Abstract
Aims/Introduction
To assess the efficacy and safety of acetyl‐L‐carnitine (ALC) on diabetic peripheral neuropathy compared with methylcobalamin (MC).
Materials and methods
This was a multicenter, randomized, parallel‐group, double‐blind, double‐dummy, positive‐controlled, non‐inferior phase II clinical trial. Diabetic patients with abnormal nerve conduction test results were randomized in a 1:1 ratio to receive oral ALC 500 mg t.i.d. or MC 0.5 mg t.i.d. for 24 weeks. The neuropathy symptom score, neuropathy disability score and neurophysiological parameters were measured during follow up.
Results
A total of 232 patients were randomized (ALC n = 117, MC n = 115), 88% of which completed the trial. At week 24, patients from both groups had significant reductions in both neuropathy symptom score and neuropathy disability score with no significant difference between two groups (neuropathy symptom score reduction: ALC vs MC 2.35 ± 2.23, P < 0.0001 vs 2.11 ± 2.48, P < 0.0001, intergroup P = 0.38; neuropathy disability score reduction ALC vs MC 1.66 ± 1.90, P < 0.0001 vs 1.35 ± 1.65, P < 0.0001, intergroup P = 0.23). Neurophysiological parameters were also improved in both groups. No significant difference was found between groups in the development of adverse events.
Conclusions
ALC is as effective as MC in improving clinical symptoms and neurophysiological parameters for patients with diabetic peripheral neuropathy over a 24‐week period with good tolerance.
Keywords: Acetyl‐L‐carnitine, Diabetic peripheral neuropathy, Methylcobalamin
Introduction
Diabetic peripheral neuropathy (DPN) is one of the most common chronic complications of diabetes mellitus1, with a 30–50% prevalence in diabetic patients2. DPN commonly presents with distal symmetric polyneuropathy, and is diagnosed and evaluated based on clinical symptoms and electrophysiological examinations. The progressive development of pain, numbness and sensory or motor disorders obviously affects patients’ quality of life, laying great clinical value on its prevention and treatment.
The pathogenic mechanisms of DPN are not fully understood. Hyperglycemia is an important etiology of DPN, and antihyperglycemic treatment is fundamental for long‐term prevention and management of DPN. However, simple blood glucose control is not always sufficient. A variety of agents with potential effect on the pathogenic pathway of DPN have been studied, including aldose reductase inhibitors (ARI)3, α‐lipoic acid4, recombinant human nerve growth factor5, angiotensin‐converting enzyme inhibitor6 and γ‐linolenic acid7. Nevertheless, current managements are still not able to achieve satisfactory neuropathic pain reduction8.
Acetyl‐L‐carnitine (ALC; also known as levacecarnine and ALCAR) deficiency plays a primary role in the development of DPN in diabetic patients9. A recent meta‐analysis of randomized controlled clinical trials showed that ALC significantly reduced neuropathic pain, especially in that caused by diabetes, compared with placebo10. Previous uncontrolled trials11, 12 also supported the efficacy and safety of ALC on DPN. However, ALC is not introduced as a treatment alternative in the latest guideline of the American Academy of Neurology8. Clinical evidence comparing ALC with active medications in DPN is lacking.
Methylcobalamin (MC), a methylated derivative of vitamin B12, has been suggested to be beneficial on alleviating neuropathic pain symptoms and on improving nerve conduction, especially in the Chinese population13, 14. It has been approved by the China Food and Drug Administration for treating peripheral neuropathy, and is recommended in the Chinese guideline for type 2 diabetes. In the current trial, we compared the efficacy and safety of ALC and MC in patients with DPN.
Methods
Study design and patients
This multicenter, randomized, parallel‐group, double‐blind, double‐dummy, positive‐controlled, non‐inferior phase II clinical trial was carried out between August 2008 and March 2011 in eight centers in China (ChiCTR‐TRC‐08000141).
Men and women with type 1 or type 2 diabetes mellitus were eligible to participate if they were aged between 18 and 70 years, had been diagnosed with DPN according to electrodiagnostic criteria from San Antonio Conference15, and had abnormal nerve conduction velocity (NCV) and/or amplitude found in at least one nerve of the extremities. Negative urine or blood test for pregnancy was an additional requirement for women of reproductive age. Diagnosis of diabetes was made according to 1999 World Health Organization criteria16.
Exclusion criteria included unstable blood glucose control or glycated hemoglobin (HbA1c) >8.5% within 2 weeks before the study; established non‐diabetic causes for peripheral neuropathy, such as HIV and chemotherapy; history of allergy or intolerance to ALC; history of or current treatment for thyroid disorders; severe hemorrhagic diseases; peptic ulcer; grade III hypertension, unstable angina pectoris, severe arrhythmia, cardiopulmonary dysfunction, cardiac pacemaker or stent, or myocardial infarction within 6 months before the study; impaired renal or hepatic function (serum concentrations of alanine transaminase or aspartate transaminase more than twice the upper limit of normal range; serum creatinine higher than the upper limit of normal range); malignant cancer; lactating or pregnant women, men or women of reproductive age refusing to use effective contraception during the study; history of alcohol or drug abuse within 1 year before the study; and participation in other clinical trials currently or within 3 months before the study. During the study, therapies known to affect the nervous system (e.g., aldose reductase inhibitors, gangliosides or acupuncture) were avoided. Oral hypoglycemic agents or insulin were maintained. Other therapies for concomitant diseases were allowed, but monitored during the trial.
All participants provided written informed consent before the study. The study was carried out in accordance with the Declaration of Helsinki and the International Conference on Harmonization Guidelines for Good Clinical Practice, and was approved by the China Food and Drug Administration (2005L01756). The study protocol was approved by the ethics committee of the West China Hospital of Sichuan University.
Randomization and masking
Computer‐generated randomization lists were produced by each center, sealed in opaque envelops and assigned to participants by physicians according to the sequence of entry to the study. ALC, MC and dummy tablets were identical in appearance, and were provided by the Liaoning Haisike Pharmaceutical Co. Ltd. in individualized patient kits with only one number on each tablet for patient matching. Patients and investigators, including the investigators assessing nerve conduction and blood tests, were masked to treatment assignment throughout the study.
Procedures
After screening, eligible patients were randomized in a 1:1 ratio to receive oral treatment with ALC (500 mg three times per day) or MC (0.5 mg three times per day) for 24 weeks. Three times per day after every meal, patients in the ALC group received two ALC tablets (250 mg ALC per tablet) plus one dummy tablet, whereas those in the MC group received one MC tablet (0.5 mg MC per tablet) plus two dummy tablets.
The primary end‐point was the changes in the neuropathic symptom and sign scores from baseline to week 24, assessed by the neuropathy symptom score (NSS), the neuropathy disability score (NDS) and the sum of both (NSS+NDS). The secondary end‐points included changes in the NSS and in the NDS from baseline at week 12, change in the NCV and amplitude from baseline to week 24, and the reversal rates of affected nerves at week 24. Measurements of the NSS and NDS at baseline, week 12, and week 24 were carried out by trained investigators using standard questionnaires, which were used as clinical assessment tools of DPN17. The measurements of the NCV and amplitude were carried out at baseline and week 24 by one neurologist in each center according to the guideline of standardized measures in diabetic neuropathy18, and were carried out on the affected side of the body for patients with unilateral symptoms or both sides for patients with bilateral symptoms. The NCV and amplitude in the median sensory and motor, ulnar sensory and motor, peroneal sensory, tibial sensory, and sural motor nerves were measured.
Safety end‐points included incidence and intensity of adverse events, withdrawals as a result of adverse events, changes in fasting blood glucose and HbA1c, abnormal electrocardiographs, and changes in vital signs, laboratory variables and background treatment.
During the study period, patients visited their local study center every 4 weeks, to receive a tablet count for compliance assessment, to report adverse events and to report changes in background treatments.
Statistical analysis
A sample size of 113 participants per treatment group was required to achieve 95% power to show non‐inferiority for treatment difference through a 1.2 score reduction of the NSS+NDS from baseline to week 24 at the level of α = 0.025 (one‐sided), taking into account a 20% dropout.
All primary and secondary efficacy analyses were carried out in both the full analysis set (FAS) and the per‐protocol set. The FAS population included all randomized patients receiving at least one dose of study treatment, and the last observation carried forward approach was used to impute missing data. Analysis of covariance (ancova) was also carried out in assessment of the change in the NSS+NDS from baseline at week 24, to explore the effect of baseline NSS+NDS, baseline HbA1c level, diabetes duration, center and treatment group on the primary end‐point.
All data analyses were carried out using the sas program system (version 9.1; SAS Institute, Cary, NC, USA). Data are presented as mean ± standard deviation. Non‐inferiority analysis for primary efficacy was carried out by one‐tailed Mann–Whitney U‐test with a level of significance being P < 0.025. Baseline parameters in both groups were compared by t‐test.
Intragroup changes from baseline were analyzed by paired samples t‐test for normal distributed data and Wilcoxon signed‐rank test for non‐normal distributed data. Intergroup comparisons were carried out by independent sample t‐test for normally distributed data and Wilcoxon rank–sum test for non‐normally distributed data. All statistical tests were two‐sided with a level of significance being α < 0.05. Dichotomous baseline characteristics, reversal rates and incidence of adverse events were compared by χ2‐test or Fisher's exact test.
Results
A total of 232 patients from eight centers were randomized to receive either ALC (n = 117) or MC (n = 115). A total of 204 patients (88%) completed the 24‐week study (ALC n = 103 [88%], MC n = 101 [88%]), and the dropout rate was not significantly different between the two groups (P = 0.96). Details are shown in Figure 1. The two treatment groups were well balanced with respect to demographic characteristics, vital signs, NSS, NDS, fasting blood glucose, HbA1c, laboratory assessments and proportion of patients with abnormal electrocardiographs (Table 1).
Figure 1.
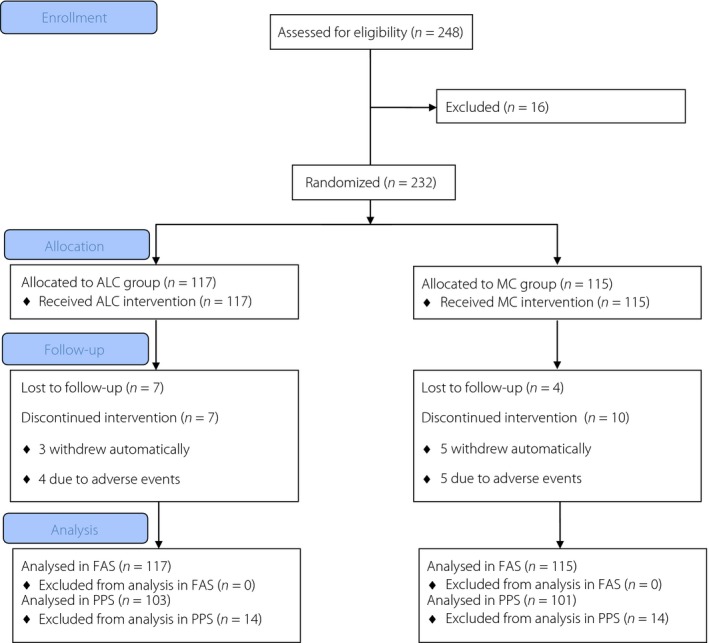
Trial profile. ALC, acetyl‐L‐carnitine; FAS, full analysis set; MC, methylcobalamin; PPS, per‐protocol set.
Table 1.
Baseline characteristics of the study population
| ALC group (n = 117) | MC group (n = 115) | P‐value | |
|---|---|---|---|
| Demographic parameters | |||
| Age (years) | 57.82 ± 8.72 | 57.75 ± 7.92 | 0.95 |
| Female (n/%) | 60/51.28 | 50/43.48 | 0.23 |
| Diabetes duration (months) | 118.36 ± 94.89 | 102.67 ± 77.90 | 0.33 |
| Vital signs | |||
| Temperature (°C) | 36.52 ± 0.35 | 36.46 ± 0.37 | 0.30 |
| Heart rate (cpm) | 77.65 ± 8.80 | 77.15 ± 9.29 | 0.67 |
| Respiratory (cpm) | 17.62 ± 1.70 | 17.58 ± 1.89 | 0.90 |
| SBP (mmHg) | 127.32 ± 14.19 | 127.90 ± 15.09 | 0.93 |
| DBP (mmHg) | 76.88 ± 8.36 | 76.74 ± 8.62 | 0.90 |
| Neurological parameters | |||
| NSS | 6.52 ± 1.52 | 6.37 ± 1.71 | 0.48 |
| NDS | 6.58 ± 2.19 | 6.43 ± 2.04 | 0.57 |
| NSS+NDS | 13.10 ± 2.80 | 12.79 ± 2.80 | 0.40 |
| Laboratory tests | |||
| TSH (mU/L) | 2.54 ± 1.97 | 2.58 ± 2.75 | 0.13 |
| WBC (109/L) | 5.86 ± 1.72 | 5.69 ± 1.57 | 0.98 |
| RBC (1012/L) | 4.46 ± 0.46 | 4.53 ± 0.48 | 0.35 |
| HB (g/L) | 134.41 ± 14.81 | 136.25 ± 15.95 | 0.42 |
| PLT (109/L) | 187.49 ± 74.02 | 178.05 ± 60.12 | 0.44 |
| ALT (U/L) | 22.35 ± 11.81 | 22.98 ± 9.96 | 0.31 |
| AST (U/L) | 23.13 ± 8.19 | 23.23 ± 8.41 | 0.94 |
| TBIL (μmol/L) | 13.68 ± 5.98 | 12.78 ± 4.92 | 0.30 |
| BUN (mmol/L) | 6.26 ± 2.09 | 5.99 ± 1.93 | 0.39 |
| Cr (μmol/L) | 68.13 ± 16.09 | 67.49 ± 14.23 | 0.75 |
| FPG (μmol/L) | 7.58 ± 2.48 | 7.44 ± 3.04 | 0.27 |
| HbA1c (%) | 7.10 ± 1.16 | 6.96 ± 1.35 | 0.52 |
| ECG | |||
| ECG, abnormal (n/%) | 30/25.86 | 28/25.00 | 0.88 |
All continuous variables are presented as mean ± standard deviation. Continuous parameters were compared by independent sample t‐test. Dichotomous parameters were compared by χ2‐test. SD, standard deviation; ALC, acetyl‐L‐carnitine; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; cpm, counts per min; Cr, creatinine; DBP, diastolic blood pressure; ECG, electrocardiograph; FPG, fasting plasma glucose; HB, hemoglobin; HbA1c, glycated hemoglobin; MC, methylcobalamin; NDS, neuropathy disability score; NSS, neuropathy symptom score; PLT, platelet; RBC, red blood cell; SBP, systolic blood pressure; TBIL, total bilirubin; TSH, thyroid‐stimulating hormone; WBC, white blood cell.
Comparison of the effects on improvement of the clinical scores of DPN
In the FAS population, the sum of NSS and NDS was reduced significantly in both ALC and MC groups at week 24 compared with baseline (Table 2), with no significant difference found between changes in the two groups (change in ALC vs MC 4.01 ± 3.25 vs 3.46 ± 3.43, intergroup P = 0.14). The change of summed NSS and NDS in the ALC group was non‐inferior to that in the MC group (U = 3.98, P < 0.025). A similar trend was observed for the individual NSS (change in ALC vs MC 2.35 ± 2.23 vs 2.11 ± 2.48, intergroup P = 0.38) and for the individual NDS (change in ALC vs MC 1.66 ± 1.90 vs 1.35 ± 1.65, intergroup P = 0.23). Analyzed covariates, including baseline NSS+NDS, baseline HbA1c level, diabetes duration, center and treatment group, did not significantly affect the change of NSS+NDS at week 24 (Table S1). At week 12, changes in the NSS and NDS were also of significance compared with baseline, whereas no significant difference was found between treatment groups (Table 2).
Table 2.
Changes in the neuropathy symptom score, the neuropathy disability score, and the sum of both comparing baseline and week 12 and week 24 in the full analysis set population
| ALC (n = 117) | MC (n = 115) | P‐value: change in ALC vs MC | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Week 12 | Week 24 | Change from baseline to week 24 | P‐value: baseline vs week 24 | Baseline | Week 12 | Week 24 | Change from baseline to week 24 | P‐value: baseline vs week 24 | ||
| NSS+NDS | 13.10 ± 2.80 | 10.50 ± 3.78 | 9.09 ± 4.24 | 4.01 ± 3.25 | <0.0001 | 12.79 ± 2.80 | 10.51 ± 3.70 | 9.33 ± 4.34 | 3.46 ± 3.43 | <0.0001 | 0.14 |
| NSS | 6.52 ± 1.52 | 4.95 ± 2.21 | 4.17 ± 2.45 | 2.35 ± 2.23 | <0.0001 | 6.37 ± 1.71 | 4.94 ± 2.12 | 4.25 ± 2.60 | 2.11 ± 2.48 | <0.0001 | 0.38 |
| NDS | 6.58 ± 2.19 | 5.55 ± 2.50 | 4.92 ± 2.62 | 1.66 ± 1.90 | <0.0001 | 6.43 ± 2.04 | 5.57 ± 2.37 | 5.08 ± 2.41 | 1.35 ± 1.65 | <0.0001 | 0.23 |
All continuous variables were presented as mean ± standard deviation. All comparisons were analyzed by t‐test. ALC, acetyl‐L‐carnitine; MC, methylcobalamin; NDS, neuropathy disability score; NSS, neuropathy symptom score.
Comparison of the effects on improvement of the electrophysiological parameters
In the FAS population, the NCV and amplitude of all investigated motor and sensory nerves were improved in the ALC group at week 24 compared with baseline (Table 3), when the majority of NCV and amplitude in the MC group were improved, except the amplitude of sural sensory and peroneal motor nerves. The reversal rates of most nerves were similar in the two groups (Table S4), except that the reversal rate of the motor ulnar nerve was significantly higher than that of the MC group (P = 0.0015).
Table 3.
Changes in nerve conduction velocity and amplitude comparing baseline and week 24 in the full analysis set population
| ALC | MC | P‐value: change in ALC vs MC | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Baseline | Week 24 | Change from baseline to week 24 | P‐value: baseline vs week 24 | n | Baseline | Week 24 | Change from baseline to week 24 | P‐value: baseline vs week 24 | ||
| Nerve conduction velocity | |||||||||||
| Sensory nerves (m/s) | |||||||||||
| Median | 75 | 41.58 ± 7.71 | 46.76 ± 10.23 | 5.03 ± 10.78 | <0.0001 | 65 | 40.35 ± 10.34 | 46.73 ± 10.71 | 6.42 ± 12.73 | <0.0001 | 0.57 |
| Ulnar | 50 | 42.89 ± 7.06 | 47.45 ± 9.50 | 5.01 ± 9.76 | 0.0002 | 41 | 40.54 ± 9.25 | 45.79 ± 9.30 | 5.72 ± 9.95 | 0.0002 | 0.81 |
| Sural | 37 | 35.47 ± 7.75 | 38.75 ± 7.23 | 3.10 ± 5.59 | 0.0001 | 28 | 33.88 ± 9.94 | 35.90 ± 10.93 | 2.02 ± 4.10 | 0.01 | 0.40 |
| Motor nerves (m/s) | |||||||||||
| Median | 61 | 47.33 ± 4.54 | 50.83 ± 8.24 | 3.49 ± 8.40 | 0.001 | 55 | 47.36 ± 4.49 | 49.47 ± 5.26 | 2.11 ± 6.25 | 0.004 | 0.78 |
| Ulnar | 50 | 45.81 ± 4.97 | 50.31 ± 7.38 | 4.49 ± 7.38 | <0.0001 | 52 | 46.83 ± 5.14 | 47.37 ± 7.20 | 0.55 ± 5.25 | 0.86 | 0.003 |
| Tibial | 40 | 39.80 ± 3.61 | 41.37 ± 6.03 | 1.72 ± 5.85 | 0.07 | 46 | 38.94 ± 4.01 | 42.03 ± 6.29 | 2.75 ± 5.18 | 0.0007 | 0.66 |
| Peroneal | 64 | 38.96 ± 4.61 | 43.97 ± 10.06 | 5.00 ± 10.25 | <0.0001 | 54 | 39.62 ± 4.50 | 42.13 ± 6.29 | 2.45 ± 5.36 | 0.0006 | 0.45 |
| Response amplitude | |||||||||||
| Sensory nerves (uV) | |||||||||||
| Median | 69 | 6.20 (2.30~9.60) | 6.80 (3.30~12.0) | 0.0 (–0.07~3.60) | 0.04 | 62 | 5.20 (2.60~10.15) | 5.90 (2.20~17.0) | 0.0 (0.0~3.50) | 0.01 | 0.65 |
| Ulnar | 44 | 6.05 (2.50~8.10) | 6.90 (3.20~11.0) | 0.0 (–0.30~1.35) | 0.38 | 44 | 4.30 (2.25~8.10) | 8.40 (2.95~18.0) | 0.50 (0.0~11.50) | 0.001 | 0.04 |
| Sural | 35 | 3.10 (1.57~5.0) | 3.25 (2.35~5.80) | 0.0 (–0.10~1.76) | 0.22 | 18 | 5.45 (2.80~6.80) | 5.30 (3.30~10.0) | 0.0 (‐1.95~1.40) | 1.0 | 0.41 |
| Motor nerves (mV) | |||||||||||
| Median | 32 | 2.38 (1.40~3.81) | 6.43 (3.05~8.48) | 1.03 (0.0~6.08) | <0.0001 | 23 | 3.74 ± 1.76 | 5.27 ± 3.52 | 1.53 ± 3.14 | 0.03a | 0.24 |
| Ulnar | 30 | 1.95 (1.23~2.70) | 4.35 (2.54~5.57) | 1.18 (0.0~2.71) | <0.0001 | 25 | 2.55 ± 1.34 | 2.80 (1.90~5.49) | 0.40 (0.0~0.95) | 0.01 | 0.24 |
| Tibial | 45 | 3.48 (1.35~5.45) | 4.75 (1.38~7.35) | 0.0 (–0.15~2.46) | 0.036 | 51 | 3.17 (1.37~7.0) | 5.31 (1.60~9.79) | 0.45 (‐0.41~3.96) | 0.0009 | 0.45 |
| Peroneal | 60 | 1.53 (0.76~2.46) | 2.0 (0.90~3.30) | 0.0 (–0.03~1.14) | 0.007 | 60 | 1.81 (1.23~2.70) | 2.28 (1.56~2.94) | 0.08 (0.0~0.88) | 0.06 | 1.0 |
Data was analyzed by paired samples t‐test and the rest intragroup comparisons were analyzed by Wilcoxon signed‐rank test. All intergroup comparisons were analyzed by Wilcoxon rank–sum test. ALC, acetyl‐L‐carnitine; MC, methylcobalamin.
All results in the per‐protocol set population were consistent with those from the FAS population (Table S2, S3, S5).
Safety and tolerance
During the study period, a total of 67 patients (ALC n = 34, MC n = 33, P = 0.95) reported adverse events, among which nine had severe adverse events (ALC n = 4, MC n = 5, P = 0.75). None of the severe adverse events were deemed related to study agents. Seven patients discontinued because of adverse events (ALC n = 4, MC n = 5). No deaths occurred. The most common adverse events in both groups were gastrointestinal symptoms, such as abdominal distension, hiccups and nausea (Table 4).
Table 4.
Adverse events in the full analysis set population
| ALC (n = 117) (%) | MC (n = 115) (%) | P‐value | |
|---|---|---|---|
| Overall | |||
| Any adverse event | 34 (29.06) | 33 (28.70) | 0.95 |
| Severe adverse event | 4 (3.42) | 5 (4.35) | 0.71 |
| Insufficient blood glucose control | 0 (0.00) | 1 (0.87) | 0.31 |
| Coronary events | 1 (0.85) | 1 (0.87) | 0.99 |
| Diabetic ketoacidosis | 0 (0.00) | 1 (0.87) | 0.31 |
| Diabetic foot induced infection | 2 (1.70) | 1 (0.87) | 0.57 |
| Benign paroxysmal positional vertigo | 1 (0.85) | 0 (0.00) | 0.32 |
| Angioedema | 0 (0.00) | 1 (0.87) | 0.31 |
| Cataract surgery | 1 (0.85) | 0 (0.00) | 0.32 |
| Drug‐related adverse event | 10 (8.55) | 19 (16.52) | 0.07 |
| Adverse event leading to discontinuation† | 4 (3.42) | 5 (4.35) | 0.71 |
| Stomachache | 1 (0.85) | 1 (0.87) | 0.99 |
| Diarrhea | 1 (0.85) | 1 (0.87) | 0.99 |
| Abdominal distension | 1 (0.85) | 2 (1.74) | 0.55 |
| Dizziness | 0 (0.00) | 1 (0.87) | 0.31 |
| Nausea | 0 (0.00) | 1 (0.87) | 0.31 |
| Waist pain | 1 (0.85) | 0 (0.00) | 0.32 |
| Pruritus | 1 (0.85) | 0 (0.00) | 0.32 |
| Death | 0 (0.00) | 0 (0.00) | NA |
| Most common adverse event (>3% in any treatment group) | |||
| Hiccups or nausea | 7 (5.98) | 3 (2.61) | 0.21 |
| Diarrhea | 6 (5.13) | 6 (5.22) | 0.98 |
| Upper respiratory tract infection | 3 (2.56) | 5 (4.35) | 0.46 |
| Dizziness | 4 (3.42) | 2 (1.74) | 0.42 |
| Adverse event of special interest | |||
| Hypoglycemia | 0 (0.00) | 2 (1.74) | 0.15 |
All events were compared by χ2‐test between groups. †One patient complained of more than one adverse event as the cause for discontinuation. ALC, acetyl‐l‐carnitine; NA, not applicable; MC, methylcobalamin.
Fasting blood glucose at week 24 was 8.01 ± 2.57 mmol/L in the ALC group and 7.65 ± 2.93 mmol/L in the MC group, without significant changes from baseline (ALC P = 0.12, MC P = 0.41). HbA1c at week 24 was 6.94 ± 1.02% in the ALC group and 7.04 ± 1.36% in the MC group, without significant changes from baseline (ALC P = 0.16, MC P = 0.26). Furthermore, no significant change at week 24 from baseline was observed in each group of vital signs, other laboratory variables (white blood cells, red blood cells, hemoglobin, platelets, alanine transaminase, aspartate transaminase, blood urea nitrogen, serum creatinine and total bilirubin) and the proportion of patients with abnormal electrocardiographs.
Discussion
The present randomized controlled trial showed that 500 mg ALC three times per day for 24 weeks was non‐inferior to MC in ameliorating neuropathic symptoms and neurophysiological parameters in adult diabetic patients, and was well tolerated. This was the first active‐controlled randomized trial of ALC on DPN, which was suggested for future research by the latest American Academy of Neurology guideline8. This is also the first trial studying the effects of ALC on DPN in the Eastern Asian population, while previous trials were conducted in the American and Canadian19, the Italian20, the Turkish11, 12 or the British21 population.
In the present trial, ALC showed similar efficacy and safety with MC, which was proven to be superior to placebo in treating DPN in a meta‐analysis14, and was approved by the China Food and Drug Administration. It suggested ALC might be a potential treatment of DPN. Furthermore, in the ALC group, the NSS and the NDS were reduced significantly at week 12 as well as at week 24, suggesting that ALC took effect within 3 months and remained effective until the end of the study period. It could be considered together with previous studies suggesting that in DPN patients, 8 weeks might be insufficient for ALC to bring detectable changes11, and once had ALC taken effect, it continuously improved clinical symptoms for at least 52 weeks19. Clinical symptoms evaluation is a common end‐point in previous trials19, 20; however, the assessments of which varied largely. In the present trial, we evaluated both the NSS and the NDS2, and summed the two scores for non‐inferiority determination, for which the assessment was supported by the American Association of Clinical Endocrinologists guideline22.
The NCV and amplitude were ameliorated similarly in patients on ALC and on MC, which was consistent with the studies carried out by De Grandis et al.20 and by Ulvi et al.12, and the change of electrophysiological parameters were of a similar scale in all studies. However, Sima et al.19 found that ALC (500 mg or 1,000 mg, three times per day) significantly improved all vibratory parameters, but not the NCV or amplitude throughout a follow‐up period of 52 weeks. Unfortunately, detailed data of the NCV and amplitude were not given in that study. Furthermore, in the present trial, changes of the NCV and amplitude in ulnar nerves from baseline to week 24 in two treatment groups were statistically different, but clinical significance could not be shown. Additionally, we carried out ancova and carried out analysis in both the FAS population and the per‐protocol set population. The consistency of results from all analyses carried out, together with the comparable baseline condition of patients in both groups, suggested reliability of the results from the present trial.
The therapeutic effect of ALC on DPN was supported by previous studies, in which ALC improved visual analog scale and other symptoms scores, as well as electrophysiological parameters12, 19, 20, 21. Additionally, ALC has also been studied to treat peripheral neuropathy induced by chemotherapy23, 24, 25, 26, 27 or antiviral treatment28, 29, 30. Most of these trials were uncontrolled or placebo‐controlled, and showed that ALC is efficacious and safe. To be noted, ALC was compared with MC in a recent trial among patients with chemotherapy‐induced peripheral neuropathy, and ALC was found less efficacious than MC in alleviating neuropathic symptoms27. This difference from the present study might be explained by a higher potency of ALC for neuropathy induced by diabetes than other etiologies, as supported by a recent meta‐analysis10.
Throughout the present 24‐week study, both ALC and MC were well tolerated and did not have a significant effect on blood glucose. This relieved to a certain extent the concern of hypoglycemia, as ALC had the potential to reduce insulin resistance. However, trials with a longer follow‐up period are required to confirm the long‐term safety.
The exact mechanisms for the therapeutic efficacies of ALC in DPN patients are not well established. ALC deficiency was noted in DPN patients9; all associated disorders, including membrane stability perturbations31 and dysfunction32, abnormal energy production in nerves33, disordered fatty acid oxidation34, and the impaired synthesis of vasoactive prostacyclin35, could be corrected by supplementation with ALC31, 32, 33, 34, 35, 36. The neuroprotective and analgesic effects of ALC are considered as the major mechanism of action, whose pharmacological pathway is not covered by any previously studied agent for DPN37. In the meantime, as a cofactor facilitating the utilization of fatty acids in the mitochondria, ALC also leads to reduced insulin resistance. However, the unchanged glucose level did not contribute to the improvement of DPN in the current study. Additionally, ALC was reported effective in neuropathic patients without abnormal blood glucose, including patients with chemotherapy‐induced peripheral neuropathy23, 24, 25, 26, 27 and with HIV‐associated antiretroviral toxic neuropathy28, 29, 30, 38.
The present trial had several limitations. First, the duration of study was 24 weeks, and thus the long‐term efficacy and safety of ALC remained unclear. However, this trial aimed at studying whether ALC was effective, instead of its long‐term action. Second, only the oral administration route was studied while ALC and MC could be administered both intramuscularly and orally. To be noted, although several studies39, 40 administered ALC or MC intramuscularly, the recent meta‐analysis10 suggested no significant difference between the two administration routes for ALC. Third, only a daily ALC dose of 1,500 mg was studied in the present trial. As a previous trial19 showed that 3,000 mg daily ALC is superior to 1,500 mg, it is not clear whether 3,000 mg daily ALC is superior to regular dose MC considering both efficacy and safety. Fourth, placebo control was lacking in our trial. However, administration of placebo was not accepted by local ethical committees, because MC is already approved in China for DPN treatment, although not in the USA and Europe. Fifth, we did not distinguish between type 1 and type 2 diabetes on patient inclusion. Sixth, only NCV and amplitude were used to measure the nerve damage, which only surveyed the large myelinated fibers. Seventh, potential confounding parameters were not studied extensively in the analysis, such as current medication of diabetes and other concomitant diseases, baseline serum ALC, vitamin B12 and lipid profiles, body mass index, smoking and drinking history, comorbidities, and genetic profiles. Eighth, blood glucose levels were only measured at several time‐points, making glucose fluctuation data unavailable. Ninth, we only analyzed the NSS and NDS, but not the detailed items in each scoring, which could not show if the positive and negative neuropathic symptoms had a similar response after intervention.
In summary, ALC is as effective as MC in improving clinical symptoms and neurophysiological parameters in diabetic patients with DPN with good tolerance. ALC is a treatment option for DPN, whereas further clinical trials and observational studies with long‐term follow up are required.
Disclosure
The authors declare no conflict of interest.
Supporting information
Table S1 ¦ Analysis of variance (ancova) of changes in the summed neuropathy symptom score and neuropathy disability score in acetyl‐L‐carnitine and methylcobalamin group comparing baseline and week 24. FAS, full analysis set; NDS, neuropathy disability score; NSS, neuropathy symptom score; PPS, per‐protocol set.
Table S2 ¦ Changes in the neuropathy symptom score (NSS), the neuropathy disability score (NDS), and the sum of both comparing baseline and week 12 and week 24 in the per‐protocol set population. All continuous variables were presented as mean ± standard deviation. All comparisons were analyzed by t‐test. ALC, acetyl‐L‐carnitine; MC, methylcobalamin; NDS, neuropathy disability score; NSS, neuropathy symptom score.
Table S3 ¦ Changes in nerve conduction velocity and amplitude comparing baseline and week 24 in the per‐protocol set population. *Data was analyzed by paired samples t‐test and the rest of the intragroup comparisons were analyzed by Wilcoxon signed‐rank test. All intergroup comparisons were analyzed by Wilcoxon rank–sum test. ALC, acetyl‐L‐carnitine; MC, methylcobalamin.
Table S4 ¦ Rate of nerves with reversed nerve conduction velocity and amplitude at week 24 from baseline in the per‐protocol set population. All comparisons were analyzed by χ2‐test. ALC, acetyl‐L‐carnitine; MC, methylcobalamin; MN, motor nerve; SN, sensory nerve.
Table S5 ¦ Rate of nerves with reversed nerve conduction velocity and amplitude at week 24 from baseline in the per‐protocol set population. All comparisons were analyzed by χ2‐test. ALC, acetyl‐L‐carnitine; MC, methylcobalamin; MN, motor nerve; SN, sensory nerve.
Acknowledgments
Liaoning Haisike Pharmaceutical Co. Ltd. provided the drugs (both ALC and MC) for this study, but did not participate in the study design, patient recruitment, data collection, data analysis or paper preparing. We are grateful to Xiangxun Zhang from the Laboratory of Endocrinology and Metabolism of West China Hospital of Sichuan University for laboratory analysis of the blood tests. We thank Zhong Zheng from the Mental Health Center of West China Hospital of Sichuan University for electrophysiological exams.
J Diabetes Investig 2016; 7: 777–785
Clinical Trial Registry
Chinese Clinical Trial Registry ChiCTR‐TRC‐08000141
References
- 1. Sugimoto K, Murakawa Y, Sima AA. Diabetic neuropathy–a continuing enigma. Diabetes Metab Res Rev 2000; 16: 408–433. [DOI] [PubMed] [Google Scholar]
- 2. Boulton AJ, Malik RA, Arezzo JC, et al Diabetic somatic neuropathies. Diabetes Care 2004; 27: 1458–1486. [DOI] [PubMed] [Google Scholar]
- 3. Schemmel KE, Padiyara RS, D'Souza JJ. Aldose reductase inhibitors in the treatment of diabetic peripheral neuropathy: a review. J Diabetes Complications 2010; 24: 354–360. [DOI] [PubMed] [Google Scholar]
- 4. Ziegler D, Nowak H, Kempler P, et al Treatment of symptomatic diabetic polyneuropathy with the antioxidant alpha‐lipoic acid: a meta‐analysis. Diabetic Med 2004; 21: 114–121. [DOI] [PubMed] [Google Scholar]
- 5. Apfel SC, Kessler JA, Adornato BT, et al Recombinant human nerve growth factor in the treatment of diabetic polyneuropathy. NGF Study Group. Neurology 1998; 51: 695–702. [DOI] [PubMed] [Google Scholar]
- 6. Malik RA, Williamson S, Abbott C, et al Effect of angiotensin‐converting‐enzyme (ACE) inhibitor trandolapril on human diabetic neuropathy: randomised double‐blind controlled trial. Lancet 1998; 352: 1978–1981. [DOI] [PubMed] [Google Scholar]
- 7. Horrobin DF. Essential fatty acids in the management of impaired nerve function in diabetes. Diabetes 1997; 46 (Suppl. 2): S90–S93. [DOI] [PubMed] [Google Scholar]
- 8. Bril V, England J, Franklin GM, et al Evidence‐based guideline: treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. PM R 2011; 3: 345–352, 52 e1‐21. [DOI] [PubMed] [Google Scholar]
- 9. Scarpini E, Doneda P, Pizzul S, et al L‐carnitine and acetyl‐L‐carnitine in human nerves from normal and diabetic subjects. J Peripher Nerv Syst 1996; 1: 157–163. [PubMed] [Google Scholar]
- 10. Li S, Li Q, Li Y, et al Acetyl‐L‐carnitine in the treatment of peripheral neuropathic pain: a systematic review and meta‐analysis of randomized controlled trials. PLoS ONE 2015; 10: e0119479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Uzun N, Sarikaya S, Uluduz D, et al Peripheric and automatic neuropathy in children with type 1 diabetes mellitus: the effect of L‐carnitine treatment on the peripheral and autonomic nervous system. Electromyogr Clin Neurophysiol 2005; 45: 343–351. [PubMed] [Google Scholar]
- 12. Ulvi H, Aygul R, Demir R. Effect of L‐carnitine on diabetic neuropathy and ventricular dispersion in patients with diabetes mellitus. Turk J Med Sci 2010; 40: 169–175. [Google Scholar]
- 13. Zhang M, Han W, Hu S, et al Methylcobalamin: a potential vitamin of pain killer. Neural Plast 2013; 2013: 424651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jia H, Tian H, Wei D. Effects of Methylcobalam in on diabetic peripheral neuropathy: a systematic review. Chinese J Evid Based Med 2005; 5: 609–618. [Google Scholar]
- 15. Report and recommendations of the San Antonio conference on diabetic neuropathy. Consensus statement. Diabetes 1988;37:1000–1004. [DOI] [PubMed] [Google Scholar]
- 16. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabetic Med 1998; 15: 539–553. [DOI] [PubMed] [Google Scholar]
- 17. Young MJ, Boulton AJ, MacLeod AF, et al A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia 1993; 36: 150–154. [DOI] [PubMed] [Google Scholar]
- 18. Proceedings of a consensus development conference on standardized measures in diabetic neuropathy. Clinical measures. Neurology 1992;42:1823–1825. [PubMed] [Google Scholar]
- 19. Sima AA, Calvani M, Mehra M, et al Acetyl‐L‐carnitine improves pain, nerve regeneration, and vibratory perception in patients with chronic diabetic neuropathy: an analysis of two randomized placebo‐controlled trials. Diabetes Care 2005; 28: 89–94. [DOI] [PubMed] [Google Scholar]
- 20. De Grandis D, Minardi C. Acetyl‐L‐carnitine (levacecarnine) in the treatment of diabetic neuropathy. A long‐term, randomised, double‐blind, placebo‐controlled study. Drugs R D 2002; 3: 223–231. [DOI] [PubMed] [Google Scholar]
- 21. Quatraro A, Roca P, Donzella C, et al Acetyl‐L‐carnitine for symptomatic diabetic neuropathy. Diabetologia 1995; 38: 123. [DOI] [PubMed] [Google Scholar]
- 22. Handelsman Y, Mechanick JI, Blonde L, et al American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for developing a diabetes mellitus comprehensive care plan. Endocr Pract 2011; 17(Suppl 2): 1–53. [DOI] [PubMed] [Google Scholar]
- 23. Bianchi G, Vitali G, Caraceni A, et al Symptomatic and neurophysiological responses of paclitaxel‐ or cisplatin‐induced neuropathy to oral acetyl‐L‐carnitine. Eur J Cancer 2005; 41: 1746–1750. [DOI] [PubMed] [Google Scholar]
- 24. Campone M, Berton‐Rigaud D, Joly‐Lobbedez F, et al A double‐blind, randomized phase II study to evaluate the safety and efficacy of acetyl‐L‐carnitine in the prevention of sagopilone‐induced peripheral neuropathy. Oncologist 2013; 18: 1190–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Maestri A, De Pasquale Ceratti A, Cundari S, et al A pilot study on the effect of acetyl‐L‐carnitine in paclitaxel‐ and cisplatin‐induced peripheral neuropathy. Tumori 2005; 91: 135–138. [DOI] [PubMed] [Google Scholar]
- 26. Hershman DL, Unger JM, Crew KD, et al Randomized double‐blind placebo‐controlled trial of acetyl‐L‐carnitine for the prevention of taxane‐induced neuropathy in women undergoing adjuvant breast cancer therapy. J Clin Oncol 2013; 31: 2627–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mondal S, Choudhury KB, Sharma S, et al Comparative study among glutamine, acetyl‐L‐carnitine, vitamin‐E and methylcobalamine for treatment of paclitaxel‐induced peripheral neuropathy. Clin Cancer Investig J 2014; 3: 213–219. [Google Scholar]
- 28. Youle M, Osio M. A double‐blind, parallel‐group, placebo‐controlled, multicentre study of acetyl L‐carnitine in the symptomatic treatment of antiretroviral toxic neuropathy in patients with HIV‐1 infection. HIV Med 2007; 8: 241–250. [DOI] [PubMed] [Google Scholar]
- 29. Herzmann C, Johnson MA, Youle M. Long‐term effect of acetyl‐L‐carnitine for antiretroviral toxic neuropathy. HIV Clin Trials 2005; 6: 344–350. [DOI] [PubMed] [Google Scholar]
- 30. Osio M, Muscia F, Zampini L, et al Acetyl‐l‐carnitine in the treatment of painful antiretroviral toxic neuropathy in human immunodeficiency virus patients: an open label study. J Peripher Nerv Syst 2006; 11: 72–76. [DOI] [PubMed] [Google Scholar]
- 31. Arduini A, Rossi M, Mancinelli G, et al Effect of L‐carnitine and acetyl‐L‐carnitine on the human erythrocyte membrane stability and deformability. Life Sci 1990; 47: 2395–2400. [DOI] [PubMed] [Google Scholar]
- 32. Brecher P. The interaction of long‐chain acyl CoA with membranes. Mol Cell Biochem 1983; 57: 3–15. [DOI] [PubMed] [Google Scholar]
- 33. Greene DA, Winegrad AI. In vitro studies of the substrates for energy production and the effects of insulin on glucose utilization in the neural components of peripheral nerve. Diabetes 1979; 28: 878–887. [DOI] [PubMed] [Google Scholar]
- 34. Sass RL, Werness P. Acetylcarnitine: on the relationship between structure and function. Biochem Biophys Res Commun 1973; 55: 736–742. [DOI] [PubMed] [Google Scholar]
- 35. Sima AA, Ristic H, Merry A, et al Primary preventive and secondary interventionary effects of acetyl‐L‐carnitine on diabetic neuropathy in the bio‐breeding Worcester rat. J Clin Investig 1996; 97: 1900–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stevens MJ, Lattimer SA, Feldman EL, et al Acetyl‐L‐carnitine deficiency as a cause of altered nerve myo‐inositol content, Na, K‐ATPase activity, and motor conduction velocity in the streptozotocin‐diabetic rat. Metabolism 1996; 45: 865–872. [DOI] [PubMed] [Google Scholar]
- 37. Onofrj M, Ciccocioppo F, Varanese S, et al Acetyl‐L‐carnitine: from a biological curiosity to a drug for the peripheral nervous system and beyond. Expert Rev Neurother 2013; 13: 925–936. [DOI] [PubMed] [Google Scholar]
- 38. Phillips TJ, Cherry CL, Cox S, et al Pharmacological treatment of painful HIV‐associated sensory neuropathy: a systematic review and meta‐analysis of randomised controlled trials. PLoS ONE 2010; 5: e14433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xu Q, Pan J, Yu J, et al Meta‐analysis of methylcobalamin alone and in combination with lipoic acid in patients with diabetic peripheral neuropathy. Diabetes Res Clin Pract 2013; 101: 99–105. [DOI] [PubMed] [Google Scholar]
- 40. Onofrj M, Fulgente T, Melchionda D, et al L‐acetylcarnitine as a new therapeutic approach for peripheral neuropathies with pain. Int J Clin Pharmacol Res 1995; 15: 9–15. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 ¦ Analysis of variance (ancova) of changes in the summed neuropathy symptom score and neuropathy disability score in acetyl‐L‐carnitine and methylcobalamin group comparing baseline and week 24. FAS, full analysis set; NDS, neuropathy disability score; NSS, neuropathy symptom score; PPS, per‐protocol set.
Table S2 ¦ Changes in the neuropathy symptom score (NSS), the neuropathy disability score (NDS), and the sum of both comparing baseline and week 12 and week 24 in the per‐protocol set population. All continuous variables were presented as mean ± standard deviation. All comparisons were analyzed by t‐test. ALC, acetyl‐L‐carnitine; MC, methylcobalamin; NDS, neuropathy disability score; NSS, neuropathy symptom score.
Table S3 ¦ Changes in nerve conduction velocity and amplitude comparing baseline and week 24 in the per‐protocol set population. *Data was analyzed by paired samples t‐test and the rest of the intragroup comparisons were analyzed by Wilcoxon signed‐rank test. All intergroup comparisons were analyzed by Wilcoxon rank–sum test. ALC, acetyl‐L‐carnitine; MC, methylcobalamin.
Table S4 ¦ Rate of nerves with reversed nerve conduction velocity and amplitude at week 24 from baseline in the per‐protocol set population. All comparisons were analyzed by χ2‐test. ALC, acetyl‐L‐carnitine; MC, methylcobalamin; MN, motor nerve; SN, sensory nerve.
Table S5 ¦ Rate of nerves with reversed nerve conduction velocity and amplitude at week 24 from baseline in the per‐protocol set population. All comparisons were analyzed by χ2‐test. ALC, acetyl‐L‐carnitine; MC, methylcobalamin; MN, motor nerve; SN, sensory nerve.


