Abstract
Generating human podocytes in vitro could offer a unique opportunity to study human diseases. Here, we describe a simple and efficient protocol for obtaining functional podocytes in vitro from human induced pluripotent stem cells. Cells were exposed to a three-step protocol, which induced their differentiation into intermediate mesoderm, then into nephron progenitors and, finally, into mature podocytes. After differentiation, cells expressed the main podocyte markers, such as synaptopodin, WT1, α-Actinin-4, P-cadherin and nephrin at the protein and mRNA level, and showed the low proliferation rate typical of mature podocytes. Exposure to Angiotensin II significantly decreased the expression of podocyte genes and cells underwent cytoskeleton rearrangement. Cells were able to internalize albumin and self-assembled into chimeric 3D structures in combination with dissociated embryonic mouse kidney cells. Overall, these findings demonstrate the establishment of a robust protocol that, mimicking developmental stages, makes it possible to derive functional podocytes in vitro.
Keywords: Induced pluripotent stem cells, Differentiation, Nephron progenitors, Podocytes
Highlights
-
•
Human iPSC differentiation into podocytes recapitulates kidney developmental stages.
-
•
The differentiation protocol is reproducible and highly efficient.
-
•
The generated podocytes reflect primary cell behaviour and are functional.
1. Introduction
Podocytes are terminally differentiated epithelial cells that have limited capacity to proliferate. This feature makes them vulnerable to critical levels of cell stress leading to detachment and progressive cell loss, a central mediator of glomerular sclerosis. Over the last decade, investigators have attempted to understand why podocyte depletion per se is sufficient to cause glomerulosclerosis, and to identify the mediators responsible for local propagation of podocyte injury. In this context, the possibility of having podocyte cultures would be a valuable tool for clarifying the molecular mechanisms underlying specific podocytopathies with a view to developing targeted therapy.
First attempts to derive primary podocytes from isolated glomeruli failed largely because podocytes cultured under standard conditions dedifferentiate rapidly, with a loss of foot processes and expression of synaptopodin, a key marker of differentiated podocytes. Changes in culture conditions resulted in cells with the characteristic arborized phenotype and rapid growth arrest, and the latter closely reflected in vivo podocyte behaviour, but limited cell culture abilities for in vitro experiments (Shankland et al., 2007). The establishment of conditionally immortalized cell lines circumvented the detrimental cell growth arrest, generating highly proliferative cells under permissive conditions, which stopped growing in non-permissive conditions. However, despite their widespread use for studying podocyte biology, these cell lines show dramatic differences in the expression of podocyte markers, response to toxins, and motility, not only between podocytes of different species but even between similarly-derived cell lines (Chittiprol et al., 2011).
A potentially exciting possibility for deriving podocytes has been created by studies by Romagnani and co-workers, who identified and isolated renal progenitor cells (RPCs) from the parietal epithelium of Bowman's capsule of the adult kidney (Ronconi et al., 2009). This CD133+ CD24+ cell population, which represents 1 to 4% of all renal cells, exhibits heterogeneous potential for differentiation into different renal cells. In this cell population, the subset of CD133+ CD24+ Podocalyxin− cells displayed the potential to differentiate into podocytes and tubular cells in vitro and to functionally improve glomerular and tubulointerstitial injury in a model of adriamycin-induced renal injury. Despite promising results, difficulties with accessing human RPCs from kidney biopsies has pushed research towards searching for a new source of RPCs. Taking into account that renal cells are naturally lost in urine, urine itself may represent a possible source of renal progenitor cells. To this end, the same group (Lazzeri et al., 2015) did establish RPC cultures from the urine of children with glomerular genetic disorders with the aim of obtaining podocytes and tubular cells. However, the major limitation of this technique is that the success rate for achieving a culture ranges from 8% to 51%, according to the phase of the disease, and drops to 0% with healthy subjects (Lazzeri et al., 2015).
The breakthrough discovery of induced pluripotent stem cells (iPSCs) makes it possible to generate cells in vitro with an overall genetic and epigenetic background identical to donor cells, making iPSCs the ideal tool for in vitro disease modelling (Ye et al., 2013). The derivation of podocytes from pluripotent stem cells is an attractive alternative and an inexhaustible source of podocytes. Recently, different protocols for iPSC commitment towards renal progenitor cells through the activation of Wnt, bone morphogenic protein (BMP), fibroblast growth factor (FGF) and retinoic acid (RA) pathways involved in the induction of the intermediate mesoderm (IM) and subsequently in the metanephric mesenchyme and ureteric bud cells have been reported (Batchelder et al., 2009, Imberti et al., 2015, Kim and Dressler, 2005, Mae et al., 2010, Mae et al., 2013, Oeda et al., 2013, Taguchi et al., 2014, Takasato et al., 2014, Xia et al., 2013). The feasibility of deriving more mature kidney cells from pluripotent stem cells has also been demonstrated (Kang and Han, 2014, Kobayashi et al., 2005, Lam et al., 2014, Song et al., 2012).
Here, we propose a simple and robust three-stage protocol based on single cell differentiation in chemically defined and feeder-free conditions, allowing for the highly efficient generation of human iPSC-derived podocytes. The podocytes generated are mature cells expressing the main podocyte markers and are able to respond to Angiotensin II, one of the major players in podocyte injury, and to internalize BSA and to integrate into WT1 positive structures in an ex-vivo model of developing kidney.
2. Materials and methods
2.1. Human iPSC culture
The human Episomal iPS cell line from Life Technologies (Grand Island, NY), hiPSC Clone IV and hiPSC#16 cell lines generated in our laboratories were cultured on hESC-qualified matrigel (BD Biosciences, New Jersey, USA) coated dishes in mTeSR1 medium (StemCell Technologies, Vancouver, Canada). Cells were routinely sub-cultured using Accutase (Life Technologies) and plated twice a week as small clumps at a dilution of 1:10 to 1:15.
2.2. Human iPSC differentiation into podocytes
For differentiation, hiPSCs were dissociated using Accutase and plated on growth factor reduced matrigel (BD Biosciences) coated dishes at a density of 30,000/50,000 cells/cm2 in mTeSR1 medium with 10 μM ROCK inhibitor Y-27632 (Sigma, Milan, Italy). After one day medium was replaced with a medium consisting of a 1:1 mixture of DMEM:F12 (1:1) plus Glutamax (Life Technologies) and neurobasal media with N2 and B27 supplements (all Life Technologies, N2B27 medium), in the presence of 1 μM CP21R7 (Roche) and 25 ng/ml BMP4 (Prepotech, USA). The first induction medium was maintained for three days and thereafter replaced with STEMdiff APEL medium (StemCell Technologies) supplemented with 100 nM Retinoic Acid (Sigma), 50 ng/ml BMP7 (Prepotech) and 200 ng/ml FGF9 (Abnova, Taipei City, Taiwan) for 2 more days. On day 6 of differentiation, cells were dissociated with Accutase and plated on type I collagen-coated plates at a density of 20,000/40,000 cells/cm2 and cultured for 7 days in VRAD medium consisting of DMEM:F12 plus Glutamax (Life Technologies) supplemented with 10% FBS, Retinoic Acid (80–100 μM depending on the cell line) and 100 nM Vitamin D3.
2.3. Derivation and characterization of human iPSC#16
2.3.1. Generation of human iPS cell line
Human iPSCs were derived from peripheral blood mononuclear cells (PBMCs) through Sendai virus-mediated reprogramming using CytoTune- iPS 2.0 Sendai reprogramming Kit (Life Technologies) following manufacturer's instruction. PBMCs were isolated from healthy donor by Ficoll-Plaque gradient. Briefly, PBMCs were thawed four days before infection and cultured in StemPro-34 medium (Life Technologies) supplemented with cytokines (PBMC medium). The day of infection around 300,000 PBMCs were infected with 5 MOI hKOS, 5 MOI hc-Myc and 3 MOI hKLF4 Sendai virus. Three days after the infection the cells were plated at different densities on MEF-feeder coated dishes (e.g. from 10,000 to 50,000 per well of six well-plate) and cultured in PBMC medium without cytokines for other four days, replacing spent medium every other day. On day seven post-infection the PBMC medium was replaced with human iPSC-medium (DMEMF12, 20% KO serum, 0.1 mM NEAA, 0.1 mM 2-mercaptoethanol and 10 ng/ml bFGF). The iPSC-medium was changed every other day until the first colonies started to appear, and then every day. iPSC colonies were manually picked about 20 days post-infection, transferred into 0.5 ml of human iPSC-medium and dissected to small clumps by pipetting and seeded onto a new MEFs feeder in a well of six well-plate. The colonies were mechanically split for 4 passages and then adapted to growth on hESC qualified- matrigel (BD Biosciences) in mTeSR1 medium and cultured as described above. hiPSC#16 clone has been chosen for characterization by conventional methods (immunostaining and qRT-PCR for pluripotency marker expression, karyotype analysis and embryoid body formation) and for differentiation studies.
2.3.2. Karyotyping
Metaphase spreads were prepared after treatment with 10 mg/ml Colcemid and processed for karyotype analysis. At least 20 metaphases of each sample were counted. Karyotype analysis was performed in collaboration with the Genetic Medicine Laboratory of Azienda Ospedaliera Papa Giovanni XXIII, Bergamo (Italy).
2.3.3. Embryoid body (EB) formation
For EB formation, human iPSC#16 cells were harvested after Accutase detachment and counted by Trypan Blue solution. Around 106 live cells were plated in each well of AggreWell 800 plate (Stem Cell Technologies) in AggreWell medium (Stem Cell Technologies) plus 10 μM ROCK inhibitor (Sigma). Forty-eight hours after plating, EBs were transferred into Ultra Low-attach six well-plate (Corning, USA) and the medium was replaced every other day. After 8 days as floating culture, EBs were transferred onto gelatin-coated plate and cultured in the same medium for another 8 days. On day sixteen the plates were fixed with 4% PFA solution and processed by immunostaining.
2.4. Immunocytochemistry
The cells were fixed with PBS containing 4% paraformaldehyde for 15 min at room temperature. After three washes with PBS, the cells were permeabilized with 0.3% Triton for 15 min at room temperature (when necessary) and then incubated for 1 h with 5% BSA as a blocking solution. For synaptopodin staining, the cells were fixed with 1:1 mixture of EtOH/Acetone for 10 min at 4 °C. The samples were stained with the primary antibody diluted in 2% BSA solution overnight at 4 °C, followed by incubation with the appropriate secondary antibody for 1 h at room temperature. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) for 5 min at RT. Images were taken using CF40 Axiovert fluorescence microscope (Zeiss, Oberkochen, Germany) and are representative of n = 3 experiments. The primary and secondary antibodies used are listed in Supplementary Table S1.
2.5. Gene expression analysis
Total RNA was isolated with Trizol (Life Technologies) and treated with DNAse (Promega) as described by the manufacturer. 2.5 μg of total RNA were used for reverse transcription reaction with Superscript II kit (Life Technologies) following the manufacturer's instructions. Quantitative real-time PCR assays were performed using Taqman gene expression assays (Life Technologies) using predesigned Taqman probes for the genes of interest according to the supplier's recommendations (Supplementary Table S2) and gene expression levels were normalized to the housekeeping gene HPRT1. To amplify cDNA of SIX2, CD2AP and ACTN4, SYBR Green PCR Master Mix (Applied Biosystems, Life Technologies) according to the manufacturer's protocol and the following primers (300 nM) were used: human SIX2 forward 5′-CTTGCCACCGTTCATTCT-3′ and reverse 5′-GGACCAGGACACAGAGTA-3′; human CD2AP forward 5′-CCCTGGATGAACTTAGAGCCC-3′ and reverse 5′-TCCAGTTCTTTCCCGTGATCC-3′; human ACTN4 forward 5′-GAGCAAGCAGCAGTCCAAC-3′, and reverse 5′-GCCCGATCTCCTCCATCTTG-3′; human HPRT forward 5′-GGCAGTATAATCCAAAGATGGTCA-3′, and reverse 5′-TCCTTTTCACCAGCAAGCTTG-3′.
2.6. Scanning electron microscopy analysis
For scanning electron microscopy analysis, nephron progenitor cells obtained on day 6 of the differentiation protocol (n = 2) were seeded on collagen Type I coated Thermanox slides (Thermo Fisher Scientific, USA) and cultured in the podocyte-differentiating medium VRAD for seven days. After differentiation, cells were fixed in 2.5% glutaraldehyde (buffered with 0.1 M sodium cacodylate buffer, pH of 7.4) for 1 h, post-fixed in 1% osmium tetroxide, and dehydrated through an increasing ethanol series. Cell monolayers were then dried with pure hexamethyldisilazane (Sigma) (twice for 30 min), sputter-coated with gold, and observed at scanning electron microscopy (Supra 55; Carl Zeiss).
2.7. Cell proliferation assay
On day 6 of the differentiation protocol cells were seeded in each well of a 96-well plate in the presence (differentiated cells) or in the absence (undifferentiated cells) of VRAD medium at a cell density of 30,000 cells/cm2. Cells were then fixed and stained with crystal violet solution (Sigma) on days 7, 11 and 13 of the differentiation protocol, rinsed, and then air-dried. The stain was eluted with an ethanol: 0.1 M sodium citrate (1:1) solution, and absorbance at 595 nm was measured. The experiments (n = 3) were performed in triplicate and data are expressed as mean ± s.e.m.
2.8. Cytoskeleton rearrangement evaluation
Human iPSC-derived podocytes were exposed to 100 nM and 500 nM Angiotensin II for 15 and 24 h in serum-free conditions. The cells were then fixed with a 4% PFA solution for 15 min at RT and stained with rhodamine phalloidin (Molecular Probes, USA) for 1 h at RT followed by DAPI staining. For each condition an average of 10 fields (around 100 cells) were counted in three different experiments. The results are expressed as the percentage of cells that showed cytoskeleton rearrangement compared to the total number of counted cells and are reported as mean ± s.e.m.
2.9. Albumin uptake assay
An albumin uptake assay was performed as previously described (Xinaris et al., 2015). Human iPSC-derived podocytes were incubated with serum-free medium overnight. After washing with Ringer's buffer pH 7.4, cells were exposed to 50 μg/ml FITC-conjugated bovine serum albumin (BSA; Sigma-Aldrich) for 90 min at 37 °C. After washing with Ringer's buffer, cells were fixed in 4% PFA for 10 min at room temperature. Nuclei were stained with DAPI. Cells were mounted using Dako Fluorescence Mounting Medium (DAKO) and examined by fluorescence microscope (ApoTome Axio Imager Z2, Zeiss).
2.10. Chimeric organoid cultures
Chimeric organoids were constructed and cultured as previously described (Xinaris et al., 2015). Briefly, embryonic day (E) 12.5 CD1 mouse (Charles River Italia SpA) kidneys were dissected in MEM (M5650; Sigma-Aldrich, St. Louis, MO), placed in 1 × trypsin/EDTA (Biochrom AG) for 5 min at 37 °C, and dissociated into single cell suspensions by trituration and then filtration through a 40 μm cell strainer (BD Falcon). A total of 1.2 × 105 mouse cells were mixed with 1.2 × 104 human iPSC-derived podocytes or undifferentiated iPSC (10:1, mouse:human) and centrifuged at 900 × g for 5 min. The resulting pellets were placed on top of the 5 μm filter (Merck Millipore Ltd) supported by a metal grid in a humidified atmosphere with 5% CO2 at 37 °C. Chimeric organoids were cultured in Advanced DMEM (12494; Gibco, Invitrogen Corporation) supplemented with 2% Embryonic Stem cells Fetal Bovine Serum (ES-FBS, Gibco), 1% l-glutamine (Invitrogen Corporation) and 1% penicillin/streptomycin (Invitrogen). For the first 24 hour organoids were cultured in the presence of 1.25 μM Glycyl-H1152 dihydrochloride (Tocris), a ROCK inhibitor.
2.11. Immunofluorescence analysis of chimeric organoids
After 2 days of in vitro culture, chimeric organoids were fixed in 4% PFA for 10 min, permeabilized with 100% cold methanol for 10 min, and incubated with mouse anti-E-cadherin (1:100, BD Biosciences), rabbit anti-WT1 (1:50, Santa Cruz Biotechnology) followed by species-specific secondary antibodies (1:50; Jackson ImmunoResearch Laboratories). To detect human cells, chimeric organoids were incubated with FITC-conjugated anti–Human Nuclear Antigen (HNA, 1:100; Merck Millipore Ltd). Chimeric organoids were mounted with Dako Fluorescence Mounting Medium (DAKO Corporation) and examined using an inverted confocal laser-scanning microscope (LS 510 Meta; Carl Zeiss). 3D images of chimeric organoids were reconstructed using the Axio Vision Imager software (Carl Zeiss).
2.12. Statistical analysis
Statistical analysis was performed using GraphPad Prism software (version 6). Data were analyzed using ANOVA followed by the Bonferroni test for multiple comparisons or t test for unpaired data, as appropriate. P < 0.05 was considered a statistically significant difference.
3. Results
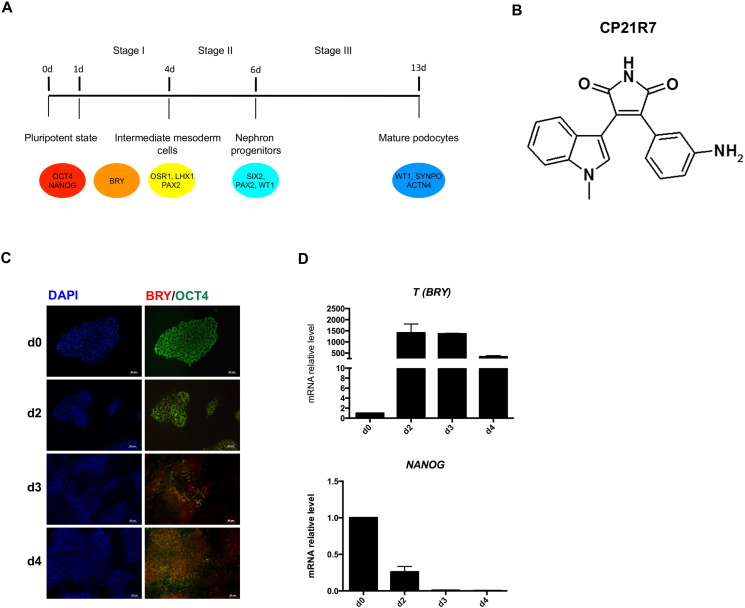
A highly efficient and chemically defined protocol for differentiating human iPS cells into fully mature podocytes was attempted by trying to recapitulate kidney developmental stages in vitro. The differentiation protocol is based on three different stages, which include the induction phase into intermediate mesoderm, commitment towards nephron precursors, and specification into podocytes (Fig. 1A).
Fig. 1.
Human iPSC induction towards mesoderm by the newly identified GSK3β inhibitor CP21R7. (A) Outline of the three-step differentiation protocol to obtain hiPSC-derived podocytes; (B) CP21R7 chemical structure; (C) immunofluorescence analysis of BRY and OCT4 displaying commitment to mesodermal fate and reduction of pluripotency. Scale bar 50 μm; (D) gene expression analysis confirmed the induction towards mesoderm through an increase in T (BRY) expression and the loss of pluripotency through a decrease in NANOG expression. Data are expressed as mean ± s.e.m.
3.1. hiPSC commitment towards intermediate mesoderm (IM) and nephron progenitor cells
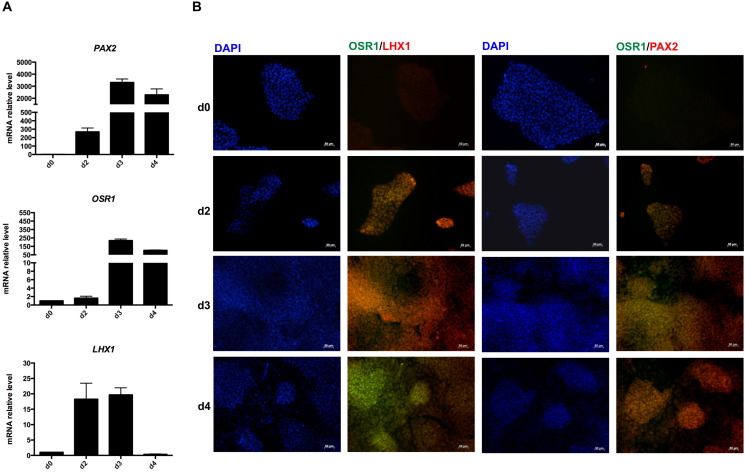
Wnt/β-Catenin and BMP pathways have been reported to direct the differentiation of human PSCs into mesoderm and IM-like precursors (Mae et al., 2013, Sumi et al., 2008, Woll et al., 2008). Here we used a new, potent, and highly selective GSK3β inhibitor as a Wnt pathway activator, to allow efficient commitment of PSCs towards a mesodermal fate. A complete characterization, in terms of Wnt signalling activation, of the novel compound 3-(3-amino-phenyl)-4-(1-methyl-1H-indol-3-yl)-pyrrole-2,5-dione, referred to as CP21R7 (structure shown in Fig. 1B), has been described in a very recent paper (Patsch et al., 2015). Notably, the TCF/LEF reporter assay in the human reporter cell line (DeAlmeida et al., 2007) showed that CP21R7 was able to potently activate canonical Wnt signalling even at the dose of 1 μM, with highest activity seen at 3 μM. Moreover, the induction into mesoderm by the combination of CP21R7 with bone morphogenic protein-4 (BMP4) was also demonstrated (Patsch et al., 2015). On the basis of these results, 24 h after plating as a single-cell monolayer, commercially available human iPSCs were exposed to N2B27 medium, supplemented with 1 μM GSK3β inhibitor CP21R7 and 25 ng/ml BMP4 for 3 days. The expression of T (also known as Brachyury, T/BRY), a meso-endoderm marker expressed in the primitive streak, was evaluated during the first stage. Its expression was strongly upregulated by day 2 and remained high until day 4 of differentiation. The concomitant loss of expression of the typical pluripotency markers was highlighted by a progressive decrease in NANOG and OCT4 at the mRNA and protein levels, respectively (Fig. 1C, D). Previous studies investigating renal differentiation of pluripotent cells have relied on PAX2, OSR1, and LHX1 as definitive markers for IM formation (James et al., 2006, Mae et al., 2013, Torres et al., 1995, Tsang et al., 2000). According to this evidence, we analyzed their expression both at the genomic and protein levels. PAX2, OSR1, and LHX1 gene expression had already increased by day 2 of differentiation, reaching peak expression on day 3 (Fig. 2A). PAX2 and OSR1 expression remained high until day 4, while LHX1 expression decreased to control value (Fig. 2A). Cellular co-localization of OSR1 and LHX1 as well as OSR1 and PAX2 protein, was observed from day 2 until day 4 of the differentiation protocol, confirming the induction of IM-like cells (Fig. 2B).
Fig. 2.
Human iPSC specification into intermediate mesoderm cells. (A) Gene expression analysis of PAX2, OSR1 and LHX1. Data are expressed as mean ± s.e.m.; (B) representative immunofluorescence images of co-staining for OSR1/LHX1 and OSR1/PAX2 up to day 4. Scale bar 50 μm.
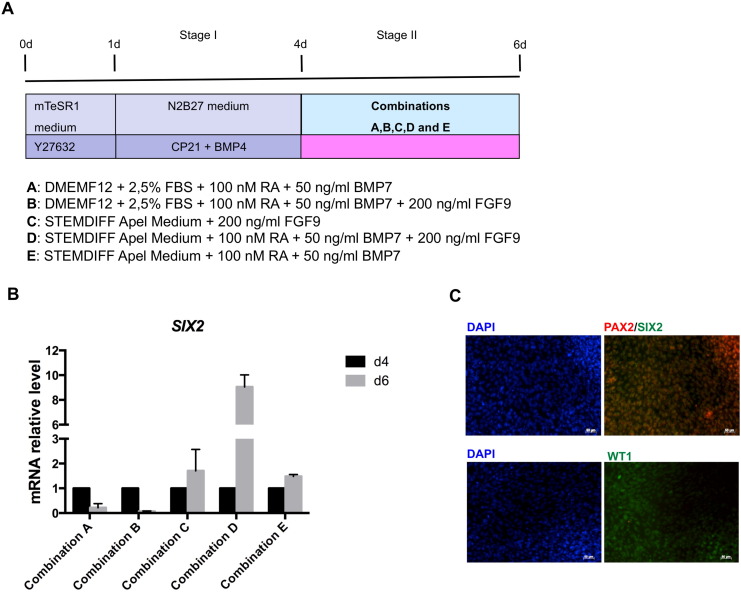
To determine whether hiPSC-derived IM cells have the capacity to give rise to more differentiated cells, IM-like cells were exposed to five different combinations of cell culture media and growth factors, including retinoic acid (RA), bone morphogenic protein-7 (BMP7) and fibroblast growth factor-9 (FGF9), as depicted in Fig. 3A. RA and BMP7 have previously been described as inducing renal lineage from mESCs (Kim and Dressler, 2005), and FGF9 can maintain nephron progenitors in vitro (Barak et al., 2012). IM-like cells were exposed to stage II differentiation media for 2 days and the induction of the expression of SIX2, a key marker of nephron progenitor cells giving rise to podocytes, was evaluated to define the best combination able to promote IM-like cell maturation into renal progenitors. As shown by gene expression analysis, combinations C, D and E were the most efficient in inducing SIX2 expression (Fig. 3B). In particular, in combination D SIX2 expression on day 6 was around nine-fold higher than that detected on day 4. Prolonged exposure to the differentiation media failed to further increase SIX2 expression, which was already downregulated by day 7 in all the conditions tested (data not shown).
Fig. 3.
Intermediate mesoderm cell commitment into nephron progenitors. (A) Schematic representation of the five conditions tested for inducing SIX2 expression; (B) SIX2 gene expression analysis showed condition D as the most efficient for inducing SIX2. Data are expressed as mean ± s.e.m.; (C) representative immunofluorescence images of co-staining for SIX2/PAX2 and WT1 expression on day 6 of condition D. Scale bar 50 μm.
We thus focused on combination D, and to further confirm the identity of cells generated in this specific combination, the expression of markers specific to the nephron progenitor cells, such as PAX2 and WT1, was also evaluated. Although PAX2 gene expression peaked in the first stage of differentiation between days 3 and 4 (Fig. 2A and Supplementary Fig. S2B), immunofluorescence analysis performed on cells on day 6 of differentiation showed the presence of cells that co-expressed SIX2 and PAX2 proteins (Fig. 3C and Supplementary Fig. S3A). As occurs in normal kidney development (Armstrong et al., 1993), at this stage of differentiation the expression of WT1 was still weak both at the gene (Fig. 4D and Supplementary Fig. S3C) and protein level (Fig. 3C and Supplementary Fig. S3A).
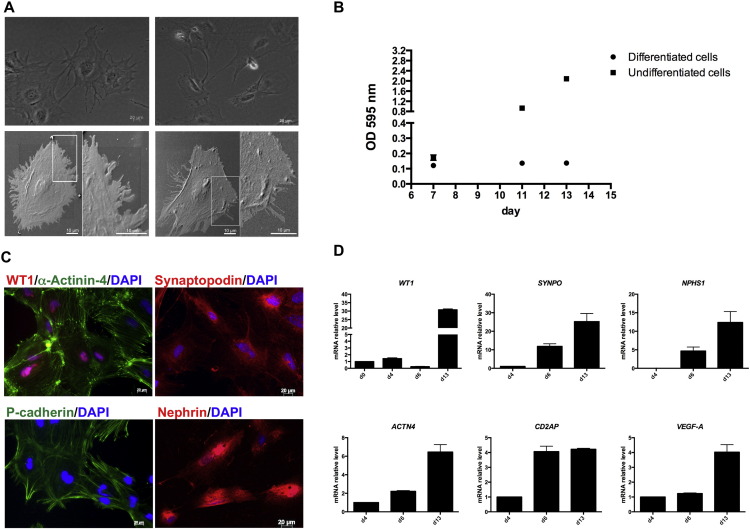
Fig. 4.
Podocyte specification. (A) Representative images of hiPSC-derived podocytes visualized by light microscopy (top, scale bar: 20 μm) or by scanning electron microscopy (bottom, scale bar: 10 μm); (B) proliferation assay showed no proliferation properties of hiPSC-derived podocytes during podocyte specification step as compared to cells not exposed to VRAD medium (undifferentiated cells). Data are expressed as mean ± s.e.m.; (C) immunostaining of the main podocyte markers. Scale bar: 20 μm; (D) gene expression analysis confirmed podocyte marker expression. Data are expressed as mean ± s.e.m.
Given the use of N2B27 medium in neural differentiation, cells at days 4 and 6 were analyzed by real-time PCR for the expression of PAX6, a specific neural progenitor marker. Compared to undifferentiated iPSCs, PAX6 expression dropped to undetectable levels in differentiated cells (data not shown).
Taken together these results demonstrate that combination D could efficiently induce the maturation of IM-like cells into nephron progenitor-like cells, and we used this condition for the following step to promote nephron progenitor differentiation towards a podocyte lineage.
3.2. Specification of hiPSC-derived nephron progenitor cells into podocytes
To obtain a podocyte-like phenotype, on day 6 of differentiation cells were split, seeded on collagen type I-coated plates and cultured for seven days in VRAD medium consisting of DMEM:F12 medium supplemented with 10% FBS, 100 nM of Vitamin D3 and 100 μM RA. VRAD medium has been reported to induce the differentiation of renal progenitors isolated from the Bowman's capsule and from urine into podocytes (Lazzeri et al., 2015, Ronconi et al., 2009).
On day 13 of differentiation, a homogeneous cell population with multinucleated cells characterized by a large cell body and arborized morphology was observed at light microscopy (Fig. 4A, top). To further evaluate the ultrastructure of our iPSC-derived podocytes, we performed scanning electron microscopy, which confirmed the typical arborized phenotype consisting of a main body surrounded by elongated processes resembling foot processes (Fig. 4A, bottom). As human primary podocytes, our iPSC-derived podocytes showed a low proliferation rate, as measured through the proliferation assay performed on days 7, 11 and 13 of differentiation. As shown in Fig. 4B, differentiated cells showed a growth arrest from days 7 to 13 when compared to undifferentiated cells, confirming that iPSC-derived podocytes behaved like mature podocytes.
In addition to confirming morphology and growth behaviour, we also studied gene and protein expression of podocyte-specific markers. After VRAD exposure, hiPSC-derived podocytes expressed several podocyte proteins, such as the nuclear marker WT1 and the actin-binding and cross-linking protein α-Actinin-4. WT1 and α-Actinin-4 co-localization showed that more than 90% of cells expressed both markers (Fig. 4C). Moreover, hiPSC-derived podocytes expressed the podocyte-specific protein synaptopodin in a filamentous arrangement typical of human podocytes. The expression of slit diaphragm protein P-cadherin and nephrin was also evaluated, and the staining displayed typical peripheral cellular localization. The strong induction of podocyte markers was also confirmed by gene expression analysis. As shown in Fig. 4D, the expression of ACTN4, SYNPO, CD2AP and NPSH1, was already upregulated by day 6, and further increased on day 13. WT1 expression was significantly upregulated on day 13. Notably, hiPSC-derived podocytes expressed higher levels of VEGF-A mRNA compared to nephron progenitors at day 6 of differentiation.
3.3. Reproducibility and robustness of the protocol across different human iPS cell lines
To evaluate the reproducibility and robustness of the protocol, the differentiation was tested on other hiPSC lines that we generated using different reprogramming systems and starting from different cell sources. The first one, hiPSC Clone IV, was generated by STEMCCA lentivirus from human neonatal dermal fibroblasts, and its pluripotency properties had previously been demonstrated (Imberti et al., 2015). The second iPS cell line, hiPSC#16, was on the other hand generated by Sendai virus starting from human peripheral blood mononuclear cells (PBMCs). As shown in Supplementary Fig. S1, the hiPSC#16 cell line showed all the properties typical of pluripotent stem cells in terms of pluripotency marker expression, at the protein and mRNA level, the ability to differentiate in vitro into derivatives of the three germ layers, and had a normal karyotype.
Both hiPSC lines showed a pattern of protein and gene expression similar to that observed with the commercial iPSCs in all three stages of the differentiation protocol. Indeed, downregulation of the pluripotency marker OCT4 was observed, going from the undifferentiated state to day 4 of differentiation. Conversely, upregulation of markers specific to the mesoderm Brachyury and for the IM, such as OSR1, LHX1and PAX2 (Supplementary Fig. S2A), was detected during the first stage of differentiation. In parallel, gene expression analysis of pluripotency and IM markers confirmed strong induction towards the intermediate mesodermal fate (Supplementary Fig. S2B). The co-localization of SIX2 with PAX2 and WT1 identified nephron progenitor cells on day 6 of differentiation, generated using combination D (Supplementary Fig. S3A). Finally, specification into podocytes was evaluated. hiPSC-derived podocytes from both cell lines expressed the main podocyte markers, WT1, α-Actinin-4, synaptopodin, P-cadherin and the tight-junction protein ZO-1, at the protein and mRNA level (Supplementary Fig. S3B, C) with efficiency estimated around 90% on the basis of WT1 and α-Actinin-4 co-localization.
Collectively, these results demonstrate that the protocol we established supports the efficient commitment of three different human iPSC lines towards podocytes and provides the basis for functional studies.
3.4. Functional traits of hiPSC-derived podocytes
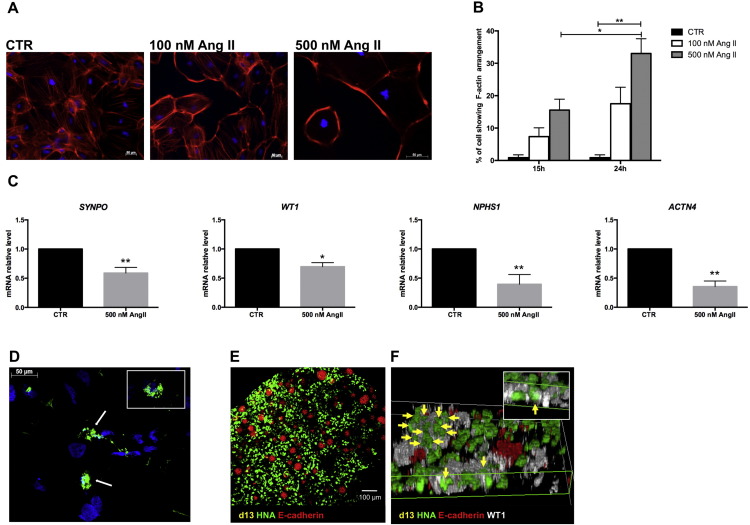
In order to test whether iPSC-derived podocytes possessed functional properties, cells were exposed to Angiotensin II (Ang II), one of the major players in podocyte injury, and the cytoskeleton rearrangement and expression of podocyte markers were evaluated. To assess the cytoskeleton rearrangement, cells were exposed to two different concentrations of Ang II (100 nM and 500 nM) for 15 and 24 h. Exposure of the cells to Ang II treatment induced significant reorganization of the F-actin containing filaments. The pattern changed into reorganization of F-actin to the cell periphery at the expense of trans-cytoplasmic microfilaments with retraction of foot processes, which compacted the cell body (Fig. 5A). Notably, the Ang II effect on the F-actin arrangement was dose- and time-dependent, with a higher percentage of cytoskeletal rearrangement (33% ± 4.6) found 24 h after exposure to the highest dose of Ang II (Fig. 5B). Moreover, these morphological changes were associated with significantly reduced expression of podocyte-specific markers such as SYNPO, WT1, NPSH1 and ACTN4, evaluated 3 h after exposure to 500 nM Ang II (Fig. 5C). It is known that podocytes are able to endocytose albumin (Eyre et al., 2007, Xinaris et al., 2015). To confirm this ability in our iPSC-derived podocytes, uptake of FITC-albumin was examined by fluorescence microscopy (Fig. 5D). After 90 min of incubation, FITC-albumin was detected in abundant large vesicles within the podocyte cell bodies near the perinuclear region.
Fig. 5.
Human iPSC-derived podocyte functionality. (A) Representative F-actin immunostaining showed peripheral localization after 24 h of Ang II exposure. Scale bar 50 μm; (B) percentage of cells showing F-actin rearrangement (*P < 0.05, **P < 0.01). Data are expressed as mean ± s.e.m.; (C) gene expression analysis showed a significant reduction in podocyte marker expression after 3 h of Ang II exposure (*P < 0.05, **P < 0.01). Data are expressed as mean ± s.e.m. (D) representative images of hiPSC-derived podocyte uptake of FITC-BSA, which accumulated in the perinuclear regions Scale bar 50 μm. (E–F) Integration of human iPSC-derived podocytes into the developing chimeric kidney organoid after 2 days in vitro. (E) iPSC-derived podocytes positive for the human marker human nuclear antigen (HNA, green) were homogeneously distributed within the chimeric organoid among ureteric bud epithelia positive for E-cadherin (red). (F) 3D reconstruction images showed iPSC-derived podocytes (HNA-positive, green) integrated into WT1-positive (white) structures (arrows). Inset: HNA-positive cell expressing WT1.
To test whether differentiated cells could functionally integrate into developing kidney tissue, human podocytes at 13 days of differentiation were re-aggregated with fully dissociated E12.5 mouse kidneys (Xinaris et al., 2015), and cultured as explants for 2 days. Human iPSC-derived podocytes were homogenously integrated into re-aggregated kidney tissue to form fine-grained chimeric organoids without having a disruptive effect on renal development (Fig. 5E). Remarkably, human cells were found abundantly in WT1-positive/E-cadherin-negative structures and co-expressed WT1 (Fig. 5F). Such integration did not occur in re-aggregation experiments performed with undifferentiated iPSCs that, on the contrary, were exclusively found in E-cadherin-positive Ureteric Bud epithelia (Supplementary Fig. S4).
4. Discussion
From the developmental perspective, the kidney is a mesodermal organ derived from the embryonic metanephros, which is composed of the ureteric bud, whose maturation produces the collecting ducts, the renal pelvis and ureters, and the intermediate mesoderm-derived metanephric mesenchyme, from which renal tubules and glomeruli originate. Over the last decade, a number of groups worldwide have tested the feasibility of deriving renal cells from embryonic stem cells or iPSCs. However, most of them generated mixed populations of progenitor cells committed to the ureteric bud or metanephric mesenchyme fate. So far, few studies have demonstrated the ability of progenitor cells to differentiate into more mature renal cells. Kobayashi et al. (2005), and more recently Lam et al. (2014), promoted stem cell differentiation into renal tubular cells. Two groups have attempted to derive podocytes from hiPSCs, but both studies have some limitations. Kang and Han described the generation of podocytes as just the ability of nephron progenitors to differentiate into more mature cells without giving any evidence of podocyte functionality (Kang and Han, 2014). Song and colleagues generated podocytes from iPSCs which, in contrast with their in vivo counterparts, still maintained proliferative capacity, a feature typical of immature cells (Song et al., 2012). Moreover, in this latter study the efficiency and robustness of the protocol were not discussed. Here, we developed a three-stage protocol that allowed us to obtain mature podocytes from iPSCs, passing through a nephron progenitor cell generation step. Our protocol induces the generation of intermediate mesoderm cells, which then acquire metanephric mesenchyme cell markers to finally mature into a podocyte-lineage cell population.
The expression of T (BRY), taken as a marker of precursors of all mesodermal tissues, was here induced through the activation of the canonical Wnt signalling which has been identified as an inducer for the primitive streak. A novel GSK3β inhibitor compound, active at a lower concentration than that reported for the commercially available analogue CHIR99021 (Lam et al., 2014, Mae et al., 2013, Takasato et al., 2014), was used in combination with BMP4 to induce robust generation of IM cells as soon as 2 days after differentiation. Strong IM commitment was gained within 3 days of differentiation, as proven by the co-expression of the specific IM markers OSR1, LHX1 and PAX2. Although expression of these markers is not unique to kidney development (Fujii et al., 1994, Puschel et al., 1992), their co-expression in the same cells identifies a very specific cell population in the developing kidney. Using a combination of RA, BMP7 and FGF9, we could direct the maturation of IM like cells into more mature cells expressing multiple markers of nephron progenitors of the cap mesenchyme (CM), particularly SIX2, PAX2 and WT1. During nephrogenesis, the CM cells, when stimulated, undergo epithelialisation to form podocytes. Studies on zebrafish pronephros formation, a model that mirrors mammalian kidney development, have shown that RA actively triggers WT1 expression (Wingert et al., 2007). RA, by binding to the retinoic acid response elements, modulates the transcription of numerous genes in stem cells, leading them to exit the self-renewing state and promoting their differentiation (Gudas, 2013). In the kidney, the retinoids act on renal progenitors by promoting their differentiation into mature podocytes. Indeed, RA is a stronger inducer of WT1 (Bollig et al., 2009), which is a transcription factor that can physically interact with different co-factors to modulate the transcriptional activity of essential podocyte genes like podocalyxin (O'Brien et al., 2011) and nephrin (Guo et al., 2004). In our study, we induced podocyte differentiation by exposing renal progenitor cells to the VRAD medium, which contains RA. At day 13 of differentiation, cells showed high expression levels for genes like WT1, SYNPO, NPHS1, ACTN4, CD2AP, and VEGF-A that have been previously identified as essential for defining a cell as a podocyte (Shankland et al., 2007). The expression of the main podocyte markers not only reveals the cellular identity of the generated cells, but also demonstrates the differentiation efficiency of our protocol, with more than 90% of the cells co-expressing WT1 and α-Actinin-4 proteins, and the overall cells expressing synaptopodin and P-cadherin. Notably, the protocol was strongly reproducible, generating comparable results across three hiPSC lines generated with different reprogramming methods (integrative and non-integrative) and from distinct cell sources (dermal fibroblasts and PBMCs).
Human podocytes are terminally differentiated cells with the typical feature of bi- and multinucleated cells, both in vitro and in vivo (Pavenstadt et al., 2003). Here, iPSC-derived podocytes share the same characteristics as primary podocytes, as shown by growth arrest and the presence of a fraction of bi- or multinucleated cells. They acquired the typical morphology of primary cells with an arborized appearance, the presence of processes extending from the cell body, and a large cytoplasmic to nuclear volume ratio (Shankland et al., 2007). Most importantly, iPSC-derived podocytes are functional. A recent study from our group demonstrated that Ang II plays an important role in perpetuating glomerular injury in experimental and human diabetic nephropathy by persistently activating Notch1 and Snail signalling in podocytes, eventually down-regulating nephrin expression, the integrity of which is crucial for the glomerular filtration barrier (Gagliardini et al., 2013). Ang II induces actin cytoskeleton rearrangement, which is instrumental to podocyte permselective dysfunction (Macconi et al., 2006). In our in vitro model, hiPSC-derived podocytes exposed to Ang II show a rearrangement of the cytoskeleton, which is also accompanied by a de-differentiation process of the podocytes detected by a marked and significant decrease in the expression of the specific podocyte markers. Notably, as previously demonstrated in conditionally immortalized podocytes (Eyre et al., 2007) and in podocytes generated in organoids from amniotic fluid stem cells (Xinaris et al., 2015), our cells behaved like functional mature podocytes, able to internalize albumin. Furthermore, iPSC-derived podocytes interacted efficiently with mouse renal progenitors to form fine-grained chimeras, and incorporated abundantly into WT1-positive induced metanephric mesenchyme and developing glomeruli, confirming their nephrogenic potential.
Compared with other protocols promoting podocyte differentiation from pluripotent stem cells, our method offers several advantages. First, the protocol allows podocyte formation in a process reminiscent of the in vivo kidney developmental stages. Second, hiPSC-derived podocytes are obtained in only two weeks with high efficiency. Lastly, the hiPSC-derived podocytes share the most important feature of human primary podocytes, not only in terms of morphology and marker expression, but also as growth behaviour and their ability to respond to stressors and to internalize albumin.
In conclusion, we have derived functional mature podocytes from human iPSCs, establishing an efficient differentiation protocol that recapitulates in vivo podocyte development and offers the methodological basis for exciting investigative approaches. Starting from iPSCs, an infinite source of cells, this protocol will hopefully enable us to better understand how genetic mutations can affect podocyte development and physiology. This could involve the introduction in healthy iPSCs of mutations in genes required for renal development, and then monitoring for a disease phenotype during the developmental stages of our protocol. On the contrary, with recent innovations in genome editing, genetic mutations can be removed from patients' iPSCs and monitored for healthy phenotype acquisition. Our findings provide a technical advance for designing investigations, which make it possible to better define the molecular and cellular mechanisms of kidney development.
Author contributions
O.C., R.I., M.G., A.B., and S.T.: designed the experiments; O.C., L.L. and V.B.: maintained iPSC culture and conducted all the experiments; C.P.: performed data collection and interpretation; O.C., R.I., M.C. M., C.X., G.R., A.B., and S.T.: analyzed and interpreted the data; O.C., R.I., G.R., A.B. and S.T.: wrote the manuscript.
Grant supports
The research leading to these results was supported by the European Research Council under the European Community's Seventh Framework Programme (FP7/2007–2013)/ERC Grant agreement 268632 and by the Innovative Medicines Initiative Joint Undertaking under grant agreement no. 115439; the resources of the latter are composed of financial contributions from the European Community's Seventh Framework Programme FP7/2007–2013 and EFPIA companies' in kind contributions. This publication reflects only the authors' views and neither the IMI JU, nor EFPIA, nor the European Commission is liable for any use that may be made of the information contained herein. This work was supported by the Roche Postdoctoral Fellowship Program (Grant number A1412744-A17).
Disclosure of potential conflicts of interest
C.X. is a co-founder and managing director of Biorenovo Ltd; however he received no compensation for this role. C.X. received research funding from Bellco Srl.
Acknowledgments
We thank Jacques Bailly, Frederic Delobel and Gerard Regine for their technical support and Sara Conti for providing scanning electron microscopy images. V.B. is recipient of a fellowship from Fondazione Aiuti per la Ricerca sulle Malattie Rare (ARMR), Bergamo, Italy. We are deeply indebted to Paolo Fruscella and Ursula Giussani for performing the karyotype analysis and to Kerstin Mierke for English language editing. Manuela Passera assisted in the preparation of the manuscript.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.scr.2016.06.001.
Appendix A. Supplementary data
Supplementary Figures and Tables.
References
- Armstrong J.F. The expression of the Wilms' tumour gene, WT1, in the developing mammalian embryo. Mech. Dev. 1993;40(1-2):85–97. doi: 10.1016/0925-4773(93)90090-k. [DOI] [PubMed] [Google Scholar]
- Barak H. FGF9 and FGF20 maintain the stemness of nephron progenitors in mice and man. Dev. Cell. 2012;22(6):1191–1207. doi: 10.1016/j.devcel.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchelder C.A. Renal ontogeny in the rhesus monkey (Macaca mulatta) and directed differentiation of human embryonic stem cells towards kidney precursors. Differentiation. 2009;78(1):45–56. doi: 10.1016/j.diff.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollig F. A highly conserved retinoic acid responsive element controls wt1a expression in the zebrafish pronephros. Development. 2009;136(17):2883–2892. doi: 10.1242/dev.031773. [DOI] [PubMed] [Google Scholar]
- Chittiprol S. Marker expression, behaviors, and responses vary in different lines of conditionally immortalized cultured podocytes. Am. J. Phys. Renal Phys. 2011;301(3):F660–F671. doi: 10.1152/ajprenal.00234.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeAlmeida V.I. The soluble wnt receptor Frizzled8CRD-hFc inhibits the growth of teratocarcinomas in vivo. Cancer Res. 2007;67(11):5371–5379. doi: 10.1158/0008-5472.CAN-07-0266. [DOI] [PubMed] [Google Scholar]
- Eyre J. Statin-sensitive endocytosis of albumin by glomerular podocytes. Am. J. Phys. Renal Phys. 2007;292(2):F674–F681. doi: 10.1152/ajprenal.00272.2006. [DOI] [PubMed] [Google Scholar]
- Fujii T. Expression patterns of the murine LIM class homeobox gene lim1 in the developing brain and excretory system. Dev. Dyn. 1994;199(1):73–83. doi: 10.1002/aja.1001990108. [DOI] [PubMed] [Google Scholar]
- Gagliardini E. Angiotensin II contributes to diabetic renal dysfunction in rodents and humans via Notch1/Snail pathway. Am. J. Pathol. 2013;183(1):119–130. doi: 10.1016/j.ajpath.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudas L.J. Retinoids induce stem cell differentiation via epigenetic changes. Semin. Cell Dev. Biol. 2013;24(10 − 12):701–705. doi: 10.1016/j.semcdb.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo G. WT1 activates a glomerular-specific enhancer identified from the human nephrin gene. J. Am. Soc. Nephrol. 2004;15(11):2851–2856. doi: 10.1097/01.ASN.0000143474.91362.C4. [DOI] [PubMed] [Google Scholar]
- Imberti B. Renal progenitors derived from human iPSCs engraft and restore function in a mouse model of acute kidney injury. Sci. Rep. 2015;5:8826. doi: 10.1038/srep08826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James R.G. Odd-skipped related 1 is required for development of the metanephric kidney and regulates formation and differentiation of kidney precursor cells. Development. 2006;133(15):2995–3004. doi: 10.1242/dev.02442. [DOI] [PubMed] [Google Scholar]
- Kang M., Han Y.M. Differentiation of human pluripotent stem cells into nephron progenitor cells in a serum and feeder free system. PLoS One. 2014;9(4):e94888. doi: 10.1371/journal.pone.0094888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Dressler G.R. Nephrogenic factors promote differentiation of mouse embryonic stem cells into renal epithelia. J. Am. Soc. Nephrol. 2005;16(12):3527–3534. doi: 10.1681/ASN.2005050544. [DOI] [PubMed] [Google Scholar]
- Kobayashi T. Wnt4-transformed mouse embryonic stem cells differentiate into renal tubular cells. Biochem. Biophys. Res. Commun. 2005;336(2):585–595. doi: 10.1016/j.bbrc.2005.08.136. [DOI] [PubMed] [Google Scholar]
- Lam A.Q. Rapid and efficient differentiation of human pluripotent stem cells into intermediate mesoderm that forms tubules expressing kidney proximal tubular markers. J. Am. Soc. Nephrol. 2014;25(6):1211–1225. doi: 10.1681/ASN.2013080831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzeri E. Human urine-derived renal progenitors for personalized modeling of genetic kidney disorders. J. Am. Soc. Nephrol. 2015;26(8):1961–1974. doi: 10.1681/ASN.2014010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macconi D. Permselective dysfunction of podocyte-podocyte contact upon angiotensin II unravels the molecular target for renoprotective intervention. Am. J. Pathol. 2006;168(4):1073–1085. doi: 10.2353/ajpath.2006.050701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mae S. Combination of small molecules enhances differentiation of mouse embryonic stem cells into intermediate mesoderm through BMP7-positive cells. Biochem. Biophys. Res. Commun. 2010;393(4):877–882. doi: 10.1016/j.bbrc.2010.02.111. [DOI] [PubMed] [Google Scholar]
- Mae S. Monitoring and robust induction of nephrogenic intermediate mesoderm from human pluripotent stem cells. Nat. Commun. 2013;4:1367. doi: 10.1038/ncomms2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien L.L. Wt1a, Foxc1a, and the Notch mediator Rbpj physically interact and regulate the formation of podocytes in zebrafish. Dev. Biol. 2011;358(2):318–330. doi: 10.1016/j.ydbio.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeda S. Induction of intermediate mesoderm by retinoic acid receptor signaling from differentiating mouse embryonic stem cells. Int. J. Dev. Biol. 2013;57(5):383–389. doi: 10.1387/ijdb.130058ma. [DOI] [PubMed] [Google Scholar]
- Patsch C. Generation of vascular endothelial and smooth muscle cells from human pluripotent stem cells. Nat. Cell Biol. 2015;17(8):994–1003. doi: 10.1038/ncb3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavenstadt H. Cell biology of the glomerular podocyte. Physiol. Rev. 2003;83(1):253–307. doi: 10.1152/physrev.00020.2002. [DOI] [PubMed] [Google Scholar]
- Puschel A.W. Comparative analysis of Pax-2 protein distributions during neurulation in mice and zebrafish. Mech. Dev. 1992;38(3):197–208. doi: 10.1016/0925-4773(92)90053-m. [DOI] [PubMed] [Google Scholar]
- Ronconi E. Regeneration of glomerular podocytes by human renal progenitors. J. Am. Soc. Nephrol. 2009;20(2):322–332. doi: 10.1681/ASN.2008070709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankland S.J. Podocytes in culture: past, present, and future. Kidney Int. 2007;72(1):26–36. doi: 10.1038/sj.ki.5002291. [DOI] [PubMed] [Google Scholar]
- Song B. The directed differentiation of human iPS cells into kidney podocytes. PLoS One. 2012;7(9):e46453. doi: 10.1371/journal.pone.0046453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumi T. Defining early lineage specification of human embryonic stem cells by the orchestrated balance of canonical Wnt/beta-catenin, Activin/Nodal and BMP signaling. Development. 2008;135(17):2969–2979. doi: 10.1242/dev.021121. [DOI] [PubMed] [Google Scholar]
- Taguchi A. Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell. 2014;14(1):53–67. doi: 10.1016/j.stem.2013.11.010. [DOI] [PubMed] [Google Scholar]
- Takasato M. Directing human embryonic stem cell differentiation towards a renal lineage generates a self-organizing kidney. Nat. Cell Biol. 2014;16(1):118–126. doi: 10.1038/ncb2894. [DOI] [PubMed] [Google Scholar]
- Torres M. Pax-2 controls multiple steps of urogenital development. Development. 1995;121(12):4057–4065. doi: 10.1242/dev.121.12.4057. [DOI] [PubMed] [Google Scholar]
- Tsang T.E. Lim1 activity is required for intermediate mesoderm differentiation in the mouse embryo. Dev. Biol. 2000;223(1):77–90. doi: 10.1006/dbio.2000.9733. [DOI] [PubMed] [Google Scholar]
- Wingert R.A. The cdx genes and retinoic acid control the positioning and segmentation of the zebrafish pronephros. PLoS Genet. 2007;3(10):1922–1938. doi: 10.1371/journal.pgen.0030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woll P.S. Wnt signaling promotes hematoendothelial cell development from human embryonic stem cells. Blood. 2008;111(1):122–131. doi: 10.1182/blood-2007-04-084186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y. Directed differentiation of human pluripotent cells to ureteric bud kidney progenitor-like cells. Nat. Cell Biol. 2013;15(12):1507–1515. doi: 10.1038/ncb2872. [DOI] [PubMed] [Google Scholar]
- Xinaris C. Functional human podocytes generated in organoids from amniotic fluid stem cells. J. Am. Soc. Nephrol. 2015 doi: 10.1681/ASN.2015030316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L. Induced pluripotent stem cells and their potential for basic and clinical sciences. Curr. Cardiol. Rev. 2013;9(1):63–72. doi: 10.2174/157340313805076278. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures and Tables.