Abstract
Background
The Instrumented Stand and Walk (ISAW) test, which includes 30 seconds of stance, step initiation, gait, and turning, results in many objective balance and gait metrics from body-worn inertial sensors. However, it is not clear which metrics provide independent information about mobility.
Objective
It was hypothesized that balance and gait represent several independent domains of mobility and that not all domains would be abnormal in individuals with Parkinson disease (PD) or would change with levodopa therapy.
Design
This was a cross-sectional study.
Methods
A factor analysis approach was used to identify independent measures of mobility extracted from the ISAW in 100 participants with PD and 21 control participants. First, a covariance analysis showed that postural sway measures were independent of gait measures. Then, the factor analysis revealed 6 independent factors (mobility domains: sway area, sway frequency, arm swing asymmetry, trunk motion during gait, gait speed, and cadence) that accounted for 87% of the variance of performance across participants.
Results
Sway area, gait speed, and trunk motion differed between the PD group in the off-levodopa state and the control group, but sway frequency (but not sway area) differed between the PD group in the off-levodopa state and the control group. Four of the 6 factors changed significantly with levodopa (off to on): sway area, sway frequency, trunk motion during gait, and cadence. When participants were on levodopa, the sway area increased compared with off levodopa, becoming more abnormal, whereas the other 3 significant metrics moved toward, but did not reach, the healthy control values.
Limitations
Exploratory factor analysis was limited to the PD population.
Conclusions
The different sensitivity various balance and gait domains to PD and to levodopa also support neural control of at least 6 independent mobility domains, each of which warrants clinical assessment for impairments in mobility.
Functional mobility requires the ability to: (1) maintain stable equilibrium during stance, (2) make appropriate anticipatory postural adjustments (APAs) prior to step initiation, (3) generate speed and temporal coordination of gait, (4) control trunk and arm displacements while walking, and (5) produce stable turns in walking direction.1 We developed the Instrumented Stand and Walk Test (ISAW) as a quick, clinical protocol that could reveal impairments in each of these aspects of functional mobility using measurements derived from inertial movement monitors attached to participants' ankles, wrists, sternum, and lumbar vertebral area.2 The ISAW requires the person to stand still for 30 seconds, initiate gait, walk 7 m, turn 180 degrees, and walk back to the starting location.3 This new, wearable technology streams synchronized body motion data to a laptop that automatically provides a myriad of balance and gait metrics during protocols such as the ISAW, but it is not clear which measures provide independent, versus redundant, information about mobility impairments.
Lord and colleagues4–6 recently used factor analysis of spatiotemporal inertial measures during walking to reveal 5 relatively independent domains of gait that accounted for 84.6% of variance of performance in both healthy elderly participants and participants with Parkinson disease (PD) in the on-levodopa state: pace, rhythm, variability, asymmetry, and postural control. They found that both groups showed the same gait domains and that gait speed, but not gait timing, was affected by PD.4 However, their analysis was limited to gait spatiotemporal measures of footfalls during straight walking on a GAITRite mat (CIR Systems Inc). We took a similar factor analysis approach to determine functional mobility domains in the ISAW, but body-worn sensors allowed us to add measures of postural sway, step initiation, turning, and trunk and arm motion to examine a broader range of mobility-related measures.
If kinematic measures of mobility can be grouped into several, independently controlled domains of mobility, we expect that neurological disease and interventions would have selective impact across these domains. Alternatively, if the underlying neural circuits for balance and gait represent one neural control system, pathologies such as PD and treatments such as dopamine replacement therapy would be expected to affect all of the underlying domains of balance and gait similarly. Based on our own and other laboratory studies, we predicted that PD would impair the speed of gait, as well as trunk and arm movement and turning, but not its timing. We also predicted, based on our laboratory studies, that levodopa would improve gait and APAs but impair postural sway during stance.7
The purposes of this study were: (1) to determine the independent domains of balance and gait and (2) to determine which domains are important for PD and levodopa. We hypothesized that the different domains of balance and gait represent separate, independent neural control systems that would respond differently to PD and levodopa.
Method
Participants
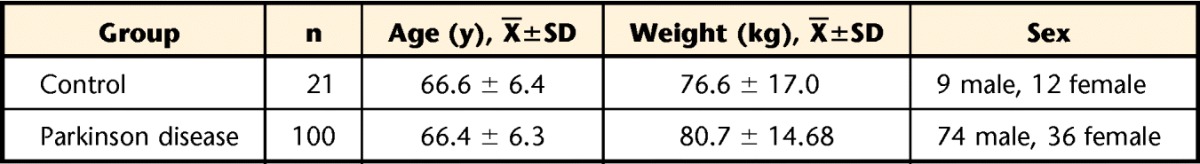
One hundred patients with idiopathic PD and 21 healthy controls participated in this study. Table 1 shows the age, weight, and sex of participants in each group. Healthy controls were either spouses of the patients or recruited from the community.
Table 1.
Characteristics of the Control and Parkinson Disease Groups
Participants with idiopathic PD were diagnosed by movement disorder clinicians, were taking levodopa medication, and were in the moderate stage of the disease (Tab. 2), with a mean duration of disease of 8.8 years (SD=5.8, range=2–29). The mean daily levodopa equivalent dose was 1,480 mg/d (SD=4,069, range=75–37,560). Many participants (54%) also took dopamine agonists, 17% took amantadine, 14% took monoamine oxidase B (MAO-B) inhibitors, and 6% took anticholinergics. Participants with PD were tested in the morning in their off-levodopa state (PD-OFF group, in the morning after withholding their antiparkinsonian medications for 12 hours, and in their on-levodopa state (PD-ON group), and 1 hour after taking their antiparkinsonian medications. To ensure quick change to the on state, participants with PD were given a standard levodopa challenge dose (approximately 1.25-fold of their regular levodopa dose).8 Control participants completed the same mobility tests but without taking levodopa. Participants with PD were recruited through the movement disorders clinics, referral from movement disorders neurologists, and recruitment from our database of control participant volunteers.
Table 2.
Severity of Parkinson Disease in the On- and Off-Levodopa Statesa

UPDRS=Unified Parkinson's Disease Rating Scale, PIGD=Postural Instability and Gait Disability subscale of the UPDRS.
As a measure of disease severity, the Unified Parkinson's Disease Rating Scale (UPDRS Part III), with its Postural Instability and Gait Disability subscale (PIGD) (posture, gait, arising from chair, and pull test items), was administered before the start of the on and off sessions. Participants were excluded if they had any neurological disorders other than idiopathic PD, orthopedic disorders, or other impairments that interfered with their gait or if they required assistive devices to stand or walk. The PD group's mean Activities-specific Balance Confidence (ABC) Scale score was 80.4 (range=42.5–100) compared with the control groups' mean score of 95.9 (range=70.3–100).9 The PD groups' mean 39-item Parkinson's Disease Questionnaire (PDQ-39) self-assessed quality of life mobility score was 20.1 (SD=16.1, range=0–62.50).10 Out of 100 participants, 17 reported 1 fall in the previous 6 months, and 19 reported 2 or more falls in the previous 6 months. All participants provided informed consent.
Measurement Protocol
Each session of tests consisted of 3 repetitions of the same mobility protocol. Each trial started with the participants standing with their arms at sides, looking straight ahead with eyes fixed on an object. The examiner used a foot template to keep a standardized initial stance position of 10 cm between the heels, with a 30-degree external ankle rotation.11 Participants stood quietly in this position for 30 seconds. After the 30-second period, the examiner gave the verbal cue “Start walking.” Participants initiated gait with their most affected leg (as determined from the UPDRS motor subscale) and walked at their comfortable pace for 7 meters. The end of 7 m was clearly marked on the ground using 3-cm-wide red tape. Participants were instructed to cross the tape, turn 180 degrees, and back to the starting location.
Participants wore 6 inertial sensors on their wrists, ankles, lumbar segment, and sternum. Each inertial sensor included a 3-dimensional accelerometer and a 3-dimensional gyroscope. The sensitivity of the inertial sensors differed based on the position of the sensor on the body: the gyroscopes on the limbs had a range of ±1,200°/s, and the accelerometers had a range of ±5 g. In contrast, the gyroscopes on the sternum had a range of ±150°/s, and the accelerometers had a range of ±1.7 g. The inertial sensors used were the MTX (Xsens North America Inc, Culver City, California) for the first 40 participants and the Opal (APDM Inc, Portland, Oregon) for the remaining participants. The MTX and Opal sensors have similar characteristics, and the interchangeability of systems was confirmed though concurrent evaluation of metrics (data unpublished). Specifically, the intraclass correlation coefficient (ICC) for absolute agreement in metrics ranged from .59 to .97, with no significant difference in Xsens-Xsens versus Opal-Xsens variability.
Signal Analysis
We used automatic processing algorithms published previously to analyze each section of the ISAW-recorded signals: gait and 180-degree turning,12 postural sway during 30 seconds of standing,13 and APA during gait initiation.14 We wrote a wrapper program to segment the data and pass the signals to each of the 3 analysis algorithms in MATLAB (The Mathworks Inc, Natick, Massachusetts). These algorithms were fully automatic (ie, the data could be segmented and analyzed without operator intervention). The 3 analysis algorithms computed more than 90 metrics related to mobility derived from data generated by the ISAW.
For further analysis, we focused only on the measures that showed high test-retest reliability across the 3 trials. We used the ICC with a cutoff of ρ=0.75 as our criterion. The ISAW metrics had ICCs in the range of .43 to .95. The number of metrics with ICCs higher than ρ=0.75 was 30 (12 metrics related to body sway, 2 measures related to APA, 14 measures related to gait, and 2 measures related to turning). To compute ICCs, we used a formulation based on linear mixed modeling,15 which is comparable to analysis of variance–based ICC (1-1). The ρ=0.75 cutoff is consistent with existing guidelines.16
Data Analysis
Descriptive statistics and univariate hypothesis tests were first used to characterize the data. Exploratory factor analysis (EFA) was used to examine the underlying structure of the 30 most reliable measures of mobility computed by the ISAW processing algorithms. For EFA, the data from the PD group in the off state was used, as it represented the practical, unmedicated, baseline level of motor abilities of the participants with parkinsonism and allowed us to determine how levodopa changed these factors. To estimate the number of factors to use in EFA, we used the nFactors package in R.17 Using this package, we used several alternative methods for selecting the number of factors to ensure correct analysis. Namely, we used the well-known Kaiser's criterion18 and reached the same estimate for the number of factors using Horn's parallel analysis19 and Cattell's optimal coordinates.20 Because we did not hypothesize that the underlying factors are necessarily orthogonal, the oblimin rotation was used for EFA.
We then determined how levodopa and PD affected the factors. Once the factors and their loadings (ie, the regression coefficients that mapped the measures to the factors) were estimated in the PD-OFF group, the same factors also were computed in the PD-ON group and in the control group. In other words, the same underlying regression equations that EFA used to compute the factors in the PD-OFF group were then applied to the PD-ON group and the control group so that we could compare the 3 groups in the same factor space. To compare the computed factor between the on and off states in the PD group, paired t tests were used. We also used t tests to compare PD and control groups.
Role of the Funding Source
This publication was made possible with support from a grant from the National Institute on Aging (AG006457); a Challenge Grant from the National Institute of Neurological Disorders and Stroke (RC1 NS068678) and from the Oregon Clinical and Translational Research Institute at Oregon Health & Science University; and grant number UL1 RR024140 from the National Center for Research Resources, a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research.
Results
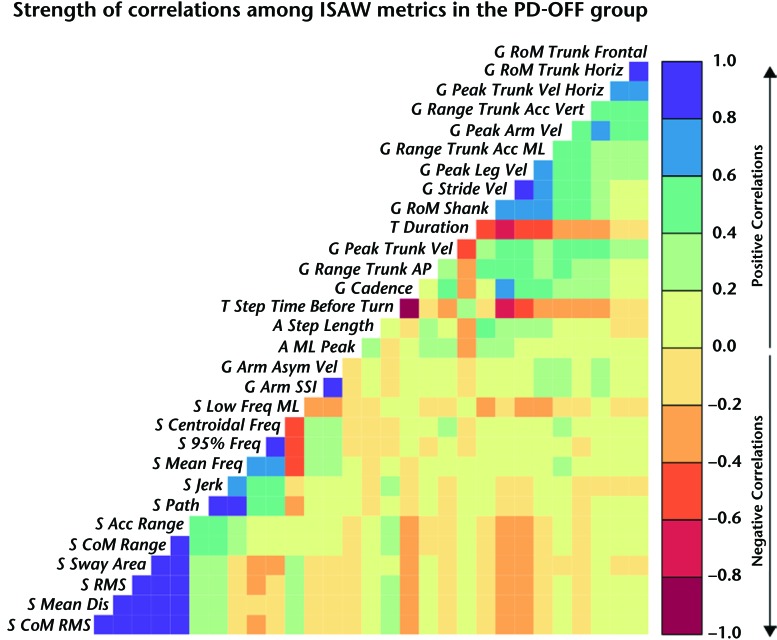
A cross-correlation matrix of all ISAW measures (using Pearson correlation) shows that gait measures were mostly correlated with other gait measures and turning but not with postural sway or APAs. Likewise, postural sway measures were mostly correlated with other postural sway measures but not with gait measures. Figure 1 illustrates the covariance matrix of the 30 ISAW measures in the PD-OFF group to determine how closely related each measure was to all of the other measures. A color code was used to illustrate the strength of correlation, where saturated blue (correlation close to 1.0) and saturated red (correlation close to −1.0) showed the 2 extremes and yellow showed the correlation close to 0. A summary of the metrics is provided in eTable 1 (for definitions, refer to Curtze et al7).
Figure 1.
Visualization of the covariance structure of the Instrumented Stand and Walk Test (ISAW) measures in participants with Parkinson disease in the off-levodopa state (PD-OFF group). Blue indicates positive, and red indicates negative correlation. Higher color saturation indicates higher correlation. Gait metrics start with G_, turning metrics start with T_, anticipatory postural adjustments metrics start with A_, and postural sway metrics start with S_. RoM=range of motion, Horiz=horizontal, Acc=acceleration, Vert=vertical, Vel=velocity, ML=mediolateral, AP=anteroposterior, Asym=asymmetrical, SSI=Symbolic Symmetry Index, Freq=frequency, CoM=center of mass, RMS=root mean square, Dis=distance.
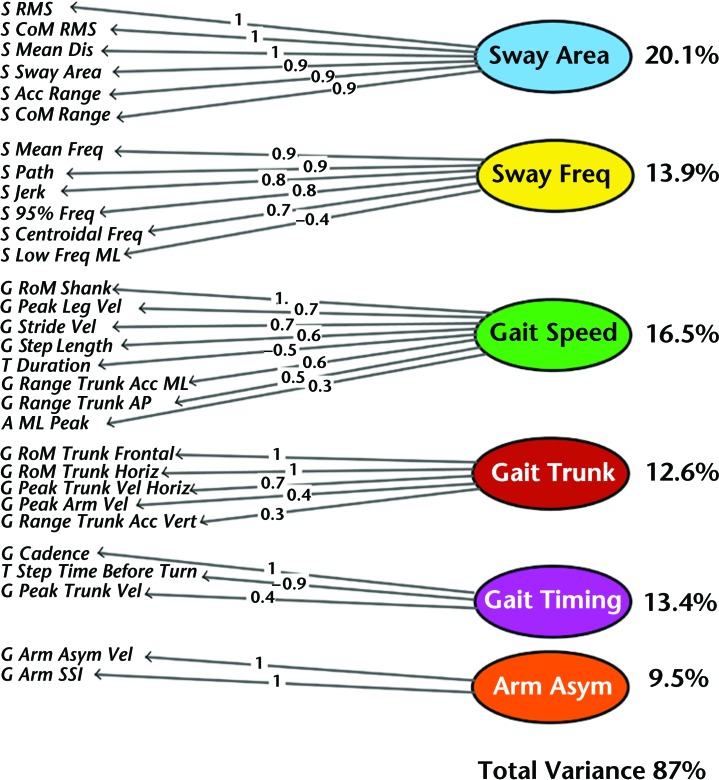
To avoid subjectivity regarding the selection of number of independent factors for factor analysis, we used Kaiser's criterion (ie, number of eigenvalues higher than 1.0). This method suggested that 6 factors should be included. Then, we used “parallel analysis” and the “optimal coordinate,” and both of these methods reported 6 factors as the optimal number of factors to explain the distribution of data. The scree plot in the eFigure shows the magnitude of the eigenvalues of the covariance matrix of the ISAW measures. In this plot, a small bend observable right before the curve starts to flatten points to 6 factors.
Together, the 6 factors explained 87% of the variance of the data set. Figure 2 shows the result of the exploratory factor analysis. Based on the measures that grouped within each factor, we named the factors: postural sway area, postural sway frequency, gait speed, gait trunk, gait timing, and arm asymmetry. The variance explained by each factor is shown on the right side of Figure 2, and loadings of each measure on their respective factors are shown on the lines.
Figure 2.
Exploratory factor analysis of 30 measures performed during the Instrumented Stand and Walk Test (ISAW) test in 100 people with Parkinson disease in the off-levodopa state. Six independent domains of gait and balance were identified: sway area, sway frequency (freq), gait speed, gait trunk, gait timing, and arm asymmetry (asym). The percentage of variance explained by each factor and the total variance is given at right, as well as the loading of each measure. RMS=root mean square, CoM=center of mass, Dis=distance, RoM=range of motion, Horiz=horizontal, Acc=acceleration, Vert=vertical, Vel=velocity, ML=mediolateral, AP=anteroposterior, Asym=asymmetrical, SSI=Symbolic Symmetry Index.
The differences in the 6 mobility factors between the PD-OFF group and the PD-ON and control groups are shown in eTable 2. Notice that, by definition, the factors computed are normalized (ie, they have a mean of 0 and a standard deviation of 1 in the PD group).
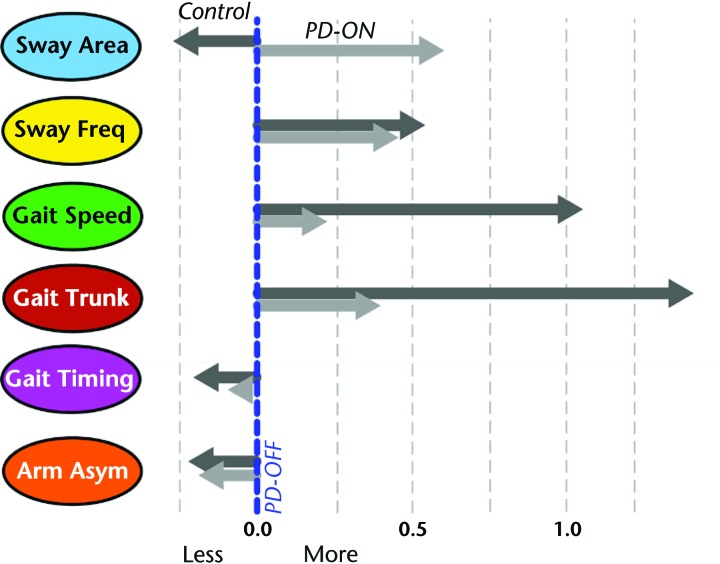
The direction (larger or smaller) and size of factor differences between the PD-OFF group and the PD-ON and control groups are illustrated in Figure 3. Interestingly, the sway area factor changed in the opposite direction from that of the control group in the PD-ON group compared with the PD-OFF group (ie, worse on levodopa). In contrast, the sway frequency factor changed toward the direction of the control group's values. Also, levodopa treatment did not change the gait cadence factor or the arm asymmetry factor. The largest differences between the PD-ON and control groups were in gait speed and gait trunk factors; that is, levodopa did not normalized these factors.
Figure 3.
Effect of Parkinson disease (PD) and levodopa replacement on each domain. Participants with PD in the off-levodopa state (PD-OFF group) are represented by the blue dotted line. Participants with PD in the on-levodopa state (PD-ON group) are represented by the light gray arrows, and the control group is represented by the dark gray arrows. Freq=frequency, Asym=asymmetry.
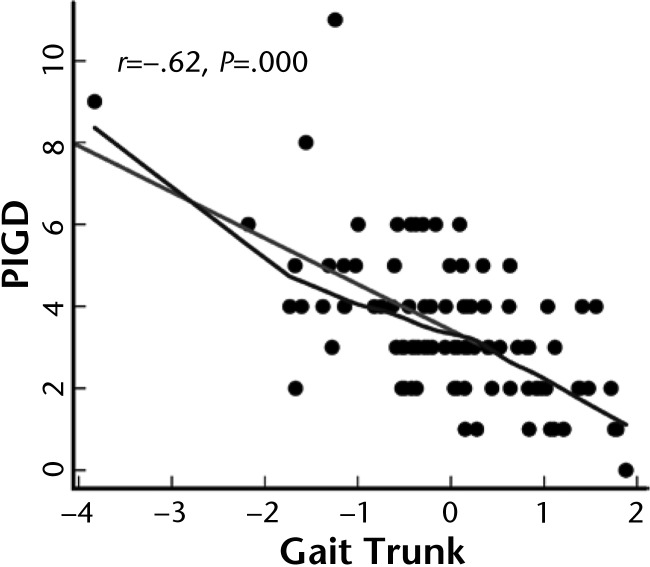
To determine whether the mobility factors were related to clinical severity of balance and gait impairments, each factor was compared with the PIGD subscale of the UPDRS. The PIGD is the only subscale of the UPDRS that directly assesses mobility in people with PD. Among the 6 factors, only gait trunk was significantly related to the PIGD subscale in participants with PD in the off state, as shown in Figure 4. No factors were significantly correlated with the total UPDRS motor subscale score.
Figure 4.
Correlation between the gait trunk domain and the Postural Instability and Gait Disability (PIGD) subscale (from the Unified Parkinson's Disease Rating Scale [UPDRS] motor subscale) in participants with Parkinson disease.
Discussion
The results of this study demonstrate that measures of postural sway during standing are not reflective of measures of gait in a large cohort of patients with PD and similar-aged controls. The 30 most reliable metrics of the ISAW could be grouped into 6 different domains of mobility: sway area, sway frequency, gait speed, trunk motion during gait, timing aspects of gait, and arm asymmetry during gait. Some, but not all, of these domains of mobility were affected by PD or levodopa therapy, confirming their relative independence. Thus, several different mobility domains should be assessed because any one measure, such as gait speed or sway area, is inadequate to characterize mobility impairments in people with PD.
Factor analysis accounted for 87% of the variance of performance for our 100 individuals with PD in the off state. This percentage compares favorably with the 85% explanation of the variance for the factor analysis on stepping parameters alone in individuals with PD in the on state in the study by Lord et al.5 Similar to Lord and colleagues, we found gait speed and gait timing to be independent factors, but they did not measure postural sway or upper body measures, so their other factors included gait variability, gait asymmetry, and postural control (similar to our gait trunk factor) during gait.
This study supports the notion that the neural control of balance and gait is relatively independent. That is, measures of postural sway while standing on a firm surface with eyes open could not predict spatial or temporal measures of gait, including trunk stability during gait, in people with PD. Although many measures of postural sway and gait were abnormal in our participants with PD, balance control in standing was not related to gait control, suggesting that postural sway in these static conditions could not predict dynamic postural instability (such as trunk displacements) while walking.
Postural sway measures were grouped into 2 independent domains: area and frequency. That is, to evaluate sway area, one can choose among the redundant measures within this factor (eg, root mean square, ellipse, distance, or range). Sway frequency, however, was independent from sway area, which has been shown previously for PD cohorts.21,22 Most postural sway occurs at very low frequencies (below 1 Hz23,24), and our results are consistent with the literature in finding higher sway frequency in patients with PD.23,25,26 Increases in postural sway in individuals with PD in the off state could reflect their resting tremor, and increases in the on state could reflect their dyskinesia.7 However, models of postural control have demonstrated that instability of the postural control loop also is reflected by increasing postural sway frequency as the nervous system increases stiffness and frequency of postural corrections.23,24,27
Gait measures grouped into 4 independent domains: speed, trunk displacement, timing, and arm swing asymmetry. Thus, gait speed alone, as commonly used, does not necessarily predict other impairments in gait (eg, temporal control, trunk stability, arm swing) in patients with PD. However, gait speed measures were partially redundant with turning duration and APA magnitude prior to step initiation as they mapped onto the same factor. The close relationship among these measures suggests that bradykinesia resulting from reduced corticospinal drive in PD may contribute similarly to how slowly patients with PD walk, turn, and initiate gait. The fact that levodopa replacement therapy improves all 3 measures (speed, turning, and APAs) is consistent with a common mechanism of control.28 However, we have shown previously that APAs are impaired by deep brain stimulation in the subthalamic nucleus, whereas other authors29 have shown improvements in gait speed, so some independent influences on these 2 measures persist.
The range of trunk motion in all 3 planes grouped into one factor, despite previous findings that lateral trunk motion during gait is particularly affected by PD.30 The factor that we call “gait timing” includes cadence, step time prior to a turn, and peak trunk velocity during gait. This grouping suggests that people who have slow cadence likely also show shorter step times prior to a turn and slower trunk velocity during gait.
Our 2 measures of arm swing asymmetry grouped separately from other domains. However, we do not know whether arm swing range of motion or speed would have grouped with arm swing asymmetry or with gait speed because arm swing range and speed were not in the 30 metrics included for factor analysis due to poor reliability in our sample. It is likely that arm swing range and velocity would group with the gait speed factor because the smaller the arm swing, the slower people walk.30,31
The impact of levodopa on the 6 domains of mobility in our participants with PD (eTab. 2, Fig. 3) supports our recent study of the relative effects of levodopa on each measure in this same cohort of people with PD.7 Levodopa improved sway frequency, gait speed, and trunk stability during gait; worsened sway area; and had no significant effect on gait timing or arm asymmetry (eTab. 2). The largest effect of levodopa was on sway area, but levodopa increased postural sway away from healthy control values. Our previous study suggests that this increase of sway with levodopa replacement therapy may be due to dyskinesia.7 However, levodopa improved the sway frequency domain, perhaps due to a reduction of tremor in the on state or a decrease in rigidity in the on state, when sway frequency no longer differed from that of the control group (eTab. 2). Although levodopa improved gait speed, trunk stability, and arm asymmetry, only arm asymmetry returned to normal levels.
In this study, we verified that impairments of balance and gait in PD are extensive and complex and that levodopa replacement therapy does not normalize all of these impairments. The largest difference between our participants with PD in the on state and our control group was in the trunk stability domain of gait, and the next largest difference was gait speed, followed by sway frequency. Thus, both balance and gait rehabilitation are needed, even in patients with PD for whom medication has been optimized.
This study suggests that a comprehensive assessment and treatment of balance and gait impairments in patients with PD should include standing balance, gait speed, gait timing, and upper body control during gait. Assessment of these relatively independent aspects of mobility should then be used to target physical therapy intervention for the specific impairments in each patient. We demonstrated the potential value of new, wearable technology to quickly quantify many, nonredundant, objective measures of balance and gait to characterize and track changes in mobility impairments with therapy.
The most common clinical evaluation of severity of PD is the UPDRS, particularly the motor subscale (Part III). Four items of the UPDRS motor subscale (posture, gait, arising from chair, and pull test), called the PIGD, clinically characterize mobility impairments. We were surprised to find that the only ISAW domain that significantly related to the PIGD was the trunk stability domain, which reflects dynamic balance control (Fig. 4). The reason for this relationship is unclear, but perhaps standing posture, gait quality, ability to rise from a chair, and postural stepping responses to a backward pull are all dependent on neural control of dynamic balance control of the trunk (ie, body center of mass). If so, the clinical implication is that therapists should focus on improving dynamic control of the trunk in patients with PD in order to improve balance, gait, and activities of daily living.
This study had several limitations. The factor analysis in this study was limited to people with PD. A study of independent objective mobility measures for elderly individuals and other people with high fall risk will require large cohorts with different types of diseases and may result in a different set of independent factors. However, PD is a good model for this approach because it impairs many domains of mobility. The factor analysis approach has its own limitations. It is valuable in explaining all of the data with fewer variables, but the results depend on the number of, and which, variables are provided for each task. For example, if more gait measures than gait initiation measures are provided, it may not identify multiple aspects of gait initiation. Future studies will use a similar approach to determine which balance and gait factors are related to prospective falls in elderly people.
In summary, no single measure of balance or gait can fully characterize mobility impairments in people with PD, but a small set of relatively independent measures is useful for strategic assessment and targeted rehabilitation. The ISAW test provides clinicians with a quick assessment of a broad range of objective balance and gait measures that can be the focus of balance and gait rehabilitation.
Footnotes
All authors provided concept/idea/research design. Dr Horak, Dr Mancini, Dr Carlson-Kuhta, and Dr Salarian provided writing. Dr Horak, Dr Carlson-Kuhta, and Dr Salarian provided data collection. Dr Mancini and Dr Salarian provided data analysis. Dr Horak and Dr Carlson-Kuhta provided project management. Dr Horak provided fund procurement, facilities/equipment, and institutional liaisons. Dr Nutt provided participants. Dr Horak, Dr Nutt, and Dr Salarian provided consultation (including review of manuscript before submission).
This study was approved by the Institutional Review Board of Oregon Health & Science University.
This publication was made possible with support from a grant from the National Institute on Aging (AG006457); a Challenge Grant from the National Institute of Neurological Disorders and Stroke (RC1 NS068678) and from the Oregon Clinical and Translational Research Institute at Oregon Health & Science University; and grant number UL1 RR024140 from the National Center for Research Resources, a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research.
Oregon Health & Science University and Dr Horak have a significant financial interest in APDM, a company that may have a commercial interest in the results of this research and technology. This potential institutional and individual conflict has been reviewed and managed by OHSU.
References
- 1. Patla AE, Shumway-Cook A. Dimensions of mobility: defining the complexity and difficulty associated with community mobility. J Aging Phys Act. 1999;7:7–19. [Google Scholar]
- 2. Horak FB, King L, Mancini M. Role of body-worn movement monitor technology for balance and gait rehabilitation. Phys Ther. 2015;95:461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mancini M, King L, Salarian A, et al. Mobility lab to assess balance and gait with synchronized body-worn sensors. J Bioeng Biomed Sci. 2011;suppl 1:007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lord S, Galna B, Coleman S, et al. Mild depressive symptoms are associated with gait impairment in early Parkinson's disease. Mov Disord. 2013;28:634–639. [DOI] [PubMed] [Google Scholar]
- 5. Lord S, Galna B, Rochester L. Moving forward on gait measurement: toward a more refined approach. Mov Disord. 2013;28:1534–1543. [DOI] [PubMed] [Google Scholar]
- 6. Lord S, Galna B, Verghese J, et al. Independent domains of gait in older adults and associated motor and nonmotor attributes: validation of a factor analysis approach. J Gerontol A Biol Sci Med Sci. 2013;68:820–827. [DOI] [PubMed] [Google Scholar]
- 7. Curtze C, Nutt JG, Carlson-Kuhta P, et al. Levodopa is a double-edged sword for balance and gait in people with Parkinson's disease. Mov Disord. 2015;30:1361–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moreau C, Delval A, Defebvre L, et al. Methylphenidate for gait hypokinesia and freezing in patients with Parkinson's disease undergoing subthalamic stimulation: a multicentre, parallel, randomised, placebo-controlled trial. Lancet Neurol. 2012;11:589–596. [DOI] [PubMed] [Google Scholar]
- 9. Powell LE, Myers AM. The Activities-specific Balance Confidence (ABC) Scale. Gerontol A Biol Sci Med Sci. 1995;50:M28–M34. [DOI] [PubMed] [Google Scholar]
- 10. Jenkinson C, Fitzpatrick R, Peto V, et al. The Parkinson's Disease Questionnaire (PDQ-39): development and validation of a Parkinson's disease summary index score. Age Ageing. 1997;26:353–357. [DOI] [PubMed] [Google Scholar]
- 11. McIlroy WE, Maki BE. Preferred placement of the feet during quiet stance: development of a standardized foot placement for balance testing. Clin Biomech. 1997;12:66–70. [DOI] [PubMed] [Google Scholar]
- 12. Salarian A, Horak FB, Zampieri C, et al. iTUG: a sensitive and reliable measure of mobility. IEEE Trans Neural Syst Rehabil Eng. 2010;18:303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mancini M, Salarian A, Carlson-Kuhta P, et al. ISway: a sensitive, valid and reliable measure of postural control. J Neuroeng Rehabil. 2012;9:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mancini M, Zampieri C, Carlson-Kuhta P, et al. Anticipatory postural adjustments prior to step initiation are hypometric in untreated Parkinson's disease: an accelerometer-based approach. Eur J Neurol. 2009;16:1028–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stanish WM, Taylor N. Estimation of the intraclass correlation coefficient for the analysis of covariance model. Am Stat. 1983;37:221–224. [Google Scholar]
- 16. Cicchetti D. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol Assess. 1994;6:284–290. [Google Scholar]
- 17. Raiche G. Package “nFactors”: Parallel Analysis and Non Graphical Solutions to the Cattell Scree Test. Version 2.3.3. 2010. Available at: https://cran.r-project.org/web/packages/nFactors/nFactors.pdf Accessed April 2016.
- 18. Kaiser HF. The application of electronic computers to factor analysis. Educ Physiol Meas. 1960;20:141–151. [Google Scholar]
- 19. Horn JL. A rationale and test for the number of factors in factor analysis. Psychometrika. 1965;30:179–185. [DOI] [PubMed] [Google Scholar]
- 20. Cattell RB. The scree test for the number of factors. Multivariate Behav Res. 1966;1:245–276. [DOI] [PubMed] [Google Scholar]
- 21. Maurer C, Peterka RJ. A new interpretation of spontaneous sway measures based on a simple model of human postural control. J Neurophysiol. 2005;93:189–200. [DOI] [PubMed] [Google Scholar]
- 22. Rocchi L, Chiari L, Cappello A, Horak FB. Identification of distinct characteristics of postural sway in Parkinson's disease: a feature selection procedure based on principal component analysis. Neurosci Lett. 2006;394:140–145. [DOI] [PubMed] [Google Scholar]
- 23. Maurer C, Mergner T, Xie J, et al. Effect of chronic bilateral subthalamic nucleus (STN) stimulation on postural control in Parkinson's disease. Brain. 2003;126(pt 5):1146–1163. [DOI] [PubMed] [Google Scholar]
- 24. Peterka RJ. Postural control model interpretation of stabilogram diffusion analysis. Biol Cybern. 2000;82:335–343. [DOI] [PubMed] [Google Scholar]
- 25. Maurer C, Mergner T, Peterka RJ. Abnormal resonance behavior of the postural control loop in Parkinson's disease. Exp Brain Res. 2004;157:369–376. [DOI] [PubMed] [Google Scholar]
- 26. Rocchi L, Chiari L, Horak FB. Effects of deep brain stimulation and levodopa on postural sway in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2002;73:267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maurer C, Mergner T, Peterka RJ. Multisensory control of human upright stance. Exp Brain Res. 2006;171:231–250. [DOI] [PubMed] [Google Scholar]
- 28. Burleigh-Jacobs A, Horak FB, Nutt JG, Obeso JA. Step initiation in Parkinson's disease: influence of levodopa and external sensory triggers. Mov Disord. 1997;12:206–215. [DOI] [PubMed] [Google Scholar]
- 29. Collomb-Clerc A, Welter ML. Effects of deep brain stimulation on balance and gait in patients with Parkinson's disease: a systematic neurophysiological review. Neurophysiol Clin. 2015;45:371–388. [DOI] [PubMed] [Google Scholar]
- 30. Zampieri C, Salarian A, Carlson-Kuhta P, et al. The instrumented Timed Up and Go test: potential outcome measure for disease modifying therapies in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2010;81:171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bruijn SM, Meijer OG, van Dieen JH, et al. Coordination of leg swing, thorax rotations, and pelvis rotations during gait: the organisation of total body angular momentum. Gait Posture. 2008;27:455–462. [DOI] [PubMed] [Google Scholar]