Abstract
Objectives
In this study, we investigated whether a high-fat diet (HFD) affected the bone implant connection (BIC) in peri-implant bone.
Materials and Methods
Four male rabbits were used in this study. Dental implant surgery was introduced into each tibia, and four implants were integrated into each animal. In both the normal diet (ND) group (n=2) and HFD group (n=2), 8 implants were integrated, for a total of 16 integrated implants. The animals continued with their respective diets for 12 weeks post-surgery. Afterward, the rabbits were sacrificed, and the BIC was assessed histomorphometrically.
Results
Histologic and histomorphometric analyses demonstrated that BIC was not impaired in the HFD group compared to the ND group.
Conclusion
Within the limitations of this study, we found that HFD did not decrease the BIC in rabbit tibias.
Keywords: Bone implant contact, Osseointegration, Titanium implants, High-fat diet, Histomorphometric analysis
I. Introduction
Atherosclerosis-vascular calcification and osteoporosis are major health problems in the aging population. High-fat diet (HFD)-induced hyperlipidemia has adverse effects on the cardiovascular system, including vascular problems. In a hyperlipidemic condition, protein-bound lipid particles pass through the endothelial wall into the subendothelial field, where they are caught and oxidatively modified by reactive oxygen species produced by metabolically active neighboring smooth muscle cells and macrophages. A similar cycle takes place in human osteoporotic bone, with oxidized protein bound lipid particles collecting in the perivascular subendothelial fields. Osteoblast cells also have the ability to oxidatively modify protein-bound lipid particles. Oxidized lipid products are detected in the bone marrow of hyperlipidemic mice1,2,3.
According to the National Health and Nutrition Examination Survey, 63% of osteoporotic patients have hyperlipidemia1. Moreover, numerous reports have stated that obesity is a risk factor of osteoporosis in humans1,2,4,5,6. Epidemiological studies have reported statistically significant inverse relationships between serum cholesterol level and bone mineral parameters and density, independent of age and body mass index. HFD consumption is also associated with decrease in bone mineral content (BMC) and bone mineral density (BMD) in experimental animal studies1.
HFDs further increase the levels of protein-bound lipid paparticles and reactive oxygen species2,5,7,8. Additionally, whether due to a genetic or dietary abnormality, excessive lipid-derived reactive oxygen species reduce osteoblastic differentiation in vitro 1,9. In addition, oxidized lipids induce osteoclastogenesis and decrease the signaling of parathyroid hormone and bone morphogenic protein-2. Experimental studies have reported that hyperlipidemia induces bone loss in mice1. Therefore, we tested the effects of HFD on the bone implant connection (BIC) in rabbits in the present study.
II. Materials and Methods
All surgical and experimental procedures were conducted at Firat University Experimental Research Center. The approval for the study was obtained from the Firat University Animal Experimental Ethics Council, Elazig, Turkey (2015/55). The animals used for testing were also supplied by the same center. The recommendations of the Helsinki Declaration related to the protection of laboratory test animals were strictly followed.
In total, four male 0.5- to 1-year-old New Zealand rabbits were used. Their average body weight was 2,200 to 2,800 g on the first day of the experimental protocol. The animals were kept in temperature-controlled cages, exposed to a 12 hour/12 hour light/dark cycle, and had ad libitum free access to food and water during the experimental period.
The rabbits were divided randomly into two study groups as follows:
Normal diet (ND) group (n=2): Dental implants were surgically inserted into the rabbit tibias, and the rabbits were fed a ND during the experimental period of 12 weeks10.
HFD group (n=2): Dental implants were surgically inserted into the rabbit tibias, and the rabbits were fed a HFD during the experimental period of 12 weeks10.
General anesthesia was administered intramuscularly using 35 mg/kg ketamine hydrochloride (Ketalar; Eczacibasi, Istanbul, Turkey) and 5 mg/kg xylazine (Rompun; Bayer, Leverkusen, Germany). All the surgical procedures were performed under sterile conditions. After general anesthesia and prior to the surgical application, the tibial skin was washed with povidone iodine and shaved. A skin incision on the tibia was made over the tibial crest. A periosteal elevator was used to lift the flap and periosteum to access the tibial bone. The tibial skin was sutured with 4/0 polyglactin resorbable sutures. Penicillin antibiotic (50 mg/kg) and an analgesic (1 mg/kg diclofenac sodium) were injected intramuscularly in all animals for three days after the procedure.
In both groups, 16 sand-blasted large acid-etched surface implants (Gulmaksan, Izmir, Turkey) 6 mm in length and 3 mm in diameter were integrated into the metaphyseal part of the tibia. Two implants in each tibia and 4 implants for each animal were integrated. In each group, 8 total implants were integrated. All surgical procedures were performed atraumatically by the same researcher.
1. Animal feeding and histological processing
The control group was fed a standard chow diet, which contained 17.3% protein, 3.9% vegetable fat, 13.6% fiber, 8.7% ash, 48.9% nitrogen-free extract, and 7.6% moisture by weight. The HFD group was fed a chow diet supplemented with 10% coconut oil10.
The rabbits were killed 12 weeks after surgical implant placement. The rabbit tibias were dissected from the muscles and soft tissues and fixed in 10% formaldehyde solution. The specimens were embedded into 2-hydroxyetylmetacrylate resin to allow the cutting of undecalcified bone and titanium with the Exakt microtome (Exakt Apparatebau, Norderstedt, Germany)11. For the histologic and histomorphometric evaluation, each section was ground with the Exakt grinder (Exakt Apparatebau), and a 50-µm-thick section was obtained for light microscope (Olympus, Tokyo, Japan) analysis. For the histological analysis, toluidine blue stain was used12. After this procedure, histologic and histomorphometrical analyses were performed to quantify the bone tissue response in the peri-implant bone. These procedures were performed at the Department of Oral and Maxillofacial Surgery in the Faculty of Dentistry of the Erciyes University, Kayseri, Turkey, and at the Department of Medical Pathology in the Faculty of Medicine of the Firat University, Elazig, Turkey. The histopathologic and histomorphometrical analyses for the BIC were performed with an image analyzer (Olympus). The BIC was defined directly from the bone connection with the implant perimeter13. The BIC was measured as the bone implant contact in the cortical and trabecular bone layers following a reported method13.
2. Biochemical analysis
Blood samples were taken from rabbit hearts under deep anesthesia. The samples were centrifuged at 3,000g for 10 minutes, and the serum was collected. The serum samples were stored at –80℃ until they were analyzed. Serum glucose, triglyceride, and alanineaminotransferase (ALT) parameters were analyzed by routine biochemical methods obtained from the serum samples of rats at the Department of Biochemistry in the Faculty of Medicine of Firat University.
3. Statistical analysis
IBM SPSS Statistics software version 22 (IBM Co., Armonk, NY, USA) was used for the statistical analysis. Mean value±standard deviation of each group for all the analyzed data were calculated. In this study, all parameters when subjected to Kolmogorov-Smirnov and homogenity (Levene test) tests had P-values >0.05. Therefore, for the statistical analysis in our study, we used independent t-test (BIC, triglyceride, glucose, and ALTs) and paired (weight) tests, and P-values <0.05 were considered statistically significant.
III. Results
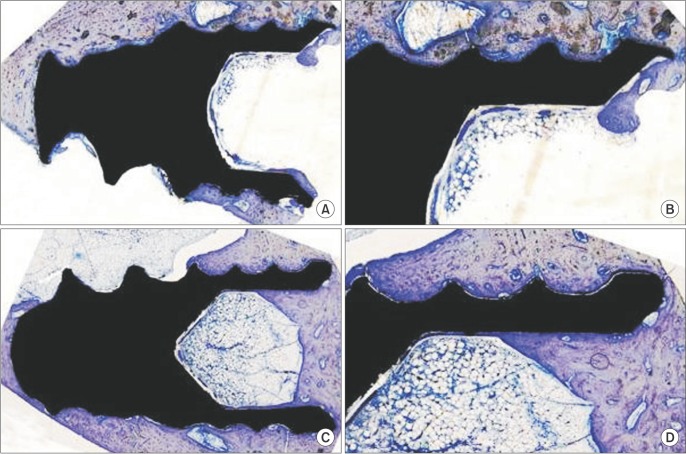
Body weight gain was observed in the HFD group compared to the ND group (P<0.05).(Table 1) Mortality, infection, or wound dehiscence was not recorded in this protocol. The results of the histomorphometric analysis of the BIC in the all groups are shown in Table 2. Statistically significant differences were not detected between the ND and HFD groups for BIC (P>0.05).(Fig. 1)
Table 1. Comparison of body weight between groups.
| Group | n | Mean±SD (g) | P-value1 | |
|---|---|---|---|---|
| HFD | First | 2 | 2.4±0.26 | 0.045* |
| 12 wk | 2 | 3.1±0.19 | 0.045* | |
| ND | First | 2 | 2.67±0.31 | 0.064 |
| 12 wk | 2 | 3.41±0.20 | 0.064 | |
(HFD: high-fat diet, ND: normal diet, SD: standard deviation)
1Paired test.
*Statistically significant difference, P<0.05.
Serkan Dündar et al: The effects of high-fat diet on implant osseointegration: an experimental study. J Korean Assoc Oral Maxillofac Surg 2016
Table 2. Comparison percentage of BIC.
| Measurement | Group | n | Mean±SD (%)1 | P-value2 |
|---|---|---|---|---|
| BIC | ND | 8 | 60.32±10.74 | >0.05 |
| HFD | 8 | 60.17±10.79 |
(BIC: bone implant connection, SD: standard deviation, ND: normal diet, HFD: high-fat diet)
1BIC area/total implant surface area×100.
2Independent t-test (P>0.05).
Serkan Dündar et al: The effects of high-fat diet on implant osseointegration: an experimental study. J Korean Assoc Oral Maxillofac Surg 2016
Fig. 1. A, B. Histologic view of the control group (A: ×2, B: ×4). C, D. Histologic view of the high-fat diet group (C: ×2, D: ×4).
Serkan Dündar et al: The effects of high-fat diet on implant osseointegration: an experimental study. J Korean Assoc Oral Maxillofac Surg 2016
The results of the biochemical analysis of ALT, triglyceride, and glucose levels in all groups are shown in Table 3. Statistically significant differences were not detected between the ND and the HFD groups for serum glucose, triglyceride, or ALT levels (P>0.05).
Table 3. Biochemical parameters.
| Group | Glucose (mg/dL) | Triglycerides (mg/dL) | ALT (U/L) | P-value1 | |
|---|---|---|---|---|---|
| ND | 12 wk | 115 | 72 | 76 | >0.05 |
| 12 wk | 106 | 49 | 69 | >0.05 | |
| HFD | 12 wk | 132 | 152 | 115 | >0.05 |
| 12 wk | 168 | 99 | 97 | >0.05 | |
(ALT: alanineaminotransferase, ND: normal diet, HFD: high-fat diet)
1Independent t-test (P>0.05).
Serkan Dündar et al: The effects of high-fat diet on implant osseointegration: an experimental study. J Korean Assoc Oral Maxillofac Surg 2016
IV. Discussion
The purpose of this study was to examine the effects of HFD on osseointegration in rabbit tibias. Our histologic and histomorphometric results did not confirm findings from previous studies about the inverse association between HFD and bone metabolism2,14.
We prepared the HFD protocol according to the studies of Ning et al.10 and Waqar et al.15. The HFD group was fed a chow diet supplemented with 10% coconut oil10,15. The HFD was rich in fat, but with relatively less protein and fiber. In this study, we did not utilize Watanabe heritable hyperlipidemic rabbits according to Ozaki and de Almeida16, who fed healthy male New Zealand rabbits a hypercholesterolemic diet.
The role of lipid and lipid-bound protein oxidation in the pathophysiology of osteoporosis has been reported by a variety of studies17,18,19. Consistent with a previous study, mice that were fed an atherogenic HFD not only became hyperlipidemic, but also exhibited significantly reduced mineral content and bone volume/trabecular volume in both the femoral and tibial bones. Moreover, Lac et al.20 demonstrated that, during the early growth period (i.e., 35 days), rats fed a HFD had lower bone BMC and BMD and exhibited a negative correlation between visceral fat and BMD when compared the normal diet fed controls. Lu et al.14 found similar results in young male mice in their experimental study. They reported that the BMC and the area of trabecular bone were significantly decreased in the HFD group compared to the controls. Moreover, Lu et al.14 reported that the number of colony-forming unit osteoblasts per bone was significantly decreased compared to the controls in a cell culture study on aspirated bone marrow cells derived from mice. Pirih et al.1 reported that oxidized lipids and/or hyperlipidemia adversely affected the mechanical strength of bone and impaired bone regeneration. Their micro-computed tomography (CT) and histologic results showed that bone regeneration was significantly impaired in HFD-fed males. In femoral bones, the cortical bone volume fraction (bone volume/tissue volume) was significantly decreased in the HFD group compared to the controls. Keuroghlian et al.21 reported that mice fed an HFD had significantly increased implant loss and decreased formation and strength of BIC in the femur. These results support the hypothesis that HFD can significantly compromise osseointegration and cause a poor outcome in dental implant therapy. Our histologic and histomorphometric results were not confirmed by these previous results. BIC osseointegration in the HFD group was not statistically significantly different compared to the controls. In this study, we used a histologic method to directly observe BIC. Although the histologic methods assured the assimilation of spatial datum in the tissue sections, micro-CTs can produce detailed three-dimensional (3D) structure scans without histologic tissue processing. Bone tissue cells cannot be identified in micro-CT sections, but discrete changes in bone architecture can be assessed 3D. The 3D micro-CT high-resolution views ensure the advantage of evaluating bone tissues without tissue-processing techniques that can result in the impairment of bone tissue architecture.
Parhami et al.22 found that oxidized lipids inhibited the differentiation of osteoblasts. Hirasawa et al.23 showed that HFD also increased osteoblast apoptosis. Increased osteoclastic resorption, as evidenced by the increased level of ctelopeptides in hyperlipidemic mice, could also contribute to bone loss. Sage et al.24 reported that lipid oxidation products compromized the inverse effects of HFD on bone tissue. In a previous study, oxidized lipids inhibited the differentiation of osteoblasts17. Moreover, epidemiological studies showed that osteoporosis could be linked to hyperlipidemia and atherosclerosis 4. This result is independent of age in some populations 25. In some studies, lipid-lowering treatments reduced osteoporosis. In an experimental animal study, low bone density was reported in atherosclerotic mice2,17. Our biochemical results did not confirm these results. The triglyceride, serum glucose, and ALT levels were higher in the HFD group than in the controls. Glucose and liver metabolism were not affected by HFD in the current study. The BIC in our study could not confirm the results found in previous studies about the association between bone metabolism and lipid metabolism.
The present results show that the BIC did not decrease in HFD-fed rabbits compared to ND-fed rabbits in the 3-month feeding period. Lipid oxidation products were previously shown to increase bone resorptive potential2,3,17,20. Cortical and trabecular bone are vascularized tissues and as a result, bone cells are exposed to lipoproteins from the vascular circulation. Our findings could not confirm that lipids in the perivascular space of the Haversian canals are associated with osteoporotic bone tissues2. In our study, serum triglyceride levels were not statistically significantly different between the two groups. We concluded that the 3-month HFD feeding period did not effect bone tissue metabolism in the experimental rabbit model.
V. Conclusion
Within the limitations of this study, we concluded that HFD did not decrease the BIC in the 3-month osseointegration period. However, further studies are needed to clarify the relationship between BIC and HFD.
Acknowledgements
The authors wish to extend their gratitude to Gulmaksan Izmir, Turkey, for providing the implants. The authors thank Dr. Cem Gurgan and Hasan Ekeer (Faculty of Dentistry, Erciyes University) for their helpful support on histomorphometric analysis and Dr. Selcuk Ilhan for his helpful support with statistical analysis (Firat University).
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Pirih F, Lu J, Ye F, Bezouglaia O, Atti E, Ascenzi MG, et al. Adverse effects of hyperlipidemia on bone regeneration and strength. J Bone Miner Res. 2012;27:309–318. doi: 10.1002/jbmr.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tintut Y, Morony S, Demer LL. Hyperlipidemia promotes osteoclastic potential of bone marrow cells ex vivo. Arterioscler Thromb Vasc Biol. 2004;24:e6–e10. doi: 10.1161/01.ATV.0000112023.62695.7f. [DOI] [PubMed] [Google Scholar]
- 3.Brodeur MR, Brissette L, Falstrault L, Ouellet P, Moreau R. Influence of oxidized low-density lipoproteins (LDL) on the viability of osteoblastic cells. Free Radic Biol Med. 2008;44:506–517. doi: 10.1016/j.freeradbiomed.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 4.Boukhris R, Becker KL. Calcification of the aorta and osteoporosis. A roentgenographic study. JAMA. 1972;219:1307–1311. [PubMed] [Google Scholar]
- 5.Halade GV, El Jamali A, Williams PJ, Fajardo RJ, Fernandes G. Obesity-mediated inflammatory microenvironment stimulates osteoclastogenesis and bone loss in mice. Exp Gerontol. 2011;46:43–52. doi: 10.1016/j.exger.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Núñez NP, Carpenter CL, Perkins SN, Berrigan D, Jaque SV, Ingles SA, et al. Extreme obesity reduces bone mineral density: complementary evidence from mice and women. Obesity (Silver Spring) 2007;15:1980–1987. doi: 10.1038/oby.2007.236. [DOI] [PubMed] [Google Scholar]
- 7.Laurila A, Cole SP, Merat S, Obonyo M, Palinski W, Fierer J, et al. High-fat, high-cholesterol diet increases the incidence of gastritis in LDL receptor-negative mice. Arterioscler Thromb Vasc Biol. 2001;21:991–996. doi: 10.1161/01.atv.21.6.991. [DOI] [PubMed] [Google Scholar]
- 8.Navab M, Hama SY, Anantharamaiah GM, Hassan K, Hough GP, Watson AD, et al. Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: steps 2 and 3. J Lipid Res. 2000;41:1495–1508. [PubMed] [Google Scholar]
- 9.Muluke M, Gold T, Kiefhaber K, Al-Sahli A, Celenti R, Jiang H, et al. Diet-induced obesity and its differential impact on periodontal bone loss. J Dent Res. 2016;95:223–229. doi: 10.1177/0022034515609882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ning B, Wang X, Yu Y, Waqar AB, Yu Q, Koike T, et al. High-fructose and high-fat diet-induced insulin resistance enhances atherosclerosis in Watanabe heritable hyperlipidemic rabbits. Nutr Metab (Lond) 2015;12:30. doi: 10.1186/s12986-015-0024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donath K, Breuner G. A method for the study of undecalcified bones and teeth with attached soft tissues. The Säge-Schliff (sawing and grinding) technique. J Oral Pathol. 1982;11:318–326. doi: 10.1111/j.1600-0714.1982.tb00172.x. [DOI] [PubMed] [Google Scholar]
- 12.Wang D, Künzel A, Golubovic V, Mihatovic I, John G, Chen Z, et al. Accuracy of peri-implant bone thickness and validity of assessing bone augmentation material using cone beam computed tomography. Clin Oral Investig. 2013;17:1601–1609. doi: 10.1007/s00784-012-0841-y. [DOI] [PubMed] [Google Scholar]
- 13.Tresguerres IF, Clemente C, Blanco L, Khraisat A, Tamimi F, Tresguerres JA. Effects of local melatonin application on implant osseointegration. Clin Implant Dent Relat Res. 2012;14:395–399. doi: 10.1111/j.1708-8208.2010.00271.x. [DOI] [PubMed] [Google Scholar]
- 14.Lu XM, Zhao H, Wang EH. A high-fat diet induces obesity and impairs bone acquisition in young male mice. Mol Med Rep. 2013;7:1203–1208. doi: 10.3892/mmr.2013.1297. [DOI] [PubMed] [Google Scholar]
- 15.Waqar AB, Koike T, Yu Y, Inoue T, Aoki T, Liu E, et al. High-fat diet without excess calories induces metabolic disorders and enhances atherosclerosis in rabbits. Atherosclerosis. 2010;213:148–155. doi: 10.1016/j.atherosclerosis.2010.07.051. [DOI] [PubMed] [Google Scholar]
- 16.Ozaki MR, de Almeida EA. Evolution and involution of atherosclerosis and its relationship with vascular reactivity in hypercholesterolemic rabbits. Exp Toxicol Pathol. 2013;65:297–304. doi: 10.1016/j.etp.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Graham LS, Tintut Y, Parhami F, Kitchen CM, Ivanov Y, Tetradis S, et al. Bone density and hyperlipidemia: the T-lymphocyte connection. J Bone Miner Res. 2010;25:2460–2469. doi: 10.1002/jbmr.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parhami F. Possible role of oxidized lipids in osteoporosis: could hyperlipidemia be a risk factor? Prostaglandins Leukot Essent Fatty Acids. 2003;68:373–378. doi: 10.1016/s0952-3278(03)00061-9. [DOI] [PubMed] [Google Scholar]
- 19.Rajamannan NM. Low-density lipoprotein and aortic stenosis. Heart. 2008;94:1111–1112. doi: 10.1136/hrt.2007.130971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lac G, Cavalie H, Ebal E, Michaux O. Effects of a high fat diet on bone of growing rats. Correlations between visceral fat, adiponectin and bone mass density. Lipids Health Dis. 2008;7:16. doi: 10.1186/1476-511X-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keuroghlian A, Barroso AD, Kirikian G, Bezouglaia O, Tintut Y, Tetradis S, et al. The effects of hyperlipidemia on implant osseointegration in the mouse femur. J Oral Implantol. 2015;41:e7–e11. doi: 10.1563/AAID-JOI-D-13-00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parhami F, Tintut Y, Beamer WG, Gharavi N, Goodman W, Demer LL. Atherogenic high-fat diet reduces bone mineralization in mice. J Bone Miner Res. 2001;16:182–188. doi: 10.1359/jbmr.2001.16.1.182. [DOI] [PubMed] [Google Scholar]
- 23.Hirasawa H, Tanaka S, Sakai A, Tsutsui M, Shimokawa H, Miyata H, et al. ApoE gene deficiency enhances the reduction of bone formation induced by a high-fat diet through the stimulation of p53-mediated apoptosis in osteoblastic cells. J Bone Miner Res. 2007;22:1020–1030. doi: 10.1359/jbmr.070330. [DOI] [PubMed] [Google Scholar]
- 24.Sage AP, Lu J, Atti E, Tetradis S, Ascenzi MG, Adams DJ, et al. Hyperlipidemia induces resistance to PTH bone anabolism in mice via oxidized lipids. J Bone Miner Res. 2011;26:1197–1206. doi: 10.1002/jbmr.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jie KG, Bots ML, Vermeer C, Witteman JC, Grobbee DE. Vitamin K status and bone mass in women with and without aortic atherosclerosis: a population-based study. Calcif Tissue Int. 1996;59:352–356. doi: 10.1007/s002239900139. [DOI] [PubMed] [Google Scholar]