0 (86.0–109.0)** |
| TG (mg/dL) | 115.0 (79.3–158.8) | 100.0 (71.0–148.0) | 102.0 (80.0–148.0) | 111.0 (74.3–148.3) | 107.0 (77.5–140.5) | 116.6 (78.0–147.0) | 116.0 (90.0–152.0) | 108.0 (85.0–148.0)* | 103.0 (79.8–137.8)* |
| HDL‐C (mg/dL) | 43.0 (36.0–51.8) | 44.0 (36.0–54.0) | 43.0 (36.0–51.8) | 46.0 (39.0–54.0) | 47.5 (39.3–56.0) | 48.0 (37.5–54.0) | 46.0 (39.0–54.0) | 48.0 (39.0–55.0) | 46.0 (41.0–53.0) |
| <40 | 35.0 (32.0–38.0) | 36.0 (33.0–39.0) | 35.0 (32.0–39.0) | 37.0 (34.0–38.0) | 38.0 (34.8–44.3)* | 37.0 (34.0–42.0) | 35.0 (30.5–37.5) | 37.5 (33.0–40.3)* | 37.0 (32.5–41.0)* |
| ≥40 | 48.0 (44.0–57.0) | 50.0 (43.8–58.3) | 48.0 (43.5–57.5) | 49.0 (43.5–61.5) | 52.0 (43.0–61.0) | 50.0 (45.0–59.0) | 49.0 (44.0–56.0) | 50.0 (45.3–55.8) | 49.0 (44.0–57.0) |
| TG/HDL‐C | 2.7 (1.7–4.1) | 2.4 (1.5–3.6) | 2.5 (1.6–3.8) | 2.4 (1.4–3.6) | 2.2 (1.6–3.1) | 2.3 (1.5–3.7) | 2.7 (1.7–3.6) | 2.2 (1.5–3.4)* | 2.1 (1.5–3.3)* |
Values are median and interquartiles. *P < 0.05 compared to the baseline values based on the Wilcoxon signed‐rank test. **P < 0.001 compared to the baseline values based on the Wilcoxon signed‐rank test. A to A group, patients who continued atorvastatin treatment; A to P group, patients who were switched from atorvastatin to pitavastatin treatment; N to P group, patients who had no medication for dyslipidemia and began pitavastatin treatment. HbA1c, glycated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; TC, total cholesterol; TG, triglyceride.
In the A to P group, there were no significant differences in total cholesterol, LDL‐C or triglycerides after changing to pitavastatin treatment. A transient increase in HDL‐C was observed in male patients with HDL‐C <40 mg/dL at 3 months after pitavastatin treatment in this group (36.5 vs 38.0 mg/dL, P = 0.019). Nevertheless, this effect was lost at 6 months of treatment.
No increase in HDL‐C was found in the patients with a normal baseline HDL‐C level in either the N to P group or A to P group. In the A to A group, there was no significant change in the levels of HbA1c, total cholesterol, LDL‐C, triglycerides, HDL‐C or triglycerides/HDL‐C at both 3 and 6 months of the observation period.
Changes in serum HbA1c
Correlations between baseline HbA1c and delta HbA1c at 6 months in the 222 patients who did not change their antidiabetic agents during the study period were analyzed according to their grouping (Figure 1). A significant correlation between delta HbA1c and baseline HbA1c was shown in the patients treated with pitavastatin. This negative correlation was found in the N to P group (Figure 1a; ρ = −0.329, P = 0.006) and more prominent in the A to P group (Figure 1b; ρ = −0.480, P < 0.001). There was no correlation between baseline HbA1c and the delta HbA1c in the A to A group (Figure 1c; ρ = −0.015, P = 0.895). The correlations remained significant after adjusting for age, body mass index, dose of pitavastatin, estimated glomerular filtration rate and HDL‐C (in the N to P group: ρ = −0.358, P = 0.016; in the A to P group: ρ = −0.478, P = 0.001; in the A to A group: ρ = 0.015, P = 0.933).
Figure 1.
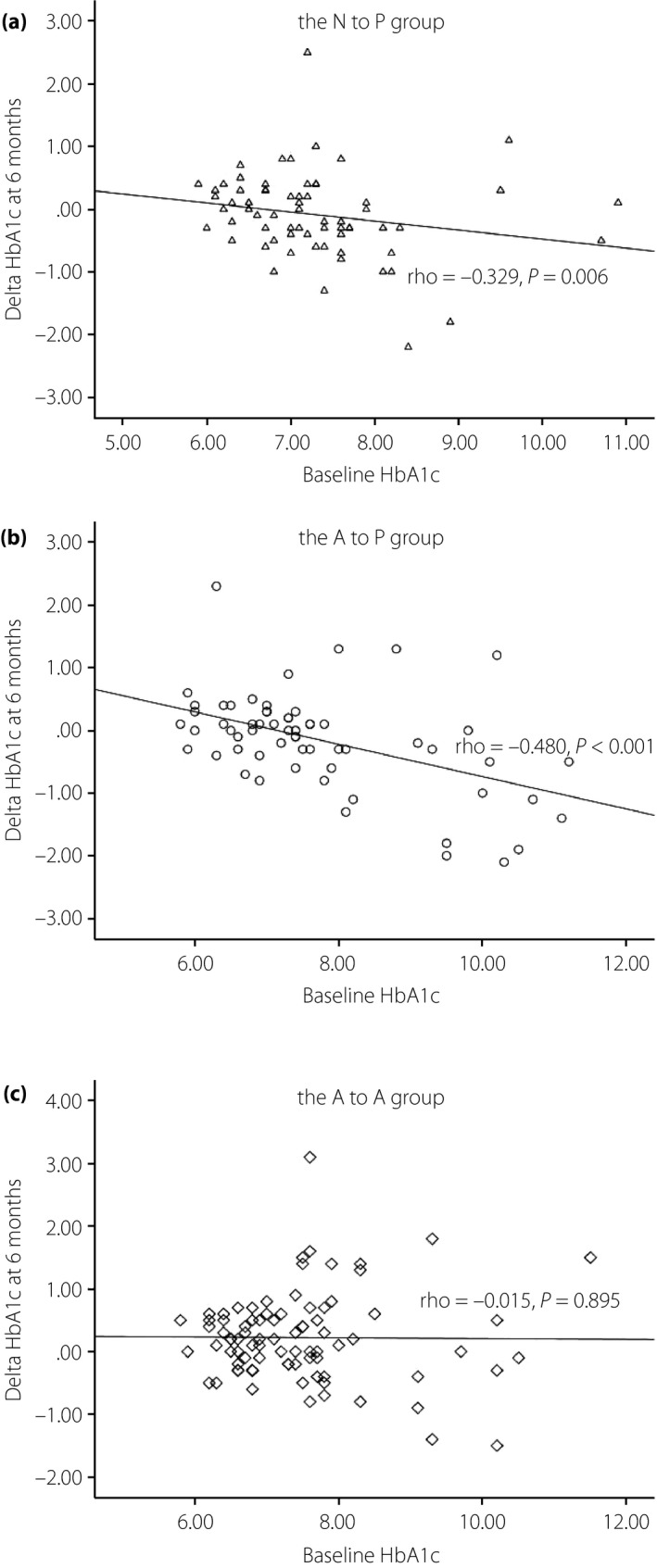
Correlations between baseline glycated hemoglobin (HbA1c) and delta HbA1c at 6 months in the three groups are shown. The baseline HbA1c and delta HbA1c of 222 patients with type 2 diabetes without adjustments for antidiabetic agents within 6 months of treatment were analyzed using Spearman's rank correlation. The plots and regression line were stratified by three groups. Negative correlations were found in (a) patients who had no medication for dyslipidemia and began pitavastatin treatment (N to P group; ρ = −0.329, P = 0.006) and (b) patients who were switched from atorvastatin to pitavastatin treatment (A to P group; ρ = −0.480, P < 0.001), (c) but not in patients who continued atorvastatin treatment (A to A group; ρ = −0.015, P = 0.895). The correlation remained significant after adjusting for age, body mass index, dose of pitavastatin, estimated glomerular filtration rate and high‐density lipoprotein cholesterol (in the N to P group: ρ = −0.358, P = 0.016; in the A to P group: ρ = −0.478, P = 0.001; in the A to A group: ρ = 0.015, P = 0.933).
To further investigate changes in serum HbA1c after pitavastatin treatment, we subgrouped the baseline HbA1c of each group into tertiles. Comparisons of baseline HbA1c and HbA1c at 3 and 6 months after pitavastatin treatment in each tertile of the three groups are shown in Table 3. There were no significant differences between baseline HbA1c and HbA1c at 3 or 6 months in all tertiles in the A to A group. There were no statistically significant differences between baseline HbA1c and HbA1c at 3 or 6 months after pitavastatin treatment among the patients in the first and second tertiles of the N to P and A to P groups. In contrast, a significant improvement in HbA1c was found in the third tertile of baseline HbA1c in both the N to P group and A to P group. In the poorest controlled tertile of patients in the N to P group, the level of HbA1c decreased from baseline 8.1 to 7.4% at 6 months (P = 0.018). A decrease in HbA1c was also observed in the poorest controlled tertile of patients in the A to P group, from 9.7% at baseline to 9.0% at 6 months (P = 0.015).
Table 3.
Comparison of glycated hemoglobin between baseline and values at 3 or 6 months in 222 patients who did not adjust their antidiabetic agents
| Tertiles of baseline HbA1c | A to A group (n = 84) | A to P group (n = 65) | N to P group (n = 73) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HbA1c (%) | HbA1c (%) | HbA1c (%) | |||||||
| Baseline | 3 months | 6 months | Baseline | 3 months | 6 months | Baseline | 3 months | 6 months | |
| 1st tertile | 6.6 (6.3–6.7) | 6.6 (6.3–6.9) | 6.6 (6.4–6.9) | 6.5 (6.0–6.8) | 6.7 (6.3–6.9) | 6.5 (6.1–6.9) | 6.5 (6.3–6.7) | 6.4 (6.2–6.8) | 6.5 (6.3–6.9) |
| 2nd tertile | 7.4 (7.1–7.5) | 7.6 (7.2–8.0) | 7.6 (7.1–7.9) | 7.4 (7.2–7.6) | 7.3 (7.2–7.5) | 7.3 (7.2–7.7) | 7.1 (7.0–7.3) | 7.0 (6.6–7.2) | 7.1 (6.7–7.7) |
| 3rd tertile | 8.3 (7.8–9.3) | 8.4 (7.9–9.6) | 8.5 (7.7–9.7) | 9.7 (8.2–10.4) | 9.1 (8.0–10.1) | 9.0 (7.7–9.8)* | 8.1 (7.6–8.5) | 8.0 (7.2–8.3) | 7.4 (7.2–8.2)* |
Values are median and interquartiles. *P < 0.05 compared with the baseline values based on the Wilcoxon signed–rank test. A to A group, patients who continued atorvastatin treatment; A to P group, patients who were switched from atorvastatin to pitavastatin treatment; N to P group, patients who had no medication for dyslipidemia and began pitavastatin treatment. The 33rd and 66th percentiles for the A to A group were 6.8 and 7.7, for the A to P group were 6.9 and 7.8, and for the N to P group were 6.7 and 7.4.
Effects of different doses of pitavastatin
In the N to P group, the total cholesterol and LDL‐C levels were higher at baseline in the patients who were prescribed with pitavastatin 2 mg daily compared with those prescribed with 1 mg daily (total cholesterol: 212.0 vs 193.0 mg/dL, P = 0.019; LDL‐C: 151.0 vs 132.0 mg/dL, P < 0.001); however, their total cholesterol and LDL‐C levels were not different at 3 and 6 months (total cholesterol: 161.0 vs 162.0 mg/dL at 3 months, P = 0.385, and 159.0 vs 165.0 mg/dL at 6 months, P = 0.501; LDL‐C: 95.0 vs 97.0 mg/dL at 3 months, P = 0.800, and 98.0 vs 95.0 mg/dL at 6 months, P = 0.997). Furthermore, the levels of HDL‐C and triglycerides at baseline, 3, and 6 months did not differ between the patients prescribed with pitavastatin 1 mg daily and 2 mg daily in the N to P group. In the A to P group, there was no significant difference between the patients taking pitavastatin 1 mg daily and 2 mg daily in the lipid profile at baseline, 3, and 6 months. In both the N to P and A to P groups, there were no significant differences in serum HbA1c level between pitavastatin 1 mg daily and 2 mg daily at baseline, 3 and 6 months.
Discussion
Previous studies have shown that pitavastatin 2 mg and atorvastatin 10 mg are equally effective in improving LDL‐C, total cholesterol, and triglycerides1, 21, 22. Pitavastatin has been reported to have the effects of decreasing LDL‐C and increasing HDL‐C in both patients with or without diabetes. However, atorvastatin has been reported to only have an effect on lowering LDL‐C, but not on increasing HDL‐C in both patients with or without diabetes1, 21, 23, 24. It has also been reported that pitavastatin increases the production of apolipoprotein A1 in HepG2 cells at lower concentrations compared with atorvastatin25. In addition, pitavastatin has been shown to facilitate an increase in HDL‐C through stimulating lipoprotein lipase activity in 3T3‐L1 preadipocytes more potently than atorvastatin26. In the current study, pitavastatin was effective in raising HDL‐C, and lowering total cholesterol, LDL‐C and triglycerides in the statin‐naïve patients with diabetes, which is consistent with previous reports1, 21, 22, 24, 27. Teramoto et al.27 reported that the effect on increasing HDL‐C was more prominent in patients with HDL‐C levels less than 40 mg/dL at baseline. Interestingly, we observed that HDL‐C was significantly increased in male patients in the A to P group who had a baseline HDL‐C level less than 40 mg/dL at 3 months, suggesting an overriding effect of pitavastatin in patients who had previously received atorvastatin treatment.
The present study is the first to identify a correlation between baseline serum HbA1c levels and the beneficial effects of lowering HbA1c in patients with type 2 diabetes receiving pitavastatin treatment. The correlation between baseline HbA1c and improvements in HbA1c remained significant after adjusting for age, estimated glomerular filtration rate, BMI, dose of pitavastatin and baseline HDL‐C. Only the patients with poorly controlled diabetes experienced a benefit from lowering glucose with pitavastatin treatment in the present study, and this might explain why the beneficial effect on glucose with pitavastatin treatment is not consistent between studies11, 12, 19, 20. Of note, an effect on lowering HbA1c with pitavastatin treatment was found in patients who had previously received atorvastatin treatment.
Improved homeostasis model assessment of insulin resistance has been reported in Japanese patients with type 2 diabetes who were treated with pitavastatin28. Another study reported that HDL‐C and apolipoprotein A1 ameliorate glucose metabolism by both improving insulin resistance and increasing homeostasis model assessment of β‐cell function in patients with type 2 diabetes29. With regard to increases in HDL‐C and apolipoprotein A1, pitavastatin might be helpful not only in preventing cardiovascular disease, but also in glucose metabolism25, 26, 29. We lacked data on homeostasis model assessment of insulin resistance and homeostasis model assessment of insulin secretion in the present study, though there was no significant difference between BMI after 6 months and at baseline in the three studied groups of 222 patients. The present results suggest that the correlation between baseline HbA1c and improvements in HbA1c was independent of age, BMI, dose of pitavastatin, estimated glomerular filtration rate, and HDL‐C. Mechanisms other than these factors are probably involved in the beneficial effects of pitavastatin treatment on glucose metabolism. Some studies have reported that angiotensin II receptor blockers, and especially telmisartan, have beneficial effects in hypertensive patients with type 2 diabetes by activating peroxisome proliferator‐activated receptor‐γ, improving insulin resistance, increasing the level of adiponectin and decreasing the level of high‐sensitivity C‐reactive protein30, 31. Because the distribution of the use of RAS inhibitors was insignificant among the three study groups including 222 patients who did not change their antidiabetic agents during the study period, the benefits on changes in HbA1c after pitavastatin treatment are probably not related to the use of telmisartan or RAS inhibitors. Atorvastatin has been reported to impair insulin secretion and suppress GLUT4 expression in 3T3‐L1 adipose cells32, 33. Interestingly, we also found that only the patients with poorly controlled diabetes had a beneficial glucose‐lowering effect from pitavastatin treatment. Although we did not identify a definitive mechanism, the beneficial effects of adding pitavastatin and/or removing atorvastatin could explain the reason why the HbA1c level was significantly reduced in the patients with poorly controlled diabetes who might have had relatively worse β‐cell function and/or insulin resistance. Compared with the patients in the A to A group, the patients in both the N to P and A to P groups were treated less commonly with sulfonylurea, dipeptidyl peptidase‐4 inhibitors, and insulin. It is not clear whether the HbA1c‐lowering effect of pitavastatin would be similar in the diabetic patients who were treated more commonly with insulin, sulfonylurea and dipeptidyl peptidase‐4 inhibitors.
The present study was limited by the retrospective design, the relatively short observation period, and the lack of data on β‐cell function and insulin resistance. Further large‐scale trials are warranted to assess the outcomes of long‐term clinical events from pitavastatin treatment.
In conclusion, other than decreasing LDL‐C in patients with type 2 diabetes who were naive to statins, pitavastatin treatment increased HDL‐C in patients with a lower baseline HDL‐C level, and decreased HbA1c in those with a higher baseline HbA1c level. A benefit with regard to HbA1c was also observed in patients who had previously received atorvastatin treatment.
Disclosure
The authors declare no conflict of interest.
Acknowledgment
We thank Hsing‐Fen Alfred Lin for assistance with statistics.
J Diabetes Investig 2016; 7: 769–776
References
- 1. Maruyama T, Takada M, Nishibori Y, et al Comparison of preventive effect on cardiovascular events with different statins. Circ J 2011; 75: 1951–1959. [DOI] [PubMed] [Google Scholar]
- 2. Nicholls SJ, Tuzcu EM, Sipahi I, et al Statins, high‐density lipoprotein cholesterol, and regression of coronary atherosclerosis. JAMA 2007; 297: 449–508. [DOI] [PubMed] [Google Scholar]
- 3. Waters DD, Ho JE, DeMicco DA, et al Predictors of new‐onset diabetes in patients treated with atorvastatin. J Am Coll Cardiol 2011; 57: 1535–1545. [DOI] [PubMed] [Google Scholar]
- 4. Carter AA, Gomes T, Camacho X, et al Risk of incident diabetes among patients treated with statins: population based study. BMJ 2013; 346: f2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Preiss D, Seshasai SRK, Welsh P, et al Risk of incident diabetes with intensive‐dose compared with moderate‐dose statin therapy. JAMA 2011; 305: 2556–2564. [DOI] [PubMed] [Google Scholar]
- 6. Sattar N, Preiss D, Murray HM, et al Statin and risk of incident diabetes: a collaborative meta‐analysis of randomised statin trials. Lancet 2010; 375: 735–742. [DOI] [PubMed] [Google Scholar]
- 7. Chen JCN. Risk‐benefit analysis of use of statins for primary prevention of cardiovascular disease in subjects without diabetes. J Diabetes Investig 2013; 4: 344–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yamazaki T, Kishimoto J, Ito C, et al Japan Prevention Trial of Diabetes by Pitavastatin in Patients with Impaired Glucose Tolerance (the J‐PREDICT study): rationale, study design, and clinical characteristics of 1269 patients. Diabetol Int 2011; 2: 134–140. [Google Scholar]
- 9. Odawara M, Yamazaki T, Kishimoto J, et al Pitavastatin for the delay or prevention of diabetes prevention in individuals with impaired glucose tolerance In: 61‐LB, 73th American Diabetes Association Annual Meeting 2013.
- 10. Choi SH, Lim S, Hong ES, et al PROPIT: a PROspective comparative clinical study evaluating the efficacy and safety of PITavastatin in patients with metabolic syndrome. Clin Endocrinol 2015; 82: 670–677. [DOI] [PubMed] [Google Scholar]
- 11. Daido H, Horikawa Y, Takeda J. The effects of pitavastatin on glucose metabolism in patients with type 2 diabetes with hypercholesterolemia. Diabetes Res Clin Pract 2014; 106: 531–537. [DOI] [PubMed] [Google Scholar]
- 12. Cho Y, Choe E, Lee YH, et al Risk of diabetes in patients treated with HMG‐CoA reductase inhibitors. Metabolism 2014; 64: 482–488. [DOI] [PubMed] [Google Scholar]
- 13. Sasaki J, Iwashita M, Kono S. Statins: beneficial or adverse for glucose metabolism. J Atheroscler Thromb 2006; 13: 123–129. [DOI] [PubMed] [Google Scholar]
- 14. Son JW, Kim DJ, Lee CB, et al Effects of patient‐tailored atorvastatin therapy on ameliorating the levels of atherogenic lipids and inflammation beyond lowering low‐density lipoprotein cholesterol in patients with type 2 diabetes. J Diabetes Investig 2013; 4: 466–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yamakawa T, Takano T, Tanaka S, et al Influence of pitavastatin on glucose tolerance in patients with type 2 diabetes mellitus. J Atheroscler Thromb 2008; 15: 269–275. [DOI] [PubMed] [Google Scholar]
- 16. Takano T, Yamakawa T, Takahashi M, et al Influence of statins on glucose tolerance in patients with type 2 diabetes mellitus. J Atheroscler Thromb 2006; 13: 95–100. [DOI] [PubMed] [Google Scholar]
- 17. Sabatine MS, Wiviott SD, Morrow DA, et al High dose atorvastatin associated with worse glycemic control: a PROVE‐IT TIMI 22 substudy. Circulation 2004; 110(Supplement III): 834. [Google Scholar]
- 18. Yokote K, Saito Y; CHIBA study investigators . Influence of statins on glucose tolerance in patients with type 2 diabetes mellitus: subanalysis of the collaborative study on hypercholesterolemia drug intervention and their benefits for atherosclerosis prevention (CHIBA study). J Atheroscler Thromb 2009; 16: 297–298. [DOI] [PubMed] [Google Scholar]
- 19. Gumprecht J, Gosho M, Budinski D, et al Comparative long‐term efficacy and tolerability of pitavastatin 4 mg and atorvastatin 20–40 mg in patients with type 2 diabetes mellitus and combined (mixed) dyslipidaemia. Diabetes Obes Metab 2011; 13: 1047–1055. [DOI] [PubMed] [Google Scholar]
- 20. Mita T, Nakayama S, Abe H, et al Comparison of effects of pitavastatin and atorvastatin on glucose metabolism in type 2 diabetic patients with hypercholesterolemia. J Diabetes Investig 2013; 4: 297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yokote K, Bujo H, Hanaoka H, et al Multicenter collaborative randomized parallel group comparative study of pitavastatin and atorvastatin in Japanese hypercholesterolemic patients‐collaborative study on hypercholesterolemia drug intervention and their benefits for atherosclerosis prevention (CHIBA study). Atherosclerosis 2008; 201: 345–352. [DOI] [PubMed] [Google Scholar]
- 22. Budinski D, Arneson V, Hounslow N, et al Pitavastatin compared with atorvastatin in primary hypercholesterolemia or combined dyslipidemia. Clin Lipidol 2009; 4: 291–302. [Google Scholar]
- 23. Hiro T, Kimura T, Morimoto T, et al Diabetes mellitus is a major negative determinant of coronary plaque regression during statin therapy in patients with acute coronary syndrome – serial intravascular ultrasound observations from the Japan Assessment of Pitavastatin and Atorvastatin in Acute Coronary Syndrome Trial (the JAPAN‐ACS Trial). Circ J 2010; 74: 1165–1174. [DOI] [PubMed] [Google Scholar]
- 24. Sasaki J, Ikeda Y, Kuribayashi T, et al A 52‐week, randomized, open‐label, parallel‐group comparison on the tolerability and effects of pitavastatin and atorvastatin on high‐density lipoprotein cholesterol levels and glucose metabolism in Japanese patients with elevated levels of low‐density lipoprotein and glucose intolerance. Clin Ther 2008; 30: 1089–1101. [DOI] [PubMed] [Google Scholar]
- 25. Maejima T, Yamazaki H, Aoki T, et al Effect of pitavastatin on apolipoprotein A‐I production in HepG2 cell. Biochem Biophys Res Commun 2004; 324: 835–839. [DOI] [PubMed] [Google Scholar]
- 26. Saiki A, Murano T, Watanabe F, et al Pitavastatin enhanced lipoprotein lipase expression in 3T3‐L1 preadipocytes. J Atheroscler Thromb 2005; 12: 163–168. [DOI] [PubMed] [Google Scholar]
- 27. Teramoto T, Shimano H, Yokoto K, et al Effects of pitavastatin (LIVALO Tablet) on high density lipoprotein cholesterol (HDL‐C) in hypercholesterolemia. J Atheroscler Thromb 2009; 16: 654–661. [DOI] [PubMed] [Google Scholar]
- 28. Katayama T. Usefulness of pitavastatin on lipid profile and glucose metabolism in hypercholesterolemia with diabetes mellitus. Prog Med 2005; 25: 3110–3115 (Japanese). [Google Scholar]
- 29. Drew BG, Duffy SJ, Formosa MF, et al High‐density lipoprotein modulates glucose metabolism in patients with type 2 diabetes mellitus. Circulation 2009; 119: 2103–2111. [DOI] [PubMed] [Google Scholar]
- 30. Yamada A, Arita M, Furuta M, et al The angiotensin II receptor blocker telmisartan improves insulin resistance and has beneficial effects in hypertensive patients with type 2 diabetes and poor glycemic control. Diabetes Res Clin Pract 2008; 82: 127–131. [DOI] [PubMed] [Google Scholar]
- 31. Kurtz TW. New treatment strategies for patients with hypertension and insulin resistance. Am J Med 2006; 119: 24S–30S. [DOI] [PubMed] [Google Scholar]
- 32. Ishikawa M, Okajima F, Inoue N, et al Distinct effects of pravastatin, atorvastatin, and Simvastatin on insulin secretion from a beta‐cell line, MIN6 cells. J Atheroscler Thromb 2006; 13: 329–335. [DOI] [PubMed] [Google Scholar]
- 33. Nakata M, Nagasaka S, Kusaka I, et al Effects of statins on the adipocyte maturation and expression of glucose transporter 4 (SLC2A4): implications in glycaemic control. Diabetologia 2006; 49: 1881–1892. [DOI] [PubMed] [Google Scholar]


