Abstract
Aims/Introduction
To explore the association between atrial fibrillation (AF) and diabetes mellitus in a general Chinese population, and the influence of hypertension.
Materials and Methods
From January 2013 to August 2013, we carried out a cross‐sectional study involving 11,956 permanent residents aged ≥35 years from the general population in the Liaoning province of China (response rate 85.3%). Each participant completed a questionnaire, had a physical examination, and underwent an electrocardiogram and echocardiogram. AF was diagnosed on the basis of the electrocardiogram findings. Logistic regression analyses were carried out to estimate the associations between diabetes mellitus and AF. The associations were also analyzed in hypertensive and normotensive subgroups.
Results
There was a higher prevalence of AF in participants with diabetes mellitus than those without diabetes mellitus (1.2 vs 0.5%; P = 0.004). In the hypertensive subgroup, the prevalence of AF in participants with diabetes mellitus was significantly higher than in participants without diabetes mellitus (1.5 vs 0.6%; P = 0.008); however, the prevalences were similar in the normotensive subgroup (0.3 vs 0.4%; P = 1.000). Similar trends were present in both men and women. After adjustment for cardiovascular risk factors, the independent association between diabetes mellitus and AF remained in the total sample (odds ratio 2.33, 95% confidence interval 1.20–4.54) and hypertensive subgroup (odds ratio 3.15, 95% confidence interval 1.52–6.56), but not in the normotensive subgroup (odds ratio 0.64, 95% confidence interval 0.08–5.31).
Conclusions
Diabetes mellitus is an independent risk factor for AF in the general population in China, this association was present in total and hypertensive participants, but not in normotensive participants.
Keywords: Atrial fibrillation, Diabetes mellitus, Hypertension
Introduction
Atrial fibrillation (AF) is one of the most common clinical cardiac arrhythmias in the general population1, and the prevalence of AF is expected to increase dramatically over the next few decades2. AF is an independent risk factor for stroke, and is also associated with all‐cause and cardiovascular disease mortality3. Therefore, identifying all the possible risk factors, and making the detailed relationship between these risk factors and AF clear will help to form population‐based strategies for this serious cardiovascular problem.
Diabetes mellitus is one of the most common metabolic diseases and has been a worldwide public health issue, and the proportion of cardiovascular diseases attributable to diabetes mellitus is increasing along with socioeconomic development4. The most recent data show that the overall prevalence of diabetes mellitus in the Chinese adult population is estimated to be 11.6% (95% confidence interval [CI] 11.3–11.8%), which is far higher than the estimated global prevalence5.
As a high‐prevalence health problem, diabetes mellitus has long been recognized as a risk factor for AF as well as advancing age, hypertension, congestive heart failure and valve disease in the Framingham study6, 7. However, recent studies have shown that the independent contribution of diabetes mellitus to the prevalence and incidence of AF is controversial. A community‐based study of Japanese adults showed that AF was independently associated with diabetes mellitus (odds ratio [OR] 1.46. 95% CI 1.20–1.78)8. On the contrary, some other cross‐sectional surveys showed that diabetes mellitus was not an independent risk factor for AF9, 10, 11, whereas one cohort study suggested that the increased AF risk associated with diabetes mellitus was mainly mediated by other confounding AF risk factors12. However, in China, only a few cross‐sectional, but not cohort, studies about the relationship between diabetes mellitus and AF have been carried out, and reported that diabetes mellitus was not associated with the prevalence of AF in the multivariate model13, 14. Furthermore, all these data in China were obtained mainly from their household registration system, and only included hospital patients, which could not reflect the reliable epidemiological information about AF in the general population of China.
Data from the Valsartan Antihypertensive Long‐term Use Evaluation trial population showed that hypertensive patients who developed diabetes mellitus had a significantly higher incidence of AF with a hazard ratio of 1.49 (1.14–1.94) compared with patients without diabetes mellitus15. However, whether blood pressure levels have an influence on the diabetes mellitus‐related risk for AF is not clear so far.
The current study was carried out in the Liaoning Province in northeastern China, which suffers a high prevalence of hypertension16. Therefore, the present study was designed to explore the association between diabetes mellitus and AF, and the influence of hypertension on the relationship in the general population aged ≥35 years in China.
Materials and Methods
Study Population
From January 2013 to August 2013, a representative sample of men and women in different areas of Liaoning Province was evaluated for the presence of cardiovascular risk factors using a multistage, randomly stratified, cluster‐sampling scheme. In the first stage, three counties (Dawa, Zhangwu and Liaoyang County) were randomly selected from Liaoning Province. In the second stage, one town was randomly selected from each county (a total of three towns). In the third stage, six to eight villages from each town were randomly selected (a total of 26 rural villages). Participants with malignant tumor and mental disorder, and pregnant women were excluded.
All the eligible permanent residents aged ≥35 years from each village (n = 14,016) were invited to participate, of which 11,956 (85.3%) completed the study. The study was approved by the ethics committee of China Medical University in Shenyang, China, and all procedures were carried out in accordance with its ethical standards. Written consent was obtained from all participants after they had been informed of the study's objectives, benefits, medical procedures and confidentiality safeguards for personal information. If the participants were illiterate, we obtained written informed consent from their proxies.
Data Collection and Measurement
Data were collected during a single clinic visit by cardiologists and trained nurses using a standard questionnaire in a face‐to‐face interview. All potential investigators had received training on the purpose of the study, how to administer the questionnaire, the standard methods of measurement, the importance of standardization and study procedures. Only those who earned a perfect score on a post‐training test were allowed to participate as study investigators. During data collection, the inspectors received further instructions and support.
Data on demographic characteristics, medical history of AF, myocardial infarction (MI), hypertension, diabetes mellitus, lifestyle risk factors and family history of AF were obtained, as described above, by interview with the standardized questionnaire. There was a central steering committee with a subcommittee for quality control that made sure all data were collected according to well‐known standards.
According to the American Heart Association17, blood pressure (BP) should be measured three times at 2‐min intervals after at least 5 min of rest using a standardized automatic electronic sphygmomanometer (HEM‐907; Omron, Kyoto, Japan). Two doctors checked the calibration of the Omron device every month using a standard mercury sphygmomanometer according to the British Hypertension Society protocol18. The participants were advised to avoid caffeinated beverages and exercise for ≥30 min before the measurement. During the measurement, the participants were seated with their arms supported at the level of their hearts. The mean of three BP measurements was calculated and used in all analyses.
Weight and height were measured to the nearest 0.1 kg and 0.1 cm, respectively, with the participants in lightweight clothing without shoes. The body mass index (BMI) was calculated as weight in kilograms divided by the square of the height in meters.
Fasting blood samples were collected in the morning after at least 8 h of fasting for all participants. Blood samples were obtained from an antecubital vein using BD Vacutainer tubes containing ethylenediaminetetraacetic acid (Becton, Dickinson and Co., Franklin Lakes, NJ, USA). Serum was subsequently isolated from whole blood, and all serum samples were frozen at −20°C for testing at a central, certified laboratory. Fasting blood glucose, total cholesterol (TC), triglycerides (TG), high density lipid cholesterol (HDL‐C), low density lipid cholesterol (LDL‐C) and other routine blood biochemical indices were analyzed enzymatically on an auto‐analyzer (Olympus AU640 Auto‐Analyzer; Olympus Corp., Kobe, Japan).
Twelve‐lead resting, 10‐s electrocardiograms (ECGs) were carried out on all participants by well‐trained cardiologists using an electrocardiography machine (MAC 5,500; GE Healthcare, Little Chalfont, UK), and analyzed automatically by the MUSE Cardiology Information System (version 7.0.0; GE Healthcare). ECG‐based diagnoses of AF were confirmed by at least two independent cardiologists.
Echocardiograms were obtained using a commercially available Doppler echocardiograph (Vivid; GE Healthcare) with a 3.0‐MHz transducer. The transthoracic echocardiogram included M‐mode, 2‐D, spectral and color Doppler with participants in the supine position. Echocardiogram analyses and readings were carried out by three doctors specialized in echocardiography, and two other specialists were called in if questions or uncertainty arose. Measurements were carried out according to the recommendations of the American Society of Echocardiography. M‐mode images were used to measure and calculate the left ventricular ejection fraction (LVEF)19.
Definitions
Atrial fibrillation was diagnosed based on the ECG findings (absence of consistent P waves, presence of rapid, irregular F waves with a frequency of 350–600 b.p.m. and an irregular ventricular response). Hypertension was defined as a systolic BP ≥140 mmHg and/or diastolic BP ≥90 mmHg, and/or the use of antihypertensive medications according to the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure Guidelines20. World Health Organization criteria were followed for defining diabetes mellitus (fasting blood glucose ≥7.0 mmol/L or 126 mg/dL, and/or receiving treatment for diabetes)21. Left ventricular systolic dysfunction was defined as LVEF <0.5 based on M‐mode echocardiography. ECG‐left ventricular hypertrophy (LVH) was defined as equal voltage × QRS duration product: (RaVL + SV3) × QRS duration >2,440 mm*ms for men and (RaVL + SV3 + 8 mm) × QRS duration >2,440 mm*ms for women according to the Cornell voltage QRS duration product formula22.
Statistical Analysis
All statistical analyses were carried out using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). Differences between groups were compared using a two‐tailed Student's t‐test for continuous variables and a chi‐squared test for categorical variables. The age‐specific prevalences of AF grouped by diabetes mellitus and hypertension were calculated separately, and the sex‐specific prevalences of AF grouped by diabetes mellitus and hypertension were also presented. Univariate and multivariate logistic regression analyses were carried out to estimate the crude and independent association between diabetes mellitus and the presence of AF in the hypertensive population, normotensive and total. Data are expressed as odds ratio (OR) and 95% confidence interval (CI), mean ± SD, or frequency and percentage; a P < 0.05 was considered as statistically significant.
Results
Characteristics of the Study Population
Of the original 11,956 participants, 615 had incomplete data and were excluded from the analysis, leaving a total of 11,341 participants (5,172 men and 6,169 women) with a mean age of 53.8 years. The participants with diabetes mellitus (n = 1,171) were older than those without diabetes mellitus (P < 0.001), and there was no significant difference in sex between the two groups (P = 0.072; Table 1). Participants with diabetes mellitus had significantly higher BMI, systolic and diastolic BPs, fasting blood glucose, TC, TG, and LDL‐C levels, and lower HDL‐C level (all P < 0.001). The diabetes mellitus group also had a lower percentage of smokers, and a higher prevalence of MI and ECG‐LVH than the group without diabetes mellitus (all P < 0.001). The prevalence of AF was significantly higher in participants with diabetes mellitus than those without diabetes mellitus (1.2 vs 0.5%, P = 0.004).
Table 1.
Comparison of the characteristics of the study sample
| Variable | Participants without DM (n = 10,170) | Participants with DM (n = 1,171) | P |
|---|---|---|---|
| Age (years) | 53.4 ± 10.6 | 57.6 ± 9.7 | <0.001 |
| Male | 4,667 (45.9) | 505 (43.1) | 0.072 |
| BMI (kg/m2) | 24.6 ± 3.6 | 26.2 ± 3.7 | <0.001 |
| SBP (mmHg) | 140 ± 23 | 153 ± 24 | <0.001 |
| DBP (mmHg) | 82 ± 12 | 85 ± 12 | <0.001 |
| FBG (mmol/L) | 5.51 ± 0.55 | 9.32 ± 3.14 | <0.001 |
| TC (mmol/L) | 5.19 ± 1.06 | 5.62 ± 1.26 | <0.001 |
| TG (mmol/L) | 1.54 ± 1.27 | 2.50 ± 2.63 | <0.001 |
| HDL‐C (mmol/L) | 1.42 ± 0.38 | 1.31 ± 0.35 | <0.001 |
| LDL‐C (mmol/L) | 2.89 ± 0.80 | 3.17 ± 0.94 | <0.001 |
| Current smoker | 3,624 (35.6) | 357 (30.5) | <0.001 |
| Current drinker | 2,280 (22.4) | 245 (20.9) | 0.244 |
| History of MI | 104 (1.0) | 27 (2.3) | <0.001 |
| LVEF <0.5 | 1,128 (11.6) | 141 (12.5) | 0.330 |
| ECG‐LVH | 846 (8.3) | 169 (14.4) | <0.001 |
| AF | 53 (0.5) | 14 (1.2) | 0.004 |
| Family history of AF | 300 (2.9) | 44 (3.8) | 0.127 |
Data are expressed as mean ± SD or n (%). AF, atrial fibrillation; BMI, body mass index; DBP, diastolic blood pressure; DM, diabetes mellitus; ECG‐LVH, left ventricular hypertrophy detected by electrocardiography; FBG, fasting blood glucose; HDL‐C, high density lipid cholesterol; LDL‐C, low density lipid cholesterol; LVEF, left ventricular ejection fraction; MI, myocardial infarction; SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides.
Age‐Specific Prevalence of AF by Diabetes Mellitus and Hypertension
There were 67 participants with AF, and the age‐specific prevalences of AF by diabetes mellitus and hypertension are summarized in Table 2. The prevalence of AF rose steeply with advancing age in each group. Apparently, it rose much more in the groups with diabetes mellitus than in the groups without diabetes mellitus. Inversely, however, it rose less in hypertensive participants than normotensive participants. The prevalences were higher in the groups aged older than 55 years with diabetes mellitus than in the groups without diabetes mellitus, although there was no significant difference at any age (all P > 0.05).
Table 2.
Age‐specific prevalence of atrial fibrillation by diabetes mellitus and hypertension
| Group | n | DM | Hypertension | ||||
|---|---|---|---|---|---|---|---|
| No | Yes | P | No | Yes | P | ||
| 35–44 years | 2,706 (23.9) | 0 (–) | 0 (–) | – | 0 (–) | 0 (–) | – |
| 45–54 years | 3,506 (30.9) | 6 (0.2) | 0 (–) | 1.000 | 2 (0.1) | 4 (0.3) | 0.420 |
| 55–64 years | 3,420 (30.2) | 24 (0.8) | 7 (1.5) | 0.179 | 12 (1.0) | 19 (0.9) | 0.791 |
| 65–74 years | 1,360 (12.0) | 17 (1.5) | 5 (2.3) | 0.371 | 7 (1.9) | 15 (1.5) | 0.578 |
| ≥75 years | 349 (3.1) | 6 (1.9) | 2 (4.9) | 0.239 | 2 (3.0) | 6 (2.1) | 0.649 |
Data expressed as n (%). AF, atrial fibrillation; BP, blood pressure; DM, diabetes mellitus.
Effect of Diabetes Mellitus and Hypertension on the Prevalence of AF
The prevalences of AF in diabetes mellitus and hypertension are summarized and compared in Figure 1. In participants with hypertension, the prevalence of AF in the group with diabetes mellitus was significantly higher than without diabetes mellitus (1.5 vs 0.6%; P = 0.008). However, the prevalences were similar in normotensive participants (0.3 vs 0.4%; P = 1.000). Similar trends were present in both men and women.
Figure 1.
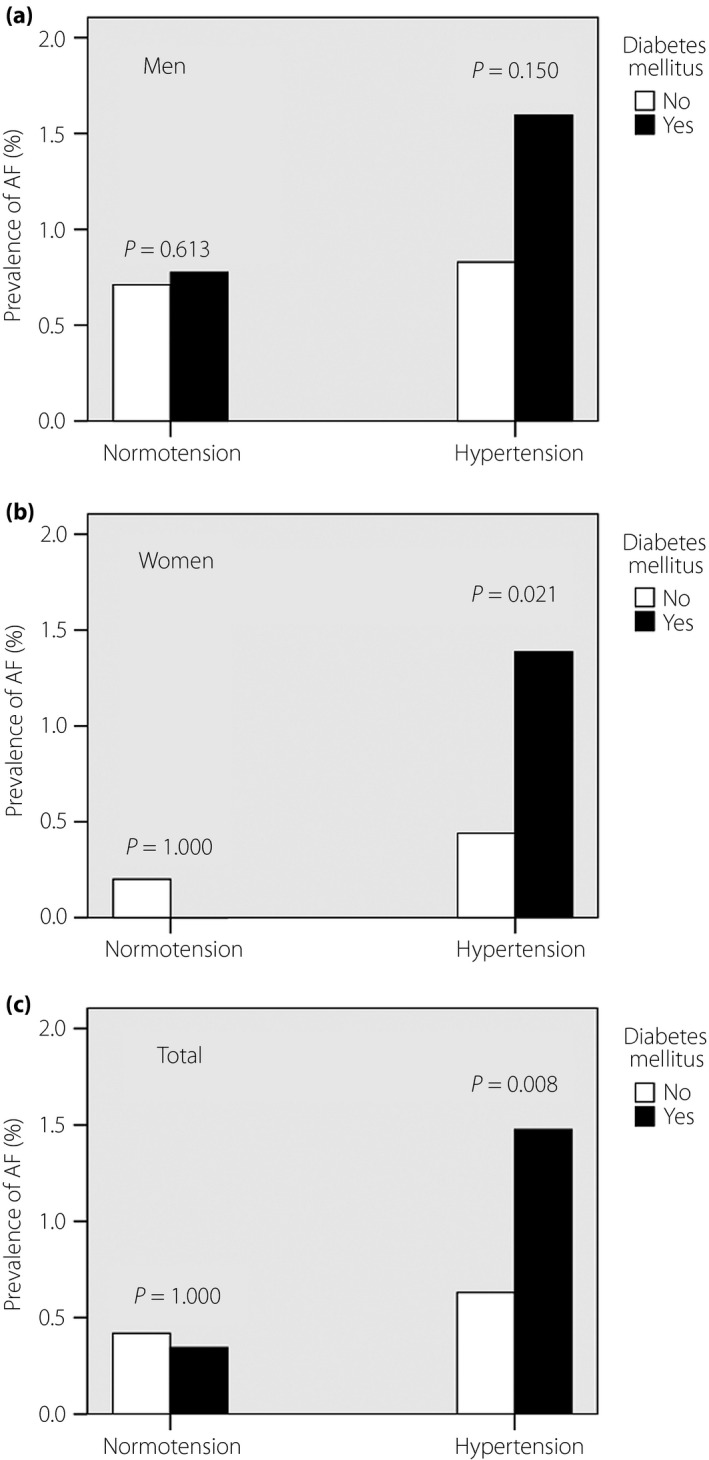
Effect of diabetes mellitus and hypertension on the prevalence of atrial fibrillation (AF). The prevalence of AF in (a) men, (b) women and (c) the total population.
Association Between Diabetes Mellitus and AF
The association between diabetes mellitus and AF was examined by logistic regression analysis (Table 3). To identify the relationship, we adjusted cardiovascular risk factors including age, sex, BMI, systolic and diastolic BP, TC, TG, LDL‐C, HDL‐C, smoking, drinking, MI, low LVEF, ECG‐LVH, and familial history of AF. In the whole sample, diabetes mellitus was positively associated with the prevalence of AF in both the univariate and multivariate model (all P < 0.05). Similar results were found in hypertensive participants (all P < 0.05), with relatively higher OR than in the whole sample. However, there was no significant association between diabetes mellitus and AF in the normotensive population (all P > 0.05).
Table 3.
Diabetes mellitus and odds ratios of atrial fibrillation, stratified by hypertension
| Group | Unadjusted model | Adjusted model 1 | Adjusted model 2 | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Total | ||||||
| Without DM | 1.00 (reference) | 0.006 | 1.00 (reference) | 0.039 | 1.00 (reference) | 0.012 |
| With DM | 2.31 (1.28–4.18) | 1.88 (1.03–3.42) | 2.33 (1.20–4.54) | |||
| Hypertension | ||||||
| Without DM | 1.00 (reference) | 0.010 | 1.00 (reference) | 0.010 | 1.00 (reference) | 0.002 |
| With DM | 2.36 (1.23–4.53) | 2.38 (1.24–4.60) | 3.15 (1.52–6.56) | |||
| Normotension | ||||||
| Without DM | 1.00 (reference) | 0.849 | 1.00 (reference) | 0.388 | 1.00 (reference) | 0.678 |
| With DM | 0.82 (0.11–6.13) | 0.40 (0.05–3.18) | 0.64 (0.08–5.31) | |||
Adjusted model 1: adjusted only for age and sex; Adjusted model 2: adjusted for age, sex, body mass index, systolic and diastolic blood pressure, total cholesterol, triglyceride, low density lipid cholesterol, high density lipid cholesterol, smoking, drinking, myocardial infarction, low left ventricular ejection fraction, left ventricular hypertrophy detected by electrocardiography, and family history of atrial fibrillation (AF). CI, confidence interval; DM, diabetes mellitus; OR, odds ratio.
Discussion
The results of the present study showed that individuals with diabetes mellitus in the general Chinese population have a significantly higher prevalence of AF than those without diabetes mellitus. These positive relationships between diabetes mellitus and AF prevalence remained statistically significant after adjustment for other cardiovascular risk factors including age, sex, BMI, SBP, DBP, TC, TG, LDL‐C, HDL‐C, smoking, drinking, MI, low LVEF, ECG‐LVH, and familial history of AF. However, hypertension showed little evidence of association with AF without significant difference. Furthermore, the positive association between diabetes mellitus and AF was significant only in the hypertensive population, but not in the normotensive population, and this specific research in subgroups has not been reported previously.
The present findings of diabetes mellitus as a risk factor for AF (OR 2.31, 95% CI 1.28–4.18) were consistent with a Japanese community‐based study8. Previous data from China reported that diabetes mellitus was not associated with the prevalence of AF in the multivariate model. There were some possible reasons for the controversial results. First, previous studies in China were based on their household registration system, whereas in the current study, a general population including 85.3% of all the eligible permanent residents participated and completed our research, which could reflect the reliable updated epidemiological characteristics of AF. Second, participants in previous studies came from both urban and rural areas in China, whereas all participants were from rural areas in the current study. Different lifestyles between them might act as confounding factors and influence the results, such as greater physical activity, which had been proven to correlate with AF23. Third, participants in the present study suffered a higher prevalence of hypertension (approximately 50%) than two previous studies in China (35–38%), which might emphasize the diabetes mellitus‐associated differences in AF prevalence.
Considering BP levels, we found the positive association between diabetes mellitus and AF presented in hypertensive participants was not significant in normotensive participants, with a rather wide CI, which was similar to one previous study24. Impaired glucose metabolism might be the underlying mechanism causing the different results between two BP groups. Insulin resistance, which plays a key role in diabetes mellitus, has been a powerful independent predictor of LVH25. In accordance with that, our results showed a 1.7‐fold increase in ECG‐LVH in diabetes mellitus compared with participants without diabetes mellitus. Furthermore, a recent meta‐analysis reported that patients with LVH had 3.4‐fold greater odds of developing supraventricular tachycardias than those without LVH26. Therefore, insulin resistance and its corresponding LVH might be the mechanisms mediating the diabetes mellitus‐related AF prevalence. However, a recent study showed that insulin resistance was not significantly associated with incident AF in the population with relatively lower blood pressure (SBP 127 ± 18 mmHg)27. Therefore, hypertension might augment the association between diabetes mellitus and AF by increasing left ventricular mass.
The present study had several limitations. First, it was cross‐sectional, and could only reflect the association between diabetes mellitus and AF. Therefore, it could not clarify the causality, and it is not clear whether diabetes mellitus was an independent predictor of progression to AF only in hypertensive participants. Second, the association between diabetes mellitus and AF in the present study might be affected by some other confounding risk factors, which were not corrected in the multivariate logistic regression analyses. Third, the generalizability of the findings is limited, as our participants were only from the Liaoning Province of China. Therefore, further large‐scale studies need to be carried out to determine the relationship between diabetes mellitus and AF prevalence, and incidence with different BP levels.
In conclusion, participants in China aged ≥35 years with diabetes mellitus have a significantly higher prevalence of AF than those without diabetes mellitus. After adjusting for various cardiovascular risk factors, diabetes mellitus was an independent risk factor for AF in total and hypertensive participants, but not in normotensive participants.
Disclosure
The authors declare no conflict of interest.
Acknowledgment
The present study was funded by National Science and Technology Support Program of China (No. 2012BAJ18B08‐7).
J Diabetes Investig 2016; 7: 791–796
References
- 1. Go AS, Hylek EM, Phillips KA, et al Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001; 285: 2370–2375. [DOI] [PubMed] [Google Scholar]
- 2. Miyasaka Y, Barnes ME, Gersh BJ, et al Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 2006; 114: 119–125. [DOI] [PubMed] [Google Scholar]
- 3. Stewart S, Hart CL, Hole DJ, et al A population‐based study of the long‐term risks associated with atrial fibrillation: 20‐year follow‐up of the Renfrew/Paisley study. Am J Med 2002; 113: 359–364. [DOI] [PubMed] [Google Scholar]
- 4. Fox CS, Coady S, Sorlie PD, et al Increasing cardiovascular disease burden due to diabetes mellitus: the Framingham Heart Study. Circulation 2007; 115: 1544–1550. [DOI] [PubMed] [Google Scholar]
- 5. Xu Y, Wang L, He J, et al Prevalence and control of diabetes in Chinese adults. JAMA 2013; 310: 948–959. [DOI] [PubMed] [Google Scholar]
- 6. Kannel WB, Abbott RD, Savage DD, et al Epidemiologic features of chronic atrial fibrillation: the Framingham study. N Engl J Med 1982; 306: 1018–1022. [DOI] [PubMed] [Google Scholar]
- 7. Benjamin EJ, Levy D, Vaziri SM, et al Independent risk factors for atrial fibrillation in a population‐based cohort, The Framingham Heart Study. JAMA 1994; 271: 840–844. [PubMed] [Google Scholar]
- 8. Iguchi Y, Kimura K, Aoki J, et al Prevalence of atrial fibrillation in community‐dwelling Japanese aged 40 years or older in Japan: analysis of 41,436 non‐employee residents in Kurashiki‐city. Circ J 2008; 72: 909–913. [DOI] [PubMed] [Google Scholar]
- 9. Jeong JH. Prevalence of and risk factors for atrial fibrillation in Korean adults older than 40 years. J Korean Med Sci 2005; 20: 26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yap KB, Ng TP, Ong HY. Low prevalence of atrial fibrillation in community‐dwelling Chinese aged 55 years or older in Singapore: a population‐based study. J Electrocardiol 2008; 41: 94–98. [DOI] [PubMed] [Google Scholar]
- 11. Thacker EL, McKnight B, Psaty BM, et al Association of body mass index, diabetes, hypertension, and blood pressure levels with risk of permanent atrial fibrillation. J Gen Intern Med 2013; 28: 247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schoen T, Pradhan AD, Albert CM, et al Type 2 diabetes mellitus and risk of incident atrial fibrillation in women. J Am Coll Cardiol 2012; 60: 1421–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li Y, Wu YF, Chen KP, et al Prevalence of atrial fibrillation in China and its risk factors. Biomed Environ Sci 2013; 26: 709–716. [DOI] [PubMed] [Google Scholar]
- 14. Zhou Z, Hu D. An epidemiological study on the prevalence of atrial fibrillation in the Chinese population of mainland China. J Epidemiol 2008; 18: 209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aksnes TA, Schmieder RE, Kjeldsen SE, et al Impact of new‐onset diabetes mellitus on development of atrial fibrillation and heart failure in high‐risk hypertension (from the VALUE Trial). Am J Cardiol 2008; 101: 634–638. [DOI] [PubMed] [Google Scholar]
- 16. Dong G, Sun Z, Zheng L, et al Prevalence, awareness, treatment, and control of hypertension in rural adults from Liaoning Province, northeast China. Hypertens Res 2007; 30: 951–958. [DOI] [PubMed] [Google Scholar]
- 17. Pickering TG, Hall JE, Appel LJ, et al Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation 2005; 111: 697–716. [DOI] [PubMed] [Google Scholar]
- 18. O'Brien E, Petrie J, Littler W, et al The British Hypertension Society protocol for the evaluation of automated and semi‐automated blood pressure measuring devices with special reference to ambulatory systems. J Hypertens 1990; 8: 607–619. [DOI] [PubMed] [Google Scholar]
- 19. Sahn DJ, DeMaria A, Kisslo J, et al Recommendations regarding quantitation in M‐mode echocardiography: results of a survey of echocardiographic measurements. Circulation 1978; 58: 1072–1083. [DOI] [PubMed] [Google Scholar]
- 20. Chobanian AV, Bakris GL, Black HR, et al The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289: 2560–2572. [DOI] [PubMed] [Google Scholar]
- 21. World Health Organization and International Diabetes Fedaration . Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia: Report of a WHO/IDF Consultation. Geneva, Switzerland: WHO, 2006; 1–3. [Google Scholar]
- 22. Dahlöf B, Devereux RB, Julius S, et al Characteristics of 9194 patients with left ventricular hypertrophy: the LIFE study. Losartan Intervention For Endpoint Reduction in Hypertension. Hypertension 1998; 32: 989–997. [DOI] [PubMed] [Google Scholar]
- 23. Azarbal F, Stefanick ML, Salmoirago‐Blotcher E, et al Obesity, physical activity, and their interaction in incident atrial fibrillation in postmenopausal women. J Am Heart Assoc 2014; 3: e001127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ostgren CJ, Merlo J, Råstam L, et al Atrial fibrillation and its association with type 2 diabetes and hypertension in a Swedish community. Diabetes Obes Metab 2004; 6: 367–374. [DOI] [PubMed] [Google Scholar]
- 25. Capoulade R, Clavel MA, Dumesnil JG, et al Insulin resistance and LVH progression in patients with calcific aortic stenosis: a substudy of the ASTRONOMER trial. JACC Cardiovasc Imaging 2013; 6: 165–174. [DOI] [PubMed] [Google Scholar]
- 26. Chatterjee S, Bavishi C, Sardar P, et al Meta‐analysis of left ventricular hypertrophy and sustained arrhythmias. Am J Cardiol 2014; 114: 1049–1052. [DOI] [PubMed] [Google Scholar]
- 27. Fontes JD, Lyass A, Massaro JM, et al Insulin resistance and atrial fibrillation (from the Framingham Heart Study). Am J Cardiol 2012; 109: 87–90. [DOI] [PMC free article] [PubMed] [Google Scholar]


